Early life experience contributes to the developmental programming
of depressive-like behaviour, neuroin
fl
ammation and oxidative stress
Gislaine Z. R
eus
a,*, Gabrielly C. Fernandes
a, Airam B. de Moura
a, Ritele H. Silva
a,
Ana Caroline Darabas
a, Thays G. de Souza
a, Helena M. Abelaira
a, Celso Carneiro
b,
Diogo Wendhausen
b, Monique Michels
b, Bruna Pescador
b, Felipe Dal-Pizzol
b,c,
Danielle S. Mac
edo
^
d, Jo
ao Quevedo
~
a,e,f,g,haLaboratory of Neurosciences, Graduate Program in Health Sciences, Health Sciences Unit, University of Southern Santa Catarina, Criciúma, SC, Brazil bLaboratory of Experimental Pathophysiology, Graduate Program in Health Sciences, University of Southern Santa Catarina, Criciúma, SC, Brazil cCenter of Excellence in Applied Neurosciences of Santa Catarina (NENASC), Graduate Program in Medical Sciences, Federal University of Santa Catarina
(UFSC), Florianopolis, SC, Brazil
dNeuropharmacology Laboratory, Department of Physiology and Pharmacology, Faculty of Medicine Federal University of Ceara Fortaleza Brazil, Brazil eCenter of Excellence on Mood Disorders, Department of Psychiatry and Behavioral Sciences, McGovern Medical School, The University of Texas Health
Science Center at Houston (UTHealth), Houston, TX, USA
fTranslational Psychiatry Program, Department of Psychiatry and Behavioral Sciences, McGovern Medical School, The University of Texas Health Science
Center at Houston (UTHealth), Houston, TX, USA
gDepartment of Clinical Medicine and Translational Psychiatry Research Group, Faculty of Medicine, Federal University of Ceara, Fortaleza, CE, Brazil hNeuroscience Graduate Program, Graduate School of Biomedical Sciences, The University of Texas Health Science Center at Houston (UTHealth), Houston,
TX, USA
a r t i c l e
i n f o
Article history:
Received 16 May 2017 Received in revised form 4 August 2017
Accepted 24 August 2017
Keywords:
Developmental programming Inflammation
Oxidative stress Maternal care deprivation Major depressive disorder
a b s t r a c t
This study used an animal model of depression induced by maternal care deprivation (MCD) to inves-tigate whether depressive behaviour, neuroinflammation and oxidative stress were underlying factors in developmental programming after early life stress. At postnatal days (PND) 20, 30, 40, and 60, individual subsets of animals were evaluated in behavioural tests and then euthanized to assess cytokine levels and oxidative stress parameters in the prefrontal cortex (PFC), hippocampus and serum. The results showed that MCD did not induce behavioural changes at PND 30 and 40. However, at PND 20 and 60, the rats displayed a depressive-like behaviour in the forced swimming test, without changes in locomotor spontaneous activity. In the brain and serum, the levels of pro-inflammatory cytokines (interleukin-1b (IL-1b), interleukin-6 (IL-6) and tumour necrosis factor-a (TNF-a)) were increased, and the anti-inflammatory cytokine (interleukin-10) level was reduced throughout developmental programming (PND 20, 30, 40 and 60). Protein carbonyl levels increased in the brain at PND 30, 40 and 60. Superoxide dismutase (SOD) activity was decreased during all developmental programming phases evaluated in the brain. Catalase (CAT) activity was decreased at PND 20, 40 and 60 in the brain. Our results revealed that “critical episodes”in early life stressful events are able to induce behavioural alterations that persist into adulthood and can stimulate inflammation and oxidative damage in both central and peripheral systems, which are required for distinct patterns of resilience against psychiatric disorders later in life.
©2017 Elsevier Ltd. All rights reserved.
1. Introduction
Major depressive disorder (MDD) is considered a critical public
health problem with an estimated 350 million individuals affected worldwide (WHO, 2017). MDD is characterized by sadness, loss of interest in daily activities and a decrease in energy. In addition, MDD is also one of the most prevalent factors involved in disability and the use of health services. The pathophysiology of MDD has been attributed to deficits in monoamine neurotransmitters, such as se-rotonin and dopamine, since antidepressant drugs increase these neurotransmitters in the synaptic cleft. However, approximately
*Corresponding author. Laboratory of Neurosciences, University of Southern Santa Catarina, Criciuma, SC 88806-000, Brazil.
E-mail address:gislainezilli@hotmail.com(G.Z. Reus).
Contents lists available atScienceDirect
Journal of Psychiatric Research
j o u r n a l h o m e p a g e :w w w . e l s e v i e r . c o m / l o c a t e / p s y c h i r e s
30% of patients undergo remission following thefirst antidepressant treatment, and approximately 50% of patients do not respond to monoaminergic antidepressants (Krishnan and Nestler, 2008). Thus, other systems appear to be involved in the pathophysiology of MDD. Several studies have shown that the immune system plays an important role in the pathophysiology of MDD, as well as in thera-peutic responses to antidepressant drugs (Abelaira et al., 2014; Reus et al., 2015a; Mocking et al., 2017). This theory is sustained by the finding that patients with inflammatory diseases, such as diabetes, cardiovascular diseases, asthma and metabolic diseases, have a high prevalence of MDD (Fenton and Stover, 2006; Maes et al., 2011;
Ceretta et al., 2012a,b). Moreover, patients with MDD have elevated peripheral levels of pro-inflammatory cytokines, such as inter-leukin-1
b
(IL-1b
), interleukin-6 (IL-6), and tumour necrosis factor-a
(TNF-
a
) (Lindqvist et al., 2017). Increased levels of inflammatory mediators in the periphery and central nervous system (CNS) are associated with more vulnerability to stress and MDD (Menard et al., 2017). In an inflammatory state, brain resident cells, such as neurons, microglia, and astrocytes, receive mediators that signal to increase the expression of genes associated with inflammation and neuro-endocrine signaling impairment (Menard et al., 2017).It is well known that an increase in inflammation can also lead to an elevation in oxidative stress biomarkers. Oxidative stress is an imbalance in reactive oxygen species (ROS) and antioxidant en-zymes that could lead to biomolecule damage. Excess ROS can culminate in the formation of pro-inflammatory molecules, such as malondialdehyde (MDA) and 8-OH 2-deoxyguanosine, and damage-associated molecular patterns (DAMP) that, in turn, could promote immune responses (Bakunina et al., 2015). Patients with MDD who were non-responders to antidepressant treatment had a significant serum increase in 8-hydroxy-20-deoxyguanosine (8-OHdG) (Lindqvist et al., 2017). In the brains of rats subjected to chronic mild stress (CMS), an increase in superoxide production (Lucca et al., 2009a), an increase in the protein and lipid peroxi-dation, and an imbalance between superoxide dismutase (SOD) and catalase (CAT) activities (Lucca et al., 2009b) was observed. Elevated oxidative stress was also found in the brains of adult rats following maternal care deprivation (MCD) (Reus et al., 2015b ).
MDD is also known to have a developmental origin. Indeed, trauma, for example, may profoundly modulate developing neural circuits, resulting in significant behavioural changes that are often permanent. Sexual abuse, physical neglect and family violence during childhood are correlated with a higher vulnerability to MDD and anxiety in adulthood (Daskalakis et al., 2013; Vranceanu et al., 2007). In addition, trauma in childhood can increase the risk of drug abuse in adolescence (Palmer et al., 2016). To study early life aversive effects, the animal model of maternal deprivation appears to be a good model with important validity criteria (Abelaira et al., 2013). We have previously demonstrated that MCD during thefirst 10 PND in rats induced cognitive impairment, depressive-like behaviour, and periphery and brain changes associated with MDD in adulthood (Reus et al., 2011; Abelaira et al., 2012; Valvassori et al., 2014; Ignacio et al., 2017). Although there are studies that document the role of the immune system and oxidative stress in MDD, this association was not evaluated at different stages of development after early life trauma. Thus, this study aimed to investigate whether early life stress induced by MCD induces oxidative stress, inflammation and depressive-like behaviour at different stages of developmental programming.
2. Materials and methods
2.1. Animals
For this study, female Wistar rats (3 months of age, weighing
250e280 g) were obtained from the breeding colony of
Uni-versidade do Extremo Sul Catarinense (UNESC, Criciúma, SC, Brazil) and were housed for one week in the presence of males for mating purposes. At the end of 7 days, the pregnant rats were housed individually withad libitumaccess to food and water. The pregnant rats were housed individually for the birth of the pups and their identification. All mothers and pups were kept on a 12 h light/dark cycle (06:00 a.m. to 06:00 p.m.) at a temperature of 23±1C. Both male and female pups were used during the maternal deprivation period. On thefirst day after birth, the litters were culled to eight pups (±four males and four females). One day after birthing occurred, the MCD protocol was applied to 50% of the male pups from days 1e10 after birth; other males were used as controls.
Females were donated to the UNESC vivarium for other studies. All experimental procedures that involved animals were performed in accordance with the NIH Guide for the Care and Usage of Labora-tory Animals, within the Brazilian Society for Neuroscience and Behavior recommendations for animal care, and with the approval of the local Ethics Committee under protocol number 058/2016-1.
2.2. Experimental groups and maternal care deprivation (MCD) protocol
The deprivation protocol consisted of removing the mother from the residence box and taking her to another room. The pups were maintained in their home cage (grouped in the nest in the presence of maternal odour). The pups were deprived of the mother for 3 h per day during the first 10 days. We prefer this MCD protocol because it does not require the manipulation of the pups (Kosten et al., 2007; Mello, 2009). At the end of each daily deprivation session, the mothers were returned to their home boxes; this procedure was carried out during the light part of the cycle, be-tween 8:00 a.m. and 12:00 p.m. The control rats remained in their resident boxes together with their mothers throughout the experiment.
After the MCD protocol, different groups of animals (MCD and controls) were subjected to behavioural tests. At PND 20, 30, 40 and 60 (infancy, adolescence, young adult and adult, respectively), different animals (N¼12 deprived; N¼12 non-deprived for each
phase; N total¼96 animals) were subjected to behavioural tests as
described in the Methods section andFig. 1. After the behavioural tests, the rats were killed, and then, the cytokine levels and oxidative stress parameters were evaluated in the brain and the serum.
2.3. Behavioural tests
2.3.1. Openfield test (OFT)
On PND 20, 30, 40 and 60, the rats were subjected to the OFT in order to evaluate possible effects on spontaneous locomotor ac-tivity. The test was performed in an apparatus that consisted of a
brown plywood 45960 cm arena surrounded by 50 cm high
wooden walls and containing a frontal glass wall. Thefloor of the openfield was divided into nine rectangles (15920 cm each)
by black lines. The animals were gently placed in the left rear quadrant and left to explore the arena, and then, the number of horizontal (crossings) and vertical (rearings) movements per-formed by each rat during the 5 min observation period was counted by an expert observer (Reus et al., 2011).
2.3.2. Forced swimming test (FST)
After the OFT, the same animals were subjected to the FST. The FST was conducted according to previous reports (Porsolt et al., 1977; Reus et al., 2011). The test involves two individual expo-sures to a cylindrical tankfilled with water, in which rats cannot touch the bottom of the tank or escape. The tank is made of transparent Plexiglas, 80 cm tall, 30 cm in diameter, andfilled with water (22e23C) to a depth of 40 cm. On PND 20, 30, 40 and 60, the
rats were individually placed in the cylinder containing water for 15 min (pre-test session). On PND 21, 31, 41 and 61, the rats were again subjected to the FST for a 5 min session (test session), and the immobility, swimming and climbing times of rats were recorded in seconds.
2.4. Brain samples and serum collection
After the behavioral tests were complete, the animals were killed by decapitation and the blood was collected and placed in microcentrifuge tubes. The skulls were removed, then the whole brain was removed and placed on afilter paper and Petri dish on ice and then and the PFC and hippocampus were quickly isolated by hand dissection using a magnifying glass, a spatula, and a thin brush by a qualified researcher. In addition, the dissection was based on the histological distinctions described by Paxinos and Watson (1986). After the removal of the structures and serum (centrifuged from blood), they were placed in microcentrifuge tubes and stored in a freezer at 70C before biochemical analysis (n¼5).
2.5. Cytokine levels
The concentrations of TNF-
a
, IL-1b
, IL-6 and IL-10 in the PFC, hippocampus and serum were determined using a commercially available sandwich ELISA kit (R&D Systems, USA) and a microplate reader.2.6. Oxidative stress parameters
2.6.1. Protein carbonyls
The oxidative damage to proteins was assessed by the deter-mination of carbonyl groups based on the reaction with dini-trophenylhidrazine (Sigma-Aldrich, Saint Louis, MO, USA) as previously described (Levine et al., 1990). Briefly, proteins were precipitated by the addition of 20% trichloroacetic acid and redis-solved in dinitrophenylhidrazine, and the absorbance was read at 370 nm. The results were expressed as protein carbonyls per mg of protein.
2.6.2. Analysis of superoxide dismutase (SOD) activity
The method for the SOD activity assay was based on the capacity of pyrogallol toautoxidize, a process highly dependent on O2e2, a
substrate for SOD (Aebi, 1984). The inhibition of autoxidation of this compound occurs when SOD is present; the enzymatic activity can then be indirectly assayed spectrophotometrically at 420 nm using a double-beam spectrophotometer with temperature control. A calibration curve was generated using purified SOD as the standard
to calculate the specific activity of SOD in the samples. A total of 50% inhibition of pyrogallol autoxidation is defined as 1 unit of SOD, and the specific activity is represented as units per mg of protein.
2.6.3. Analysis of catalase (CAT) activity
The CAT activity was assayed using a double-beam spectro-photometer with temperature control. This method is based on the disappearance of H2O2at 240 nm in a reaction medium containing
20 mM H2O2, 0.1% Triton X-100, 10 mM potassium phosphate buffer
and 0.1e0.3 mg protein/mL at a pH of 7.0 (Bannister and Calabrese, 1987). One CAT unit is defined as 1 mol of hydrogen peroxide consumed per minute, and the specific activity is reported as units per mg protein.
2.6.4. Protein determination
All biochemical measures were normalized to the protein con-tent using bovine albumin as the standard (Lowry et al., 1951).
2.7. Statistical analysis
Data were analysed by Student's t-test for unpaired data and are expressed as the mean± standard error of the mean (S.E.M).p
values of<0.05 were considered to be statistically significant. The analyses were performed using the Statistical Package for the Social Science (SPSS) software, version 21.0.
3. Results
3.1. Effects of MCD on behaviour in different phases of developmental programming
Figure 2 illustrates the effects of MCD on depressive-like behaviour in the FST. At PND 20, the immobility and climbing time did not differ in deprived rats. However, in deprived rats, a decrease in the swimming time was observed compared to the non-deprived group, suggesting a depressive-like behaviour (t¼2.118;df¼22;p¼0.046;Fig. 2A). At PND 60, there was an
increase in the immobility time (t¼3.224;df¼18.11;p¼0.005; Fig. 2D) and a decrease in the climbing time (t¼2.373;df¼25; p¼0.026;Fig. 2D) indicating a depressive-like behaviour. MCD did
not alter the swimming time at PND 60. At PND 30 and 40 no alteration was found in the FST for the parameters analysed, including immobility, swimming, and climbing.
The spontaneous locomotor activity results are presented in
Fig. 3. At PND 20, the rats subjected to the MCD protocol did not exhibit an alteration in the number of crossings and rearings. The number of crossings did not differ at PND 30; however, there was an increase in the number of rearings at PND 30 (t¼2.705;df¼18; p ¼0.014; Fig. 3B) in deprived rats compared to non-deprived
group. No significant alterations were found in the number of crossings and rearings at PND 40.
3.2. Effects of MCD on cytokine levels in the PFC, hippocampus and serum in different phases of developmental programming
Fig. 4presents the results for IL-1
b
levels in the PFC, hippo-campus and serum. In deprived rats at PND 20, there was a decrease in IL-1b
levels in the PFC (t¼4.688;df¼6;p¼0.003;Fig. 4A), andan increase in IL-1
b
levels in the hippocampus (t¼2.531;df¼6; p¼0.045;Fig. 4A); the serum levels of IL-1b
in deprived rats did notFig. 2.Effects of the MCD on the immobility, climbing and swimming time of rats subjected to the FST at 20 (A), 30 (B), 40 (C) and 60 (D) PND, respectively. Values are expressed as mean±S.E.M. (n¼12). Different from non-deprived; *p<0.05, according to Student t-test.
Fig. 4D) and an increase in IL-1
b
levels in the serum (t¼3.465; df¼14;p¼0.004;Fig. 4D) and PFC at PND 60 (t¼2.629;df¼6; p¼0.039;Fig. 4D).The levels of TNF-
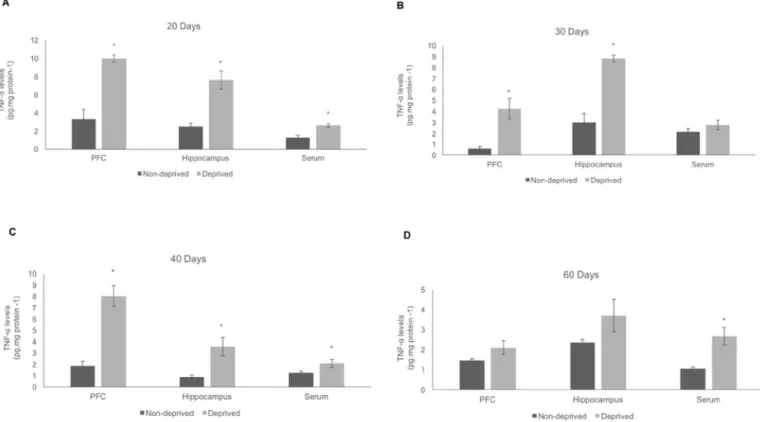
a
are illustrated inFig. 5. At PND 20, elevated levels of TNF-a
induced by MCD in the PFC (t¼5.487;df ¼7; p¼0.001;Fig. 5A), hippocampus (t¼5.248;df¼7;p¼0.001; Fig. 5A), and serum (t¼3.864;df¼11;p¼0.003;Fig. 5A) wereobserved. The TNF-
a
levels were increased at PND 30 in the PFC (t¼3.303;df¼5;p¼0.021;Fig. 5B) and hippocampus (t¼7.345; df ¼ 5; p¼ 0.001;Fig. 5B) in deprived rats, compared tonon-deprived rats. At PND 40, there was an increase in the TNF-
a
levels in the PFC (t¼6.238;df ¼4.31;p¼0.003;Fig. 5C) and
hippocampus (t¼3.236;df¼3.35;p¼0.041;Fig. 5C), The serum
levels of TNF-
a
were not altered at PND 30 and 40, but it was increased at PND 60 (t¼3.687;df¼6.48;p¼0.009;Fig. 5D). In thePFC and hippocampus of deprived rats at PND 60, there was no change in the TNF-
a
levels. Thus, the TNF-a
levels were increased in all brain structures analysed at PND 20, 30 and 40, suggesting a pro-inflammatory effect induced by MCD.In deprived rats at PND 20, a decrease in the IL-6 levels in the hippocampus (t¼4.554;df¼3.22;p¼0.017;Fig. 6A) was found,
and no change was found in the PFC or serum. At PND 30, in the PFC (t¼2.734; df¼6; p¼0.034;Fig. 6B) and in the hippocampus
(t¼4.110;df¼6;p¼0.006;Fig. 6B), the IL-6 levels were increased,
and it were decreased in the serum (t¼3.728;df¼8.85;p¼0.005; Fig. 6B) of deprived rats, compared to non-deprived rats. The IL-6 levels were elevated in maternally deprived rats at PND 40 in the PFC (t¼7.775;df¼6;p<0.0001;Fig. 6C), hippocampus (t¼5.427; df¼6;p¼0.002;Fig. 6C), and serum (t¼5.512;df¼14;p<0.0001;
Fig. 6C). At PND 60, IL-6 levels were not altered in the serum and in the PFC, but IL-6 levels were increased in the hippocampus (t¼4.119;df¼3.42;p¼0.020;Fig. 6D). Therefore, the results of
IL-6 levels suggest that MCD could induce a pro-inflammatory effect,
in the hippocampus and PFC of rats at PND 30, 40 and 60. The levels of IL-10 were decreased at PND 20 in the PFC (t¼6.778;df¼6;p¼0.001;Fig. 7A), hippocampus (t¼6.227; df ¼4.64;p ¼0.002; Fig. 7A), and serum (t ¼2.666; df ¼11; p¼0.022;Fig. 7A) of deprived rats, compared to the control group.
At PND 30, the IL-10 levels were also decreased in the hippocampus (t ¼ 3.602;df ¼6; p¼ 0.011; Fig. 7A), and serum (t ¼6.337; df¼7.56;p<0.0001;Fig. 7A). At PND 30, 40 and 60, the levels of IL-6 were not altered in the PFC; however, in the hippocampus, a decrease in the IL-6 levels at PND 40 (t ¼ 6.320; df ¼ 3.38; p¼0.006;Fig. 7C) and at PND 60 (t¼3.252;df¼6;p¼0.017; Fig. 7D), as well as in the serum at PND 40 (t ¼4.128;df ¼14; p¼0.001;Fig. 7C) and at PND 60 (t¼2.983;df¼7.68;p¼0.018; Fig. 7D), it was observed. Thus, these results suggest that the hip-pocampus and serum may have potent pro-inflammatory activity induced by MCD in rats at 30, 40 and 60 PND.
3.3. Effects of MCD on oxidative stress parameters in the PFC and hippocampus in different phases of developmental programming
Fig. 8demonstrates the results associated with protein damage in the PFC and hippocampus. At PND 20, the carbonyl protein levels were not altered in deprived rats in either the PFC. On the other hand, at PND 30, the carbonyl protein levels were increased in the PFC (t ¼ 3.90; df ¼5.68;p ¼0.014; Fig. 8B) and hippocampus
(t¼4.253;df¼8;p¼0.003;Fig. 8B) in deprived rats, compared to
the control group. At PND 40, MCD induced an increase in the carbonyl protein levels in the hippocampus (t ¼2.656;df ¼6; p¼0.038;Fig. 8C), but not in the PFC. The carbonyl protein levels
were also elevated in the PFC (t¼3.926;df ¼5.44;p ¼0.009; Fig. 8D) and hippocampus (t ¼11.043;df ¼5.201; p< 0.0001;
Fig. 8D) at PND 60 in maternally deprived rats, compared to the control group. At PND 30 and 60, MCD induced an increase on
Fig. 5.Effects of the MCD on the tumor necrosis fator-a(TNF-a) levels in the PFC, hippocampus and serum at 20 (A), 30 (B), 40 (C) and 60 (D) PND, respectively. Values are expressed as mean±S.E.M. (n¼5). Different from non-deprived; *p<0.05, according to Student t-test.
Fig. 7.Effects of the MCD on the interleukin-10 (IL-10) levels in the PFC, hippocampus and serum at 20 (A), 30 (B), 40 (C) and 60 (D) PND, respectively. Values are expressed as mean±S.E.M. (n¼5). Different from non-deprived; *p<0.05, according to Student t-test.
carbonyl protein levels in the PFC and hippocampus, suggesting an oxidative damage in these phases of developmental programming. SOD activity was decreased in all phases of developmental programming, suggesting a pro-oxidant effect induced by MCD. The MCD exhibited decreased SOD enzyme activity at PND 20 in the PFC (t ¼ 8.592; df ¼ 4.88; p < 0.0001; Fig. 9A) and hippocampus (t¼19.957;df¼5.85;p<0.0001;Fig. 9A), at PND 30 in the PFC (t¼4.674;df¼7;p¼0.002;Fig. 9B) and hippocampus (t¼6.026; df¼5.508;p¼0.001;Fig. 9B), at PND 40 in the PFC (t¼7.142; df¼12;p<0.0001;Fig. 9C) and hippocampus (t¼7.822;df¼12; p<0.0001;Fig. 9C), and at PND 60 in the PFC (t¼6.199;df¼10;p< 0.0001;Fig. 9D) and hippocampus (t¼9.866;df¼10;p<0.0001;
Fig. 9D).
MCD induced a decrease in the CAT activity at PND 20 in hip-pocampus (t¼4.972;df¼2.18;p¼0.032;Fig. 10A), but not in the
PFC, as well as at PND 30 in the hippocampus and PFC. At PND 40, MCD led to a decrease in CAT activity in the PFC (t ¼ 4.302; df¼6.66;p¼0.004;Fig. 10C), and an increase in CAT activity in the
hippocampus (t¼3.781;df¼12;p¼0.003;Fig. 10C). In the PFC of
deprived rats at PND 60, a decrease in the CAT activity in the PFC (t¼3.474;df¼5.81;p¼0.014;Fig. 10D) was found; however, in the
hippocampus, CAT activity was not altered at PND 60.
4. Discussion
Prenatal adverse experience, such as maternal deprivation, mainly during sensitive periods early in life, can lead to alterations in brain development and contribute to the development of psy-chiatric disorders, including anxiety and MDD. Increasing evidence shows that immune changes can impact the brain and lead to behavioural alterations associated with vulnerability or resilience to stress-related emotional disorders (Menard et al., 2017 ). In the
present study, we demonstrated that rats subjected to maternal care deprivation (MDD) in thefirst 10 PND did not have behavioural changes at PND 30 and 40. At PND 20 (infancy stage), a decrease in swimming time was demonstrated in the FST in deprived rats. At PND 30 (adolescence stage), an increase in the rearing time was found, indicating an alteration in locomotor spontaneous activity. At PND 60 (adulthood), the rats that were maternally deprived displayed depressive-like behaviour in the FST, as evidenced by an increase in immobility time and a decrease in climbing time. These results suggest that events that occur during the postnatal period could alter the normal course of development, resulting in adult outcomes. Our previous studies also showed that MCD in thefirst 10 PND induced an increase in immobility time, a decrease in climbing time, and no change in swimming time at PND 60 (Reus et al., 2011). Other studies also associated MCD with depressive-like behaviour (Reus et al., 2013a,b) and memory impairment (Valvassori et al., 2014) in adulthood.
Although behavioural changes in the FST induced by MCD occur in adulthood, in the present study, an inflammatory and oxidative status was observed during different stages of developmental programming, providing evidence that maternal deprivation pro-grams inflammatory system and thus influences behavioural and brain functions responses in later life periods. A mechanism that can elucidate the effects of early life it is epigenetic changes that could contribute to the individual's disease risk and susceptibility to MDD across the lifetime or generations (Jawahar et al., 2015).
Smith et al. (2011)revealed that prolonged immune dysregulation enduring after early in stress is secondary to epigenetic changes in immunoregulatory genes and associated to post-traumatic stress. Thus, we suggest that the behavioural changes in adult life found in the present study were due of an inflammatory process during development. In fact, an increase in pro- and a decrease in
inflammatory cytokines were found in both the periphery and brain at PND 20, 30, 40 and 60. It is known that balanced neuro-inflammation is an essential innate immune response in the CNS that can react against pathogens and damage (Song et al., 2017). Conversely, uncontrolled neuroinflammation may lead to an important impairment in brain resident cells (Choi et al., 2009; Abo-Ouf et al., 2013). In response to inflammation, the organism releases cytokines (endogenous peptide immunomodulators) (Allan and Rothwell, 2001). Cytokines interact with the CNS in numerous ways in which it is not relevant whether these mediators originate from the peripheral immunocompetent cells or are pro-duced within the CNS (Szelenyi, 2001). Generally, it can be accepted that the peripheral and central compartments appear to be inte-grated, their effects might synergize or inhibit each other; however, it should always be taken into account that they are differentially regulated (Szelenyi, 2001). In addition, the cytokines in the CNS are also recruited under physiological conditions and play an impor-tant role in neurogenesis, synaptic plasticity, memory and learning (Levin and Godukhin, 2017; Stepanichev and Yu, 2005; Vitkovic et al., 2000).
Some studies have described MDD as an inflammatory disease. In an animal model, it was reported that IL-1 can induce sickness behaviour, and depression-like symptoms can accompany the infection response (Kent et al., 1992). On the other hand, the administration of an IL-1 receptor antagonist reversed the sickness behaviour (Kent et al., 1992). Acute inflammatory responses are initiated by pro-inflammatory cytokines, such as IL-1
b
, TNF-a
, and IL-6. In MDD patients, lower levels of the anti-inflammatory cyto-kine IL-10 were reported (Dhabhar et al., 2009). Moreover, higher levels of pro-inflammatory cytokines, such as TNF-a
, IL-1b
, and IL-6, were associated with depressed mood (Reichenberg et al., 2001; Strike et al., 2004; Wright et al., 2005). In a meta-analysis study,Müller (2014) confirmed that IL-6 levels are elevated in MDD patients.
Interestingly, in the present study, we demonstrated that early life stress induces an increase in TNF-
a
, IL-1b
, and IL-6 levels and a decrease in IL-10 levels in both serum and in the brain in infancy, adolescence and adulthood. It is difficult to conclude whether early life stress induced by maternal deprivation in our study induced an inflammatory statusfirst in the periphery or in the brain. Increased levels of inflammatory mediators in the periphery could increase inflammation in the CNS. Peripheral immune responses commu-nicate with the brain through vagal nerves, cross the bloodebrainbarrier (BBB) linked to macrophages, and are associated with IL-1 receptors located on perivascular macrophages and the endothe-lial cells of brain venules (Dantzer et al., 2008). In addition, acti-vated microglia cells in an inflammatory response can release chemokine ligand 2 (CCL2) in the blood, which, in turn, can bind to chemokine receptor type 2 (CCR2) in monocytes, cross the BBB, penetrate into the brain areas involved in stress and MDD, and increase the expression of cytokines, such as IL-1
b
(Brevet et al., 2010; Menard et al., 2017). Thus, both peripheral and brain inflammation can contribute to more inflammation.Previously, we found increased levels of TNF-
a
and IL-1b
in the serum and cerebrospinalfluid (CSF) and decreased levels of IL-10 in the serum of rats maternally deprived at PND 60 (Reus et al., 2013b, 2015c). Additionally, treatment with the antidepressant imipra-mine (Reus et al., 2013) and ketamine, an antagonist of N-methyl-D-aspartate (NMDA) receptor (Reus et al., 2015c), reversed pe-ripheral cytokine alterations induced by MCD. Other study, demonstrated that a single dose of ketamine normalized the higher serum levels of IL-1b
and IL-6 in the rat with depression-like phenotype (Xie et al., 2017). Avolio et al. (2016)showed an in-crease in IL-1b
and NF-kB in the hippocampus, amygdala, and PFCof hamsters subjected to chronic stress. IL-10 levels were also increased in the same brain areas, but the higher levels of IL-10 were associated with resilience (Avolio et a. 2016). An over-production of IL-1
b
in the PFC, hippocampus, amygdala and nucleus accumbens of rats was associated with memory impairment and depressive-like behaviour in an animal model of neuropathic pain (Gui et al., 2016).Our present findings demonstrated that IL-1
b
levels were reduced in the PFC at PND 20 and in the hippocampus at PND 60, and IL-6 levels were decreased in the serum at PND 30; however, we typically found high levels of pro-inflammatory cytokines and low levels of anti-inflammatory cytokines. In the brain, both microglia and astrocytes influenced by stressful stimuli can pro-duce high levels of pro-inflammatory cytokines and lower levels of anti-inflammatory cytokines, and these impairments in the normal balance of cytokines can impact neuroplasticity in the whole brain (Levin and Godukhin, 2017). Under chronic conditions, microglia cells can be primed and produce elevated levels of inflammatory mediators. Reduced levels of IL-10 contributed to an increase in the expression of IL-6 in the brains of aged mice (Ye and Johnson, 2001). In a human study, prenatal maternal stress was associated with a reduction in of CD4þlymphocyte proportions, and higher levels of TNF-
a
, IL-1b
, and IL-6 were demonstrated in the blood of adoles-cents, indicating that prenatal maternal stress is a programming factor that leads to long-lasting consequences on immunity (Veru et al., 2015). Although the present study has been shown changes on the TNF-a
levels at PND 20, 30, 40 in the brain and serum, in the animals with 60 PND there was an increase in the TNF-a
levels only in the serum. Differently from this,Tang et al. (2017)reported that MCD induced an increase on TNF-a
levels in rats with 60 PND in the paraventricular nucleus. Still,Silva et al. (2010)showed that MCD rats also show high circulating levels of TNF-a
and increased expression of TNF-a
mRNA in the spleen and liver. Therefore, the results of the present study suggest that the effects under TNF-a
levels induced by MCD, could be related, at least, in part, with the brain structures and time that were analyzed.
DNA methylation of Th1 cytokines in children was also associ-ated with prenatal maternal stress (Cao-Lei et al., 2016). Thus, environment stressors, such as maternal deprivation, independent of genes, can lead to inflammatory responses throughout devel-opment and culminate in a depressive phenotype in adult life. In fact, a neuroinflammatory environment can lead to a disruption in brain cells and alter the trajectory of brain development (Jones and Friedman, 1982). Enhanced levels of pro-inflammatory cytokines can result in NMDA receptor impairment and elevated ROS pro-duction (Koriauli et al., 2015).
Brain cells are particularly more vulnerable to the effects of ROS, since 20% of the total consumption of oxygen is used for brain ac-tivity (Gandhi and Abramov, 2012). ROS in the brain also can be produced by the activity of monoamine oxidase, which is required for the inactivation of monoamines, including serotonin, noradrenaline and dopamine (Gandhi and Abramov, 2012), which are involved in the pathophysiology of MDD. Oxidative stress can result in mitochondrial dysfunction (Gandhi and Abramov, 2012) and neuroinflammation (Bakunina et al., 2015). Following oxidative stress, proteins can be damaged and protein structures can suffer fragmentation and degradation. Thus, protein changes can lead to enzymatic activity loss and alterations in physiological functions (Kohen and Nyska, 2002). Our presentfindings demonstrated an increase in protein carbonyl levels at PND 30, 40 and 60 and sug-gested that protein damage in the brain occurred in adolescence and adulthood as a result of maternal deprivation. Elevated carbonyl protein levels also were detected in the PFC, hippocampus, striatum and cortex of rats subjected to chronic mild stress (Lucca et al., 2009b). Following maternal deprivation, an increase in the
carbonyl protein levels was demonstrated in the hippocampus, amygdala and nucleus accumbens in adult rats at PND 60 (Reus et al., 2015b). Interestingly, a single injection of ketamine 14 days early reduced the carbonyl protein levels of deprived rats (Reus et al., 2015b).Liu et al. (2015)confirmed in a meta-analysis that depressed patients, when compared to healthy controls, had oxidative damage products, including MDA, 8-F2-isoprostanes and red blood cell (RBC) damage.
ROS are neutralized by antioxidant enzymes, such as SOD and CAT. Our present findings demonstrated that SOD activity was decreased at all stages of development in both the PFC and hip-pocampus. CAT activity was reduced in the hippocampus at PND 20 and in the PFC at PND 40 and 60. However, at PND 40, CAT activity was increased in the hippocampus. An imbalance in the SOD/CAT ratio could be an indicator of an increase in ROS (Maes et al., 2011). An increase in CAT activity in the hippocampus was also reported in chronically stressed and diabetic rats which displayed depressive-like behaviour (Lucca et al., 2009b; Ceretta et al., 2012a,b; Reus et al., 2016). Other stress factors can also lead to antioxidant changes during development; for example, an imbalance in maternal micronutrients also was associated with a reduction in antioxidant enzymes, SOD and glutathione peroxidase (GPx) in the brain of pups (Roy et al., 2014). At PND 21, the micronutrient imbalance was increased and MDA levels and GPx were elevated. In addition,Tsai and Huang (2015)demonstrated that patients with acute phase MDD had elevated levels of SOD and CAT in the serum. Thus, the oxidative stress parameters could be mediated by different signaling pathways, however its depend on the brain area, stressor and stage of development, proposing that the results of the oxidative stress present in the present study could be associated, at least in part, to development of depressive-like behavior in adulthood.
The evidence in this study suggests that early life stress induced by maternal care deprivation has negative developmental pro-gramming effects on offspring. Although behavioural alterations were more evident in adults, neuroinflammation and oxidative stress were detected early in adolescence, suggesting that both inflammation and oxidative stress induced by early life stress impact nervous system leading to long-term behavioural changes. Further studies are necessary to elucidate the mechanism by which these alterations occur. In addition, future studies investigating the use of potential antidepressant interventions to shift the thera-peutic approach from adulthood to early life, before depressive symptoms appear, could be an important strategy.
Conflicts of interest
None.
Acknowledgements
The Translational Psychiatry Program (USA) is funded by the Department of Psychiatry and Behavioral Sciences, McGovern Medical School, The University of Texas Health Science Center at Houston (UTHealth). Laboratory of Neurosciences (Brazil) is one of the centers of the National Institute for Molecular Medicine (INCT-MM) and one of the members of the Center of Excellence in Applied Neurosciences of Santa Catarina (NENASC). Its research is sup-ported by grants from CNPq (JQ), FAPESC (JQ); Instituto Cerebro e Mente (JQ) and UNESC (JQ, GZR and FDP). JQ is a 1A CNPq Research Fellow.
References
Streck, E.L., Quevedo, J., 2012. Lamotrigine treatment reverses depressive-like behavior and alters BDNF levels in the brains of maternally deprived adult rats. Pharmacol. Biochem. Behav. 101, 348e353.
Abelaira, H.M., Reus, G.Z., Quevedo, J., 2013. Animal models as tools to study the pathophysiology of depression. Ver. Bras. Psiquiatr. 35, 112e120.
Abelaira, H.M., Reus, G.Z., Petronilho, F., Barichello, T., Quevedo, J., 2014. Neuro-immunomodulation in depression: a review of inflammatory cytokines involved in this process. Neurochem. Res. 39, 1643e1649.
Abo-Ouf, H., Hooper, A.W., White, E.J., van Rensburg, H.J., Trigatti, B.L., Igdoura, S.A., 2013. Deletion of tumor necrosis factor-aameliorates neurodegeneration in Sandhoff disease mice. Hum. Mol. Genet. 22, 3960e3975.
Aebi, H., 1984. Catalase in vitro. Mater. Enzym 105, 121e126.
Allan, S.M., Rothwell, N.J., 2001. Cytokines and acute neurodegeneration. Nat. Rev. Neurosci. 2, 734e744.
Avolio, E., Fazzari, G., Mele, M., Alo, R., Zizza, M., Jiao, W., Di Vito, A., Barni, T., Mandala, M., Canonaco, M., 2016. Unpredictable chronic mild stress paradigm established effects of pro- and anti-inflammatory cytokine on neurodegeneration-linked depressive states in hamsters with brain endothelial damages. Mol. Neurobiol. in press.
Bakunina, N., Pariante, C.M., Zunszain, P.A., 2015. Immune mechanisms linked to depression via oxidative stress and neuroprogression. Immunology 144, 365e373.
Bannister, J.V., Calabrese, L., 1987. Assays for superoxide dismutase. Mater. Biochem. Anal. 32, 279e312.
Brevet, M., Kojima, H., Asakawa, A., Atsuchi, K., Ushikai, M., Ataka, K., Inui, A., Kimura, H., Sevestre, H., Fujimiya, M., 2010. Chronic foot-shock stress potenti-ates the influx of bone marrow-derived microglia into hippocampus. J. Neurosci. Res. 88, 1890e1897.
Cao-Lei, L., Veru, F., Elgbeili, G., Szyf, M., Laplante, D.P., King, S., 2016. DNA methylation mediates the effect of exposure to prenatal maternal stress on cytokine production in children at age 13½ years: project Ice Storm. Clin. Epigenetics 8, 54.
Ceretta, L.B., Reus, G.Z., Abelaira, H.M., Ribeiro, K.F., Zappellini, G., Felisbino, F.F.,
Steckert, A.V., Dal-Pizzol, F., Quevedo, J., 2012a. Increased oxidative stress and imbalance in antioxidant enzymes in the brains of alloxan-induced diabetic rats.Exp. Diabetes Res. 2012, 302e682.
Ceretta, L.B., Reus, G.Z., Abelaira, H.M., Ribeiro, K.F., Zappellini, G., Felisbino, F.F.,
Steckert, A.V., Dal-Pizzol, F., Quevedo, J., 2012b. Increased oxidative stress and imbalance in antioxidant enzymes in the brains of alloxan-induced diabetic rats. Exp. Diabetes Res. 2012, 8 pages.
Choi, D.Y., Liu, M., Hunter, R.L., Cass, W.A., Pandya, J.D., Sullivan, P.G., Shin, E.J., Kim, H.C., Gash, D.M., Bing, G., 2009. Striatal neuroinflammation promotes Parkinsonism in rats. PLoS One 4, e5482.
Dantzer, R., O'Connor, J.C., Freund, G.G., Johnson, R.W., Kelley, K.W., 2008. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat. Rev. Neurosci. 9, 46e56.
Daskalakis, N.P., Bagot, R.C., Parker, K.J., Vinkers, C.H., de Kloet, E.R., 2013. The three-hit concept of vulnerability and resilience: toward understanding adaptation to early-life adversity outcome. Psychoneuroendocrinology 38, 1858e1873. Dhabhar, F.S., Burke, H.M., Epel, E.S., Mellon, S.H., Rosser, R., Reus, V.I.,
Wolkowitz, O.M., 2009. Low serum IL-10 concentrations and loss of regulatory association between IL-6 and IL-10 in adults with major depression. J. Psychiatr. Res. 43, 962e969.
Fenton, W.S., Stover, E.S., 2006. Mood disorders: cardiovascular and diabetes co-morbidity. Curr. Opin. Psychiatry 19, 421e427.
Gandhi, S., Abramov, A.Y., 2012. Mechanism of oxidative stress in neuro-degeneration. Oxid. Med. Cell Longev. 2012, 11.
Gui, W.S., Wei, X., Mai, C.L., Murugan, M., Wu, L.J., Xin, W.J., Zhou, L.J., Liu, X.G., 2016. Interleukin-1b overproduction is a common cause for neuropathic pain, memory deficit, and depression following peripheral nerve injury in rodents. Mol. Pain 12, 1e15.
Ignacio, Z.M., Reus, G.Z., Abelaira, H.M., Maciel, A.L., de Moura, A.B., Matos, D., Demo, J.P., da Silva, J.B., Gava, F.F., Valvassori, S.S., Carvalho, A.F., Quevedo, J., 2017. Quetiapine treatment reverses depressive-like behavior and reduces DNA methyltransferase activity induced by maternal deprivation. Behav. Brain Res. 320, 225e232.
Jawahar, M.C., Murgatroyd, C., Harrison, E.L., Baune, B.T., 2015. Epigenetic alterations following early postnatal stress: a review on novel aetiological mechanisms of common psychiatric disorders. Clin. Epigenetics 7, 122.
Jones, A.P., Friedman, M.L., 1982. Obesity and adipocyte abnormalities in offspring of rats undernourished during pregnancy. Science 215, 1518e1519.
Kent, S., Bluthe, R.M., Kelley, K.W., Dantzer, R., 1992. Sickness behavior as a new target for drug development. Trends Pharmacol. Sci. 13, 24e28.
Kohen, R., Nyska, A., 2002. Invited review: oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quan-tification. Toxicol. Pathol. 30, 50e620.
Koriauli, S., Natsvlishvili, N., Barbakadze, T., Mikeladze, D., 2015. Knockdown of interleukin-10 induces the redistribution of sigma1-receptor and increases the glutamate-dependent NADPH-oxidase activity in mouse brain neurons. Biol. Res. 9, 48e55.
Kosten, T.A., Lee, H.J., Kim, J.J., 2007. Neonatal handling alters learning in adult male and female rats in a task-specific manner. Brain Res. 18, 144e153.
Krishnan, V., Nestler, E.J., 2008. The molecular neurobiology of depression. Nature 455, 894e902.
Levin, S.G., Godukhin, O.V., 2017. Modulating effect of cytokines on mechanisms of
synaptic plasticity in the brain. Biochem. (Mosc.) 82, 264e274.
Levine, R.L., Garland, D., Oliver, C.N., 1990. Determination of carbonyl content in oxidatively modified proteins. Meth. Enzymol. 186, 464e478.
Lindqvist, D., Dhabhar, F.S., James, S.J., Hough, C.M., Jain, F.A., Bersani, F.S., Reus, V.I., Verhoeven, J.E., Epel, E.S., Mahan, L., Rosser, R., Wolkowitz, O.M., Mellon, S.H., 2017. Oxidative stress, inflammation and treatment response in major depres-sion. Psychoneuroendocrinology 76, 197e205.
Liu, T., Zhong, S., Liao, X., Chen, J., He, T., Lai, S., Jia, Y., 2015. A Meta-Analysis of Oxidative Stress Markers in Depression. PLoS One 10, e0138904.
Lowry, O.H., Rosebrough, N.J., Farr, A.L., Randall, R.J., 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193, 265e275.
Lucca, G., Comim, C.M., Valvassori, S.S., Reus, G.Z., Vuolo, F., Petronilho, F., Dal-
Pizzol, F., Gavioli, E.C., Quevedo, J., 2009a. Effects of chronic mild stress on the oxidative parameters in the rat brain. Neurochem. Int. 54, 358e362. Lucca, G., Comim, C.M., Valvassori, S.S., Reus, G.Z., Vuolo, F., Petronilho, F.,
Gavioli, E.C., Dal-Pizzol, F., Quevedo, J., 2009b. Increased oxidative stress in submitochondrial particles into the brain of rats submitted to the chronic mild stress paradigm. J. Psychiatr. Res. 43, 864e869.
Maes, M., Kubera, M., Obuchowiczwa, E., Goehler, L., Brzeszcz, J., 2011. Depression's multiple comorbidities explained by (neuro)inflammatory and oxidative and nitrosative stress pathways. Neuro Endocrinol. Lett. 32, 7e24.
Mello, P.B., 2009. Physical exercise can reverse the deficit in fear memory induced by maternal deprivation. Neurobiol. Learn Mem. 92, 364e369.
Menard, C., Pfau, M.L., Hodes, G.E., Russo, S.J., 2017. Immune and neuroendocrine mechanisms of stress vulnerability and resilience. Neuropsychopharmacology 42, 62e80.
Mocking, R.J., Nap, T.S., Westerink, A.M., Assies, J., Vaz, F.M., Koeter, M.W., Ruhe, H.G., Schene, A.H., 2017. Biological profiling of prospective antidepressant response in major depressive disorder: associations with (neuro)inflammation, fatty acid metabolism, and amygdala-reactivity. Psychoneuroendocrinology 79, 84e92.
Müller, N., 2014. Immunology of major depression: neuroimmunomodulation. Neuroimmunomodulation 21, 123e130.
Palmer, R.H., Nugent, N.R., Brick, L.A., Bidwell, C.L., McGeary, J.E., Keller, M.C., Knopik, V.S., 2016. Evidence of shared genome-wide additive genetic effects on interpersonal trauma exposure and generalized vulnerability to drug depen-dence in a population of substance users. J. Trauma. Stress 29, 197e204. Paxinos, G., Watson, C., 1986. The Rat Brain: Stereotaxic Coordinates, second ed.
Academic Press, Australia.
Porsolt, R.D., Le Pichon, M., Jalfre, M., 1977. Animal model of depression. Nature 266, 730e732.
Reichenberg, A., Yirmiya, R., Schuld, A., Kraus, T., Haack, M., Morag, A., Pollm€acher, T., 2001. Cytokine-associated emotional and cognitive disturbances in humans. Arch. Gen. Psychiatry 58, 445e452.
Reus, G.Z., Stringari, R.B., Ribeiro, K.F., Cipriano, A.L., Panizzutti, B.S., Stertz, L., Lersch, C., Kapczinski, F., Quevedo, J., 2011. Maternal deprivation induces depressive-like behaviour and alters neurotrophin levels in the rat brain. Neurochem. Res. 36, 460e466.
Reus, G.Z., Abelaira, H.M., dos Santos, M.A., Carlessi, A.S., Tomaz, D.B., Neotti, M.V., Liranço, J.L., Gubert, C., Barth, M., Kapczinski, F., Quevedo, J., 2013a. Ketamine and imipramine in the nucleus accumbens regulate histone deacetylation induced by maternal deprivation and are critical for associated behaviors. Behav. Brain Res. 259, 451e456.
Reus, G.Z., Dos Santos, M.A., Abelaira, H.M., Ribeiro, K.F., Petronilho, F., Vuolo, F., Colpo, G.D., Pfaffenseller, B., Kapczinski, F., Dal-Pizzol, F., Quevedo, J., 2013b. Imipramine reverses alterations in cytokines and BDNF levels induced by maternal deprivation in adult rats. Behav. Brain Res. 242, 40e46.
Reus, G.Z., Fries, G.R., Stertz, L., Badawy, M., Passos, I.C., Barichello, T., Kapczinski, F., Quevedo, J., 2015a. The role of inflammation and microglial activation in the pathophysiology of psychiatric disorders. Neuroscience 300, 141e154. Reus, G.Z., Nacif, M.P., Abelaira, H.M., Tomaz, D.B., dos Santos, M.A., Carlessi, A.S., da
Luz, J.R., Gonçalves, R.C., Vuolo, F., Dal-Pizzol, F., Carvalho, A.F., Quevedo, J., 2015b. Ketamine ameliorates depressive-like behaviors and immune alterations in adult rats following maternal deprivation. Neurosci. Lett. 584, 83e87. Reus, G.Z., Carlessi, A.S., Titus, S.E., Abelaira, H.M., Ignacio, Z.M., da Luz, J.R.,
Matias, B.I., Bruchchen, L., Florentino, D., Vieira, A., Petronilho, F., Quevedo, J., 2015c. A single dose of S-ketamine induces long-term antidepressant effects and decreases oxidative stress in adulthood rats following maternal depriva-tion. Dev. Neurobiol. 75, 1268e1281.
Reus, G.Z., Dos Santos, M.A., Abelaira, H.M., Titus, S.E., Carlessi, A.S., Matias, B.I., Bruchchen, L., Florentino, D., Vieira, A., Petronilho, F., Ceretta, L.B., Zugno, A.I., Quevedo, J., 2016. Antioxidant treatment ameliorates experimental diabetes-induced depressive-like behavior and reduces oxidative stress in brain and pancreas. Diabetes Metab. Res. Rev. 32, 88e278.
Roy, S., Sable, P., Khaire, A., Randhir, K., Kale, A., Joshi, S., 2014. Effect of maternal micronutrients (folic acid and vitamin B12) and omega 3 fatty acids on indices of brain oxidative stress in the offspring. Brain Dev. 36, 219e227.
Silva, S.V., Garcia-Souza, E.P., Moura, A.S., Barja-Fidalgo, C., 2010. Maternal protein restriction during early lactation induces changes on neutrophil activation and TNF-alpha production of adult offspring. Inflammation 2010 (33), 65e75. Smith, A.K., Conneely, K.N., Kilaru, V., Mercer, K.B., Weiss, T.E., Bradley, B., Tang, Y.,
Gillespie, C.F., Cubells, J.F., Ressler, K.J., 2011. Differential immune system DNA methylation and cytokine regulation in post-traumatic stress disorder. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 156, 700e708.
diseases, from functions to therapies. Front. Cell Neurosci. 11, 63.
Stepanichev, M., Yu, 2005. Cytokines as neuromodulators in the central nervous system. Neurochemistry 22, 5e11.
Strike, P.C., Wardle, J., Steptoe, A., 2004. Mild acute inflammatory stimulation in-duces transient negative mood. J. Psychosom. Res. 57, 189e194.
Szelenyi, J., 2001. Cytokines and the central nervous system. Brain Res. Bull. 54, 329e338.
Tang, H.L., Zhang, G., Ji, N.N., Du, L., Chen, B.B., Hua, R., Zhang, Y.M., 2017. Toll-like receptor 4 in paraventricular nucleus mediates visceral hypersensitivity induced by maternal separation. Front. Pharmacol. 8, 309.
Tsai, M.C., Huang, T.L., 2015. Increased activities of both superoxide dismutase and catalase were indicators of acute depressive episodes in patients with major depressive disorder. Psychiatry Res. 235, 38e42.
Valvassori, S.S., Varela, R.B., Arent, C.O., Dal-Pont, G.C., Bobsin, T.S., Budni, J., Reus, G.Z., Quevedo, J., 2014. Sodium butyrate functions as an antidepressant and improves cognition with enhanced neurotrophic expression in models of maternal deprivation and chronic mild stress. Curr. Neurovasc. Res. 11, 359e366.
Veru, F., Dancause, K., Laplante, D.P., King, S., Luheshi, G., 2015. Prenatal maternal stress predicts reductions in CD4þlymphocytes, increases in innate-derived
cytokines, and a Th2 shift in adolescents: project Ice Storm. Physiol. Behav 144, 137e145.
Vitkovic, L., Bockaert, J., Jaque, C., 2000. “Inflammatory” cytokines: neuro-modulators in normal brain? J. Neurochem. 74, 457e471.
Vranceanu, A.M., Hobfoll, S.E., Johnson, R.J., 2007. Child multi-type maltreatment and associated depression and PTSD symptoms: the role of social support and stress. Child. Abuse Negl. 31, 71e84.
World Health Organization, 2017. Media Centre Fact Sheet. www.who.int/ mediacentre/factsheets/fs369/en/.
Wright, C.E., Strike, P.C., Brydon, L., Steptoe, A., 2005. Acute inflammation and negative mood: mediation by cytokine activation. Brain Behav. Immun 19, 345e350.
Xie, Z.M., Wang, X.M., Xu, N., Wang, J., Pan, W., Tang, X.H., Zhou, Z.Q., Hashimoto, K., Yang, J.J., 2017. Alterations in the inflammatory cytokines and brain-derived neurotrophic factor contribute to depression-like phenotype after spared nerve injury: improvement by ketamine. Sci. Rep. 7, 3124.