www.jped.com.br
ORIGINAL
ARTICLE
Breast
milk
supplementation
and
preterm
infant
development
after
hospital
discharge:
a
randomized
clinical
trial
夽
,
夽夽
Roxana
Desterro
e
Silva
da
Cunha
a,b,∗,
Fernando
Lamy
Filho
c,
Eremita
Val
Rafael
a,b,
Zeni
Carvalho
Lamy
c,
André
Luiz
Guimarães
de
Queiroz
d,eaHospitalUniversitárioMaterno-Infantil,SãoLuís,MA,Brazil bUniversidadeFederaldoMaranhão(UFMA),SãoLuís,MA,Brazil
cDepartamentodeMedicina,UniversidadeFederaldoMaranhão(UFMA),SãoLuís,MA,Brazil dFaculdadedeMedicina,UniversidadeFederaldoMaranhão(UFMA),SãoLuís,MA,Brazil eInstitutionalProgramforScientificInitiationScholarships(PIBIC),Brazil
Received7January2015;accepted6May2015 Availableonline25September2015
KEYWORDS
Breastfeeding; Preterm;
Hospitaldischarge; Development; Humanmilk
Abstract
Objectives: Toassesstheeffectofmaternalbreastmilksupplementationonthedevelopment ofexclusivelybreast-fedverylowbirthweightpreterminfantsat12monthsofcorrectedage. Methods: A randomized clinical trial with 53 infants followed-up after discharge from the neonatalunituntilacorrected gestationalageof12 months.Newborns intheintervention groupwerebreastfedexclusivelywithmaternalmilkandreceived2gofamultinutrient sup-plement(Pré-Nan®,Nestlé,Vevey,Switzerland)addedtoexpressedbreastmilktwiceadayuntil acorrectedageof4---6months.Thecontrolgroupwasexclusivelybreastfedwithout supplemen-tation.Aftermonthlyfollow-up,developmentalassessmentwasperformedusingtheBayleyIII Scale.
Results: TherewasnostatisticallysignificantdifferenceontheBayleyIIIScalebetweenthe interventionandcontrolgroupsinanyoftheassesseddomains:motor,cognitive,and commu-nication.However,scoresinthethreedomainswerealwayshigherinthegroupthatreceived thesupplement.Therewereasimilarnumberofcasesofdevelopmentaldelayinbothgroups: seven(28%)inthegroupthatreceivedthesupplementandnine(33.3%)inthegroupthatwas exclusivelybreastfed.
夽 Pleasecitethisarticleas:daCunhaRD,LamyFilhoF,RafaelEV,LamyZC,QueirozAL.Breastmilksupplementationandpreterminfant
developmentafterhospitaldischarge:arandomizedclinicaltrial.JPediatr(RioJ).2016;92:136---42.
夽夽StudyconductedatUniversidadeFederaldoMaranhão(UFMA),SãoLuís,MA,Brazil.
∗Correspondingauthor.
E-mail:roxanacunha@hotmail.com(R.D.e.S.daCunha).
http://dx.doi.org/10.1016/j.jped.2015.04.004
Conclusions: Theresultsfailedtoshowanassociationbetweenpost-dischargemultinutrient supplementationanddevelopmentintheassessedinfants.
©2015SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Allrightsreserved.
PALAVRAS-CHAVE
Amamentac¸ão; Prematuro; Altahospitalar; Desenvolvimento; LeiteMaterno
Suplementac¸ãodoleitematernoedesenvolvimentodelactentespré-termoapósalta hospitalar:ensaioclínicorandomizado
Resumo
Objetivos: Avaliaroefeitodasuplementac¸ãodoaleitamentomaternoexclusivocomaditivo multicomponentenodesenvolvimentodelactentesnascidospré-termodemuitobaixopesoaos 12mesesdeidadegestacionalcorrigida.
Método: Ensaio Clínico Randomizado com 53 lactentes, acompanhados da alta hospitalar na Unidade Neonatal até o 12◦ mês de idade gestacional corrigida. Aqueles alocados no grupointervenc¸ãopermaneciamemaleitamentomaternoexclusivoerecebiam02gramasde suplemento multicomponente em pó (Pré-Nan®, Nestlé,Vevey, Suíc¸a),adicionados ao leite ordenhadoduasvezesaodia,porumperíodode4a6mesesdeidadegestacionalcorrigida. O grupo controlepermaneciaem aleitamento materno exclusivosem suplementac¸ão. Após acompanhamentomensal,foifeitaavaliac¸ãododesenvolvimentopormeiodaEscaladeBayley III.
Resultados: Na comparac¸ão do desenvolvimento pela Escala de Bayley III entre os grupos intervenc¸ãoecontrole,nãohouvediferenc¸aestatísticasignificanteemnenhumdosdomínios estudados:motor,cognitivoelinguagem.Porém,osvaloresdosescoresforamsempremaiores nogrupointervenc¸ãoquenogrupocontrolenostrêsdomínios.Oatrasodedesenvolvimentose distribuiudeformasimilarnosgrupos:setecasos(28%)nogrupointervenc¸ãoenove(33,3%)no grupocontrole.
Conclusões: Osresultadosnão mostraramassociac¸ãoentresuplementac¸ãomulticomponente pós-altaeodesenvolvimentodoslactentesanalisadospelaEscaladeBayleyIII.
©2015SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Todososdireitos reservados.
Introduction
The developing brain is particularly vulnerable to nutri-tionaldeficienciesduetoseveralfastneurologicalprocess pathways,suchassynapseformationandmyelination.Itis regulatedbynutrientssuchasprotein,energy,fat,minerals andgrowthfactors.1---3
A deficiency of these elements can have later conse-quences, progressing to cerebral dysfunction associated withfuture alterations in child development.2 Therefore,
adequate nutrition, especially in preterm newborns is of greatimportancetoprevent,amongotherproblems, neu-rodevelopmentaldelays.4---6
Breastmilkisconsideredtheidealfoodintheneonatal period,promoting gastrointestinal maturation,generating immunologicalbenefits,andleadingtoincreasedlevelsof docosahexaenoicacid,animportantcomponentforcerebral development.5,7---9 Nonetheless, its exclusive use in
cer-tainsituations can lead tonutrient deficiencies andbone demineralization.10,11
The hospitalizationperiodof preterm newborns, espe-cially those with very low birth weight, is one of the situations where breast milk supplementation is a well-established and current practice during the in-hospital period,promotingbetterweightgain,increasedlengthand headcircumferenceintheshortterm,andbetter develop-mentindexesinthemediumandlongterms.12---14
Even though the benefits of human milk supplemen-tationforhospitalizedpreterminfantsarewelldocumented in the literature, thereis noconsensus on the effective-nessofthispracticeafterhospitaldischarge.6,10,15Recently,
theEuropean Societyfor PediatricGastroenterology, Hep-atology, andNutrition (ESPGHANCommittee onNutrition) recommendedthatexclusivelybreast-feedinfantswhoare underweightfortheirpost-conceptualagewhendischarged fromthehospitalshouldreceive supplementationtomeet theirnutritionalneeds.16
Inrecentyears,someauthorshaveattemptedtoclarify thisissue by assessing theeffectiveness of post-discharge supplementationinstudieswithexperimental design.Ina systematicreview,Youngetal.17selectedonlythe
random-izedtrialsbyO’Connoretal.18 andbyZachariassenetal.19
These authors observed good results of supplementation in some specific situations, such as higher length during thestudyperiodandhigherheadcircumferenceinpreterm infantswithbirthweight<1250g.Inspiteofthat,thereview authorsconcludedthatthereisnoconsensusonthebestway offeedingpreterminfantspost-dischargeinordertoprovide bettergrowthresults.
Regardingtheeffectsofbreastmilksupplementationon thepost-dischargedevelopment,Aimoneetal.,20 studying
thesamepopulationasO’Connoretal.,19observedatrend
thosefromthecontrolgroupafter12weeks.When evalu-atedat18monthsofcorrectedgestationalage(CGA),the infants from the intervention group had better scores on theBayleyIIScaleinthelanguageandmotordomains,but withoutstatisticalsignificance.
Giventhelackofevidenceinthisarea,thepresentstudy aimedtoinvestigatewhetherchildrendischargedfromthe neonatal unit (NICU), born preterm, with very low birth weight, and who were fed breast milk and a multinutri-entsupplementhadbetterpsychomotordevelopmentscores whencomparedtothosewhowerefednon-supplemented breastmilk.
Methods
Thiswasarandomizedclinicaltrialconductedfrom Decem-berof2010toOctoberof2013with53preterminfantswith birthweight<1500g,admittedattheNICUofHospital Uni-versitárioUnidadeMaterno-InfantilofUniversidadeFederal doMaranhão,andwhoweredischargedonexclusive breast-feeding(EBF).Afterdischarge,allchildrenthatmetthese criteriawere followed-up until 12 months of CGA. Those withconditionsthatcouldinterferewithneuropsychomotor development(NPMD):majormalformations;hydrocephalus; chromosomalabnormalities;fetalhydrops;congenital infec-tions;maternal use of illicit drugs, tobacco,alcohol, and continuoususeofcorticosteroids;twinpregnancy; necrotiz-ingenterocolitissequelae;andcerebralpalsywereexcluded fromthestudy.
Beforedischarge,themotherswereinstructed tooffer the breast to their children, on demand, prioritizing the hindmilk. Seven to ten days after discharge on EBF, the dyads returnedto the first assessment. If theycontinued onEBF,therandomizationwasperformed.This isjustified becauseofthelargenumberofmotherswhogiveuponEBF soonafterdischarge,whenfacedwithreal-lifesituationsat home.
Randomnumbersweregeneratedforchildrenallocation inthecontrolgrouporinterventiongroup.For randomiza-tion,anopaqueandsealedenvelopewasused.Forethical reasons,placebocouldnotbeused,anditwasnotpossible toblindthemothersandprofessionals.
To calculate the sample size, the valuesobtained in a pilotstudywereused.Forthatpurpose, ameanvalue(90 points)wasassumedforthelanguagedomainoftheBayley ScaleIIIofthecontrolgroupat12monthsofCGA,whereas the scorein the intervention group was 10 pointshigher, witha standard deviation of 12 points in both groups. A powerof80andtheprobabilityoftype1errorof0.05were alsoassumed, thuscalculating23infants pergroupasthe necessarynumber.
The intervention consisted of breast milk supplemen-tationforaperiodoffourtosixmonthsandwasconducted asfollows.
Afterrandomization,themothersfromtheintervention group were instructed on how toadd the supplement to breastmilk:10mLofbreastmilkwereexpressedby man-ual milkingand 2g of PRE-NAN® powder formula(Nestlé) wereadded.A50mLgraduatedplasticcupwasused.After homogenization, the mixture was offered to the child. The procedure wasconductedbeforetwofeedingsduring
the day, one in the morning and another in the after-noon. The addition of the supplement corresponded to an increase of 20kcal/day.On average, thisincrease was enough to raise the caloric intake of the child at dis-charge (considering 1.8---2.0kgof weightat discharge) to approximately140kcal/kg/day.21 Furthermore,italso
cor-responded to a daily increase of 2.12gof carbohydrates, 1.04goffats,0.56gofproteins,1.04goftotalfat,0.44g ofsaturatedfats,0.164goflinoleicacid,19.6mgofalpha linoleicacid,10.76gofsodium,30.4mgofcalcium,0.4mg of iron,24.2mg of potassium, and 1.6mg of taurine and vitamins.
The infants were followed-up monthly to assess their general and nutritional status,as well asdevelopment in abreastfeedingfollow-upandmotivationoutpatientclinic. The Bayley IIIScale wasappliedtoevaluatethe NPMDup to 12 months of CGA. The evaluation was performed by anoccupationaltherapistwhohadspecialtrainingwithan authorized team in properly equipped room to apply the test. The evaluator wasnot informedabout thegroup to whichthechildbelonged.
To verify the effectiveness of randomization, charac-teristics oftheassessed infantsuptorandomizationwere compared in both groups. The compared variables were: weight,gender,gestationalage,headcircumference(HC), lengthatbirth,weightatrandomization,weightfor gesta-tionalage(SGA/AGA),lengthofhospitalstay,andseverity score(ScoreforNeonatalAcutePhysiology,Perinatal Exten-sion,VersionII [SNAPPEII]).22 The hypothesistestwasnot
appliedwhencomparingthesevariables,inaccordancewith the standards of the Consolidated Standards of Reporting Trials(CONSORTStatement).23
Scoresofmotor, cognitive,andlanguagedomainswere assessed by the Bayley Scale III at 12 months of CGA werealsodescribed.Themeanscoresofeachdevelopment domainwerecalculated.
Multivariateanalysis(simplelinearregression)wasused, despite therandomization,sincesome variablescollected before that were not balanced between the two groups. Among them, the variables birth weight and gender, which have significant influence on the future develop-ment of preterm newborns, were used in the model.24,25
Although they were not balanced, the SNAPPE II varia-bles and the length of hospital stay were not used in the model because they are considered collinear with birth weight. Moreover, as the outcome was measured several months after the end of the intervention, exist-ing elements in this period could influence the final result.
Onlyonepost-randomizationvariablewaschosento com-posethemodelduetoitsimportanceforchilddevelopment: ‘‘mother asprimary caregiver of the child’’ (yes/no cat-egories). It is known that the family unit is one of the mostimportantfactorinthechilddevelopment.26Resegue
etal.27 givespecialemphasistothematernalpresence in
thecareofchildrenintheirfirstyearsoflife,andconsider maternal deprivation a risk factor for child development delay.
Maternalschoolingwas alsoconsidered tobe essential fordevelopment26;however,havingbeencollectedbefore
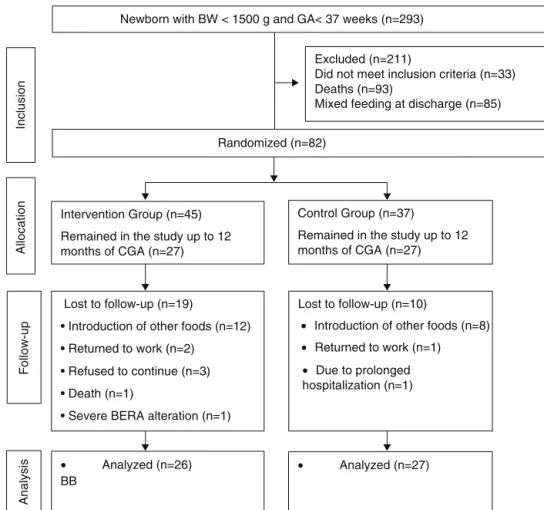
Newborn with BW < 1500 g and GA< 37 weeks (n=293)
Excluded (n=211)
Did not meet inclusion criteria (n=33) Deaths (n=93)
Mixed feeding at discharge (n=85)
Randomized (n=82)
Intervention Group (n=45)
Remained in the study up to 12 months of CGA (n=27)
Control Group (n=37)
Remained in the study up to 12 months of CGA (n=27)
Lost to follow-up (n=19)
• Introduction of other foods (n=12)
• Returned to work (n=2)
• Refused to continue (n=3)
• Death (n=1)
• Severe BERA alteration (n=1)
Lost to follow-up (n=10)
• Introduction of other foods (n=8)
• Returned to work (n=1)
• Due to prolonged
hospitalization (n=1)
• Analyzed (n=26)
BB
• Analyzed (n=27)
Inclusion
Allocation
Follow-up
Analysis
Figure1 Flowchartofpreterm infantrandomizationafter hospitaldischarge.23 BW,birthweight;GA, gestationalage;CGA,
correctedgestationalage;BERA,brainevokedresponseaudiometry.
Thesimplelinearregressionanalysiswasperformed,at first, in an unadjusted way only with the group variables (intervention or control)andscore onthe BayleyIII scale for the threedevelopmentdomains(motor, cognitive and language).Then,asecondanalysisofsimplelinear regres-sionwascarriedout,adjustedbythevariablesgender,birth weightandmotherasprimarycaregiverofthechild.
The variable corresponding to the scores of Bayley III Scaleinthethreedomainshaditsnormalityverifiedbythe Shapiro---Wilktest.
DatatabulationwascarriedoutinEpiInfo3.5software (Epi InfoTM, Centers for Disease Control and Prevention,
Atlanta,USA)and thestatisticalanalyses wereperformed in STATA 11 (StataCorp. 2009. Stata Statistical Software: Release11.CollegeStation,TX:StataCorpLP).
The researchwasapprovedthroughEdictN.302/10of the Research Ethics Committee of Hospital Universitário daUniversidadeFederaldoMaranhão.Thisstudyconsisted of a randomized clinical trial that followed the interna-tionalstandardsestablishedbytheCONSORTStatement.23
The study was registered with the Brazilian Registry of Clinical Trials (REBEC) of the Brazilian Ministry of Health underNo.U1111-1131-8413.It wasbasedontheresearch project ‘‘Multicomponentsupplementation of human milk fornutrition,growth,anddevelopmentofpreterminfants discharged from Neonatal Intensive Care Units’’, edict MS/CNPq/FAPEMA---No.477848/2007-9.
Results
During the study period, 293 preterm infants were born weighing less than 1500g. A total of 118 newborns did not meet the inclusion criteria and 93 died. Randomiza-tionwasperformedin82 infants.Fromtherandomization to the end of the intervention, 29 children were lost to follow-up.Mostofthelosseswereduetothemother’s dif-ficultyin maintaining EBF during the intervention period. These losses were statistically similar in both groups (p=0.12).TheBayleyIIIscale forthedevelopment assess-ment wasused toassess 53 children. The mean duration of the intervention was similar between the control and intervention groups: 5.84±0.54 months; 5.64±0.73 months; (p=0.42). Of the assessed children, 27 were in the control group and 26 in the intervention group (Fig.1).
Of the children who completed the intervention, 20 (37.7%) were boys and 33 (62.3%) girls; most infants had mothersagedbetween20and30years(60.4%)witheightto 11yearsofschooling(69.2%),andparentslivingin common-lawmarriage(60.4%).
Table 1 Distribution of perinatal characteristics of very low birth weight preterm infants. São Luís, Maranhão, Brazil, 2010---2014.
Variable Control Intervention
Mean±SD(f) Mean±SD(f)
Birthweight(g) 1250.2±231.1(27) 1309.4±181.9(26)
HCatbirth(g) 27.7±2.1(27) 27.7±1.4(26)
Birthlength(cm) 37.9±3.4(25) 38.0±2.5(25)
Weightrandomization(g) 1946.2±134.8(27) 1915.4±167.3(25)
GAatbirth(weeks) 30.6±2.4(26) 31.1±2.1(26)
SNAPPE-IIscore 14.9±10.6(25) 10.7±13.1(24)
Hospitallengthofstay(days) 50.2±17.5 43.3±15.4
%(f) %(f)
SGANB 38.5(10) 38.5(10)
Malegender 33.3(09) 42.3(11)
Maternalschooling<8years 50(21) 50(21)
SD,standarddeviation;f,frequency;HC,headcircumference;GA,gestationalage;SNAPPE-II,Scorefor NeonatalAcute Physiology-PerinatalExtension,VersionII;NB,newborn;SGA,smallforgestationalage.
The mother wasthe primary caregiver of the child in 73.6%ofcases.Only18.9%ofthefamilieshadmorethanone childlivinginthesamehousehold.Mosthomes(88.9%)had floorcovering,allowingthechildtoplayonthefloor.Just overhalfofthepopulation(52.8%)hadaccesstoeducational toys(fittingandstackingtoys).
Intheanalysisofpre-randomizationcharacteristics,the infants from the control and intervention groups showed similar profiles, except for the variables birth weight, SNAPPE-II,lengthofhospitalstay,andgender(Table1).
Theassessmentresultsofthe53childrenusingtheBayley IIIScaleinthethreedomainsofdevelopmentat12months ofCGAareshowninTable2.In16cases(30.8%),someNPMD delaywasobserved:nine(33,3%)inthecontrolgroupand seven(28%)intheinterventiongroup.
Intheanalysisbysimplelinearregression,therewereno statisticallysignificantassociationsinanyofthemodels cor-respondingtothethreedomains,althoughthemeanvalues of the Bayley Scale score among the supplemented chil-dren werealways higherthan those ofnon-supplemented children(Table3).
Table2 Normalityclassificationinthethreedevelopment
domainsassessedbytheBayleyScaleIII.SãoLuís,Maranhão, Brazil,2010---2014.
Domains Control Intervention
n % n %
Cognitive
Normal(>85) 24 (88.9) 22 (84.6)
Delayed(<84) 3 (7.4) 4 (15.4)
Language
Normal(>85) 21 (77.8) 23 (88.5) Delayed(<84) 6 (22.2) 3 (11.5)
Motor
Normal(>85) 24 (88.9) 24 (92.3) Delayed(<84) 3 (11.1) 2 (7.7)
Discussion
The comparisonof the NPMD assessed by Bayley III Scale between the control and intervention groups showed no statistically significant difference in any of the studied domains:motor,cognitive,andlanguage.The scorevalues were always higher in the intervention group than in the controlgroupinthethreedomains.
Only one study similar to the present trial has been identified in the literature to date. Both studied similar populationsconsistingofpreterminfants,dischargedfrom theNICU,withmeanbirthweightof1279g(presentstudy) and1287g,20 onEBFat hospitaldischarge,andboth
stud-iesaimedatusingmultinutrientsupplementationtoprovide more energy and protein intake. However, the way the supplementationwasadministereddifferedbetweenthem. Unlikethepresentstudy,Aimoneetal.20added
supplemen-tationtoapproximatelyhalfthedailyintakeofbreastmilk, whichrepresented10%moreenergyand20%moreprotein. Inboth studies,breastmilkwasobtainedby themother’s own milking. In the present trial, a special formula for preterminfantswasused(Pré-Nan®,Nestlé,Vevey,
Switzer-land),differentfromthatstudy,whichusedahumanmilk multinutrientfortifierinpowderform(SimilacHMfortifier, AbbottNutrition®,Ohio,USA).
Inbothtrials,therewasnoinfluenceofsupplementation onthedurationofEBF.
ThestudybyAimoneetal.20 assessedthedevelopment
at 18 months of CGA through the Bayley II Scale, finding nostatisticallysignificantdifferencesbetweengroups.They found, however, borderline associations in the language (0.053) and motor (0.067) domains. Similar percentages of children withdevelopmental delay werefound inboth groups.
The present study confirmed these results, as similar meanscoreswereobtained ontheBayleyIII Scaleinboth groups, in the threeevaluated domains, at 12 months of CGA,withnostatisticallysignificantdifference.Inthestudy by Aimone et al.,20 developmental delay was distributed
Table3 Adjustedandunadjustedanalysesoftheassociationbetweenbreastmilksupplementationanddevelopmentassessed bytheBayleyIIIscaleforthemotor,cognitive,andlanguagedomains.SãoLuís,Maranhão,Brazil,2010---2014.
Variables MeanBayley Unadjustedanalysis Adjustedanalysis
Coefficient 95%CI p Coefficient 95%CI p
Motordomain
Group
Control 94.55
Intervention 99.19 −4.64 −11.38to2.10 0.174 −4.019 −10.77to2.73 0.237
Birthweight 0.015 −0.0006to0.03 0.059
<1000g ≥1000g
Gender −3.21 −10.47to4.04 0.377
Male Female
Motherasprimarycaregiver 0.37 −2.03to2.78 0.755
Yes No
Cognitivedomain
Group
Control 95.55
Intervention 98.15 −2.59 −9.34to4.14 0.443 −2.92 −9.89to4.04 0.404
Birthweight 0.0014 −0.015to0.018 0.867
<1000g ≥1000g
Gender −3.26 −10.76to4.22 0.385
Male Female
Motherasprimarycaregiver 1.37 −1.11to3.85 0.273
Yes No
Languagedomain
Group
Control 94.14
Intervention 97.50 −3.35 −10.39to3.69 0.344 −3.25 −10.27to3.76 0.356
Birthweight 0.012 −0.004to0.029 0.153
<1000g ≥1000g
Gender −6.42 −13.97to1.11 0.093
Male Female
Motherasprimarycaregiver 0.66 −1.83to3.16 0.596
Yes No
Some factors mayhave contributedtothis result.The non-monthlycorrectionofsupplementationmayhave rela-tivelyreducedtheeffectofthe4gofsupplementoninfant development.Anotherpointthatmayhavecontributedto thereductionofdifferencesbetweenthegroups,whichis characteristicofinterventionstudieswithfollow-up,isthat, despitethecarefulandsystematicfollow-upofthedyads,it isvirtuallyimpossibletoensurethatthecorrectfrequency ofsupplementedbreastmilkadministrationfordomesticuse hadbeenfollowed.
Athirdfactoristhattheinfantsinbothgroupsprobably ingestedsimilaramountsofcaloriesandnutrients,asthose whodidnotreceivesupplementsupposedlywouldnursefor
alongerperiod,receivingalargeramount ofhindmilk.As forthoseinthesupplementedgroup,theywouldtendtobe satiatedearlier,nursingforashorterperiodoftimethanthe controlgroup.
Finally,althoughtheinitialsamplecalculationindicated thatthenumberofindividualswassufficienttodetect dif-ferencesbetween groups,it ispossible thattheextent of thesedifferenceshasbeenoverestimated,andlarger sam-plesarerequiredtodetectthem.
This study faced some obstacles. The difficulties of follow-upandtheintroductionofotherfoodsmadeit impos-sibletofollowalargernumberofchildren.The possibility ofbiasduetothelongtimebetweentheendofthe inter-ventionandthedevelopmentassessmentandthedifferent percentageoflossesinbothgroupswereminimizedbythe useofadjustedanalysis.
Consideringtheresultsofthisstudy,itcanbeconcluded that,accordingtothecurrentstateofknowledge,thereis stillnoevidenceofanassociation betweenpost-discharge multicomponent supplementation and NPMD of very low birthweightpreterminfants.
Funding
Financialsupportfor theequipmentandmaterialsusedin thestudy:MS/CNPq/FAPEMA---n.477848/2007-9.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.CaseyPH,Whiteside-MansellL,BarrettK,BradleyRH,Gargus R.Impactofprenataland/orpostnatalgrowthproblemsinlow birthweightpreterminfantsonschool-ageoutcomes:an8-year longitudinalevaluation.Pediatrics.2006;118:1078---86.
2.GeorgieffMK.Nutritionandthedevelopingbrain:nutrient pri-oritiesandmeasurement.AmJClinNutr.2007;85:614S---20S.
3.RodriguesMC,MelloRR,SilvaKS,CarvalhoML.Cognitive devel-opmentofprematurechildrenatschoolage:aproposalfora hierarchicalmodeltoinvestigateriskfactors.CadSaude Pub-lica.2011;27:1154---64.
4.StephensBE,WaldenRV,GargusRA,TuckerR,McKinleyL,Mance M,etal.First-weekproteinandenergyintakesareassociated with18-monthdevelopmentaloutcomesinextremelylowbirth weightinfants.Pediatrics.2009;123:1337---43.
5.EhrenkranzRA.EarlynutritionalsupportandoutcomesinELBW infants.EarlyHumDev.2010;86:21---5.
6.Bhatia J. Human milk and the premature infant. Ann Nutr Metab.2013;62:8---14.
7.Tanaka K, Kon N, Ohkawa N, Yoshikawa N, Shimizu T. Does breastfeedingin theneonatal periodinfluence thecognitive functionofvery-low-birth-weightinfantsat5yearsofage?Brain Dev.2009;31:288---93.
8.CameloJSJr,MartinezFE.Nutritionaldilemmasinextremely lowbirthweightinfantsandtheireffectsonchildhood, adoles-cenceandadulthood.JPediatr(RioJ).2005;81:S33---42.
9.LapillonneA,O’ConnorDL,WangD,RigoJ.Nutritional recom-mendationsforthelate-preterminfantandthepreterminfant afterhospitaldischarge.JPediatr.2013;162:S90---100.
10.Thomaz DM, Serafim PO, Palhares DB, Melnikov P, Venhofen L, Vargas MO.Comparison between homologous humanmilk supplementsandacommercialsupplementforverylowbirth weightinfants.JPediatr(RioJ).2012;88:119---24.
11.ArslanogluS,MoroGE,ZieglerEE,TheWapmWorkingGrooupOn Nutrition.Optimizationofhumanmilkfortificationforpreterm infants:newconceptsand recommendations.JPerinat Med. 2010;38:233---8.
12.Kuschel CA, Harding JE. Protein supplementation of human milkforpromotinggrowthinpreterminfants.CDSRev.2000. CD000433.
13.HenriksenC,HaugholtK,LindgrenM,AurvågAK,RønnestadA, GrønnM,etal.Improvedcognitivedevelopmentamongpreterm infantsattributableto earlysupplementationof humanmilk withdocosahexaenoic acid and arachidonic acid. Pediatrics. 2008;121:1137---45.
14.EhrenkranzRA,DusickAM,VohrBR,WrightLL,WrageLA,Poole WK.Growthintheneonatalintensivecareunitinfluences neu-rodevelopmentalandgrowthoutcomesofextremelylowbirth weightinfants.Pediatrics.2006;117:1253---61.
15.TudehopeDI.Humanmilkandthenutritionalneedsofpreterm infants.JPediatr.2013;162:S17---25.
16.AgostoniC,BuonocoreG,CarnielliVP,DeCurtisM,Darmaun D,DecsiT,etal.Enteralnutrientsupplyforpreterminfants: commentaryfromtheEuropeanSocietyofPaediatric Gastroen-terology,HepatologyandNutritionCommitteeonNutrition.J PediatrGastroenterolNutr.2010;50:85---91.
17.YoungL,EmbletonND,McCormickFM,McGuireW.Multinutrient fortificationofhumanbreastmilkforpreterminfantsfollowing hospitaldischarge.CDSRev.2013;2:CD004866.
18.O’ConnorDL,JacobsJ,HallR,AdamkinD,AuestadN,Castillo M,et al.Growth and developmentofpremature infantsfed predominantlyhuman milk, predominantlypremature infant formula,oracombinationofhumanmilkandpremature for-mula.JPediatrGastroenterolNutr.2003;37:437---46.
19.ZachariassenG, FaerkJ,GrytterC,EsbergBH,HjelmborgJ, MortensenS,etal.Nutrientenrichmentofmother’smilkand growthofverypreterminfantsafterhospitaldischarge. Pedi-atrics.2011;127:e995---1003.
20.AimoneA,RovetJ,WardW,JefferiesA,CampbellDM,Asztalos E,etal.Growthandbodycompositionofhumanmilk-fed pre-matureinfantsprovidedwithextraenergyandnutrientsearly afterhospitaldischarge:1-yearfollow-up.JPediatr Gastroen-terolNutr.2009;49:456---66.
21.FalcãoMC.Nutritionsupport intheillorpremature newborn infant.RevMed(SãoPaulo).2003;82:11---21.
22.RichardsonDK,CorcoranJD, EscobarGJ,LeeSK.SNAP-IIand SNAPPE-II:simplifiednewbornillnessseverityandmortalityrisk scores.JPediatr.2001;138:92---100.
23.SchulzKF,AltmanDG,MoherD,CONSORTGroup.CONSORT2010 statement:updatedguidelinesforreportingparallelgroup ran-domisedtrials.BMJ.2010;340:c332.
24.ArgolloN,LessaI,RibeiroS,AbreuKC,PintoJM,FariaRP,etal. Birthweightaspredictorfortheseverityofneonatalbrainwhite matterlesion.ArqNeuropsiquiatr.2006;64:287---94.
25.JohnstonMV,HagbergH.Sexandthepathogenesisofcerebral palsy.DevMedChildNeurol.2007;49:74---8.
26.Andrade SA, Santos DN, Bastos AC, Pedromônico MR, de Almeida-FilhoN,BarretoML.Familyenvironmentandchild’s cognitive development: an epidemiological approach. Rev SaudePublica.2005;39:606---11.