Research paper
Use of
1
H NMR STD, WaterLOGSY, and Langmuir monolayer techniques
for characterization of drug–zein protein complexes
F.F.O. Sousa
a,b, A. Luzardo-Álvarez
a, J. Blanco-Méndez
a,c, F.J. Otero-Espinar
a,c, M. Martín-Pastor
d,
I. Sández Macho
e,⇑aDepartment of Pharmacy and Pharmaceutical Technology, School of Pharmacy, University of Santiago de Compostela, Santiago de Compostela, Spain bDepartment of Biological and Health Sciences, School of Pharmacy, University Federal of Amapá, Macapá, Brazil
cInstitute of Industrial Pharmacy, University of Santiago de Compostela, Santiago de Compostela, Spain dUnidade de Resonancia Magnética, RIAIDT, University of Santiago de Compostela, Santiago de Compostela, Spain
eDepartment of Physical-Chemistry, School of Pharmacy, University of Santiago de Compostela, Santiago de Compostela, Spain
a r t i c l e
i n f o
Article history:
Received 10 April 2013
Accepted in revised form 14 July 2013 Available online 24 July 2013
Keywords:
Biopolymers Zein Maize protein Tetracycline Indomethacin Drug–protein complexes NMR
p–Aisotherm
Brewster angle microscopy
a b s t r a c t
Zein is a protein based natural biopolymer containing a large amount of nonpolar amino acids, which has shown the ability to form aggregates and entrap solutes, such as drugs and amino acids to form stable protein–drug complexes. In this work,p–Aisotherm, NMR, and Dynamic light scattering were used to detect the formation of protein aggregates and the affinity between zein and two different drugs: tetra-cycline and indomethacin. An effective interaction of zein and the two drugs was evidenced by means of liquid NMR reinforced by means of changes in the surface pressure byp–Aisotherm.
The effective interactions zein/drugs under air/water interface were evidenced as a change in the sur-face pressure of thep–Aisotherm of zein in the presence of drug solutions. The presence of tetracycline in the subphase decreased the area occupied by the monolayer at the expanded region until pressures of 12 mN/m were the areas became similar, but indomethacin produces an increment of the area in both expanded and collapsed region.
The feasible methodology employed, focused in the functionality of the protein–drug interaction, can be very promising in the drug delivery field.
Ó2013 Elsevier B.V. All rights reserved.
1. Introduction
The technological advances in drug delivery demand constant innovation on biomaterials and polymer science, mostly regarding special functionalities that may interest for the transport and re-lease of the ligand from the carrier systems. Recently, zein has gained attention because of its potential applications as a biopoly-mer [1], being considered in the development of drug delivery systems (DDS) such as microspheres, films, and other devices for the release of antimicrobials, anticancer, anti-coagulants, and also parasitic drugs, mainly due to its cost and good standards of biocompatibility[2–8].
Zein, a prolamin rich protein, is found in protein bodies in the endosperm of the corn kernel (Zea maysL.). There are four classes
of this protein as follows:
a
,b,c
, anddzein. The componenta
-zein represents 35% of these proteins that include two prominent bands of 22 and 24 kDa. Reducing SDS–PAGE analysis shows thatb-zeinhas three major bands of 24, 22, and 14 kDa. The amino acid se-quences have been published by Phillips et col[9]. Approximately 70% of zein is composed by glutamine (20%), leucine (20%), alanine (14%), and proline (9%), and due the high proportion of nonpolar amino acids, it is neither soluble in pure water or alcohol, but sol-uble in hydroalcoholic and acetone water mixtures. The amphiph-illic nature comes from the hydrophilic and hydrophobic residues distributed in the protein structure.
Its properties and structure allow zein to be a filmogenic mate-rial that is able to form stable, biodegradable, and biocompatible films with great potential for they use in DDS, implants, food pack-aging, and electrochemical devices (selective sensors, biosensors, etc.). The average hydrophobicity of zein is reported to be 50 times larger than albumin, fibrinogen, etc.[10]. The properties of zein films, such as biodegradability, mechanical resistance, water absorption, and barrier ability, largely depend on the interaction among protein, plasticizers, and other functional groups[11]. It has been shown the ability of zein to form aggregates and entrap
0939-6411/$ - see front matterÓ2013 Elsevier B.V. All rights reserved.
http://dx.doi.org/10.1016/j.ejpb.2013.07.008
⇑Corresponding author. Department of Physical-Chemistry, School of Pharmacy, University of Santiago de Compostela, Campus Vida s/n, 15782 Santiago de Compostela, Spain.
E-mail addresses: Phabio_oliveira@yahoo.com.br (F.F.O. Sousa), asteriam. luzardo@usc.es (A. Luzardo-Álvarez), jose.blanco.mendez@usc.es
(J. Blanco-Méndez), francisco.otero@usc.es (F.J. Otero-Espinar), manuel.martin@ usc.es(M. Martín-Pastor),bebel.sandez@usc.es(I. Sández Macho).
Contents lists available atScienceDirect
European Journal of Pharmaceutics and Biopharmaceutics
via Infrared spectroscopy, Circular dichroism (CD), SDS–PAGE electrophoresis, and Dynamic light scattering (DLS), suggesting that the monomer adopts an extended structure in solution, a result that is also consistent with an NMR qualitative study of H/D exchange [17–29]. However, the structure of this protein has not been fully elucidated in the literature neither by high resolution methods such as X-ray crystallography or liquid NMR.
Due to the complex structure of zein, its amphiphilic character and solubility characteristics, it is difficult to establish a homoge-neous distribution of drugs in zein-based DDS. Drugs are able to establish interactions with zein and form complexes[17]; thus, gaining insight into these interactions would improve in the devel-opment of zein devices, concerning the drug distribution or their release behavior. Its amphiphilic nature also permits to use
p
–A isotherm to study the interaction in the air/water interface be-tween drugs and zein. Most recent, advances in NMR techniques have provided tools to probe specificity, affinity, and structural as-pects of receptor–ligand interaction[30], an adequate approach to understand the molecular recognition between drugs and biomaterials.Our main focus in this paper is to characterize by the aforemen-tioned NMR,
p
–A isotherm techniques, and Dynamic light scattering the interactions of zein with small molecules of pharma-cological interest and study the potential of zein as biomaterial to elaborate multiparticulate drug delivery systems.2. Materials and methods
2.1. Materials
Zein isolated from maize and tetracycline hydrochloride (TCL) were purchased from Sigma. Indomethacin (IND) was obtained from Guinama. Ultrapure water was obtained by reverse osmosis (Millipore Corp.) and purified with a 0.22
l
m zetapore filter. The resistivity of the purified water was checked and kept at 18 MXcm. All the other reagents were pure grade and used asre-ceived. Deuterated solvents D2O 99.9%, CD3OD 99.8%, and CDCl3
99.8% were purchased from Eurisotop.
Inc.)[31].
2.2.2.1H NMR signal assignment
Proton NMR signal assignment of the pure drugs TCL or IND was performed using a combination of three standard NMR spectra: 1D
1H, TOCSY (Total Correlation Spectroscopy), and 2D NOESY
(Nucle-ar Overhauser Enhancement Spectroscopy) acquired in the DRX-500 spectrometer. For these experiments, a sample of the pure drug IND or TCL was prepared at 7.5 mM concentration in the mix-ture CDCl3:CD3OD 90:10 (v/v). The1H NMR signal assignment was
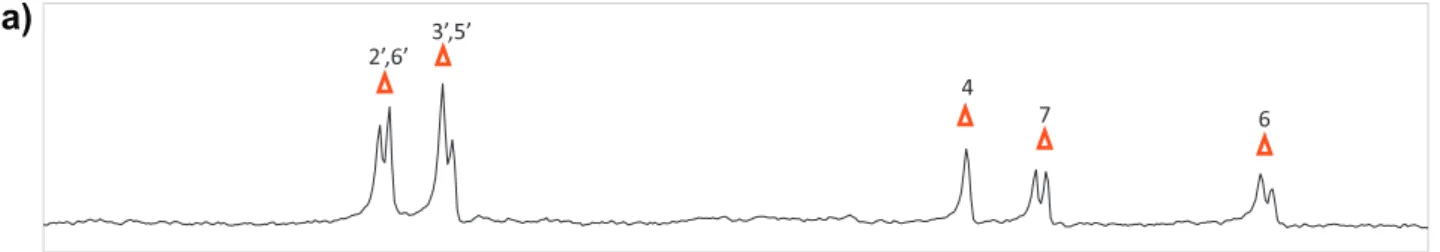
obtained from the analysis of the spectra assisted by the compari-son with previous assignments found in the literature for these molecules[34]and the theoretical prediction based in the molec-ular structure using MestreNovaÒsoftware. The signal assignment and numbering scheme used thorough the text for TCL and IND molecules is given inFig. 2.
2.2.3. Saturation Transferred Difference (STD) and water-ligand-observed via gradient spectroscopy (waterLOGSY)
These spectra were acquired in the Inova-750 spectrometer. Samples containing a mixture of a drug and zein protein, IND:zein and TCL:zein, were dispersed in H2O:CD3OD 4:5 v/v. For discerning
the role of water, control samples IND:zein and TCL:zein were prepared identically by replacing the protic solvent H2O by D2O
(i.e. mixture of solvents D2O:CD3OD 4:5 v/v). The drug and protein
concentrations used were 7.5 mM and 0.15 mM, respectively, equivalent to the ligand:protein molar ratio of50:1.
Samples IND:zein were prepared by dissolving IND in 0.5 mL CD3OD. After stirring, 0.1 mL of water (either D2O or H2O) was
added, and then, zein was added. Finally, 0.3 mL of water (either D2O or H2O) was added. The final product was transferred to an
NMR tube for measurement.
Samples TCL:zein were prepared by dissolving of TCL in 0.1 mL of water (either H2O or D2O). After stirring, 0.5 mL of CD3OD was
added and then zein was added. Finally, 0.3 mL of water (either H2O or D2O) was added. The final product was transferred to an
NMR tube for measurement. A sample was prepared with the mix-ture of the two drugs, TCL and IND, and zein protein dissolved in H2O:CD3OD 4:5 v/v. The total ratio drug:zein in this sample was
the same as in the other four samples.
Saturation Transferred Difference spectra (STD) [32,33] were acquired for each one of the four samples prepared drug:zein. The selective saturation was placed at 0.87 ppm, a region of the proton spectrum where exclusively appear signals of zein but not TCL or IND. It provided the so-called STDonspectrum. Besides, a
ref-erence 1D STD spectrum was acquired under the same conditions except for the selective saturation that was placed in an empty re-gion of the spectrum at 15 ppm, providing the so-called STDoff
spectrum. The total duration of the saturation was 2 s. It consisted in a train of low power selective Gaussian pulses of 50 ms sepa-rated by a 0.1 ms delay. The scans corresponding to the STDon
and STDoff experiments were interleaved during the acquisition,
and the correspondingly, FIDs are subtracted automatically by the phase cycling providing the STDoff–onspectrum that is finally
(a)
(b)
reported. Each STDoff–onspectrum was acquired with 128 scans and
a total duration of each scan of 4 s.
WaterLOGSY experiments[34,35] were acquired for the three samples dissolved in H2O:CD3OD 4:5 v/v. They are the two samples
that contain zein and a single drug, TCL or IND, and the sample that contains the mixture of two drugs and zein. The experiments were performed with a 180° inversion pulse applied over the water
signal at4.7 ppm by means of a sinc shaped selective pulse of 25 ms. The mixing time of the experiment was set to 2 s. Each waterLOGSY spectrum was acquired with 128 scans and a total duration of each scan of 5 s.
2.3. Compression isotherms
Isotherms were carried out using a surface balance single barrier NIMA 611 (U.K.) with total area 550 cm2 placed on an
anti-vibration table. Prior to experiments, the trough was cleaned with chloroform and ethanol and further rinsed with water. The monolayer stability was verified by monitoring the change in sur-face pressure while holding the area constant. The subphases were purified water, TCL, or IND solutions. Also, the temperature was kept at 20°C. Solutions of zein (0.1 mg mL1) were prepared in
chloroform/methanol 9:1 v/v, To record the
p
–Aisotherms, a vol-ume of 30l
L of the protein solution was deposited by means of a microsyringe (Hamilton, USA) at the air/water interface and al-lowed to stand for at least 10 min in order to ensure the complete evaporation of the solvent. The monolayers were compressed at a speed of 15 cm2min1. The surface pressure was measured withan accuracy of ±0.1 mN/m, using a Wilhelmy plate as a pressure
sensor, and the surface pressure,
p
, was recorded as a function of the area of the monolayer. To carry out relaxation measurements, monolayers were compressed at a speed of 15 cm2/min until thedesired surface pressure was achieved. The relationship between the time and the area was recorded as the surface pressure was maintained automatically.
2.4. BAM images and thickness of monolayers
Brewster angle microscopy (BAM) images and ellipsometric measurements were performed with a BAM 2 Plus (NFT, Göttingen, Germany) equipped with a 30 mW laser emittingp-polarized light at 532 nm wavelength which was reflected off the air/water inter-face at the Brewster angle (53.1°). This reflected beam pass through
a focal lens, into an analyzer at a known angle of incident polariza-tion and finally to a CCD camera to measure gray levels (GL) in-stead of relative intensity (I). The light intensity at each point in the BAM image depends on the local thickness and film optical properties. At the Brewster angle:I¼ jR2pj ¼Cd2, where Iis the relative intensity or relative reflectivity (defined as the ratio of the reflected intensity (Ir) and the incident intensity (I0),I=Ir/I0),
Rp is thep-component of the light, Cis a constant, anddis the relative film thickness. The relative reflectivity of the film was measured with a calibrated charge-coupled device (CCD) camera. Therefore, it was determined the relationship between the gray levels (GL) and the relative reflectivity (I). The procedure used for this calibration was described in previous articles [36], but cur-rently, with the BAM 2 Plus equipment, the calibration is automat-ically performed for each measurement at different shutter speeds,
Fig. 3.NMR spectra of the mixture IND:zein dissolved in H2O:CD3OD 4:5 v/v. (a)1H reference spectrum. (b) STDoff–onspectrum and (c) water-LOGSY spectrum. The signal selectively saturated or inverted is indicated with a ray symbol. The assignment of the signals of IND is indicated, it is based in the NMR assignment of the pure compound in
providing the relative thickness values of the monolayer as it is compressed. The lateral resolution of the microscope was 2
l
m, the shutter speed used was 1/50 s, and the images were digitalized (768572 pixels) and processed to optimize the quality (Iris v5.34 software, Christian Buil, France).2.5. Dynamic light scattering
Drug–protein aggregates were characterized by means of size and zeta potential measurements using Dynamic light scattering (ZetasizerÒNano-ZS, Malvern Instruments). In order to obtain the aggregates, we prepared a hydroalcoholic (70%) solution of zein of a concentration of 8 mM. From this solution, an aliquot of 60
l
L was dropped to a vial containing a drug solution of 8 mM (TCL or IND) and stirred vigorously. By this means, it was obtained a ratio zein:drug at approximately 1:10 each.3. Results and discussion
There are available several NMR experiments for the detection or characterization of macromolecule-ligand or supramolecular-li-gand interactions that relies in the observation of the NMR signals of the ligand [32–34,37–39]. The Saturation Transfer Difference (STD)[32,33]and water-LOGSY[34,38]experiments are two sensi-tive and reliable experiments of this class. Both experiments are based in the Nuclear Overhauser Effect (NOE) and are able to detect ligand binding to a macromolecule or supramolecular aggregate in
the range of medium-to-weak affinity, corresponding to an equilib-rium dissociation constant (Kd) from
l
M to mM[33].The STD experiment provides a simpler access to the ligand binding epitope and to other relevant aspects such as the ligand binding affinity [40]. A previous study performed in our group had used the STD methodology and found that in the complexes TCL–zein and IND–zein, the aromatic ring of TCL and the aromatic ring containing chlorine of IND are, respectively, the most relevant parts of these drugs for its interaction with zein in D2O:CD3OD
solutions [17]. The same study had also determined that both drugs present medium-to-weak affinity for zein, whereas the affin-ity of IND is an order of magnitude higher than TCL.
The water-LOGSY experiment is an alternative method to STD for the detection of binding for ligands to macromolecular receptors[34,35]. It relies in the transference of NOE from the water resonance. In a sample containing a small molecule and a macromolecular receptor, the appearance in the spectrum of sig-nals of the small molecule with the same phase than the water peak proves that there is binding affinity among the two molecules and that water is present at the macromolecule binding site[41].
In this section, solution NMR methods are used to gain insight about the drug:protein mechanism of interaction and the role of the water co-solvent in the complexes TCL–zein and IND–zein. STD and water-LOGSY spectra were used to obtain information about the intermolecular interactions between a drug, TCL or IND, and zein under the relevant conditions in which the sample is forming a colloidal dispersion, as it is required for the formation
(b)
(a)
(c)
Fig. 4.NMR spectra of the mixture TCL:zein dissolved in H2O:CD3OD 4:5 v/v. (a)1H reference spectrum. (b) STD off–on spectrum and (c) water-LOGSY spectrum. The signal selectively saturated or inverted is indicated with a ray symbol. The assignment of the signals of TCL is indicated, it is based in the NMR assignment of the pure compound in
of monolayers. For this mean, samples IND:zein and TCL:zein were prepared in the mixture of solvents H2O:CD3OD 4:5 v/v. To discern
the role of water, two analog samples were also prepared replacing the water protic solvent (H2O) by deuterated water (D2O).
The STDoff–on spectra obtained for samples IND:zein and
TCL:zein by placing the on-saturation in the aliphatic signal of zein at0.87 ppm are given inFigs. 3 and 4, respectively. It is clear from the comparison of the spectra ofFig. 3b and c (orFig. 4b and c) that the STD signals corresponding to the drug are much weaker when the sample is prepared co-solvent H2O than when they are
pre-pared in co-solvent D2O. This unexpected result indicates that for
these colloidal samples, the water co-solvent remarkably affects to the mode of interaction of the drugs TCL or IND with zein. The most plausible interpretation suggests that the form of the water co-solvent, D2O or H2O, affects to the conformation of zein. Since
it is known that the strength of hydrogen-bonding interactions weakens in D2O respect to H2O, zein could be more unfolded in
D2O than in H2O co-solvent and therefore have different residues
exposed for the interaction with the drug. The STD spectra suggest that the direct interaction of the drug with zein is diminished in H2O respect to D2O co-solvent.
To obtain further information about the mode of binding of the drug, waterLOGSY spectra were acquired for the colloidal disper-sions drug:zein prepared with the H2O co-solvent. In the
water-LOGSY spectrum of samples IND:zein and TCL:zein (Fig. 5a and b), most of the signals of the drug have considerably intensity and the same phase as the water peak. This is an indication that with the H2O co-solvent, there is binding between the drug and
the zein mediated by a layer of water molecules that have relative long residence times attached to the hydrophilic surface of the pro-tein (bound water fraction). Qualitatively, considering the poor transference of saturation from the protein to the drug in the STDoff–on spectra obtained with the H2O co-solvent (Fig. 3a and
(b)
(c)
Fig. 5.Aromatic region of the water-LOGSY spectrum of colloidal samples drug:zein in the mixture of solvents H2O:CD3OD. (a) IND:zein, (b) TCL:zein, and (c) IND:TCL:zein in a ratio IND:TCL 1:1. The signals of TCL and IND are indicated with filled squares and open triangles, respectively.
0 5 10 15 20 25 30 0
5 10 15 20 25 30
IND TCL Water
A2/residue
(m
N/m
)
Water TLC IND
Water TLC IND
Water TLC IND
Water TLC IND
Fig. 6.Zeinp–Aisotherm and BAM images obtained in water and in presence of the
b), the mode of binding mediated by the water molecules must be predominant when the sample is prepared in H2O co-solvent.
A waterLOGSY competition experiment was performed with a sample containing the two drugs, TCL and IND, and zein in a colloi-dal dispersion in H2O co-solvent (Fig. 5c). This spectrum shows the
presence of the signals the two drugs with the same phase as the water peak. Moreover, the signals of each drug appear with similar intensity as that previously seen in the waterLOGSY spectrum of the sample containing each individual drug (Figs. 3 and 4). This re-sult indicates that the two drugs IND and TCL do not compete for the binding to zein in the colloidal dispersions with H2O
co-sol-vent, a very different situation of what it was observed by us in the co-solvent D2O [17]. The later suggests that the binding of
the drug in H2O co-solvent is rather unspecific, and possibly to a
number of water solvated and relative amphiphilic residues ex-posed at the surface of zein.
Zein molecules are able to extend in the A/W interface forming a stable monolayer. It is represented in Fig. 6, the compression curve obtained for this protein. The shape of the isotherm denotes
the existence of a liquid condensate state monolayer in the range of surface pressure between 12 mN/m and 27 mN/m, as indicated in the value of the compressibility modulus (C1
s ,Fig. 7)[2]which represents the slope of the
p
–Acurve. The area liftoff (where the surface pressure starts to increase) is 26 Å2/residue. The limit areaof the monolayer (extrapolated area to pressure 0) is 20 Å2/amino
acid residue. This is a typical value for proteins monolayers in room conditions when they are well extended in air/water inter-face (A/W) [42,43]. Assuming an average molecular weight of 27 kDa for the protein molecule and 113 Da and for each amino acid residue and a number of residues of amino acid by protein molecule of 239, the liftoff value of 26 Å2/residue corresponds
approximately to an area of 600 Å2/molecule[44]. In accordance
with Mitchell[45], a value of 26 Å2/residue for the area liftoff
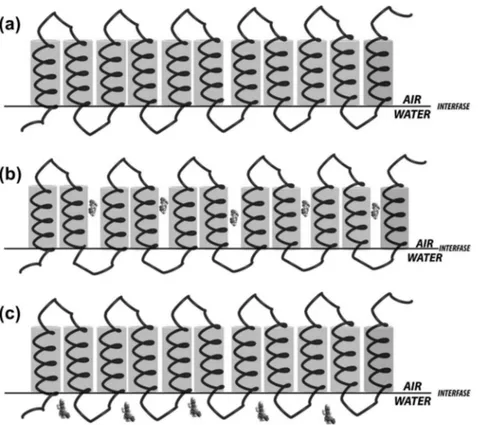
cor-responds to the area of zein molecules when a reorientation of the side chains occurs due to the effect of the monolayer com-pression: polar residues are oriented toward the water subphase under the main chain and nonpolar residues toward the air (Fig. 8a).
0 60 120 180 240 300 360 420 480 540 600 0
5 10 15 20
0 5 10 15 20 25 30 water
TCL IND
Isoterm-time
Thickness-time
Time (sec.)
thickness (nm)
(m
N
/m
)
Fig. 7.Compressibility values and thickness–time curves of zein molecules extends in the air/water, air/IND and air/TCL solution interface.
Above the equilibrium extension pressure, estimated as 19.5 mN/m[45], an inflection in the
p
–A isotherm is observed, remaining relatively stable until pressures of 24.5 mN/m. This behavior could be due that helical parties and unroll chains of zein that are rearranged to an upright position up to an area at which they start to compress, or due to the formation of a bilayer of coil parts. Such behavior was already described for cyclic molecules such as cyclosporine, which featured a ‘‘quasi plateau’’[46]. In or-der to determinate the transition values more accurately, the com-pressibility modulus was obtained[47], showing a reduction of about a quarter in the value of the area from the beginning until the end of theplateauwhich is incompatible with the formation of the bilayer. To gain more insight into the interpretation, the thickness-time curves were obtained (Fig. 7), showing a constant thickness during the entireplateau, indicating that a bilayer was not formed. BAM images taken throughout the plateau (Fig. 6) show a homogeneous system without appearance of collapsed monolayer, confirming that theplateaucan be attributed to a chain reorientation, forming ‘‘loops’’ and ‘‘tails’’ in the air/water inter-face. This behavior can also explain the quasi-reversible processes observed in the hysteresis cycles of compression curves (Fig. 9).Zein
p
–Aisotherm obtained when TCL and IND are added to the subphase showed significant changes compared to water alone (Fig. 6). These modifications are not related to the physical state of monolayer, but rather in the occupied area. TCL produces a declining in the area occupied by the monolayer at low pressure until values around 12 mN/m when the area becomes similar to the one obtained with pure water. Contrary to TCL, IND produces an increment in the area of the monolayer compared to water, along all the pressure values assayed. These differences in mono-layer area are indicative that both TCL and IND interact with zein, but possibly at different places in the structure of the protein, as it has been confirmed by the NMR studies.In the air interface, zein molecules oriented their polar residues toward water subphase and nonpolar residues toward the air. In this situation, IND molecules interact with the hydrophobic parts of the protein (Fig. 8b) bringing the area occupied by the monolayer of zein larger than the same in water alone. So the
Zein–drug aggregates were also studied in size and superficial charge. The results obtained using DLS show that aggregates have differences in diameter depending to the drug entrapped (Table 1) that is in accordance with the assembly of the drug in the protein complex proposed in monolayer experiments. Thus, it seems that IND is involved in the expansion of the polymeric chains of the pro-tein, while TCL keeps the morphology and size distribution closer to what is found with the zein aggregates in absence of drugs. In any case, the aggregates obtained were uniform in size, with poly-dispersity index (PdI) ranging from 0.027 to 0.070. Zeta potential was also determined found to be positive and quite similar in aggregates of zein and in IND/zein aggregates but lower in the case of TCL/zein aggregates.
4. Conclusion
The affinity between a drug and its carrier is a key parameter on the development of a drug delivery system since it affects impor-tant aspects such as loading efficiency, release, and distribution. The formation of a protein–drug complex is the interplay of a num-ber of molecular interactions that utterly contributes to the ther-modynamics of the process such as the establishment of hydrogen bonds, van der Waals forces[48], the release of solvent molecules and the entropic balance. The net contribution of all of them must be favorably in order to form the complex. Even when the structure of the two counterparts is known experimentally, the prediction of the affinity is still limited[49]. Some of the reasons that make difficult such prediction are the fact that the interactions does not only depends on the structure but also depends on the dynamics of both components. In addition, there are difficulties for the evaluation of the enthalpy and entropic contribution of the solvent. In this study is show that at difference that in solution
[17], were aromatic–aromatic interactions are the driving force for the stabilization of drug/zein complexes, in colloidal state the interactions drug–protein are rather unspecific and strongly dependent on drug hydrophilic–hydrophobic residues, the hydra-tion of the water solvated amphiphilic residues of zein and also the interaction with the hydrophobic parts of the protein in the air interphase. Moreover, molecular affinity and the protein/drug/ water interactions are important parameters affecting the incorpo-ration of the drug into the protein complex.
Current NMR and
p
–AIsotherms techniques are suitable tools to presume and determine binding interactions and thermody-namic parameters such as the affinity among molecules. This strategy can be useful for predicting the drug incorporation charac-teristics from a polymeric drug delivery system. This feasible methodology, focused in the functionality of the protein–drug complex, does not require any knowledge of its three-dimensional structure and can be very promising within the drug delivery field.Acknowledgement
Francisco Fábio Oliveira de Sousa was supported by the Brazil-ian Agency Capes, Grants No. 4668/06-5.
10 15 20 25
0 5
A2/residue
Fig. 9.Hysteresis cycle ofp–Aisotherms of zein molecules extends in the air/water
interface.
Table 1
Mean size and superficial charge properties of zein and zein/drug aggregates. Values are means ± SD (n= 3).
Formulation Particle size (nm) Zeta potential (mV)
Zein 288.0 ± 3.7 17.1 ± 0.1
Zein–TCL 380.0 ± 25.5 9.1 ± 0.1
References
[1]A. Gennadios, L.C. Weller, Edible films and coatings from wheat and corn proteins, Food Technol. 44 (1990) 63–69.
[2]Y. Matsuda, T. Suzuki, E. Sato, M. Sato, S. Koizumi, K. Unno, T. Kato, K. Nakai, Novel preparation of zein microspheres conjugated with P S-K available for cancer immunotherapy, Chem. Pharm. Bull. 37 (1989) 757–759.
[3]T. Suzuki, E. Sato, Y. Matsuda, H. Tada, K. Unno, T. Kato, Preparation of zein microspheres conjugated with antitumor drugs available for selective cancer chemotherapy and development of a simple colorimetric determination of drugs in microspheres, Chem. Pharm. Bull. 37 (1989) 1051–1054.
[4]X. Liu, Q. Sun, H. Wang, L. Zhang, J.Y. Wang, Microspheres of corn protein, zein, for an ivermectin drug delivery system, Biomaterials 26 (2005) 109–115. [5]H.J. Wang, Z.X. Lina, X.M. Liub, S.Y. Shenga, J.Y. Wang, Heparin-loaded zein
microsphere film and hemocompatibility, J. Control. Release 105 (2005) 120– 131.
[6]J. Dong, Q. Sung, J.Y. Wang, Basic study of corn protein, zein, as a biomaterial in tissue engineering, surface, morphology and biocompatibility, Biomaterials 25 (2004) 4691–4697.
[7]F.F. Sousa, A. Luzardo-Alvarez, A Pérez-Estévez, R. Seoane-Prado, J Blanco-Méndez, Development of a novel AMX-loaded PLGA/zein microsphere for root canal disinfection, Biomed. Mater. 5 (2010) 1–10. 055008.
[8]F.F. Sousa, J. Blanco-Méndez, A. Pérez-Estévez, R. Seoane-Prado, A. Luzardo-Álvarez, Effect of zein on biodegradable inserts for the delivery of tetracycline within periodontal pockets, J. Biomater. Appl. 2 (2012) 187–200.
[9]R.L. Phillips, B.A. McClure, Elevated protein-bound methionine in seeds of a maize line resistant to lysine plus threonine, Cereal Chem. 62 (1985) 213–218. [10]K.A. Tilley, R.E. Benjamin, K.E. Bagorogoza, B.M. Okot-Kotber, O. Prakash, H. Kwen, Tyrosine cross-links: molecular basis of gluten structure and function, J. Agric. Food Chem. 49 (2001) 2627–2632.
[11]Y. Wang, A.M. Rakotonirainy, G.W. Padua, Thermal behavior of zein-based biodegradable films, Starch – Stärke 55 (2003) 25–29.
[12]B.L. Hsu, Y.M. Weng, Y.H. Liao, W. Chen, Structural investigation of edible zein films/coatings and directly determining their thickness by, J. Agric. Food Chem. 53 (2005) 5089–5095.
[13]Q. Wang, P Geil, G. Padua, Role of hydrophilic and hydrophobic interactions in structure development of zein films, J. Polym. Environ. 12 (2004) 197–202. [14]E. Forgács, T. Cserháti, Z. Deyl, I. Miksík, Binding of substituted phenol and
aniline derivatives to the corn protein zein studied by high-performance liquid chromatography, J. Chromatogr. B, Biomed. Sci. 753 (2001) 79–86. [15]T. Cserháti, E. Forgács, Effect of pH and salts on the binding of free amino acids
to the corn protein zein studied by thin-layer chromatography, Amino acids 28 (2005) 99–103.
[16]N. Matsushima, G. Danno, H. Takezawa, Y. Izumi, Three-dimensional structure of maize alpha-zein proteins studied by small-angle X-ray scattering, Biochim. Biophys. Acta 1339 (1997) 14–22.
[17]F.F. Sousa, A. Luzardo-Álvarez, J. Blanco-Méndez, M. Martí-n-Pastor, NMR techniques in drug delivery: application to zein protein complexes, Int. J. Pharm. 439 (2012) 41–48.
[18]B.L. Hsu, Y.M. Weng, Y.H. Liao, W. Chen, Structural investigation of edible zein films/coatings and directly determining their thickness by FT-Raman spectroscopy, J Agric. Food Chem. 53 (2005) 5089–5095.
[19]M.R. Bugs, L.A. Forato, R.K. Bortoleto-Bugs, H. Fischer, Y.P. Mascarenhas, R.J. Ward, L.A. Colnago, Spectroscopic characterization and structural modeling of prolamin from maize and pearl millet, Eur. Biophys. J. 33 (2004) 335–343. [20]L.A. Forato, C. Bicudo Tde, L.A. Colnago, Conformation of alpha zeins in solid
state by Fourier transform IR, Biopolymers 72 (2003) 421–426.
[21]V. Cabra, R. Arreguin, R. Vazquez-Duhalt, A. Farres, Effect of temperature and pH on the secondary structure and processes of oligomerization of 19 kDa alpha-zein, Biochim. Biophys. Acta 1764 (2006) 1110–1118.
[22]C. Gao, M. Stading, N. Wellner, M.L. Parker, T.R. Noel, E.N. Mills, P.S. Belton, Plasticization of a protein-based film by glycerol: a spectroscopic, mechanical, and thermal study, J. Agric. Food Chem. 54 (2006) 4611–4616.
[23]L.A. Forato, R. Bernardes-Filho, L.A. Colnago, Protein structure in KBr pellets by infrared spectroscopy, Anal. Biochem. 259 (1998) 136–141.
[24]D.J. Sessa, A. Mohamed, J.A. Byars, Chemistry and physical properties of melt-processed and solution-cross-linked corn zein, J. Agric. Food Chem. 56 (2008) 7067–7075.
[25]K. Shi, J.L. Kokini, Q. Huang, Engineering zein films with controlled surface morphology and hydrophilicity, J. Agric. Food Chem. 57 (2009) 2186–2192. [26]D.M. Georget, S.A. Barker, P.S. Belton, A study on maize proteins as a potential
new tablet excipient, Eur. J. Pharm. Biopharm. 69 (2008) 718–726. [27]Y. Mizutani, Y. Matsumura, H. Murakami, T. Mori, Effects of heating on the
interaction of lipid and zein in a dry powder system, J. Agric. Food Chem. 52 (2004) 3570–3576.
[28]S. Subramanian, S. Sampath, Adsorption of zein on surfaces with controlled wettability and thermal stability of adsorbed zein films, Biomacromolecules 8 (2007) 2120–2128.
[29]P. Argos, K. Pedersen, M.D. Marks, B.A. Larkins, A structural model for maize zein proteins, J. Biol. Chem. 257 (1982) 9984–9990.
[30]B. Meyer, T. Peters, NMR spectroscopy techniques for screening and identifying ligand binding to protein receptors, Angew. Chem. Int. Ed. Engl. 42 (2003) 864–890.
[31]J.C. Cobas, F. Sardina, Nuclear magnetic resonance data processing. MestRe-C: a software package for desktop computers., J, Concepts Magn. Reson. 19A (2003) 80–96.
[32]M. Mayer, B. Meyer, Characterization of ligand binding by saturation transfer difference NMR spectroscopy, Angew. Chem. Int. Ed. 38 (1999) 1784–1788. [33]B. Meyer, T. Peters, NMR spectroscopy techniques for screening and
identifying ligand binding to protein receptors, Angew. Chem. Int. Ed. 42 (2003) 864–890.
[34]C. Dalvit, G. Fogliatto, A. Stewart, M. Veronesi, B. Stockman, WaterLOGSY as a method for primary NMR screening: practical aspects and range of applicability, J. Biomol. NMR 21 (2001) 349–359.
[35]E.C. Johnson, V.A. Feher, J.W. Peng, J.M. Moore, J.R. Williamson, Application of NMR SHAPES screening to an RNA target, J. Am. Chem. Soc. 125 (2003) 15724– 15725.
[36]P. Toimil, G. Prieto, J. Miñones Jr., J.M. Trillo, F.l. Sarmiento, Monolayer and Brewster angle microscopy study of human serum albumin/dipalmitoyl phosphatidyl choline mixtures at the air/water interface, Colloids Surf. B: Biointerf. 92 (2012) 64–73.
[37]C.B. Post, Exchange-transferred NOE spectroscopy and bound ligand structure determination, Curr. Opin. Struct. Biol. 13 (2003) 581–588.
[38]C. Dalvit, P. Pevarello, M. Tato, M. Veronesi, A. Vulpetti, M. Sundström, Identification of compounds with binding affinity to proteins via magnetization transfer from bulk water, J. Biomol. NMR 18 (2000) 65–68. [39]P. Thordarson, Determining association constants from titration experiments
in supramolecular chemistry, Chem. Soc. Rev. 40 (2011) 1305–1323. [40]J. Angulo, P.M. Enríquez-Navas, P.M. Nieto, Ligand–receptor binding affinities
from saturation transfer difference (STD) NMR spectroscopy: the binding isotherm of std initial growth rates, Chem. – Eur. J. 16 (2010) 7803–7812. [41]C. Ludwig, P.J.A. Michiels, X. Wu, K.L. Kavanagh, E. Pilka, A. Jansson, U.
Oppermann, U.L. Günther, SALMON: solvent accessibility, ligand binding, and mapping of ligand orientation by NMR spectroscopy, J. Med. Chem. 51 (2007) 1–3.
[42]D.F. Cheesman, J.T. Davies, M.L. Anson, T.E. John, Physicochemical and biological aspects of proteins at interfaces, in: Advances in Protein Chemistry, Academic Press, 1954, pp. 439–501.
[43]J.M. Trillo, E.I. Jado, S.G.a. Fernández, S.S. Pedrero, Monolayers of human serum albumin, Kolloid-Z. Z. Polym. 250 (1972) 318–324.
[44]I. Pezron, L. Galet, D. Clausse, Surface interaction between a protein monolayer and surfactants and its correlation with skin irritation by surfactants, J. Colloid Interface Sci. 180 (1996) 285–289.
[45]J.S. Mitchell, The structure of protein monolayers, Trans. Faraday Soc. 33 (1937) 1129–1139.
[46]T.S. Wiedmann, T. Trouard, S.C. Shekar, M. Polikandritou, Y.-E. Rahman, Interaction of cyclosporin A with dipalmitoylphosphatidylcholine, Biochim. Biophys. Acta (BBA) – Biomembr. 1023 (1990) 12–18.
[47]J.T. Davies, E.K. Rideal, Interfacial Phenomena, Academic Press, 1963. [48]F.A. Quiocho, Carbohydrate-binding proteins: tertiary structures and protein–
sugar interactions, Annu. Rev. Biochem. 55 (1986) 287–315.