31
ACUTE PANCREATITIS ASSOCIATED
WITH EXENATIDE AND LIRAGLUTIDE:
A META-ANALYSIS
Carlos Alves, PharmD
Health Technology Assessment (HTA) Centre, AIBILI, Coimbra. School of Pharmacy, University of Coimbra. CICS-UBI – Health Sciences Research Centre, University of Beira Interior, Covilhã Francisco Batel-Marques, PharmD, PhD
Health Technology Assessment (HTA) Centre, AIBILI, Coimbra. School of Pharmacy, University of Coimbra
Ana Filipa Macedo, PharmD, PhD
CICS-UBI – Health Sciences Research Centre, University of Beira Interior, Covilhã
Abstract
Aims: The association between GLP-1 receptor agonists (GLP-1 RA) and acute pancreatitis (AP) has been a matter of discussion. Post-marketing cases of AP were reported to FDA, which recommended a product labelling change for exenatide in 2009. This meta-analysis was conducted in order to evaluate the risk for AP associated with both GLP-1 RA, exenatide and liraglutide, in type 2 diabetic patients.
Study type: Meta-analysis of longitudinal studies.
Local: Health Technology Assessment (HTA) Centre, AIBILI, Coimbra, Portugal
Methods: Medline, Cochrane Library and clinicaltrials.gov were searched in order to identify RCTs or observational studies evaluating exenatide or liraglutide and reporting data on AP. Odds ratios (ORs) with 95% CIs were calculated using a random-effects model. Between-study heterogeneity was assessed using I2 statistics.
Results: Of the 178 possible eligible articles, 22 studies were included. Neither exenatide (OR 0.84 [95%CI 0.58 - 1.22], I2=30%) or liraglutide (OR 0.97, [95% CI 0.21 - 4.39], I2=0%) were associated with an increased risk for AP.
Conclusions: No association between GLP-1 RA and AP has been identified. However, and since AP is a rare adverse effect that can present a long-term latency, monitoring for AP in future studies evaluating GLP-1 RA remains advised.
Keywords: GLP-1 analogues, pancreatitis, meta-analysis.
Resumo
Objetivos: A associação entre os fármacos agonistas dos recetores GLP1 e a pancreatite aguda (PA) tem sido objeto de discussão. Notificações espontâneas de PA levaram a FDA, em 2009, a recomendar alterações no RCM e FI do exenatide. A presente meta-análise foi realizada com vista a avaliar a associação entre os fármacos agonistas dos recetores GLP1 e o aparecimento de PA em doentes diabéticos do tipo 2.
Tipo de estudo: Meta-análise de estudos longitudinais, experimentais (RCT) e observacionais (OBS). Local: Health Technology Assessment (HTA) Centre, AIBILI, Coimbra, Portugal.
Métodos: As bases de dados Medline, Cochrane Library e clinicaltrials.gov foram pesquisadas, com vista a identificar RCT ou OBS que estudassem o exenatido ou o liraglutido e avaliassem PA. Odds ratios (OR), IC 95%, foram calculados utilizando um modelo de efeitos aleatórios. A heterogeneidade entre estudos foi avaliada através do teste estatístico do I2.
Resultados: Dos 177 estudos identificados na pesquisa, 22 foram incluídos. Não foi identificado um risco acrescido para PA associado ao exenatido (OR 0,84 [95% CI 0,58-1,22], I2 = 30%) ou ao liraglutido (OR 0,97, [95% CI 0,21-4.39], I2 = 0%).
32
Introduction
Pharmacological treatment of type 2 diabetes mel-litus usually requires the sequential addition of antihyperglycemic agents1. Both the American
Di-abetes Assocation (ADA) and the European Asso-ciation for the Study of Diabetes (EASD) consen-sus algorithm for the treatment of type 2 diabetes mellitus recommends the initiation of metformin and a lifestyle modification program at the time of diagnosis1. Sulphonylureas, thiazolidinediones and
insulin can be subsequently added to the therapy1.
Glucagon-like peptide-1 (GLP-1) receptor agonists are a new class of blood-glucose lowering drugs indicated for the treatment of type 2 diabetes mel-litus2,3. The first in class, exenatide twice-daily
(BID) (Byetta™), was approved by the U.S. Food and Drug Administration (FDA) and by the Euro-pean Medicines Agency (EMA) in 2005 and 2006, respectively4,5. A once-weekly (QW) presentation
of exenatide (Bydureon™) was lately approved6,7.
Liraglutide (Victoza™) was authorized by EMA and FDA in 2009 and 2010, respectively8,9. During
the clinical development programmes, the GLP-1 receptor agonists have demonstrated the potential to address fasting and postprandial glucose con-trol with weight loss and low risk of hypoglycae-mia5,7,9. However, this new class of
antihypergly-caemic drugs has demanded some attention since potentially, although rare, serious adverse events have been associated with their use10.
Post-marketing spontaneous reports of acute pan-creatitis among patients treated with exenatide BID have been submitted to FDA’s Adverse Event Reporting System (FDA-AERS) since 200511.
Sig-nal generation aSig-nalyses of this database identified an increased risk for acute pancreatitis associ-ated with exenatide12,13. However, further
obser-vational longitudinal studies didn’t confirm such findings14-16. The post-marketing case reports led
to an update of the exenatide’ product labelling,
on request of FDA17. Acute pancreatitis was also
reported in randomized controlled clinical trials (RCTs) with liraglutide18.
This study was aimed at evaluating the risk of acute pancreatitis associated with GLP-1 receptor agonists, exenatide and liraglutide, by carrying out a meta-analysis based on both experimental and observational published studies.
Materials and Methods
Literature SearchThis meta-analysis was conducted according to the PRISMA criteria19. Medline and Cochrane
Li-brary were searched from its inception until April 27, 2012 in order to identify relevant studies which evaluated GLP-1 receptor agonists holding a mar-ket authorisation4-9. Text words, brand names and
manufacturer’s coded designations were used to identify the medicines. Only literature published in the English language was considered for inclusion in this analysis. In order to ensure that all studies were identified, a second electronic search in the Medline was performed. Search terms related with pancreatitis were combined with the medicines designations priori stated. The search terms were identified by consulting the MedDRA dictionary20.
Bibliographic references list of all relevant studies, meta-analyses and reviews were hand searched in order to identify additional eligible articles. The registration site clinicaltrials.gov was searched in order to identify all studies with available results that evaluated exenatide or liraglutide in type 2 diabetes mellitus. We did not seek to identify safe-ty information of GLP-1 receptor agonists beyond published studies. Studies that did not provide complete data on effect estimates (e.g. odds ratio or relative risk) for the outcomes of interest were excluded. The electronic databases search strategy is available in the Supplemental, Table 1.
Conclusões: Não foi identificada uma associação entre o tratamento com fármacos agonistas dos recetores GLP-1 e a ocorrência de PA. No entanto, e uma vez que a PA se considera um evento adverso raro que pode apresentar um longo tempo de latência, é aconselhável um cuidado acrescido na monitorização de segurança dos estudos que serão conduzidos no futuro.
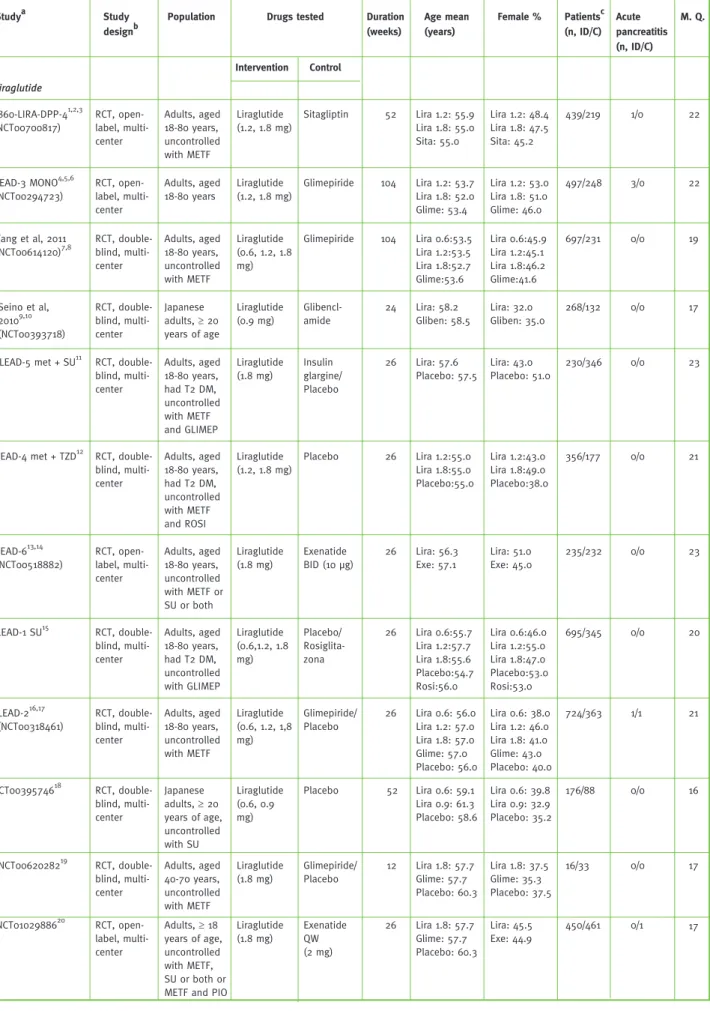
Table 1 - Characteristics of the studies included in the meta-analysis
Population Studya Study
designb
Drugs tested Age mean (years) Female % Patientsc (n, ID/C) Acute pancreatitis (n, ID/C) 1860-LIRA-DPP-41,2,3 (NCT00700817) Duration (weeks) M. Q. Intervention Control Liraglutide RCT, open-label, multi-center Adults, aged 18-80 years, uncontrolled with METF Liraglutide (1.2, 1.8 mg) Sitagliptin 52 Lira 1.2: 55.9 Lira 1.8: 55.0 Sita: 55.0 Lira 1.2: 48.4 Lira 1.8: 47.5 Sita: 45.2 439/219 1/0 22 LEAD-3 MONO4,5,6 (NCT00294723) RCT, open-label, multi-center Adults, aged 18-80 years Liraglutide (1.2, 1.8 mg) Glimepiride 104 Lira 1.2: 53.7 Lira 1.8: 52.0 Glime: 53.4 Lira 1.2: 53.0 Lira 1.8: 51.0 Glime: 46.0 497/248 3/0 22 Yang et al, 2011 (NCT00614120)7,8 RCT, double-blind, multi-center Adults, aged 18-80 years, uncontrolled with METF Liraglutide (0.6, 1.2, 1.8 mg) Glimepiride 104 Lira 0.6:53.5 Lira 1.2:53.5 Lira 1.8:52.7 Glime:53.6 Lira 0.6:45.9 Lira 1.2:45.1 Lira 1.8:46.2 Glime:41.6 697/231 0/0 19 Seino et al, 20109,10 (NCT00393718) RCT, double-blind, multi-center Japanese adults, ≥ 20 years of age Liraglutide (0.9 mg) Glibencl-amide 24 Lira: 58.2 Gliben: 58.5 Lira: 32.0 Gliben: 35.0 268/132 0/0 17
LEAD-5 met + SU11 RCT, double-blind, multi-center Adults, aged 18-80 years, had T2 DM, uncontrolled with METF and GLIMEP Liraglutide (1.8 mg) Insulin glargine/ Placebo 26 Lira: 57.6 Placebo: 57.5 Lira: 43.0 Placebo: 51.0 230/346 0/0 23
LEAD-4 met + TZD12 RCT, double-blind, multi-center Adults, aged 18-80 years, had T2 DM, uncontrolled with METF and ROSI Liraglutide (1.2, 1.8 mg) Placebo 26 Lira 1.2:55.0 Lira 1.8:55.0 Placebo:55.0 Lira 1.2:43.0 Lira 1.8:49.0 Placebo:38.0 356/177 0/0 21 LEAD-613,14 (NCT00518882) RCT, open-label, multi-center Adults, aged 18-80 years, uncontrolled with METF or SU or both Liraglutide (1.8 mg) Exenatide BID (10 μg) 26 Lira: 56.3 Exe: 57.1 Lira: 51.0 Exe: 45.0 235/232 0/0 23 LEAD-1 SU15 RCT, double-blind, multi-center Adults, aged 18-80 years, had T2 DM, uncontrolled with GLIMEP Liraglutide (0.6,1.2, 1.8 mg) Placebo/ Rosiglita-zona 26 Lira 0.6:55.7 Lira 1.2:57.7 Lira 1.8:55.6 Placebo:54.7 Rosi:56.0 Lira 0.6:46.0 Lira 1.2:55.0 Lira 1.8:47.0 Placebo:53.0 Rosi:53.0 695/345 0/0 20 LEAD-216,17 (NCT00318461) RCT, double-blind, multi-center Adults, aged 18-80 years, uncontrolled with METF Liraglutide (0.6, 1.2, 1,8 mg) Glimepiride/ Placebo 26 Lira 0.6: 56.0 Lira 1.2: 57.0 Lira 1.8: 57.0 Glime: 57.0 Placebo: 56.0 Lira 0.6: 38.0 Lira 1.2: 46.0 Lira 1.8: 41.0 Glime: 43.0 Placebo: 40.0 724/363 1/1 21 NCT0039574618 RCT, double-blind, multi-center Japanese adults, ≥ 20 years of age, uncontrolled with SU Liraglutide (0.6, 0.9 mg) Placebo 52 Lira 0.6: 59.1 Lira 0.9: 61.3 Placebo: 58.6 Lira 0.6: 39.8 Lira 0.9: 32.9 Placebo: 35.2 176/88 0/0 16 NCT0062028219 RCT, double-blind, multi-center Adults, aged 40-70 years, uncontrolled with METF Liraglutide (1.8 mg) Glimepiride/ Placebo 12 Lira 1.8: 57.7 Glime: 57.7 Placebo: 60.3 Lira 1.8: 37.5 Glime: 35.3 Placebo: 37.5 16/33 0/0 17 NCT0102988620 RCT, open-label, multi-center Adults, ≥ 18 years of age, uncontrolled with METF, SU or both or METF and PIO
Liraglutide (1.8 mg) Exenatide QW (2 mg) 26 Lira 1.8: 57.7 Glime: 57.7 Placebo: 60.3 Lira: 45.5 Exe: 44.9 450/461 0/1 17 33
Exenatide Wenten et al, 201221 Retrospective cohort study Patients with ≥ 9 months of enrolment without claims for prior pan-creatitis and a claim for a new antidia-betic between 03/2005 - 03/2009 Exenatide BID
Other Ads 212 Exe: 52.0 Others Ads: 51.0 46/802 16 Exe: 58.0 Others Ads: 53.0 24237/ 457797 Dore et al, 201122,23 (NCT01077323) Retrospective cohort study Patients with-out claims for prior pancre-atic disease and complete medical and pharmacy ben-efits between 09/2004 - 12/2007 Exenatide BID
Other Ads 123 Exe: UTD Others ADs: UTD Exe: 55.9 Others ADs: 49.0 25719/ 234536 11/223 16 Buse et al, 201124,25 (NCT00765817) RCT, double-blind, multi-center Adults, ≥ 18 years of age, had T2 DM, uncontrolled with IG with or without METF and/ or PIO Exenatide BID Placebo 30 Exe:59.0 Placebo:59.0 Exe:49.0 Placebo:36.0 137/122 0/0 24 Gill et al, 201026,27 (NCT00516074) RCT, double-blind, multi-center Adults, aged 18-75 years, had T2 DM, uncontrolled with METF or a THIAZ or both Exenatide BID (5 to 10 μg) Placebo 12 Exe: 57.0 Placebo:54. Exe: 32.0 Placebo:58.0 28/26 0/0 20
Garg et al, 201028 Retrospective cohort study Patients aged 18-63 years, with pharmacy and medical claims data for a continu-ous between 01/2007 - 06/2009 Exenatide BID
Others Ads 76 Exe:51.4 Others ADs: 52.9 Exe: 56.5 Others ADs: 42.8 6545/ 16244 22/65 15 DURATION-329,30 RCT, open-label, multi-center Adults, ≥ 18 years of age, uncontrolled with METF or METF and SU Exenatide QW (2 mg) Insuline glargine 84 Exe:58.0 IG:58.0 Exe:48.0 IG:45.0 233/223 1/0 23 DURATION-231 RCT, double-blind, multi-center Adults, ≥ 18 years of age, uncontrolled with METF Exenatide QW (2 mg) Sitagliptin/ Pioglitazone 26 Exe: 52.0 Sita: 52.0 Pio: 53.0 Exe: 44.0 Sita: 48.0 Pio: 52.0 160/331 0/2 25 LEAD-613,14 (NCT00518882) RCT, open-label, multi-center Adults, aged 18-80 years, uncontrolled with METF or SU or both Exenatide BID (10 μg) Liraglutide (1.8 mg) 26 Exe: 57.1 Lira: 56.3 Exe: 45.0 Lira: 51.0 232/235 0/0 23 Kadowaki et al, 200932 RCT, double-blind, multi-center Japanese adults, aged 20-75 years, had T2 DM, uncontrolled with SU alone or in addition to a BIG or a THIAZ Exenatide BID (2.5, 5, 10 μg) Placebo 12 Exe 2.5: 62.2 Exe 5: 60.7 Exe 10: 57.8 Exe 2.5: 29.7 Exe 5: 32.4 Exe 10: 37.8 111/40 0/0 21 34
Bunck et al, 200933,34 (NCT00097500) RCT, open-label, multi-center Adults, aged 30-75 years, uncontrolled with METF Exenatide BID (5 to 10 μg) Insuline glargine 52 Exe: 58.4 IG: 58.3 1/0 21 Exe: 36.1 IG: 33.3 36/33 NCT0057782435 RCT, double-blind, multi-center Japanese adults, aged 20-75 years, uncontrolled with SU alone or in addition with a BIG or a THIAZ Exenatide BID (5, 10 μg) Placebo 24 Exe 5: 58.5 Exe 10: 59.4 Placebo: 56.3 0/0 17 Exe 5: 31.9 Exe 10: 31.9 Placebo: 31.4 144/35 NCT0102988620 RCT, open-label, multicenter Adults, ≥ 18 years of age, uncontrolled with METF, SU or both or METF and PIO
Exenatide QW (2 mg) Liraglutide (1.8 mg) 26 Exe: 56.6 Lira: 56.7 1/0 17 Exe: 44.9 Lira: 45.5 461/450 a
References for the studies displayed in this table are listed in the Supplement 2. bConcealment assignment is referring to the most recent studies´ publi-cation. Studies could be initially double-blinded and then become open-label in the extension phase. c Number of patients included in the safety analysis; ID – Intervention drug; C – Control; n for “Acute Pancreatitis” and “Cancer” represents the number of patients; M.Q. – Methodological quality assessment; NR – Not reported; The studies LEAD-6 and NCT01029886 compared liraglutide with exenatide. Data from this study was not included in the GLP-1 receptor agonists drug class outcomes estimates; s.c. – subcutaneously; p.o. – orally; Lira – Liraglutide; Sita – Sitagliptin; T2 DM – Type 2 diabetes mellitus; Glime – Glimepiride; SU – Sulphonylurea; Gliben – Glibenclamide; ROSI – Rosiglitazone; Exe – Exenatide; METF – Metformin; GLIMEP - Glimepiride; PIA – Premixed Insulin Aspartat; UTD – unable to determin; IG – Insulin glargine; Sita – Sitagliptin; PIO – Pioglitazone; BIAsp – Biphasic insulin aspartat; BIG; - Biguanide; THIAZ; thiazolidinedione; Ins – Insuline; ADs: antidiabetic drugs.
Study Selection and Quality Assessment
Literature was searched and relevant studies were selected for further assessment. The studies in-clusion criteria were: 1- published in English lan-guage; 2- randomized, controlled clinical trials or longitudinal observational studies (case-control or cohort studies); 3- patients of all ages with type 2 diabetes mellitus; 4- comparison of GLP-1 recep-tor agonists with a placebo or active control (oral hypoglycaemic agents or insulin); 5- effect mea-sure estimates of GLP-1 receptor agonists use on pancreatitis. Only studies with duration of at least 12 weeks were included.
The quality of the retrieved studies was assessed using the checklist proposed by Downs and Black21, which can be used to assess both
random-ized and non-randomrandom-ized studies. Studies’ meth-odological quality was assessed as high, moderate or low when the total score was ≥ 20, from 10 to 19, and < 10, respectively. When more than one refer-ence was found for the same study, methodologi-cal quality evaluation was based on the total set of information. Two investigators scored the studies independently. Disagreement was resolved by dis-cussion and consensus with a third investigator.
Data Extraction and Outcomes Assessed
Data on study characteristics (design, demographic
characteristics of patients and number of subjects evaluated) and antihyperglycaemic therapy (dos-age and treatment duration) was extracted. Only cases of acute pancreatitis were considered in this meta-analysis.
Statistical Analysis
The Odds Ratio (OR) and its 95% confidence terval (CI) were calculated for the outcomes of in-terest, according to the Mantel-Haenszel weight-ing procedure. It was assumed that OR was an unbiased estimate of the relative risk (RR). When more than one adjusted effect estimate was report-ed, the most adjusted estimate was used. Crude ef-fect estimates were calculated whenever they have not been reported. For studies with more than one intervention-arm, the number of events and the number of exposures were added. The same was applied when studies with multiple controls were the case. Statistical heterogeneity was assessed by calculating a chi-square test and the I2 measure of
inconsistency22. We planned to pool data across
studies using the DerSimonian and Laird random-effects model23. This model was chosen since the
validity of tests of heterogeneity can be limited with a small number of component studies and it is more conservative than a fixed-effect model in the presence of between-studies heterogeneity.
36
When no events were reported in one or both groups, a continuity correction of 0.5 was added to each cell.
The publication bias was visually examined by a funnel plot and statistically evaluated by Egger’s regression asymmetry test24,25.
A sensitivity analysis was conducted to explore the influence of the following variables on the summa-ry estimates: studies’ design, studies’ methodologi-cal quality scores, the nature of the comparators (placebo or active control) and different GLP-1 receptor agonists dose regimens (weekly or daily). All reported p values are 2-sided with significance being set as less than 0.05.
Review Manager (RevMan) version 5.1.6 (Cochrane Collaboration, Oxford, UK) and Comprehensive Meta-analysis Version 2 (Biostat, Englewood, NJ, USA) were used for all statistical analysis.
Results
The flowchart of the search strategy criteria is pre-sented in Figure 1. The electronic databases search-es returned 3133 referencsearch-es. After searched for duplicates and reviewing the titles and abstracts, 2956 citations were refused and 177 bibliographic references were selected for further evaluation. Af-ter application of inclusion criAf-teria, 35 references were eligible for inclusion, corresponding to 22 studies. No further studies meeting the inclusion criteria were identified throughout the studies ref-erences lists’.
The main characteristics of the studies are pre-sented in Table 1. More than one article can be referred to one study. Results from the public database clinicaltrials.gov were able to comple-ment the results from published papers (e.g. follow-up results). Twenty-five studies met the inclusion criteria, of which three are retrospec-tive cohort studies, the remaining being RCTs. Two studies directly compared exenatide and liraglutideSuppl.13,14,20.
The quality assessment of the included studies is presented in Table 1. The methodological quality of 13 studies was found to be “high” while 9 studies were found as “moderate”.
Acute Pancreatitis
Thirteen studies of exenatide reported acute
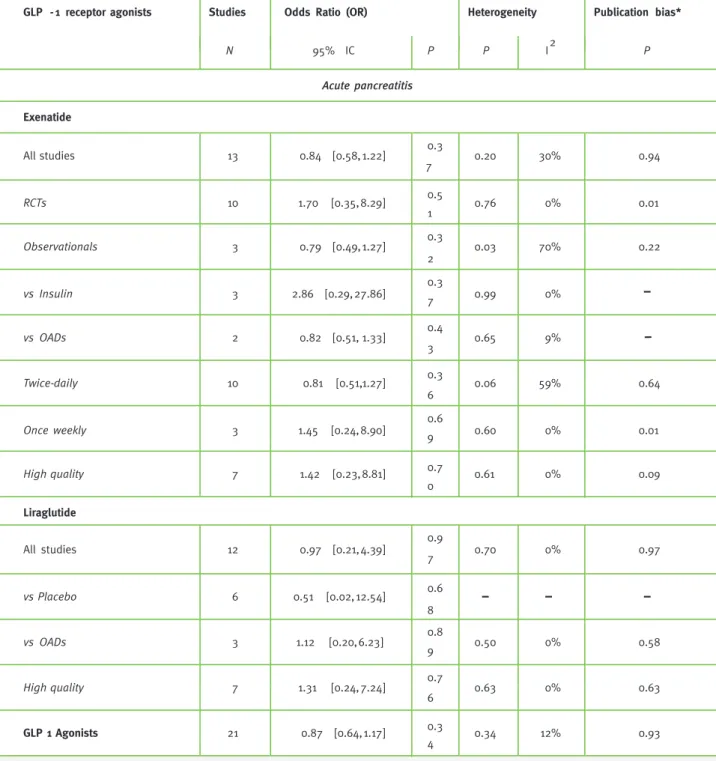
pancreatitis outcomes (Figure 2). Pool-ing their estimates yielded an OR of 0.84 (95% CI 0.58 - 1.22). Similar results were found in the sensitivity analysis stratified ac-cording to studies’ design for both RCTs (OR 1.70, 95% CI 0.35 - 8.29) and retrospective cohorts (OR 0.79, 95%CI 0.49 - 1.27) (Table 2). The results didn’t significantly change from the initial estimates when stratification according to different controls, exenatide dose regimens or when only high methodological quality studies were considered (Table 2).
Twelve liraglutide RCTs reported acute pancre-atitis as an outcome (Figure 2). The estimated OR for liraglutide and acute pancreatitis was 0.97 (95% CI 0.21 - 4.39). The sensitivity analysis according to different controls and the method-ological quality of the studies didn’t significantly change the results (Table 2).
Pooled estimates for both GLP-1 receptor ago-nists was found to be associated with a non-sta-tistically significant decrease in the risk for acute pancreatitis (OR 0.87, 95% CI 0.64 - 1.17).
Test of Heterogeneity and Publication Bias Assessment
Between-studies heterogeneity accounted for 30%, P = 0.20, of variation in treatment effect among studies that evaluated exenatide and reported acute pancreatitis outcomes (Table 2). Analysis of acute pancreatitis was stratified by study design, comparison drugs and exenatide therapeutic regi-men. Between-studies heterogeneity was deter-mined to be significant (I2=70%, P = 0.03) among the
observational studies but not between the RCTs (I2=0%, P = 0.76). Statistically non-significant
be-tween-studies heterogeneity was observed among studies using oral antidiabetic drugs as compara-tors (I2 = 9%, P = 0.65) and among studies which
evaluated exenatide administered twice-daily (I2 = 59%, P = 0.06). Between-studies
heterogene-ity accounted for 18%, P = 0.49 in treatment effect among studies of both GLP-1 receptor agonists. No significant between-studies heterogeneity was observed among studies evaluating lira-glutide and acute pancreatitis.
Egger’s asymmetry test was not statistically sig-nificant for the primary or and most subgroup
Figure 1 - Flow diagram of identification of studies for inclusion
analyses but was significant for the analysis among exenatide RCTs (P=0.01) and for once-weekly ex-enatide regimen studies (P=0.01) (Table 2). Subjec-tive evaluation of publication bias was based on the visual inspection of funnel plot. Few studies were considered for both the analyses, not allowing firm conclusions about the potential publication bias.
Conclusions
The results of this meta-analysis suggest that nei-ther exenatide nor liraglutide increase the risk for acute pancreatitis, when used in the treatment of type 2 diabetes mellitus. Our findings are in line with those reported in longitudinal observational studies which evaluated the risk for acute pan-creatitis associated with exenatide14-16. The rates
of acute pancreatitis in those observational studies were lower (less than 0.5%), indicating that this is a rare adverse event. Three retrospective cohort
studies evaluating acute pancreatitis associated with exenatide were included in this meta-analysis without significantly changing the risk. Our search didn’t find post-market observational studies for liraglutide.
Although evidence of association hasn’t been es-tablished between GLP-1 receptor agonists and acute pancreatitis, a few potentially confounding factors should be considered. Nausea, abdominal discomfort and vomiting are adverse drug reactions known to be associated with GLP-1 receptor ago-nists use5,7,9. Since these events are also symptoms
of acute pancreatitis, its recognition and appropri-ately diagnose may become difficult26. We only
in-cluded studies with patients diagnosed with type 2 diabetes mellitus. It has been documented that the incidence of acute pancreatitis is higher in diabetic patients, independent of the drug therapy27. Since
patients receiving GLP-1 receptor agonists are more
3133 references retrieved
1751 duplicates
1962 articles screened
1800 articles excluded 163 full-text articles
assessed for eligibility
142 full-text articles excluded: 5 evaluated obese patients 6 evaluated type 1 diabetes patients 10 evaluated healthy patients 1 evaluated metabolic syndrome 31 < 12 weeks interventional period 8 interin analysis of RCT(s) 2 analysis of disproportion 57 no relevant outcomes 22 uncontrolled studies 35 references included:
Exenatide: 15 references; 10 studies Liraglutide: 17 references; 10 studies Exenatide + Liraglutide: 3 references; 2 study
14 references from clinicaltrials.gov
4 studies not published as full-papers
10 duplicates
- 0 relevant studies found by hand search of reference lists,
* Egger’s regression asymmetry test. For overall pooled results, both LEAD-6 and NCT01029886 studies weren’t included.
38
Table 2 - Pooled odds ratios (OR) and 95% CIs of acute pancreatitis and cancer associated with GLP-1 agonists
likely to be at more advanced stages of the disease, which increases the risk for pancreatitis, the po-tential for confounding by indication may be in-creased, particularly when observational studies are the case. Based on spontaneous reports of adverse drug reactions, FDA recommended that the prescribing information of exenatide should include a warning about the risk of acute
pan-creatitis17. Liraglutide’ prescribing information
also includes a warning about the risk of pan-creatitis, without a specific mention to its on-set, type or severity28. This meta-analysis didn’t
find any increased risk for acute pancreatitis associated with both GLP-1 receptor agonists. Labelling change of exenatide regarding acute pancreatitis required by FDA was supported by
GLP -1 receptoragonists Studies Odds Ratio (OR) Heterogeneity Publication bias*
N 95% IC P P I2 P Acute pancreatitis Exenatide All studies 13 0.84 [0.58, 1.22] 0.3 7 0.20 30% 0.94 RCTs 10 1.70 [0.35, 8.29] 0.5 1 0.76 0% 0.01 Observationals 3 0.79 [0.49,1.27] 0.3 2 0.03 70% 0.22 vs Insulin 3 2.86 [0.29, 27.86] 0.37 0.99 0% vs OADs 2 0.82 [0.51, 1.33] 0.4 3 0.65 9% Twice-daily 10 0.81 [0.51, 1.27] 0.3 6 0.06 59% 0.64 Once weekly 3 1.45 [0.24, 8.90] 0.69 0.60 0% 0.01 High quality 7 1.42 [0.23, 8.81] 0.7 0 0.61 0% 0.09 Liraglutide All studies 12 0.97 [0.21, 4.39] 0.9 7 0.70 0% 0.97 vs Placebo 6 0.51 [0.02, 12.54] 0.6 8 vs OADs 3 1.12 [0.20, 6.23] 0.8 9 0.50 0% 0.58 High quality 7 1.31 [0.24, 7.24] 0.7 6 0.63 0% 0.63 GLP 1 Agonists 21 0.87 [0.64, 1.17] 0.3 4 0.34 12% 0.93
39
Figure 2 - Pooled odds ratios (OR) and 95% CIs of acute pancreatitis associated with GLP-1 agonists
* For GLP-1 receptor agonists overall pooled results, LEAD-6 and NCT01029886 studies were not included.
spontaneous reports. Therefore, if the increased risk exists, the meta-analysis is unable to iden-tify such risk, since spontaneous reporting data is not considered in the meta-analysis method-ology. Similarly the FDA required the market authorisation holder of liraglutide to conduct post-approval mechanistic animal studies along with a pharmacoepidemiologic study in order to better assess the risk of acute pancreatitis18.
Several studies were conducted aiming to ex-plain the mechanisms by which acute pancrea-titis could be developed. Butler el al. presented a theoretical model on which GLP-1 receptor agonists could amplify the pancreatic ductal replication already increased by type 2 diabe-tes mellitus or obesity29,30. This would increase
the risk for low grade chronic pancreatitis that predisposes to acute pancreatitis or pancreatic
carcinoma. However, the results of preclinical studies were contradictory, remaining unknown if GLP-1 receptor agonists are associated with a specific pharmacological mechanism that may cause pancreatitis31,32. In order to avoid
mis-classification bias, and since the results of pre-clinical studies have shown to be contradictory, only cases reported as acute pancreatitis were included in this meta-analysis.
This meta-analysis may be subject to several limitations. Of the 22 RCTs included, only one included the clinical evaluation of pancreatitis. Pancreatitis wasn’t defined as an initially out-come measure of RCTs. This event was recorded as serious adverse event. The absence of pancrea-titis as a pre-defined diagnostic criteria can lead to missing events. Moreover, patients enrolled in the RCTs are usually younger and with less
40
co-morbidities, therefore being at a lower risk for developing the adverse event studied in this meta-analysis when compared with the aver-age diabetic patient observed on routine clinical practice.
Residual confounding in the included observa-tional studies may extend to the results of this meta-analysis. Data was extracted from pub-lished studies and patient-level data wasn’t extracted. Therefore no more adjustments for confounding were possible. Demographic char-acteristics of patients included in observational studies may not be comparable with those from patients included in the RCTs. Clinical data such as type and duration of diabetes, glycae-mic or lipid control, alcohol consumption, and acute pancreatitis prior to baseline period must be considered in observational designs aimed at studying this outcomes. Additionally, the link-age between pharmacy submission of claims and consumption of the medication is assumed and not directly determined, although prior work suggests that medication exposure can be iden-tified from pharmacy claims16.
Different controls were identified in the RCTs included in this meta-analysis and they might be associated with different risks for acute pancrea-titis, such as the case of gliptins or pioglitazone. Because of the heterogeneity of comparators and the relatively small number of acute pancreatitis events reported in the studies, the stratification of the results at this level is difficult.
Publication bias with regard to acute pancreati-tis is difficult to assess with few studies. In two acute pancreatitis analyses, the results were sig-nificant. This may be the case of RCTs unpow-ered to detect rare events and subsequently cre-ating difficulties in adverse events assessments. In line with the current available published evi-dence, the present results didn´t identify an as-sociation between acute pancreatitis and GLP-1 receptor agonists exposure14-16. However,
phy-sicians, pharmacists, health professionals in general and patients should remain vigilant for episodes of acute pancreatitis and any events should be reported to the correspondent phar-macovigilance system.
References
1. Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Associa-tion for the Study of Diabetes. Diabetes Care. 2009;32:193-203. 2. Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly ver-sus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372:1240-1250. 3. Madsbad S, Schmitz O, Ranstam J, Jakobsen G, Matthews DR, NN2211-1310 International Study Group. Improved glycemic control with no weight increase in patients with type 2 diabetes after once-daily treatment with the long-acting glucagon-like peptide 1 analog liraglutide (NN221): a 12-week, double-blind, randomized, controlled trial. Diabetes Care. 2004;27:1334-1342. 4. US Food and Drug Administration. Centre for Drug Evaluation and Research. Byetta Medical Review, 2005. Available from URL www.accessdata.fda.gov/drugsatfda_docs/nda/2005/021773_ ByettaTOC.cfm [accessed 25 April 2012].
5. European Medicines Agency. Committee for Medicinal Pro-ducts for Human Use. European public assessment report (EPAR) for Byetta, 2006. Available from URL www.ema.europa.eu/ema/ index.jsp?curl=pages/medicines/human/medicines/000698/hu-man_med_000682.jsp&mid=WC0b01ac058001d124 [accessed 25 April 2012].
6. US Food and Drug Administration. Centre for Drug Evaluation and Research. Bydureon Medical Review, 2012. Available from URL www.accessdata.fda.gov/scripts/cder/drugsatfda/index. cfm?fuseaction=Search.LaLab_ApprovalHistory#apphist [acces-sed 25 April 2012].
7. European Medicines Agency. Committee for Medicinal Pro-ducts for Human Use. European public assessment report (EPAR) for Bydureon, 2011. Available from URL www.ema.europa.eu/ ema/index.jsp?curl=pages/medicines/human/medicines/002000/ human_med_001457.jsp&mid=WC0b01ac058001d124 [accessed 25 April 2012].
8. US Food and Drug Administration. Centre for Drug Eva-luation and Research. Victoza Medical Review, 2010. Avai-lable from URL www.accessdata.fda.gov/drugsatfda_docs/ nda/2010/022341s000TOC.cfm [accessed 25 April 2012]. 9. European Medicines Agency. Committee for Medicinal Products for Human Use. European Public Assessment Report (EPAR) for Victoza, 2009. Available from URL www.ema.europa.eu/ema/ index.jsp?curl=pages/medicines/human/medicines/001026/hu-man_med_001137.jsp&mid=WC0b01ac058001d124 [accessed 25 April 2012].
10. Drucker DJ, Sherman SI, Gorelick FS, Bergenstal RM, Sherwin RS, Buse JB. Increatin-based therapies for the treatment of type 2 diabetes: evaluation of the risks and benefits. Diabetes Care. 2010;33:428-433.
11. US Food and Drug Administration. FDA alert: Information for healthcare professionals: exenatide (marketed as Byetta), 2007.
41
Available from URL www.fda.gov/Drugs/DrugSafety/Postma-rketDrugSafetyInformationforPatientsandProviders/ucm124713. htm [accessed 25 April 2012].
12. Elashoff M, Matveyenko AV, Gier B, Elashoff R, Butler PC. Pancreatitis, pancreatic, and thyroid cancer with glucagon-like peptide-1-based therapies. Gastroenterology. 2011;141:150-156. 13. Raschi E, Piccinni C, Poluzzi E, Marchesini G, De Ponti F. The association of pancreatitis with antidiabetic drug use: gai-ning insight through the FDA pharmacovigilance database. Acta Diabetol. 2011 doi: 10.1007/s00592-011-0340-7.
14. Dore DD, Seeger JD, Chan KA. Use of a claims-based active drug safety surveillance system to assess the risk of acute pan-creatitis with exenatide or sitagliptin compared to metformin or glyburide. Curr Med Res Opin. 2009;25:1019-1027.
15. Garg R, Chen W, Pendergrass M. Acute pancreatitis in type 2 diabetes treated with exenatide or sitagliptin: a retrospec-tive observational pharmacy claims analysis. Diabetes Care. 2010;33:2349-2354.
16. Dore DD, Bloomgren GL, Wenten M, et al. A cohort study of acute pancreatitis in relation to exenatide use. Diabetes Obes Metab. 2011;13:559-566.
17. US Food and Drug Administration. MedWatch. Byetta (exe-natide) injection-detailed view: safety labelling changes appro-ved by FDA Centre for Drug Evaluation and Research, 2009. Available from URL www.fda.gov/Safety/MedWatch/SafetyIn-formation/ucm190571.htm [accessed 25 April 2012].
18. Parks M, Rosenbraugh C. Weighting risks and benefits of li-raglutide – The FDA’s review of a new antidiabetic therapy. N Engl J Med. 2010;362:774-777.
19. Moher D, Liberatu A, Tetzlaff J, Altman DG. The PRISMA Group (2099). Prefered Reporting Items for Systematic Re-views and Meta-analysis: The PRISMA Statement. PLoS Med. 6(6):e1000097. doi:10.1371/journal.pmed1000097.
20. MSSO Medical Dictionary for Regulatory Activities. Me-dDRA v14.0. Available from URL www.meddramsso.com/index. asp [accessed 25 April 2012].
21. Downs SH, Black N. The feasibility of creating a checklist for assessment of the methodological quality of both the randomised and nonrandomised studies of health care interventions. J Epide-miol Community Health. 1998;52:377-384.
22. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analysis. BMJ. 2003;327:557-560.
23. DerSimonian R, Laird N. Meta-analysis in clinical trials. Con-trol Clin Trials. 1986;7:177-188.
24. Publication bias. Borenstein M, Hedges LV, Higgins JPT, Ro-thstein HR: Introduction to Meta-analysis. Chichester, England: John Wiley & Sons, 2009.
25. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629-634.
26. Balani AR, Grendell JH. Drug-induced pancreatitis: inciden-ce, management, and prevention. Drug Saf. 2008;31:823-837.
27. Noel RA, Braun DK, Patterson RE, Bloomgren GL. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes. Diabetes Care. 2009;32:834-838.
28. US Food and Drug Administration. MedWatch. Victoza (lira-glutide [rDNA origin]) Injection: REMS - Risk of Thyroid C-cell Tumors, Acute Pancreatitis, 2011. Available from URL www.fda. gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHu-manMedicalProducts/ucm258826.htm [accessed 25 April 2012]. 29. Butler PC, Dry S, Elashoff R. GLP-1-based therapy for dia-betes: what you do not know can hurt you. Diabetes Care. 2010;33:453-455.
30. Butler AE, Galasso R, Matveyenko AV, Rizza RA, Dry S, Bu-tler PC. Pancreatic duct replication is increased with obesity and type 2 diabetes in humans. Diabetologia. 2010;53:21-26.
31. Koehler JA, Baggio LL, Lamont BJ, Ali S, Drucker DJ. Glucagon-like peptide-1 receptor activation modulates pancreatitis-asso-ciated gene expression but does not modify the susceptibility to experimental pancreatitis in mice. Diabetes. 2009;58:2148-2161. 32. Nachnani JS, Bulchandani DG, Nookala A, et al. Biochemical and histological effects of exendin-4 (exenatide) on the rat pan-creas. Diabetologia. 2010;53:153-159.
Acknowledgments
The authors would like to acknowledge Lilly Portugal - Produtos Farmacêuticos, Lda. and Novo Nordisk Portugal, for their help in providing all the articles which could not be found in the Portu-guese public libraries. Unpublished material was not requested from these companies.
Conflict of Interest
All the authors declare to have no conflict of interest relevant to the subject matter or materials discussed in the article. Carlos Alves is a PhD candidate and is supported by a research grant from Foundation for Science and Technology (FCT), Portugal, reference: SFRH/BD/64957/2009. Ana Filipa Macedo and Fran-cisco Batel-Marques didn’t receive any support to conduct this study.
Contributions
Carlos Alves performed the electronic search, analyzed data, inter-preted the results, contributed to the critical discussion and wrote the manuscript. Ana Filipa Macedo and Francisco Batel-Marques supervised data analysis, interpreted results, contributed to the critical discussion, reviewed and edited the manuscript. The opi-nion of Ana Filipa Macedo was requested in case of disagreement between Carlos Alves and Francisco Batel-Marques.