Ciências
Testing the application of new antioxidant
chemical formulations to prevent neuronal
degeneration
Marta Cristina Valério Afonso
Dissertação para obtenção do Grau de Mestre em
Biotecnologia
(2º ciclo de estudos)
Orientador: Doutora Ana Clara Cristóvão
Co-orientador: Doutora Ana Catarina Sousa
Declaro por minha honra que o trabalho aqui apresentado para obtenção do grau de mestre em Biotecnologia, resultado da investigação que realizei e que a utilização de contribuições ou textos de autores alheios estão devidamente referenciados obedecendo aos princípios e regras dos Direitos de Autor e Direitos Conexos.
_______________________________________ (Marta Afonso)
Acknowledgments
Terminada esta etapa da minha vida, não poderia deixar de agradecer a todos aqueles que me acompanharam e fizeram deste percurso uma magnifica etapa da minha vida.
À UBI e à Covilhã por me terem recebido durante estes 6 anos e por me terem permitido evoluir pessoalmente e profissionalmente.
À doutora Ana Clara Cristóvão por me ter dado a oportunidade de trabalhar com ela, por me ter mostrado o que é realmente fazer ciência e como a ver, por me ter apoiado em todos os momentos, me ter transmitido muitos dos seus conhecimentos e me ter mostrado o quão bom é fazer investigação.
À doutora Ana Catarina Sousa, por me ter dado a oportunidade de trabalhar com ela, por me ter mostrado quão versátil e bela pode ser a ciência e quão infinitas são as possibilidades de a encararmos e por me apoiar em todas as circunstâncias.
À Universidade de Aveiro, mais especificamente à Doutora Mara Freire e ao seu grupo de trabalho, Path, pela colaboração que permitiu a que este trabalho pudesse ser realizado.
Aos meus amigos de sempre, com quem vivi muitas aventuras e desventuras, sendo que todas elas me ajudaram a ser a pessoa que sou hoje. Pela nossa amizade, por todo o apoio, por todos os cafés, conselhos, viagens e guaridas.
Aos meus amigos que encontrei nesta cidade que é a Covilhã e que me mostraram que amigos também podem ser família, não de sangue, mas de coração. Obrigada por todo o apoio ao longo destes anos em todas as circunstâncias e por toda a amizade.
Aos meus colegas de laboratório por todo o apoio e por todas as partilhas.
À minha família, por todos os momentos que passamos juntos, por me apoiarem sempre, por estarem sempre disponíveis e por todo o dinamismo que com eles consigo ter.
Às minhas irmãs por serem as excelentes pessoas que são, cheias de vivacidade e alegrias! Por convosco poder partilhar tantos pedacinhos da minha vida e por me aturarem nos bons e maus momentos sem nunca me virarem as costas.
Abstract
Parkinson’s disease (PD) is a neurological disorder characterized by the degeneration of the nigrostriatal pathway and the consequent decrease levels of dopamine production, leading to both motor and non-motor symptoms. Although the exact cause behind the development of PD pathogenesis is not clarify, it is known that several factors individually or in combination, may have a role, being why this pathology is known to have a multifactorial etiology. Nevertheless, increased levels of oxidative stress have been described as a strong contributor factor to PD development. In this line, antioxidant compounds may be of great help to prevent the deleterious effect of oxidative stress on dopaminergic neurons and so capable to halt the progression of the disease. So far, several antioxidant compounds have shown to have limited effect in preventing dopaminergic neurodegeneration, probably due to the fact that several of those antioxidant compounds exhibit low solubility in aqueous solutions and consequently low bioavailability to the target organ. Moreover, many of these compounds require the use of vehicles that, in turn, may be neurotoxic, like ethanol or DMSO. Therefore, the development of new delivery systems capable to overcome these problems and prompt the concentration of antioxidant compound in the brain is urgent. In this line, the development of ionic liquids (IL) based drugs for pharmaceutical and medical application may represent a new era for therapeutic strategies.
In the present study, we tested the cytotoxicity of common antioxidant compounds like caffeine, gallate and apocynin converted to ILs ([Chol][Caf], [Chol][Gal], [Chol][Apo]) and also their precursors (caffeic and gallic acids and apocynin) in two cell lines (N27 neurons and N9 microglial cells) and in three primary cultures (microglia, astrocytes and microglia plus astrocytes cultures). Their capability to prevent neurodegeneration in different in vitro models of PD (MPP+, 6-OHDA and PQ) were further evaluated. Our cytotoxicity results demonstrated that [Chol][Caf] was more toxic than its precursor whereas [Chol][Gal] and [Chol][Apo] was less toxic. In what concerns their neuroprotection ability, we found that [Chol][Caf] can protect dopaminergic neurons against MPP+, while [Chol][Gal] and [Chol][Apo] can prevent 6OHDA toxicity in neurons. [Chol][Caf] induced glial cytotoxicity and the rest of new formulation don’t, since [Chol][Gal] and [Chol][Apo] not induce reduction in either astrocytes and microglia viability.
We have further evaluated in vivo the capability of [Chol][Apo] in protecting dopaminergic neurons against the toxicity of 6-OHDA. Our results revealed that the infusion of
x
Altogether our results show a promising new application of antioxidant ILs formulation launching a new avenue of research developments to make available a new therapy to halt the progression of PD.
Key words
Parkinson disease, Oxidative stress, Antioxidants, Ionic liquids, [Chol][Gal], [Chol][Caf], [Chol][Apo]
Resumo
A doença de Parkinson é uma doença neurológica caracterizada pela degeneração da via nigrostriatal e pela consequente diminuição dos níveis de dopamina produzidos levando a sintomas motores e não motores. Embora a causa exata por trás do desenvolvimento desta doença não estar esclarecida, é sabida da existência de vários fatores que individualmente ou combinados têm uma função, justificando o porque desta patologia ter uma etiologia multifactorial. Em todo o caso, níveis elevados de stress oxidativo têm sido descritos como um fator com fortes contribuições para o desenvolvimento da doença de Parkinson. Neste sentido, os compostos antioxidantes têm sido uma grande ajuda na prevenção dos efeitos deletérios do stress oxidativo nos neurónios dopaminérgicos e capazes de parar a progressão da doença. Até agora, muitos compostos antioxidantes têm mostrado um efeito limitado na prevenção da neurodegeneração dopaminérgica, provavelmente devido ao facto de muitos desses antioxidantes exibirem uma baixa solubilidade em soluções aquosas e consequentemente baixa biodisponibilidade para o órgão alvo. Além disso, muitos dos compostos requerem o uso de transportadores que, por sua vez, podem ser neurotóxicos, tal como o etanol e DMSO. Em todo o caso, o desenvolvimento de novos sistemas de entrega capazes de ultrapassar estes problemas e aumentar a concentração de compostos antioxidantes no cérebro é urgente. Neste sentido, o desenvolvimento de fármacos com base em líquidos iónicos para aplicação ao nível farmacêutico e médico pode representar uma nova era para as estratégias terapêuticas.
No presente estudo, testámos a toxicidade de compostos antioxidantes comuns como cafeina, galato e apocianina convertidos em líquidos iónicos ([Chol][Caf], [Chol][Gal], [Chol][Apo]) e também os seus precursores (ácido cafeico, ácido gálico e apocianina) em duas linhas celulares (neurónios N27 e células microgliais N9) e em três culturas primárias (microglia, astrócitos e culturas de microglia e astrócitos). A sua capacidade para prevenir neurodegeneração em diferentes modelos da doença de parkinson in vitro (MPP+, 6-OHDA e PQ) foi também avaliada. Os nossos resultados de citotoxicidade demonstraram que [Chol][Caf] era mais tóxico que o seu precursor enquanto que [Chol][Gal] e [Chol][Apo] era menos tóxico. No que diz respeito à sua capacidade de capacidade de neuroprotecção, descobrimos que [Chol][Caf] pode proteger os neurónios dopaminérgicos contra o MPP+, enquanto que [Chol][Gal] e [Chol][Apo] pode prevenir a toxicidade da 6-OHDA nos neurónios. [Chol][Caf] induziu citotoxicidade glial sendo que o resto das novas formulações não o fizeram, uma vez que [Chol][Gal] e [Chol][Apo] não induziram à redução da viabilidade da
xii
significativo do efeito neurotóxico da 6-OHDA em neurónios dopaminérgicos na SNpc. Além disso, descobrimos que [Chol][Apo] não tem efeitos neurotóxicos em neurónios dopaminérgicos quando usado sozinho, sugerindo que este tipo de formulações é seguro para aplicações neurológicas.
Em conjunto, os nossos resultados mostraram uma promissora nova aplicação de formulações antioxidantes de líquidos iónicos, lançando uma nova linha de desenvolvimentos de pesquisa para tornar disponível uma nova terapia para interromper a progressão da doença de Parkinson.
Palavras-chave
Doença de Parkinson, Stress oxidativo, Antioxidantes, Líquidos Iónicos, [Chol][Gal], [Chol][Caf], [Chol][Apo]
Resumo alargado
A doença de Parkinson é a segunda doença neurológica mais conhecida, associada à idade, sendo maioritariamente predominante em homens acima dos 60 anos. É caracterizada pela perda dos neurónios dopaminérgicos na substancia nigra e pela degeneração da via nigroestriatal e consequentemente, pela diminuição da produção de dopamina no estriado. Esta doença tem sintomas motores e não motores que se agravam com o passar do tempo. Não há uma causa bem definida para o surgimento desta doença, embora algumas tenham já sido propostas. Fatores ambientais, como exposição a pesticidas, e fatores genéticos, como mutações genéticas, têm sido fortemente associados ao desenvolvimento da doença de Parkinson, sendo ainda possível o envolvimento de uma interação combinada entre estes dois fatores. Mais especificamente, foram propostas duas hipóteses uma das quais sugere que o misfold e a agregação de proteínas é responsável pelo despoletar desta doença, enquanto que outra sugere a disfunção mitocondrial e o stress oxidativo inerente como responsáveis pela neurodegeneração característica da DP. No entanto sabe-se também que o desenvolvimento desta doença pode ser resultado do conjunto das duas hipóteses. i.e., o stress oxidativo pode induzir mutações/agregação proteica culminando na degeneração dos neurónios dopaminérgicos. Infelizmente não há ainda curas definidas para esta doença, mas várias terapias têm sido propostas para atrasar os sintomas, mas nenhuma delas tem conseguido responder a todos os sintomas. Várias abordagens, como moléculas antioxidantes tem sido sugerida como possíveis terapias para atrasar a progressão da doença de parkinson. Mesmo assim, a eficácia das formulações antioxidantes disponíveis tem-se mostrado limitada. Isto pode ser associado à sua baixa solubilidade e consequentemente à baixa biodisponibilidade no cérebro, limitando o seu uso como terapias que tenham como objetivos alcançar doenças neurológicas. O desenvolvimento de novos fármacos baseados em formulações de líquidos iónicos tem sido feito para ultrapassar esses problemas. Nisto, testamos o potencial de três de três formulações antioxidantes de líquidos iónicos, Choline Galato, Choline Cafeato e Choline Apocianato, para prevenir neurodegeneração em modelos pré-clínicos da doença de parkinson in vitro e in vivo. Primeiro caracterizamos o comportamento neurotóxico destas formulações de líquidos iónicos pela realização de curvas de EC50 na linha de células neuronais N27. A concentração não tóxica mais baixa (abaixo da concentração de EC50) foi então testada noutras células neuronais, como culturas primárias de microglia e astrócitos. Os resultados foram então comparados com os obtidos pelos precursores de cada liquido iónico antioxidante. Uma vez que o efeito citotóxico de cada composto foi caracterizado, testamos
xiv
verificou-se que cada um dos líquidos iónicos protegia em algum dos modelos, mais especificamente, [Chol][Gal] protegia no modelo de 6-OHDA, [Chol][Caf] protegia no modelo
de MPP+ e [Chol][Apo] protegia no modelo de 6-OHDA. Provou-se assim o efeito neuroprotetor
dos líquidos iónicos impelindo-nos a estudar os seus efeitos in vivo. Baseados nos resultados obtidos in vitro, seguimos para a avaliação in vivo da capacidade neuroprotetora da [Chol][Apo] usando modelo de rato da doença de Parkinson, 6-OHDA. A infusão continua de 50 mg/Kg/dia deste liquido iónico no ventrículo por 8 dias foi capaz de prevenir a neurodegeneração dopaminérgica na SNpc induzida pela 6-OHDA. Uma redução de 23% nos níveis de perda de células neuronais dopaminérgicas foi descoberto nos animais expostos à toxina e ao liquido iónico, comparativamente com aqueles expostos apenas com 6-OHDA. Além disso, não observamos um efeito neurotóxico da [Chol][Apo] quando infundida sozinha, tornando-a uma formulação segura para os neurónios dopaminérgicos.
Tudo junto, os resultados obtidos sugerem que as formulações antioxidantes de líquidos iónicos podem ser usadas no desenvolvimento de novas abordagens terapêuticas que visam alcançar a patogénese da doença de Parkinson. Além disso, a translação da química tecnológica dos líquidos iónicos para o desenvolvimento de fármacos, pode ser a próxima geração de estratégias que visam aumentar a biodisponibilidade e os problemas de atividade biológica levando a um aumento da eficácia terapêutica dos compostos.
Index
List of figures ... xvii
List of abbreviations ... xix
Chapter 1 – Introduction ... 3
1.1 Parkinson Disease ... 3
1.1.1 Neuropathology of PD ... 3
1.1.1.1 Genetic hypothesis ... 4
1.1.1.2 Environmental hypothesis ... 5
a. Experimental model using 1-methyl-4-phenylpyridinium (MPP+) ... 6
b. Experimental model using 6-hidroxy dopamine (6- OHDA) ... 6
c. Experimental model using Paraquat ... 7
1.1.2 Neuroinflammation ... 7
1.1.2.1 Role of glial cells: Microglia and Astrocytes ... 7
1.1.3 Oxidative Stress damage in PD ... 9
1.1.4 Current Therapeutic approaches in PD ... 9
1.2 Ionic Liquids ... 10
1.2.1 IL as component of antioxidant drug formulations for PD therapy ... 10
1.2.1.1 Choline Gallate ... 11
1.2.1.2 Choline Caffeate ... 11
1.2.1.2 Choline Apocynate ... 11
Chapter 2 – Materials and Methods ... 15
2.1 Cell cultures ... 15
2.1.1 Cell lines ... 15
2.1.1.2 Immortalized rat mesencephalic dopaminergic cell (N27 cell line) cultures and murine N9 microglial cell line cultures ... 15
2.1.2 Primary cell cultures ... 15
2.1.2.1 Astrocytes and Microglial primary cell culture from rat ventral midbrain ... 15
2.2 Cell treatments ... 16
2.2.1 Cytotoxicity tests... 16
2.2.2 Neuroprotection tests ... 17
xvi
3.1 Evaluation of Choline Gallate, Choline Caffeate and Choline Apocynate in the CNS cells
viability ... 23
3.1.1 Cytotoxicity of IL and their precursors ... 23
3.1.2 Neuroprotection of IL in N27 cell line against induced toxicity of in vitro models of PD, evaluated trough MTT assay ... 28
3.1.2.1 – Evaluation of the neuroprotective effect of [Chol][Gal] in in vitro models of PD ... 28
3.1.2.2 – Evaluation of the neuroprotective effect of [Chol][Caf] in in vitro models of PD ... 29
3.1.2.3 – Evaluation of the neuroprotective effect of [Chol][Apo] in in vitro models of PD ... 30
3.2 Evaluation of Choline Apocynate in an in vivo model of PD, 6-OHDA ... 31
Chapter 5 – Future Perspectives ... 41
Chapter 6 – References ... 45
Chapter 7 – Attachments ... 53
List of figures
Figure 1 - Neuropathology of PD... 4
Figure 2 - Pathways for neurodegeneration in PD ... 5
Figure 3 - Actuation mode of models of PD (MPTP, Paraquat and 6-OHDA).. ... 6
Figure 4 - Inflammatory mechanisms involved in PD ... 8
Figure 5 - Schematic representation of the experimental procedure used in N27 cell culture stimulation for neuroprotection tests with every IL and 6OHDA or MPP+ or Paraquat. ... 17
Figure 6 – Cytotoxicity of the ILs and their respective precursors in Rat dopaminergic neural cell line (N27 cells) measured by the MTT assay ... 24
Figure 7 - Choline Gallate and Gallic Acid cytotoxicity in N9 Microglial cell line and Primary Microglia, Astrocytes and Microglia plus Astrocytes measure by the MTT assay... 25
Figure 8 - Choline Caffeiate and Caffeic Acid cytotoxicity in N9 Microglial cell line and Primary Microglia, Astrocytes and Microglia plus Astrocytes measure by the MTT assay ... 26
Figure 9 - Choline Apocynin and Apocynin cytotoxicity in N9 Microglial cell line and Primary Microglia, Astrocytes and Microglia plus Astrocytes measure by the MTT assay ... 27
Figure 10 - Effect of [Chol][Gal] in in vitro models of Parkinson Disease ... 29
Figure 11 - Effect of [Chol][Caf] in in vitro models of Parkinson Disease ... 30
Figure 12 - Effect of [Chol][Apo] in in vitro models of Parkinson Disease ... 31
Figure 13 - Effect of [Chol][Apo] in an in vivo model of Parkinson Disease, 6-OHDA ... 32
Figure 14 - Apocynate (Apo): 1H NMR... 53
List of abbreviations
PD - Parkinson’s Disease ILs - Ionic liquids
SN – Substantia nigra ST – Striatum
SNpc – Substantia nigra pars compacta DA - Dopamine
MPTP – 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine MPP+ - 1-methyl-4-phenylpyridinium
6-OHDA – 6-hidroxydopamine or 2,4,5-trihydroxy phenylethylamine PQ - Paraquat
ROS – Reactive oxygen species DAT – Dopamine transporters BBB – Blood brain barrier MMP-3 – Metalloproteinase-3 NO – Nitric oxide
GFAP – Glial fibrillary acid protein Nox - NADPH oxidase
[Chol][Gal] – Choline Gallate [Chol][Caf] – Choline Caffeiate [Chol][Apo] – Choline Apocynate RT- Room temperature
xx
Chapter 1 - Introduction
1.1
Parkinson’s Disease
Chapter 1 – Introduction
1.1 Parkinson Disease
Parkinson Disease (PD) is a chronic, progressive, disabling 1 and the second most common neurodegenerative disorder, after Alzheimer disease. It is characterized by motor symptoms as bradykinesia (slow movements and reflexes), dyskinesia (involuntary muscle movements) and akinesia (absence, loss, or impairment of the power of voluntary movement) and non-motor symptoms as sleep disturbance and cognitive impairment which increase with disease severity 1,2. It is more common in people above 60 years, being age the biggest risk factor for developing Parkinson disease. Even though in lower number, this disease can also be developed by younger persons 2.
PD is caused by the degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) that projects into striatum and other nuclei within the basal ganglia and by an accumulation of lewy bodies composed mainly by α-Synuclein 3,4. Presently there is no treatment for PD, only possibilities to decrease it’s signs and symptoms 5.
1.1.1 Neuropathology of PD
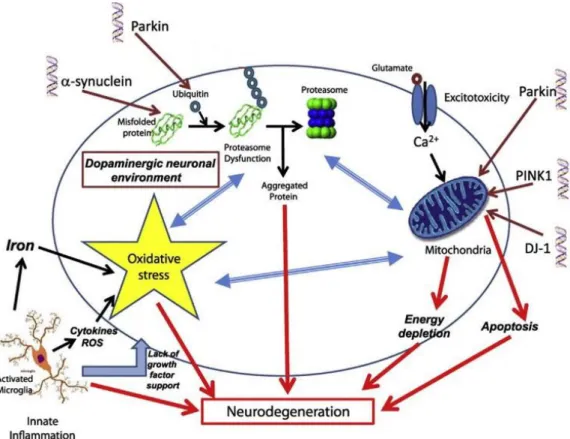
PD is characterized for affecting nigrostriatal dopaminergic pathway, being this pathway composed by dopaminergic neurons in SN and correspondent projected axons and nerve terminals in Striatum (ST). So, the loss of dopaminergic neurons in substantia nigra (SN) and consequently decrease of production of dopamine in striatum (ST)6. But dopaminergic cells are not the only cells affected, because, it is possible to find histologic abnormalities in other regions of the brain, with many other dopaminergic and non-dopaminergic cell groups 6. Additionally, it is possible to find in all the affected regions of brain, aggregated proteins, called Lewys bodies (LB), composed by α- synuclein, parkin, ubiquitin and neurofilaments 6.
The causes of the disease are still under investigation but environmental, genetics and endogenous factors are an important response 7. In this line of thought, there are two possible hypothesis that suggest two different ways of action of those factors, responding to the central issue that is the causes of the disease. One suggests that misfolding and aggregation of proteins are responsible for the death of dopaminergic neurons; the other hypothesis suggests that mitochondrial dysfunction and oxidative stress, including oxidized dopamine (DA) species are responsible for the emergence of this illness 2. It is possible as
4
Figure 1 – Neuropathology of PD. Adapted from 9. PD is characterized by affect nigrostriatal
dopaminergic pathway influencing dopaminergic neurons in SN and consequently the concentration of dopamine in ST. More specifically, in representation A there is a normal function and conditions of Nigrostriatal pathway and in B, is depicted the decrease in the axons and nerve terminal in ST as a consequence of loss of dopaminergic neurons in SN.
1.1.1.1 Genetic hypothesis
Between 10% to 20% of the cases of PD have genetic causes10. There are several genes and genetic loci involved in PD, being five genes frequently target as disease models namely α-synuclein, LRRK 2, Parkin, DJ-1 and Pink1 1. α-synuclein is a native unfold protein and the major component of Lewis Bodies commonly found in brains of PD patients. LRRK 2 is a large multi-domain protein that when mutated causes an autosomal familial form of PD. Parkin is a cytoplasmic and nuclear protein, that when mutated it usually doesn’t causes loss of dopaminergic neurons (DA) in SN, but it was found in cases of familial PD. DJ-1 is a molecular chaperon which function is to regulate anti-oxidant, anti-apoptotic, anti-inflammatory pathways and, it is involved in control of α-synuclein aggregation. When affected, it is possible to observe a decrease of DA in striatum, but not DA neurons death in SN. Pink1 (PTEN- induced putative kinase 1) is a Mitochondrial targeted serine-threonine kinase that suffers specific mutations it induces a form of early beginning autosomal PD, but with no observed DA neuron abnormalities or Lewis bodies formation 10,11. All of this genes are associated with mitochondria and its dynamics 12.
Only few studies prove the effectiveness of genetic factors as the principal factor of emergence of PD because, only a few mutations can be related with this illness, 90% of PD patients don’t have any family records of PD and when it happens, only increase appearance in family members of people who develop this at younger ages 1.
Figure 2 – Pathways for neurodegeneration in PD. Adapted from 2. There are many genes known to
affect normal function of the cell in PD, including α-synuclein, LRRK 2, Parkin, DJ-1 and Pink1. Each gene has one pathway of action in cells, Parkin, PINK1 and DJ-1 act in mitochondria, α-synuclein by misfolded proteins and Parkin by ubiquitin production. Activated microglia by exogeneous compounds induces oxidative stress influencing the other normal processes in cells. Neurodegeneration is a consequence of these processes.
1.1.1.2 Environmental hypothesis
As previously stated, genetic factors may in some cases be responsible for the development of PD. However, in many cases, the genetic predisposition coupled with environmental insults are the responsible for this illness 10. The environmental hypothesis gained strength with the discovery of toxins that induce PD, in fact using specific toxins it is possible to reproduce some PD features. These toxins which include 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or 1-methyl-4-phenylpyridinium ion (MPP+), 6-hidroxy dopamine (6- OHDA) and paraquat (PQ) are now models for PD 13.
6
Figure 3 – Actuation mode of models of PD (MPTP, Paraquat and 6-OHDA). Adapted from 14. MPTP,
6-OHDA, PQ are environmental toxins that induce PD. MPTP can transpose BBB, it is converted to MPP+ and
enters in the cells by dopamine transporters (DAT) and posteriorly induces ROS production by inhibition of complex one of the electron transport chain. 6-OHDA enters in the brain only by intracerebral injection, enters in cells bydopamine transporters (DAT) and induces oxidative stress. PQ can transpose BBB by neutral aa transporter and by redox cycling can induce oxidative stress. All these toxins consequently induce cell death.
a. Experimental model using 1-methyl-4-phenylpyridinium (MPP+)
In 1982 intravenous drug users exhibited severe and irreversible parkinsonism responsive to dopamine therapy. A toxin drug analog to narcotic meperidine (Demerol) was identified as 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) 15. This toxin transposes the blood brain barrier and it is converted in 1-methyl-4-phenylpyridinium ion (MPP+) which affects dopaminergic neurons because of its high affinity for dopamine transporters. Consequently, Complex 1 of the electron transport chain is inhibited by the binding of MPP+, concentrated in mitochondrial matrix, which causes an increase of production of reactive oxygen species (ROS) and cell death occurs 6,8. MPTP model doesn’t entirely represents PD disease because despite the DA cell death, this toxin doesn’t affect other regions that are affected in idiopathic PD, and because the symptoms don’t reveal progression. However, post mortem tissue tests reveal inflammation possibly due to oxidative stress.
b. Experimental model using 6-hidroxy dopamine (6- OHDA)
6-hidroxydopamine or 2,4,5-trihydroxy phenylethylamine is a toxin analog of noradrenaline and it was the first neurotoxin to be identified and to be associated with dopamine neuron death through the entry into the cell by dopamine transporters (DAT) and noradrenaline transporters. This toxin is unable to cross blood-brain barrier (BBB) but due to its hydrophilicity, in contact with neuronal cells it triggers the production of toxic species such as ROS 10,13,14. As disadvantage, this toxin affects other parts of the brain, not affected in PD 10.
c. Experimental model using Paraquat
N,N’-dimethyl-4-4’-bipyridinium dichloride known as paraquat (PQ), is an herbicide that has been implicated as a possible inductor of PD. It has a structure similar to MPP+, it is responsible for the production of ROS and consequently for DA neurons loss in SN 10. More specifically, in vivo studies demonstrate that PQ induces α-synuclein, aggregates formation, and microglial activation, and consequently, DA neuron cell death in basal ganglia.
1.1.2 Neuroinflammation
PD is characterized by a set of characteristics that contributes for motor and non-motor symptoms. Why neuronal degeneration in this disease occurs is not totally understood, but several cellular and molecular events have been proposed. Neuroinflammation has also been implicated in PD 16. This conclusion was obtained when in post-mortem brains of patients, pro-inflammatory mediators were found. More specifically, in PD the abnormal function of brain triggers the activation of microglia within the parenchyma and the release of numerous inflammatory mediators and changes astrocytes reactivity 17.
1.1.2.1 Role of glial cells: Microglia and Astrocytes
Evidences from post-mortem brains of patients with PD demonstrates that microglia the resident immune cells of the central nervous system (CNS), modulate cellular responses after DA cells injury, resulting in an amplification of neuroinflammatory responses and consequently decreasing neurodegenerative progress, leading to a cycle of neurodegeneration processes. Many molecules are responsible for microglial activation. They result from dying dopaminergic neurons and include α-synuclein aggregates, ATP, matrix metalloproteinase-3 (MMP-3) and others 16,18,19.
After neurodegeneration and microglial activation, α-synuclein is known for maintaining activated microglial cells accumulated around is molecular aggregates. At the same time, α-synuclein induce glial activation and release pro-inflammatory cytokines. With this is possible to affirm that possible neurotoxicity attributed to these molecules can be partially caused by activated microglia 19.
ATP is released from damaged neurons and astrocytes and it controls the migration of microglia to the injury and to the inflamed regions. Next ATP binds to the P2Y receptor and induces the production of several molecules such as IL-1β, TNF-α and nitric oxide (NO) 19.
MMP-3 is also responsible for microglial activation, it induces this activation by overexpression and consequently increases oxidative stress reaction 19.
8
cytokines (TNF-α, IL-1β, IL-6, IL-12, NO and ROS) whereas IL-4/IL13 induces M2 activation, producing cytokines as IL-4, IL13, IL-10 and TGF-β and consequently contradicting M1 and inducing tissue repair 19.
Figure 4 – Inflammatory mechanisms involved in PD. Adapted from 19. Aging, protein aggregation,
genetic mutations and environmental factors affects microglia and activates it. Consequently, microglia release pro-inflammatory mediators that communicates with astrocytes that then become reactive and consequently release IL-1β, TNFα and ROS that will act in healthy DA neurons. On the other side activated microglia releases pro-inflammatory mediators and ROS that affects directly healthy DA neurons. By these two ways, degeneration of DA neurons occurs. This degeneration induces the release of ATP, α-synuclein, ROS, NO and cell debris affecting healthy microglia. Microglia can also be affected by T cells.
Not only in microglia but all glial cells are affected and react tightly in PD. In the same way as microglia, astrocytes, which have been observed to protect neurons cultures from oxidative damage reaction in SN 20 are known as a neuropathological characteristic of
the disease 18. They respond to inflammatory stimulus such as LPS, IL-1β TNF-α and produce
pro-inflammatory cytokines. By activation of astrocytes, glial fibrillary acid protein (GFAP) expression is increased and consequently a hypertrophy of cell body and cell extensions occurs. It is possible to also occur astrogliosis, microglial activation and degeneration of DA neurons and motors neurons by a specific overexpression of mutant α-synuclein. On the other side, astrocytes may exhibit immune responses. Microglia is activated and releases inflammatory responses that activate astrocytes which, in turn, will amplify immunosignals 19.
1.1.3 Oxidative Stress damage in PD
As described above, PD is a neurodegenerative disease characterized in many cases by oxidative stress. This happens because cells can’t control ROS production and detoxification of reactive intermediates, contributing to cellular damage. However, ROS also have a fundamental function in cells. At low concentrations, ROS are responsible for regulation of intracellular signal transduction pathways by redox-dependent mechanisms, facilitating the process of tissue repair 21. ROS can be originated from many pathways, some directs as Fenton and Haber-Weiss reactions and some indirect pathways involving the activation of enzymes as NADPH oxidase. It is possible to consider ROS at three different forms, superoxide anion radical (O22-), hydroxyl radical (*OH) and hydrogen peroxidase (H2O2). Superoxide anion radical is produced in mitochondria in complex 1 and 3 and can transpose mitochondrial membrane, being reduced to hydrogen peroxidase. Hydrogen peroxidase can be generated in peroxisomes and converted to water. If peroxisomes are damaged, ROS are released to cytosol and oxidative stress occurs. Besides ROS, there are studies that suggest the existence of other type of reactive species, namely reactive nitrogen species 11.
Mitochondria is a fundamental organelle for cell function, being responsible for energy metabolism, calcium buffering, ROS generation and apoptosis regulation 12. As previously mentioned, one pathway by witch ROS is formed is through mitochondrial pathway, being as well a target for ROS. Consequently, being mitochondria normal function altered an alteration in ROS process and cell damage, related with neurodegeneration will occur 11. Mitochondria can be affected by environmental factors and by genetic factors, as mutations in α-synuclein, LRRK 2, Parkin, DJ-1 and Pink1 22.
1.1.4 Current Therapeutic approaches in PD
PD is a disease with no effective cure and therefore the treatments available are for symptom management. Unfortunately, numerous therapies induce adverse symptoms to patients and may react with other medications causing even mortality and thus risk and benefits must be carefully evaluated. It is also important to start the medication at initial stages of disease 23. Of the available treatments, most deal with treatment of motor symptoms. Levodopa-carbidopa, which is a precursor drug to dopamine, has low cost and exhibits high initial efficacy, however it has long term side effects including motor symptoms as instability and dyskinesia and in later stages of disease it may exhibit neuropsychiatric symptoms, being necessary the use of complementary medication. Dopamine agonists (DAs) are also used in the treatment of motor symptoms. DAs act in postsynaptic receptors to reduce dopamine turnover, they are less effective as levodopa and also exhibit some side
10
gene therapies) and cell based therapies (Fetal ventral mesencephalic tissue, embryonic, neural, mesenchymal and pluripotent stem cells) 25.
Therefore, it is important to find a therapy that can address the progression of PD 24. Recently some studies proved the efficacy of natural compounds and new synthetized substances in symptomatic treatments for neurogenerative diseases 26, however the solubility of these compounds is typically very low which limits their future use.
1.2 Ionic Liquids
The therapeutic efficiency of drugs depends of their bioavailability, more specifically, depends of their permeability and solubility. As described above, many drug formulations have low solubility being in many cases only soluble in organic solvents becoming necessary found strategies to avoid these solvents. In response to that problem, some alternatives are tested as for example ionic liquid (ILs) formulations 27,28.
Ionic Liquids (IL) has been then proposed as a promising alternative for drug formulations according as they are composed entirely of ions, non-volatile, can be design environmentally benign and acts in substrate solubility 29.
Ionic liquids (ILs) are molten salts who was an extraordinary flexibility and a vast number of possible combinations. They are solid at temperatures up until 100ºC and possible to modulate in nano and microscale levels of organization 28.
1.2.1 IL as component of antioxidant drug formulations for PD therapy
The attenuation of ROS generation, apoptotic signal or inflammatory response, are important mechanisms to control in neurodegenerative diseases 26. There are several studies that demonstrate the efficacy of antioxidants such as vitamins, minerals and phenolic compounds, as neuroprotectors in neurodegeneration. Some of these antioxidants have already been quantified in animal brains 26. This neuroprotection is achieved due to their antioxidant power that prevents the generation of ROS, oxidation of proteins and lipid peroxidation 26,27.
Antioxidants was already study in neurodegenerative diseases showing a promising therapeutic, however problems as low solubility decrease this possibility of being use. As example, we have phenolics compounds who have antioxidant and anti-inflammatory properties as gallic, caffeic, syringic, and vanillic acid but with low solubility or their absorption and transport in body fluids 27.
Due to the problems of solubility and for being more biocompatible, cholinium-based salts new formulations are a promising to overcome these problems. The cholinium-based salts tested in this work was Choline Gallate, Choline Caffeate and Choline Apocynate.
1.2.1.1 Choline Gallate
Gallic acid (3,4,5-trihydroxylbenzoic acid) is a phenolic compound present in plants and fruits extracts, including nuts, tea, grapes and sumacs. It exhibits strong antioxidant activities that can provide an effective response to oxidative damage 30,31. It is as well, anticarcinogenic, inflammatory, antibacterial, melanogenic, angiogenic, anti-mutation, anti-fungal and many other activities 32.
Gallic acid acts as an antioxidant by donating a hydrogen atom or by acting as electron donors, and thus acting directly in ROS. In an indirect way gallic acid can restore endogenous levels of antioxidants, which were before inhibited by high concentrations of ROS, and gallic acid can prevent as well lipid peroxidation by endogenous ROS control 30. However the therapeutic application of gallic acid has some problems; namely poor absorption, fast metabolism and consequently rapid drug elimination 33. Furthermore, the solubility of gallic acid is low and choline gallate ([Chol][Gal]) was produced to overcome these problems 27.
[Chol][Gal] was already study revealing antioxidant, anti-inflammatory and low ecotoxicity features and higher solubility in water than gallic acid 27.
1.2.1.2 Choline Caffeate
Caffeine is a central nervous system stimulant, commonly consumed in our society, it may cross the blood brain barrier, being already detected in the brain 34,35,36. Some studies have proposed caffeine as a possibility for PD treatment or prevention, although the mechanism by which caffeine acts are not yet fully understood. Some studies revealed that caffeine interfere with adenosine receptors responsible for cell signaling and possibly also with BBB structure and function 35. More specifically, caffeine is described as an inhibitor of lipid peroxidation and reducer of ROS production, mainly if there is a chronic caffeine intake. There are evidences that suggest caffeine is a neuroprotector in dopaminergic neurons, influencing the onset and progression of PD 34,36. In the field of adenosine receptors, caffeine act as an antagonist and decreases the activation of adenosine receptors, responsible for activation of adenylyl cyclase, which activates protein kinase A (PKA) and thus inducing an increase of extracellular calcium inside the cell and an increase in glutamate release conducing consequently to neuroinflammation 36,34. Being caffeic acid a phenolic compound who as defined above as being limited aqueous solubility, choline caffeate was formulated to respond to this problem 27.
12
reducing ROS production. This process is not totally understood. Apocynin is activated by ROS and microglia myeloperoxidase (MPO) inducing the formation of an apocynin radical who will interfere with thiols in NADPH oxidase, oxidizing them, and posteriorly thiols oxidizing agents will prevent NADPH oxidase activation and subsequently decrease levels of ROS 37,38,39. We already prove and described that apocynin reveal neuroprotection in vitro and in vivo models, however this compound have low solubility, becoming only soluble in organic solvents like DMSO and ethanol 40.
In line, the present project was design to understand if the use of IL chemical technology, could overcome the solubility and therefore the bioavailability of three antioxidant compounds and evaluate the neuroprotective potential of these new formulation in relevant pre-clinical models of PD. In this sense, this project contemplates three aims: 1) Evaluate the cytotoxic effect of the antioxidants IL formulations in N27 and N9 cell line and in primary cultures of mixed glial cells and in pure microglia and astrocytes cultures; 2) Analyze the neuroprotective effect of the new antioxidants IL formulations in in vitro models of PD; 3) To test the pharmacological efficacy of the new IL formulations in in vivo models of PD.
Chapter 2 – Materials and
Methods
2.1
Cell cultures
2.2
Cell treatments
2.3
MTT assay
2.4
In vivo experiments
2.5
Immunohistochemistry
Chapter 2 – Materials and Methods
2.1 Cell cultures
2.1.1 Cell lines
2.1.1.2 Immortalized rat mesencephalic dopaminergic cell (N27 cell line) cultures
and murine N9 microglial cell line cultures
N27 Rat dopaminergic neural cells were cultured in cell culture dishes 100mm x 20mm in RPMI-1640 medium (Sigma) containing 10% FBS, 1mL/L Pen/Strep (10 units penicillin, and 10 μg/ml streptomycin; Gibco), and kept in a humidified atmosphere of 5 % CO2 at 37 °C. For experiments, cells were trypsinized with a Trypsin solution (0.004g Trypsin in a solution of 0.04g EDTA dissolved in 200mL of PBS) at approximately 70% of confluence. For experiments, the cells were plated in in 96 and 24 well culture plates with a density of 1x104cells/well and 0.5x105cells/well, respectively. For treatment, cells were left to grown until 40 to 60% of confluency and subsequently two types of stimulus were applied.
Muricine N9 microglial cell line was stored and grown in the same conditions than N27 cell line, except for the fact that they were grown in cell culture flasks of 25 cm2.
2.1.2 Primary cell cultures
2.1.2.1 Astrocytes and Microglial primary cell culture from rat ventral midbrain
The animals used to prepare the astrocytes and microglial cell cultures were obtained following the approved protocols in accordance with the ethical requirements of animal research and with the European convention for protection of vertebrate animal used for research experiments or other scientifically proposes (Directive 2010/63/EU).Postnatal ventral midbrain was obtained from postnatal wistar rats with 2 to 5 days. The brain was extracted and the midbrain dissected and carefully peeled off of meninges. The tissues were mechanically dissociated using a scalpel and by the sequential passage through a 5 mL pipette, 1 ml micropipette (P1000) coupled with blue tips with size holes of 20G, 23G and 25G. Posteriorly the cell suspension was filtered through a 70µm mesh and the filtrate was centrifugated 10 min at 230G, room temperature and the supernatant removed. To the pellet 5 mL of Dulbecco’s Eagle’s medium (DMEM-HG; Sigma) with 10% FBS, 1mL/L
16
density, approximately 10 to 15 days after seed, culture flask was shacked for 2 hours at 200 rpm in medium culture, to detach microglial cells. The microglial cells were then collected, centrifuged for 8 min at 230g, the pellet re-suspended in medium and the cells were counted for plating in tissue culture plates. The remaining cells in the culture flask were trypsinized with a trypsin solution (0.004g Trypsin in a solution of 0.02g EDTA dissolved in 100mL of PBS) for approximately 15 minutes to recover astrocytes. Trypsinization was stopped by adding culture medium and the cell suspension collected and centrifuged for 8 min at 230g. The resultant pellet was re-suspended and cells counted. Both microglia and astrocytes were seeded at a density of 2.5x104cells/well and 5x104 cells/well in 96 and 48 well culture plates respectively. Cell cultures were kept allowed to rest and reach 40 to 60% confluence in a humidified atmosphere of 5 % CO2 at 37 °C before being exposed to treatments.
2.2 Cell treatments
The cells were exposed to ILs and they precursors, gallic and caffeic acid (Acofarma) and Apocynin. The ILs used in the present work, Choline gallate [Cho][Gal], Choline caffeate [Chol][Caf] 27 and Choline Apocynate [Chol][Apo], were synthetized and kindly provided by Dr. Mara Freire. Of note, the [Chol] [Apo] was synthetized de novo in Path laboratory, CICECO, University of Aveiro by Dr. Freire team and the respective RMN spectra is provided in attachments.
2.2.1 Cytotoxicity tests
First to evaluate IL and their precursor’s cytotoxicity, N27 cells were exposed to both precursors and their respective ILs, and the EC50 curves were obtained for each compound. Based on the previously reported characterization of [Cho][Gal] and [Chol][Caf] and respective precursors 27, the following concentrations were selected for the cytotoxic studies of these compounds in N27 cells. For Gallic Acid the concentrations were: 5.0, 24.0, 48.0, 72.0, 96.0, 385.0, 771.0, 1541.0 nM whereas for [Cho][Gal] the tested concentrations were: 3.0, 17.0, 34.5, 52.0, 69.0, 276.0, 553.0, 1106.0 nM. For caffeic acid concentrations were: 71.0, 189.0, 377.5, 566.0, 755.0, 1509.0 nM and for [Chol][Caf]: 45.0, 120.0, 240.0, 360.0, 480.0, 960.0, 3840.0 nM. The concentrations selected to evaluate the effect of [Chol][Apo] were based on previous reports showing the effect of apocynin in the brain40. In this case, the concentration selected were the same for both, apocynin and [Chol][Apo], being: 1.0, 2.5, 5.0, 10.0, 50.0, 100.0 µM and 5.0, 10.0, 15.0, 20.0 mM.
The effect of a 24 hr exposure of all the above mentioned compounds in N27 cells viability was evaluated using MTT assay.
In order to understand if our ILs formulations could have a cytotoxic effect on other cells of the CNS, we have also evaluated the effect of [Cho][Gal], [Chol][Caf], [Chol][Apo] and respective precursors in microglia (both N9 cells line and primary cultures), astrocytes and
mixed glial cells. For this, the concentrations used of each IL and precursors were selected based on the results of the EC50 curves obtained from N27 cells. In line, those concentrations were: for gallic acid 17.5nM, 24nM and 34.6nM; for [Chol][Gal] 17.5nM and 34.6nM; for caffeic acid 45nM, 71nM and 120nM; for [Chol][Caf] 45nM; for apocynin 5µM and 50µM; and for [Chol][Apo] 5µM and 50µM. In primary microglia and astrocytes cells and mixed astrocytes and microglia cultures only was study the major concentration talked above, except for [Chol][Caf] and caffeic acid that the concentration study was 45nM.
2.2.2 Neuroprotection tests
In order to investigate the putative neuroprotective effect of ILs, two different concentrations of each IL were selected from the EC50 curves and from the results obtained in microglia and astrocytes. In this way, N27 cells were pre-exposed to 17.0 and 34.5 nM of [Chol][Gal], 45.0 and 120.0 nM of [Chol][Caf] and 5.0 and 50.0 µM of [Chol][Apo] and the neuroprotective effect of each IL against the neurotoxicity of 6OHDA, MPP+ and PQ was
evaluated using MTT assay. The following concentrations of the neurotoxins used were: 50µM
of 6-OHDA, 1mM of MPP+ and 400µM of PQ. The experimental exposure paradigm is described
in Figure 6. ILs were applied 2h30 before the neurotoxins. For cell viability tests 24 hrs after the exposure to the neurotoxins 0.5 mg/ml of MTT was added to the cells and for the immunocytochemistry evaluations cells were fixed using buffered formalin and kept at 4ºC in PBS until immunostaining was performed.
2.3 MTT assay
Cell viability in the cytotoxicity and neuroprotection tests was evaluated using the MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium) assay. MTT simple and colorimetric assay that evaluates the living cells present in a culture, by assessing cell
Figure 5 - Schematic representation of the experimental procedure used in N27 cell culture stimulation for neuroprotection tests with each IL and 6OHDA or MPP+ or Paraquat toxins. At hour
0, ILs were applied and after 2h30 the toxin (6OHDA, MPP+ and Paraquat) were added. Stimulus was stopped after 24 hours.
Stop of Stimulus
Time (h)
2
3
24h
26
0
Toxin Stimulus
IL Stimulus
18
each well and incubated protected from light during 24h in the incubator in a humidified atmosphere of 5 % CO2 at 37 °C. After this period, the MTT solution was carefully removed (in order to prevent the removal of the formazan crystals produced by viable cells) and 10% SDS was added to each well. The multiwell plate was gently shaked until all crystals were dissolved. Finally, the absorbance of the resultant solution was measured at 570 nm in a Microplates spectrophotometer (xMark Microplate Absorbance Spectrophotometer, BIO-RAD).
2.4 In vivo experiments
All animal experiments were performed in agreement with the institutional animal house regulations as well as with the national and European Community guidelines (86/609/ECC; 2010/63/EU). Male C57BL/65 mice (10 to 12 weeks) were kept in a temperature/humidity controlled environment under 12h light/dark cycle with free access to food and water. These experiments aimed to evaluate the possible neuroprotective effect of [Chol][Apo] against 6-OHDA dopaminergic neurotoxicity in a pre-clinical model of PD, as well as to evaluate is [Chol][Apo] alone was neurotoxic for dopaminergic cells in vivo.
Two groups were used: one group received a stereotaxically injection of 6-OHDA (10µg/2µL of Ascorbic acid 0.1%) and the other group received stereotaxically injection of 6-OHDA (10µg/2µL of Ascorbic acid 0.1%) and a direct intraventricular delivery by a Alzet osmotic pump of [Chol][Apo] (50 mg/kg of body weight). In the second group, the intraventricular and stereotaxic procedures were performed in same day. All the procedures were performed in mice anesthetized with an intraperitoneal injection of ketamine (0.67 mL/Kg of body weight), xylazine (0.33 mL/Kg of body weight) in saline and placed in a stereotaxic apparatus. Using a digital coordinates system, the injection and infusion site were determined using bregma as zero reference. In that way the coordinates for the stereotaxic injection of 6-OHDA in the right striatum (ST) were: mediolateral (ML): -2 for right; anteroposterior (AP): 0.6 for posterior; dorsoventral (DV): -3.0 42. In the same way, for the intraventricular delivery of [Chol][Apo] by infusion in right ventricle using a minipump connected to a catheter, the coordinates were: ML: -1.1 for right; AP: 0.5 for posterior. The injections of 6OHDA in the ST were performed with a Hamilton syringe at speed of 0.2µL/min. The direct intraventricular delivery of [Chol][Apo] was performed using Alzet osmotic pump has previously described 43. 8 days after the surgeries, animals were transcardially perfused with saline and then with buffered 4% formalin and the brains collected for immunohistochemistry evaluations.
2.5 Immunohistochemistry
To evaluate the number of dopaminergic neurons we performed free floating immunohistochemistry against tyrosine hydroxylase (TH) as marker for those cells 44. For that, after collection brains were immersion-fixed in buffered 4% formalin overnight and cryoprotected in 30% sucrose.
Afterwards, the brains were sliced in a cryostat (Leica CM3050 S) with a thickness of 30µm and the SN were collected and preserved at -20ºC in an anti-freezing solution (50% Glycerin, 37.5% H2O and 12.5% of phosphate buffer). All steps performed during immunohistochemistry were with gentle agitation on a rotating platform. The section were washed 3 times with PBS-T to remove antifreeze solution. Posteriorly slices were permeabilized and blocked with a blocking solution (0,1% Triton and 10% FBS in PBS) at RT for 1h. Then slices were washed twice, 10 min in PBS-T and an inhibition of endogenous peroxidase activity was performed with 3% H2O2 in water for 10 min, followed by washing with PBS-T two times for 10 min. Next, the tissues were incubated with primary antibody in a concentration of 1:2500 (Mouse anti-TH, Vector) in a solution of 5% FBS in PBS, overnight at 4ºC. After the overnight incubation, the sections of tissues were washed three times for 10 min in PBS-T, incubated for 1h at RT with biotinylated antibody (Biotinylated goat anti-mouse, Vector) in a concentration of 1:200 diluted in 1% FBS in PBS and washed again three times 10 min in PBS-T, for an after application of avidine/biotine kit (Vectastain ABC Elite kit) that corresponded to an incubation with AB solution (1:500 solution A plus 1:500 solution B in PBS) 45 min at RT. Afterwards, the tissues were washed three times 10 min in PBS-T and next 10 min in PBS and was then incubated with DAB solution (Horseradish Peroxidase (HPR) and 2% DAB substrate, DAKO) until color develops, 5 to 10 min. This last reaction was stopped by removing the solution and washed two times for 10 min in PBS-T and 10 min in PBS. Next, the brain slices were placed on Superfrost slides (Knittel glass), allowed to dry and posteriorly dehydrated by sequential immersion of 1min in dH20, 3 min in 50% ethanol, 3 min in 75% ethanol, 3 min in 95% ethanol, 3 min in 100% ethanol, allowed to dry for few min, then 5 min in xylene and finally mounted using mounting medium (Surgipath, SelecTech MM24) and cover slipped (Microscope cover glass; histostar).
For each experimental condition 4 animals were used, and six coronal sections, serially selected with 180 m apart representing the SNpc, were analyzed for the number of TH+ neurons using a 10 magnification. The unbiased counting of TH+ dopaminergic neurons within the SNpc was performed as described previously 45.
Chapter 3 – Results
2.1
Evaluation of Choline Gallate,
Choline
Caffeate
and
Choline
Apocynate in the CNS cells viability
2.2
Evaluation of Choline Apocynate in
an in vivo model of PD, 6-OHDA
Chapter 3 – Results
3.1 Evaluation of Choline Gallate, Choline Caffeate and Choline
Apocynate in the CNS cells viability
The effect of the ILs as well as their precursors in the dopaminergic viability was studied N27 cells, microglia (N9 cell line and primary cultures), in astrocytes and in mixed glial cells cultures. The tested precursors included Gallic Acid, Caffeic Acid and Apocynin and the tested ILs were [Chol][Gal], [Chol][Caf] and, [Chol][Apo]. At first, dose response curves of dopaminergic neurons (N27 cell line) to both ILs and respective precursors were obtained for a range of selected concentration and then based on those curves the toxicity of two different concentrations of the ILs and precursors was studied in N9 cell line, Microglial cells, Astrocytes cells and mixed glial cells cultures. After the cytotoxicity test, the evaluation of the neuroprotective effects of ILs were evaluated in N27 cells exposed to different dopaminergic toxins, 6-OHDA, MPP+ and PQ.
3.1.1 Cytotoxicity of IL and their precursors
Cell viability was evaluated by MTT assay that allows the quantification of viable cells. Being the ILs a new chemical formulation constituted by a precursor and a substrate, in this case, choline, to start we studied the toxicity of these new compounds and compared the values obtained with the ones obtained for their precursors. Figure 6 shows the dose response curve obtained in N27 cell line. The EC50 concentration is the concentration capable to kill 50% of cell in the culture when compared with control condition and was calculated for each IL and precursor. The obtained results showed that [Chol][Gal] and [Cho][Apo] have higher EC50 than their respective precursors whereas [Chol][Caf] has lower EC50 than his precursor. More specifically, Gallic acid EC50 was 40.39 nM and the correspondent IL ([Chol][Gal]) EC50 was 60.19 nM (Figure 6 a) and b)), Apocynin EC50 was 3176 nM and [Chol][Apo] EC50 was 10772 nM (Figure 6 e) and f)). Caffeic acid shown an EC50 of 567.0 nM and [Chol][Caf] of 188.0 nM (Figure 6 c) and d)). Given that, we could conclude that both [Chol][Gal] and [Chol][Apo] induce a lower toxic effect on dopaminergic neurons then they respective precursor, which is in line with our hypothesis that the formulation of this compounds to ILs to increase they solubility, avoiding the use of higher concentrations of organic solvents may improve its performance reducing secondary cytotoxic effect. As for [Chol][Caf] its toxic effect on dopaminergic neurons was higher than its precursor. The reason for these last results is still
24 0 50 100 150 5 24 48 72 96 385.0 770.0 EC50= 40.89 nM Gallic Acid
Log10of percursor concentration (nM)
C e ll v ia b ill it y (% o f c o n tr o l) 0 50 100 150 3.5 17.5 34.5 52.0 69.0 276.5 553.0 EC50= 60.19 nM [Chol][Gal] Log10of IL concentration (nM) Ce ll v ia b il li ty (% o f c o n tr o l) 0 50 100 150 70 188 377 566 755 1510 EC50= 567.0 nM Caffeic Acid
Log10of percursor concentration (nM)
C e ll v ia b ill it y (% o f c o n tr o l) 0 50 100 150 45 120 240 360 480 960 Log10of IL concentration (nM) EC50= 188.0 nM C e ll v ia b il li ty (% o f c o n tr o l) a) b) c) d) [Chol][Caf]
Based on the obtained results, two concentrations of each IL were selected and their toxicity towards N9 Microglial cell line, and different primary cells as Microglia, Astrocytes and Microglia plus Astrocytes (mixed glial cells) cultures was evaluated. This last culture was important to evaluate in order to evaluate if the intercommunication between microglia and astrocytes when together may induce a different behavior then when in separated cultures. The same or similar concentrations of IL precursors were also tested in the same types of cells individually cultured.
Figure 6 – Cytotoxicity of the ILs and their respective precursors in Rat dopaminergic neural cell line (N27 cells) measured by the MTT assay. The data represents the dose response curves of N27 to a)
Gallic acid EC50 40.39 nM; b)[Chol][Gal] EC50 60.19 nM; c) Caffeic acid EC50 567.0 nM; d)[Chol][Caf] EC50
188.0 nM; e) Apocynin EC50 3176 nM f)[Chol][Apo] EC50 10772 nM. Data are represented as % of control
(untreated cells). Apocynin 0 50 100 150 EC50= 3176M 1 2.5 5 10 50 100 1000 5000 20000 Log10of precursor concentration (nM)
Ce ll v ia b ill it y (% o f c o n tr o l) [Chol][Apo] 0 50 100 150 EC50= 10772M 1 2.5 5 10 50 100 1000 5000 20000 Log10of IL concentration (nM) Ce ll v ia b ill it y (% o f c o n tr o l)
e)
f)
N9 Microglial Cells [CHOL ][G AL] 17. 5nM [CHOL ][G AL] 34. 6nM Gallic Aci d 1 7.5n M Gallic Aci d 2 4nM Gallic Aci d 3 4.6n M 0 50 100 150 Control Ce ll v ia b il li ty (% o f c o n tr o l)
Primary Microglia Cells
[Cho l][Ga l] 34 .6nM Gal lic A cid 34.6 nM 0 50 100 150 Control C e ll v ia b il li ty (% o f c o n tr o l)
Primary Astrocytes Cells
[Cho l][Ga l] 34 .6nM Gal lic A cid 34.6 nM 0 50 100 150 Control C e ll v ia b il li ty (% o f c o n tr o l)
Astrocytes + Microglia cultures
[Cho l][Ga l] 34 .6nM Gal lic A cid 34.6 nM 0 50 100 150 Control C e ll v ia b il li ty (% o f c o n tr o l) a) b) c) d)
Figure 7 – Choline Gallate and Gallic Acid cytotoxicity in N9 Microglial cell line and Primary Microglia, Astrocytes and Microglia plus Astrocytes measured by the MTT assay. The data represents
the cell viability of: a) N9 Microglial cells with two different concentrations of [Chol][Gal] (17.5 and 34.6 nM) and, with three different concentrations of Gallic Acid (17.5, 24, 34.6 nM); and b) Primary Microglia cells, c) Primary Astrocytes cells and d) Primary Astrocytes plus Microglia cultures with the highest concentration of Choline Gallate and Gallic Acid tested in N9 microglial cells (34.6 nM). Data are represented as % of control (untreated cells).
As observed in figure 7 [Chol][Gal] at concentrations 17.5 and 34.6 nm were tested in N9 Microglial cells and the higher concentration of the two of them, was tested in the rest of cultures mentioned above. As results, in N9 cell line, the two concentrations of [Chol][Gal] showed a nontoxic effect and even thought the lowest concentration of its precursor induced some toxicity this was not statistically significant and the higher concentration were not toxic but seem to increase cell viability. Even though this increase was not statically significant it
26
behavior. Although this decrease was not statistically significant it may suggest the higher susceptibility of this cultures compared with N9 cells. As for primary astrocytes cells we observed that the exposure to [Chol][Gal] or gallic acid did not induce a toxic effect (Figure 7 c)). [Chol][Gal] and gallic acid in astrocytes plus microglia cultures had no cytotoxic effect (Figure 7 d)). Comparing this result with the one showed in figure 7 b), of microglia viability in the presence of [Chol][Gal] or gallic acid, we may suggest that in the presence of both cells, microglia depict a lower toxic response to these compounds and speculate that in this culture condition astrocytes may have a protective role toward microglia cells.
N9 Microglial Cells [CHOL ][CAF ] 45n M Caf feic Aci d 4 5nM Caf feic Aci d 7 1nM Caf feic Aci d 1 20n M 0 50 100 150 Control Ce ll v ia b il li ty (% o f c o n tr o l)
Primary Microglia Cells
[Chol][Caf] 4 5nM Caffeic Acid 45nM 0 50 100 150 Control Ce ll v ia b il li ty (% o f c o n tr o l)
Primary Astrocytes Cells
[Cho l][C af] 4 5nM Caf feic Aci d 45 nM 0 50 100 150 Control # # C e ll v ia b ill it y (% o f c o n tr o l)
Astrocytes + Microglia cultures
[Cho l][C af] 4 5nM Caf feic Aci d 45 nM 0 50 100 150 Control C e ll v ia b il li ty (% o f c o n tr o l) a) b) c) d)
Figure 8 - Choline Caffeiate and Caffeic Acid cytotoxicity in N9 Microglial cell line and Primary
Microglia, Astrocytes and Microglia plus Astrocytes measured by the MTT assay. The data shown
represents the dose response graphs of: a) N9 Microglial cells with one concentration of [Chol][Caf] (45 nM) and, with three different concentrations of Gallic Acid (45, 71, 120 nM); and b) Primary Microglia cells, c) Primary Astrocytes cells and d) Primary Astrocytes plus Microglia cultures with the chose concentration of Choline Caffeiate and Caffeic Acid tested in N9 microglial cells (45 nM). Data are shown as the mean±SEM and statistical analysis was performed by using one-way ANOVA followed by Bonferroni’s comparison Test. # # p <0.05 when compared with control. This data is represented as % of
control.
In figure 8 are depicted the results obtained when evaluating [Chol][Caf] and caffeic acid for the same propose and the same way as describe above to [Chol][Gal] and Gallic Acid. In N9 microglial cells line we tested one concentration of 45 nM of [Chol][Caf] and three






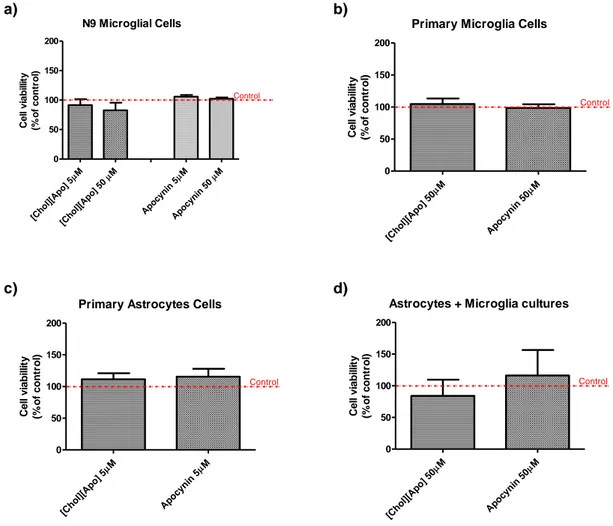
![Figure 20 - Effect of [Chol][Gal] in in vitro models of Parkinson Disease. A total of three independent experiments were performed](https://thumb-eu.123doks.com/thumbv2/123dok_br/19172779.941919/49.892.184.750.115.691/figure-effect-models-parkinson-disease-independent-experiments-performed.webp)
![Figure 11 - Effect of [Chol][Caf] in in Vitro models of Parkinson Disease. A total of three independent experiments were performed](https://thumb-eu.123doks.com/thumbv2/123dok_br/19172779.941919/50.892.139.722.282.876/figure-effect-vitro-parkinson-disease-independent-experiments-performed.webp)