MaryAnn Liebert, Inc., Publishers
Pp. 429-436
Production
of
Human
Recombinant
Proapolipoprotein
A-I
in Escherichia coli:
Purification
and Biochemical Characterization
NICOLE
MOGUILEVSKY,*
CORNELISROOBOL.t
ROSETTELORIAU,*
JEAN-PAUL
GUILLAUME,*
PAULJACOBS,*
ALFREDOCRAVADOR,*
ALBERTHERZOG,*
LÉON
BROUWERS.t
ALAINSCARSO.t
PASCALGILLES.t
LEIFHOLMQUIST.t
LARS A.
CARLSON.Î
and ALEX BOLLEN*ABSTRACT
A human liver cDNA
library
was usedtoisolateaclonecoding
forapolipoprotein
A-I(Apo
A-I).
The clonecarries thesequence forthe
prepeptide (18
aminoacids),
thepropeptide
(6
aminoacids),
and themature pro-tein(243
aminoacids).
Acoding
cassette forthe proapo A-I moleculewas reconstructedby
fusing
synthetic
sequences,chosento
optimize expression
andspecifying
the amino-terminal methionine and aminoacids -6to
+14,
to alarge fragment
of the cDNAcoding
for amino acids 15-243. The module wasexpressed
inpOTS-Nco,
anEscherichia coliexpression
vectorcarrying
theregulatable
XP^
promoter,
leading
to thepro-duction of
proapolipoprotein
A-I at up to 10% oftotal solubleproteins.
The recombinantpolypeptide
waspurified
and characterizedintermsofapparent
molecularmass, isoelectricpoint,
andby
bothchemical andenzymatic
peptide
mapping.
Inaddition,
it wasassayed
in vitro for the stimulation ofthe enzyme lecithin:cholesterol
acyltransferase.
The data showfor
the first time thatproapo A-Icanbeproduced efficiently
inE. colias a stable and
undegraded protein
having
physical
and functionalproperties
indistinguishable
fromthose ofthe natural
product.
INTRODUCTION is
responsible
for the formationofmost ofthecholesteryl
estersin
plasma
(Fielding
etal,
1972).
Apo
A-I and HDLHuman
apolipoprotein A-I(apo A-I),
themajor
pro- mayparticipate
in "reverse cholesteroltransport,"
frompe-tein constituent of
high-density lipoproteins
(HDL),
ripheral
tissues to the liver for excretion from thebody
isproduced
in liver and intestine as a precursorprotein
(Glomset, 1968).
(preproapo A-I)
whichundergoes
intracellularcleavage
Since accumulation of cholesterol in the arteries is theandis released into the
plasma
andlymph
asaproprotein
hallmark andpresumably
animportant
cause forathero-(Wu
andWindmueller,
1979).
Proapo
A-Icarries six addi-sclerosis,
stimulation of reverse cholesterol transportby
tional amino acids
(Arg-His-Phe-Trp-Gln-Gln)
at thesupplementing
apo A-Imight delay
the atherosclerotic amino-terminalof the matureprotein.
It is cleaved inthe process.Techniques
toproduce
thisprotein
inlarge
quan-vascular space
by
aspecific
protease toyield
the mature tities have beendeveloped
using
bloodplasma
asstarting
molecule
(Gordon
etal, 1983;
Zannis etal,
1983).
Apo
material(Ross
andCarson,
1985; Breweretal, 1986).
At-A-I is asingle
nonglycosylated polypeptide
ofknown se- tempts toexpressmatureapoA-I in recombinant bacteriaquence,
composed
of 243 amino acid residues(Brewer
etproved
difficult due to theinstability
of theprotein
ai, 1978).
Inthepresenceofsynthetic liposomes,
it serves(Lorenzetti
etal,
1986;Mallory
etal., 1987);
moreover,as cofactor for lecithin ¡cholesterol
acyltransferase,
which even as afusionprotein,
apoA-I wasstillverysensitiveto•Service deGénétique Appliquée, Université Libre deBruxelles, 24rue del'Industrie, B-1400 Nivelles, Belgium.
tu.C.B.
and U.C.B. Bioproducts,chemin du Foriest, B-1420 Braine-l'AUeud,Belgium.ÍKing
GustafVResearch Institute, KarolinskaInstitute, Box60004, S-10400Stockholm, Sweden.degradation
and could not be cleavedefficiently
from thefusion
(Lorenzetti
etal., 1986;
Monaco etal,
1987).
Tocircumvent these
problems,
weexpressed
apo A-I as theproprotein.
We show here that proapo A-I can bepro-duced
efficiently
in Escherichiacolias astable andunde-graded molecule,
having physical
andfunctionalproperties
identical to those of the naturalproduct.
MATERIALS AND METHODS
Plasmids, strains,
and mediaThree
plasmids, pBR322 (Bolivar
etal.,
1977),
pULB1221
(a
multi-cloning
site derivative ofpULB1219,
Cravador et
al., 1985),
pOTS-Nco
(Devare
etal,
1984),
and three E. coli
strains,
MM294(Lawn
etal.,
1981),
AR58,
and AR120(Mott
etal,
1985),
were used. M33minimal medium is described
by Fujimoto
et al.(1988).
RNA
preparation; synthesis,
cloning,
screening,
andsequencing of
cDNAProcedurestoprepareandscreenthe humanlivercDNA
library
were as described before(Jacobs
etal,
1985).
Oligonucleotides
weresynthesized
by
thephosphoramidite
method on an
Applied Biosystem
Synthesizer,
model380A(Matteucci
andCaruthers, 1981).
DNA sequenceanalysis
was
performed by
the methods of Maxam and Gilbert(1977)
andSanger
et al.(1977).
Immunological
detectionof
proapoA-I in bacterialextractsTransformed bacterial strains were grown in 20 ml of
rich
(LB)
or minimal mediumsupplemented
withampicil-lin
(50
/ig/ml)
to an OD630 of 0.6. Induction of the XPL
promoter was achieved either
by
shifting
the temperature from30°Cto42°C,
orby
adding
nalidixic acid(60
fig/ml)
to cells grown at 37°C
(Mott
etal., 1985).
Induction wasperformed
forvariousperiods
of time as indicated in thefigure legends.
One-milliliter
aliquots
of cultures were collected andcentrifuged
at10,000
x g for 5 min. Pellets wereresus-pended
in 50¡A
ofNaDodS04sample
buffer(50
mM TrisHC1
pH
6.8containing
2%NaDodS04,
6 Murea, 5%2-mercaptoethanol,
and 10%glycerol),
boiled for 3 minat100°C,
andcentrifuged
for 10 minat 15,000 x g.Samples
werethen fractionatedby electrophoresis
on 12.5or7.5%NaDodS04-polyacrylamide gels
as describedby
Laemmli(1970).
The
proteins
wereblotted ontonitrocellulose sheetsand tested for the presence of proapo A-I with antibodies raisedagainst plasma
apo A-I.Theprocedure
follows Bol-len et al.(1984),
except that the secondantibody,
goatantimouse,
was labeled with alkalinephosphatase
insteadof
peroxidase,
and that thechromogenic
substrates wereBCIP
(5-bromo-4-chloro-3-indolyl-phosphate, Boehringer)
and NBT(4-nitrotetrazolium-chloride blue, Sigma),
dis-solved in 100 mM Tris-HClpH 9.5,
100 mMNaCl,
and 50 mMMgCl2
at 66and 33fig/ml, respectively.
Purification of
recombinantproapoA-IPacked bacterial cells
(usually
35grams),
derived from 15-liter fermentations in M33medium,
wereresuspended
intwovolumes ofice-cold extractionbuffer
(20
mMTris-HCl
pH
7.5, 100mMNaCl,
1mMEDTA,
1 mMPMSF,
and 100
¡ig/ml merthiolate)
anddisrupted by
asingle
pas-sage
through
a French'spressure cell at 1,000 PSIG. Theextractwas
centrifuged
for 15 min at4,000
x gtosedi-ment membranes and aggregates then
applied
onto aPhenyl Sepharose
column CL-4B(bed volume,
25ml;
sample,
100ml).
The column wasthensequentially
elutedwith 250 mlof extraction
buffer,
250 mlof thesamebuffersupplemented
with 60%(vol/vol) propylene glycol,
and 250 ml of thesamebuffer without NaCl andsupplemented
with80%
(vol/vol) propylene glycol.
Fractionscontaining
proapo A-Iwere
pooled
and thenapplied
onto a PBE 74chromatofocusing
column(0.8
x 60cm),
which waselutedwithtwobed volumes ofabuffer
containing
25 mMimidazole-HCl
pH
6.0 and 6 Murea, followedby
10 bedvolumes of
polybuffer
74(dilution
1/10) pH 4.0,
6 Murea. The columnwasregenerated by washing
with 50 mMNa-citrate
pH
4.0,
1 MNaCl,
and 6Murea. Fractions wereassayed
for totalprotein
content and apoA-I-relatedim-munoreactivity,
andanalyzed by
isoelectricfocusing.
Chemical
peptide mapping
with BNPS-SkatolChemical
cleavage
with BNPS-Skatolwasperformed
ac-cording
to theprocedure
ofFontana(1972). Briefly,
5-10 fig ofprotein
were dissolved in 100¡A
of a solution of0.15%
(vol/vol) phenol
in50%(vol/vol)
acetic acid.Then,
50
fA
ofasolutionof 4.8mgBNPS-Skatolpermilliliter ofglacial
acetic acid wereadded,
followedby
an incubationof 72 hr at25°C.
Subsequently,
50¡A
of2-mercaptoetha-nol was
added,
followedby
a second incubationof 5 h at37°C. The
samples
wereevaporated,
redissolved in 100fA
of water, andextractedthree times with 200^tl
ofethyl
ace-tate.The
organic
phases
werediscarded,
whereas the aque-ousphases
werelyophilized
andanalyzed
by
acrylamide
gel electrophoresis.
Stimulation
of
lecithin:cholesterolacyltransferase
activity by
recombinantproapo A-IHuman
plasma
apo A-I waspurified according
toHolmquist
and Carlson(1977)
andHolmquist
andBro-strom
(1979).
Simple
bilayer
vesiclescontaining
egg leci-thinand7(/i)-3H-labeled
cholesterol(molar
ratio4:1)
werepurified
humanplasma
lecithin:cholesterolacyltransferase
(35,000
units/mg)
wasprepared
according
toHolmquist
(1987).
Incubations and enzymeactivity
determinationswerecarried outin 50 mM
phosphate
bufferpH
7.4asde-scribed
by
Piran and Morin(1979).
Incubation mixtures(250
fA)
contained perliter:240/¿moles
lecithin,
60/¿moles
cholesterol,
and 3 or 6/¿moles apolipoprotein.
Miscellaneous
procedures
Protein concentrationsweredetermined
according
totheprocedure
of Bradford(1976),
using
ovalbumin asastan-dard. Partial
tryptic
hydrolysis
wasperformed
according
tothe
procedure
ofAmonsetal.(1983).
Human apo A-I-relatedimmunoreactivity
wasquantified
by
radialimmu-nodiffusion
(Cheung
andAlbers, 1977),
using
commercialapo A-I
(Sigma)
as astandard. The relativeimmunoreac-tivity profile
were determinedby
immunoturbidimetry
(Shapiro
etal, 1980).
Isoelectricfocusing
was carriedoutaccording
to theprocedure
ofCatsimpoolas (1968)
in aBioradrod
electrophoresis
apparatus,applying
aself-gen-erating gradient
frompH
4topH
6. Linear and nonlinearregression analyses
wereperformed
withtheStatViewpro-gram
(Brain
Power Inc.,USA).
RESULTS
Molecular
cloning of
Apo
A-IcDNA andscreening
of
the cDNAlibrary
Total
poly(A)*RNA
(25
fig)
fromhuman liverwastran-scribed into double-strandedcDNA
(ds
cDNA).
Moleculesresistant to S, nuclease were then fractionated on a
10-30% sucrose
gradient
to enrich for moleculeslarger
than 0.8 kb. This ds cDNA fractionwasinserted into thePst Isiteof
pBR322 by
thedC:dGtailing
procedure
andusedtotransform E. coli MM294 cells. The
library
was screenedby
thecolony hybridization
methodusing
a 22-basesyn-thetic
oligonucleotide probe
derivedfromthesequence for apo A-I(Cheung
andChan, 1983)
andcoding
for aminoacids -22 to -15 ofthe
signal
peptide.
Of the 30 cloneshybridizing
with the labeledprobe,
one,pNIV1602,
waschosen for further
analysis.
Characterization
of
theclonepNIV1602
Clone
pNIV1602
carriedaninsertof 878bp
whichcouldbe isolated as a
single fragment by digesting
theplasmid
with Pst I.The insert wasmapped
with variousrestrictionenzymesand
fully sequenced
oneach strand. Thedata(not
shown)
showed thatpNIV1602
carries(i)
a19-bp
5'non-coding
sequence,(ii)
a75-bp
sequencecorresponding
tothe known
signal
andpropeptide
region
of apoA-I,
in-cluding
the ATGinitiationcodon,
(iii)
thecomplete 729-bp
sequence
corresponding
tothematureprotein,
followedby
aTGAstop
codon,
and(iv)
a55-bp
3'noncoding
sequencethat did notcontain the
poly(A)
stretch. The sequence of thecDNAinsertwasidenticaltothesequencepublished
by
Cheung
and Chan(1983),
but differedfrom the sequenceof Seilhameretal.
(1984)
by
afew bases andby
thelength
of the 5' and 3'
noncoding regions.
The deduced aminoacid sequence,
however,
wasconsistentwith thepublished
data(Cheung
andChan, 1983;
Seilhamer etal.,
1984).
Construction
of
the recombinantplasmid
pNIVl617
Theconstruction
proceeded
in twosteps. Thefirst usedthe
multi-cloning
siteplasmid
pULB1221,
linearized withBal
I,
as anintermediatevehicletosubcloneablunt-ended744-bp
Bal l-Pst I DNAfragment
derivedfrom the clonepNIV1602
andcoding
for amino acids 15-243 ofthe apoA-I
protein.
Theresulting plasmid
wasdigested
with Eco RI and Bal I and fused to(i)
the1,880-bp
Eco Rl-Nco I DNAfragment
containing
theregulatory regions
derivedfromthe
expression
vectorpOTS-Nco (Devare
etal., 1984)
and
(ii)
a65/61-bp
Nco l-Bal Isynthetic
DNAadaptor
en-coding
theamino-terminal methionineand amino acids -6to 14 of proapo A-I
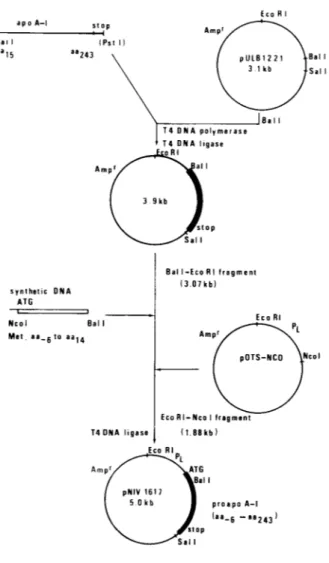
(Fig.
1).
Theresulting
recombinantplasmid,
pNIV1617,
thus carried the strongregulatable
XPl
promoter upstreamfromthecassettecoding
for proapo A-I.The
synthetic
adaptor
wasassembled fromfouroligonu-cleotides
(Fig. 2)
whose base sequence wasdesigned by
computertominimize
secondary
structuresatthe5' endofthe
coding
sequence(S.A.S.I.P.
softwarepackage;
Clav-erie,
1984). Triplets
encoding
aminoacidresidues -6, -1, 1,3, 4,
5, 6,7, 10, 11,
and14,
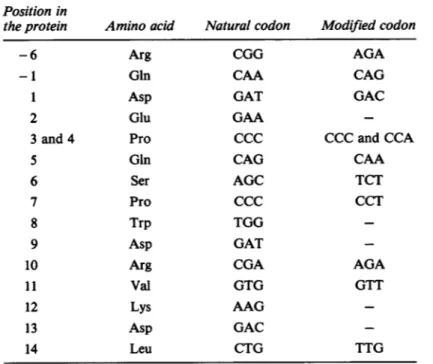
therefore differed fromthe natural ones(Table 1),
although
theresulting
amino acidsequence was
preserved.
Plasmid
pNIV1617
wascharacterizedby
restrictionanal-ysis,
hybridization
with labeledsynthetic
DNAfragments,
andsequenced throughout
theadaptor
region.
Itwasthenintroduced into E. coliAR58
(for
thermalinduction)
andAR120
(for
chemicalinduction).
Expression
of
proapoA-ICells
carrying
either the controlplasmid,
pOTS-Nco,
orthe
pNIV1617 plasmid
weregrownat30°C inminimalme-dium,
for thermalinduction,
or at 37°C in richmedium,
forchemical induction.
Derepression
of the XPL
promoterwasachievedeither
by
shifting
thetemperatureto42°Corby
adding
nalidixic acid. Inductionwasperformed
forvar-ious
periods
of time as indicated in thelegend
toFig.
3.Pelletedbacteriawere
lysed
andtheresulting
extractswereanalyzed
by
NaDodS04-polyacrylamide gel
electrophore-sis,
Westernblotting,
andimmunodetectionusing
antibod-ies raisedagainst plasma
apo A-I.Figure
3 shows that asingle
product, specifically
recognized by
anti-apo
A-Iantibodies,
isexpressed
in the bacterial strainscarrying
plasmid
pNIV1617,
irrespective
ofthe modeofinduction.apoA-l BalI "15 synthetic DNA ATG Ncol Met.aa_ T40NA ligase Antpr
Ball-EcoR Ifragment
13 07kbl
Amp
EcoRl-Nco Ifragment
(1 BBkb)
proapoA-l
"»-6 -aa243'
The
protein
hadamolecularmassofabout28,000
daltonswhich,
withinexperimental
limits,
fittedthecalculatedmo-lecular
weight
ofproapo A-I.Levels of proapo A-I
produced
in bacteria wereesti-mated
by
single
radial immunodiffusion. Under thermalinduction conditionsin M33 minimal
medium,
proapo A-Iwas
expressed
at about 10% of total solubleproteins
versus 3.5% inLB medium. Chemical induction
appeared
tobe less
efficient, yielding
expression
levels on theorderof2% in LB mediumand no
expression
in M33 medium.Purification of
recombinantproapo A-IWe used
hydrophobic
interactionchromatography
topurify
the recombinant proapo A-I. Thisapproach
al-lowed an excellent
separation
of recombinant moleculesfrom otherbacterial constituents
(Fig.
4,peak
III).
Chro-matofocusing
on aPBE-74 columnwasusedtopurify
theproduct
further. As shown inFig.
5, immunoreactivema-FIG. 1. Construction of
plasmid
pNIV1617.
A744-bp
Bal l-PstIfragment
derivedfromclonepNIV1602,
coding
for amino acids 15-243 of apoA-I,
wasligated
into theunique
Bal I site ofpULB1221.
From theresulting
plas-mid,
thelarge
Eco Rl-Bal Ifragment
was fused to the1,880-bp
Eco Rl-Nco Ifragment
derived frompOTS-Nco
andtothe
synthetic
adaptor
Nco l-Bal Itoregeneratease-quence
coding
for proapo A-I.Hybrid
molecules wereusedto transformE. colicellsandtransformantswere
se-lected for
ampicillin
resistance. The nucleotide sequence of thejunctions
betweenfragments
and ofthe fullsynthetic
fragment
were determined.Ncol site
5-- -35 mer
BalIsite
-3'5' -30
mer-CATG AGA CATTTCTGG CAG CAG GAC GAA CCT CCA C AA TCT CCTTGG GATAGA GTTAAGGAC TTG G TCT GTA AAGACC GTC GTC CTG CTTGGA GGT G TT AGAGGAACC CTATCT CAATTCCTGAAC C
3"-18 met--53-"- 43 mer-»5
Met Arg His Phe Trp Gin Gin
6 -3 1
NH-, terminal propeptide
methionine " 6 aa
1 '
Asp Glu Pro Pro Gin Ser Pro Trp Asp Arg Val Lys Asp Leu A(la)
»1 +3 +6 +9 +12 +14
mature apo A-l (aa.
-"14
cleavage site for apoA Ipropeptidase
FIG. 2.
Synthetic adaptor
usedtoreconstruct the proapo A-I cDNA. Fouroligomers
weresynthesized
andassembledin vitrotogeneratea
65/61-bp
fragment
flankedby
aNcoIandaBal Isitesonthe5'and3'ends,
respectively.
The de-duced aminoacidsequenceisindicated. The arrowshowsthe knowncleavage
site forapo A-Ipropeptidase
(Gordon
etTable 1. Relevant Changesin CodonsUsed toReconstruct
theProapo A-I cDNA Position in
the
protein
Aminoacid NaturalcodonModified
codon-6 -1 1 2 3 and4 5 6 7 8 9 10 11 12 13 14
Arg
GinAsp
Glu Pro Gin Ser ProTrp
Asp
Arg
ValLys
Asp
Leu CGG CAÁ GAT GAA CCC CAG AGC CCC TGG GAT CGA GTG AAG GAC CTG AGA CAG GAC CCC and CCA CAA TCT CCT AGA GTT TTGModifications in the codon usageweredone in ordertominimizesecondary
structures in the 5' end of the proapo A-I cDNA. The software package
S.A.S.I.P.developed bythe Institut Pasteur(Paris)wasused(Claverie,1984).
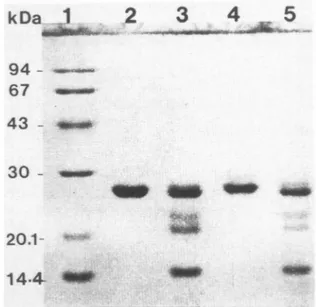
11 10 987 65432 1 kDa
A
.43 13 12 11 10 9 8 7 6 5 4 3 2 1 kDaB
_29 .18.4 29 .18.4FIG. 3. Immunodetection of proapo A-I on Western blots. Protein extracts from induced and noninduced bacteria
carrying
plasmid pNIV1617
wereprepared
forelectrophoresis
onNaDodS04-polyacrylamide gels.
Nitrocellulose blots wereincubated withmouseantiapoA-Iserum, followedby
alkalinephosphatase-labeled
goatanti-mouse and thechro-mogenic
substrate. A. Timecourseof thermalinduction ofbacteriacarrying
pNIV1617
and growninM33minimalme-dium. Lane
1,
Plasma derived apoA-I, 500 ng; lanes2-6,
samples
takenat 1,20, 40, 60,
and 120 minin noninduction conditions(30°C);
lanes 7-11,samples
taken at1, 20,
40,60,
and 120 minafter induction(42°C).
B. Time courseofchemicalinduction
(nalidixic acid)
ofbacteriacarrying
pNIV1617
andgrowninrichLBmedium.Lane 1,Plasma derived apoA-I,
500 ng; lanes2-7,
samples
taken at1, 30, 60, 120, 180,
and240minin noninduction conditions(37°C);
lanesterial
(fractions 37-48)
was resolved from contaminantsandwasidentified
by
isoelectricfocusing
mainly
asproapoA-I.
Moreover,
chromatofocusing
resolved most apo A-Irelatedisoforms.
Characterization
of
purified
recombinantproapoA-I
Pure recombinant proapo A-I was
analyzed by
NaDodS04-polyacrylamide
gel
electrophoresis
and isoelec-tricfocusing.
Recombinant proapo A-Ibehavedasasingle
Material Eluted. Arbitrary Units
h.
I
Ccccccccctíí?
<ccccCrccCco°co_
20 30 FractionNumber -•rft>-40FIG. 4.
Hydrophobie
interactionchromatography.
A total of 100 ml ofclearedextractof bacterial cells derivedfrom 15-liter fermentation in M33 medium was
subjected
to
hydrophobic
interactionchromatography
as describedinMaterials and Methods. The eluatewas
assayed
fortotalprotein (O)
and apo A-I-relatedimmunoreactivity
(•).
PeakIIIcontains the
majority
of therecombinantprotein.
polypeptide
chain with an apparent molecular mass of(27.3 ±1.1)
kD.Matureapo A-Iwasslightly
smaller,
hav-ing
an apparent molecularweight
of(26.8
±1.1)
kD. Inisoelectric
focusing (data
notshown),
the recombinantprotein
showedonemajor
isoformandoneminorisoform,
having
isoelectricpoints
of 5.20 and4.95,
respectively.
Therelative
migration
distances of these isoforms coincidedexactly
with those ofplasma
proapo A-I andapoA-I,
re-spectively.
Partial chemicalcleavage
attryptophan
resi-dues,
wasperformed
on recombinant proapo A-I(major
isoform)
and onplasma-derived
apo A-I.Figure
6 showsthe
large peptides
ofexpected
molecularweight produced
in both cases
by cleavage
atpositions
8, 50, 72,
and 108 inthe
proteins.
Inaddition,
digestions
of recombinantproapo A-I and of
plasma-derived
apo A-I withtrypsin
inverymild conditions
yielded
similarpatterns offragments
(data
notshown).
Thus,
both types ofexperiments
suggest that the recombinant molecule isstructurally
identical toits natural counterpart.
Functional
analysis
of
recombinantproapoA-IRecombinant proapo A-I
(pi 5.20)
and its minor iso-form(pi 4.95)
were both ableto transformsynthetic
leci-thin-cholsterolliposomes
into substrates for the enzymelecithin:cholesterol
acyltransferase.
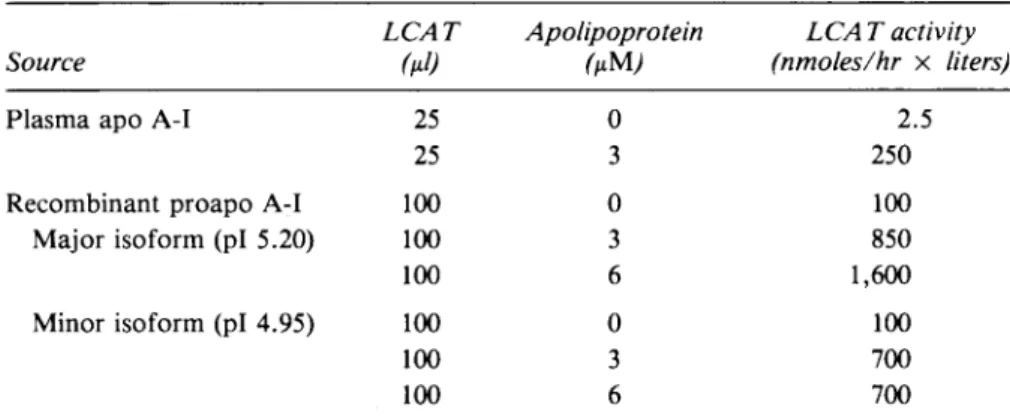
AsseeninTable2,
theactivation
capacity
of recombinantapoproteins
wassimilartotheoneobservedwithauthentic human
plasma
apo A-I.kDa
J
Material Eluted, Arbitrary Units T-6 +5 30 40 50 Fraction NumberFIG. 5.
Phenyl-sepharose chromatography.
Peak III of thephenyl sepharose
eluate wassubjected
tochromatofo-cusing
as described in Materials and Methods. The eluatewas
assayed
for totalprotein (O),
apoA-I-relatedimmu-noreactivity (•),
andpH
(A).
Fractions 37-48 contain thepurified
recombinantproduct.
43
30
20.1"
****'**14-4-FIG. 6. Chemical and
enzymatic
characterizationofpro-apo A-I. Purified recombinant proapo A-I and human
plasma
apoA-I weresubjected
topartial
chemicalpeptide
mapping
as described in Materials and Methods. Lane1,
Molecularmass standards
(Pharmacia,
14,400-94,000
dal-tons)
indicatedby
arrows; lane2,
humanplasma
apoA-I,
untreated;
lane3,
humanplasma
apoA-I, cleaved;
lane4,recombinant proapo
A-I, untreated;
lane5,
recombinantTable 2. Stimulation of Lecithin ¡Cholesterol Acyltransferase Activity by
Recombinant Proapo A-I Source
LCAT
Apolipoprotein
LCATactivity
(nmoles/hr
xliters)
Plasma apo A-I
Recombinant proapo A-I
Major
isoform(pi 5.20)
Minor isoform
(pi 4.95)
25 25 100 100 100 100 100 100 0 3 0 3 6 0 3 6 2.5 250 100 850
1,600
100 700 700Synthetic liposomes (lecithin/cholesterol, 4:1)wereincubatedat37°C for 2-4hr,inatotal vol-umeof 250(A,with LCAT(25units/ml)and variousamountsofapoA-I. The initialrateof
chole-sterylesterformation (LCAT activity)wasmeasuredaccordingtoPiran and Morin(1979).
DISCUSSION
Wehave cloned a cDNA
coding
for human preproapoA-I;
its deduced amino acid sequence fits thepublished
data
(Cheung
andChan, 1983;
Seilhameretal.,
1984)
andis almost identicaltothat
reported
by
Breweretal.(1978).
Weconstructedamodulecoding
fortheproapoA-I mole-cule andexpressed
it in E. colt under the control of theregulatable
XPL
promoter.Proapo
A-I,produced
athigh
level,
wasstable,
largely undegraded,
andindistinguishable
from the natural
product
in terms ofapparent molecularmass,isoelectric
point,
andpartial
peptide
map. Asexpected,
mostof the material
produced
in E. coliwasindiepropro-tein
form,
although
thepresenceofanamino-terminalme-thioninecannot be excluded yet. The
purified
protein
appeared
to contain a minor isoform which coincided withmature apo A-I
by
isoelectricfocusing. However,
it isun-certain if this is duetodeamidationortoconversion ofthe recombinant
protein
to matureapo A-I. Thehigh
level ofexpression
observed for proapo A-I inbacteriacontrastedwith the relative failure
(Lorenzetti
etal, 1986;
ourdata,
not
shown)
to achievesignificant expression
of astable,
undegraded
mature apo A-Ispecies
in the samehost. Thedramatically
increasedexpression
associatedwith thepres-enceofthe
propeptide
sequencemight
beexplained
insev-eral ways.
Transcriptional
or translational efficienciesmight
be increased due to the extra sequence.Alterna-tively,
theproprotein species
might
fold morestably
inbacteria thanthe mature
protein
andhencebemoreresis-tant to
degradation.
This lasthypothesis
seems moreprobable
sincepulse-chase
experiments
have shown that thehalf-life ofmature apo A-I inbacteria is less than 10min
(Mallory
etal, 1987).
Ourdata,
inaddition,
offerdi-rect evidence for the first time that recombinant proapo
A-I is able to stimulate in vitro the
enzymatic
activity
of lecithin:cholesterolacyltransferase,
asefficiently
asplasma-derived
apoA-I. In this respect,proapo A-Imight
constitute anappropriate
substituteto matureapo A-I forreplacement
therapy, provided
thatpharmacological
stud-ies in animal models confirm its functionalefficiency.
ACKNOWLEDGMENTS
This work was
performed
under a contract between U.C.B. and theUniversity
ofBrussels andsupported by
agrant from the Swedish Medical Research Council
(19X-04)
The authorsthank Drs. A. Shatzmanand M. Rosen-L i¡Smith
KlineandFrench,
Philadelphia,
PA)
forpro-viding
thepOTS-Nco
vector and the E. coli AR58 and AR120strains.REFERENCES
AMONS, R., PLUYMS, W., ROOBOL, C,andMOLLER, W.
(1983). Sequence homology between EF-la, the a-chain of
elongationfactor1 from Artemia salina andelongation factor EF-Tu from Escherichia coli. FEBS Lett. 153,37-42.
BATZRI, S.,andKORN,E.(1973).Single bilayer liposomes
pre-pared without sonication. Biochim. Biophys. Acta298, 1015-1019.
BOLIVAR, F., RODRIGUEZ, R.L., GREENE, P.J.,
BET-LACH, M.C., HEYNECKER, H.L., BOYER, H.W., CROSA, J.H., and FALKOW, S. (1977). Construction and
characterization of new cloning vehicles. IL A multipurpose cloning system. Gene2,95-113.
BOLLEN, A., LORIAU, R., HERZOG, A., and
HÉRION,
P.(1984). Expressionof humana,-antitrypsininEscherichiacoli. FEBS Lett. 166,67-70.
BRADFORD,M.M. (1976).Arapidand sensitivemethodfor the
quantitation of microgram quantities of protein utilizing the
principleofprotein-dyebinding.Ann. Biochem. 72, 248-254.
BREWER, H.B., FAIRWELL, T., LARUE, A., ROÑAN, R., HOUSER,A.,andBRONZERT,T.(1978).The amino acid
se-quence of human apo A-I, an apolipoprotein isolated from
high density lipoproteins. Biochem. Biophys. Res. Commun.
80, 623-630.
BREWER, H.B., RONAN, R., MENG, M., and BISHOP, C.
(1986). Isolation and characterization ofapolipoproteinsA-I,
A-II and A-IV. MethodsEnzymol. 128,223-246.
CATSIMPOOLAS, N. (1968). Microisoelectrofocusing in
poly-acrylamidegels columns. Ann. Biochem.26,480-482.
CHEUNG, M.C., and ALBERS, J.J. (1977). The measurement
ofapolipoproteinA-IandA-IIlevels inmenandwomenby im-munoassay. J. Clin. Invest. 60,43-50.
CHEUNG, P., and CHAN, L. (1983). Nucleotide sequence of
clonedcDNA of humanapolipoproteinA-I.NucleicAcids Res.
11, 3703-3715.
CLAVERIE, J.-M. (1984). A common philosophy and
FOR-TRAN77softwarepackageforimplementingandsearching
se-quence databases. Nucleic Acids Res. 12, 397-407.
CRAVADOR, A., JACOBS, P., VANELSEN, A., LACROIX,
C, COLAU, B., VAN ALPHEN, P., HERZOG, A., and
BOLLEN, A. (1985). Total DNA synthesis andcloning in E. coli ofagenecodingfor the human Growth Hormone
Releas-ing Factor. Biochimie67, 829-834.
DEVARE, S.G., SHATZMAN, A., ROBBINS, K.C.,
ROSEN-BERG, M.,andAARONSON,S.(1984).Expressionof
PDGF-relatedtransforming proteinof simiansarcomavirus in E. coli. Cell36,43-49.
FIELDING, C.J., SHORE, V.G.,andFIELDING,P.D.(1972). Aproteincofactor of lecithin: Cholesterolacyltransferase. Bio-chem. Biophys. Res. Commun. 46, 1493-1498.
FONTANA, A. (1972).Modification oftryptophanwith BNPS-skatol. MethodsEnzymol.25, 419-423.
FUJIMOTO, S., NAKATSU, M., KATO, K.,andKITANO,K.
(1988).Factorsaffectingthesynthesisof the N-terminal methi-onine-free molecule of recombinant human interleukin-2 by Escherichia coli. J. Ferment. Technol. 66, 181-185.
GLOMSET, J.A. (1968). The plasma lecithin: Cholesterol acyl-transferase reaction. J. Lipid Res.9, 155-167.
GORDON, J.I., SIMS, H., LENTZ, S.R., EDELSTEIN, C, SCANU, A.M., andSTRAUSS,A.W.(1983). Proteolytic
pro-cessing of human preproapolipoprotein A-I. J. Biol. Chem.
258,4037-4044.
HOLMQUIST, L., andCARLSON, K.(1977). Selective
extrac-tion of humanserumverylowdensityapolipoproteinswith
or-ganicsolvents. Biochim. Biophys. Acta493,400-409.
HOLMQUIST, L.,andBROSTROM, H. (1979).Asimple
Beck-manDUaccessoryfor location ofproteinsseparatedby isoelec-tricfocusingingranulated gel layers:Isolation of humanserum
verylow densityapolipoproteins C-II and C-III. J. Biochem.
Biophys. Methods 1, 117-127.
HOLMQUIST, L.(1987).Purification of humanplasmalecithin:
cholesterol acyltransferase by covalent chromatography. J. Biochem. Biophys. Methods 14,323-333.
JACOBS,P., CRAVADOR, A., LORIAU, R., BROCKLY, F., COLAU, B., CHUCHANA,P.,VANELSEN, A.,HERZOG, A., and BOLLEN, A. (1985). Molecularcloning, sequencing
and expressionin Escherichia coli of human preprourokinase cDNA. DNA 4, 139-146.
LAEMMLI, U.K.(1970). Cleavageof structural proteins during the assembly of the head of bacteriophage T4. Nature 227,
680-685.
LAWN, R.M., ADELMAN, J., BOCK, S.C., FRANKE, A.E., HOUCH, CM., NAJARÍAN, R.O., SEEBURG, P.H., and
WION, K.L. (1981). The sequence of human serum albumin cDNA and itsexpressioninE. coli. Nucleic Acids Res.9, 6103-6114.
LORENZETTI, R., SIDOLI, R., PALOMBA, R., MONACO, L., MARTINEAU, D., LAPPI, D.A.,andSORIA, M.(1986).
Expressionof the humanapolipoproteinA-I genefusedtothe E. coligenefor/3-galactosidase. FEBS Lett. 194, 343-346.
MALLORY, J.B., KUSHNER, P.J., PROTTER, A.A., COFER, CL., APPLEBY, V.L., LAU, K., SCHILLING, J.W., and VIGNE, J.-L. (1987). Expressionand characteriza-tion of human apolipoprotein A-I in chínese hamster ovary
cells. J. Biol. Chem. 262, 4241-4247.
MATTEUCCI, M.D.,andCARUTHERS, M.H.(1981).
Synthe-sis of deoxyoligonucleotides on a polymer support. J. Am.
Chem. Soc. 103,3185-3191.
MAXAM, A.M.,andGILBERT, W. (1977).Anewmethod for
sequencingDNA. Proc. Nati. Acad. Sei. USA 74, 560-564.
MONACO, L., BOND, H.M., HOWELL, K.E.,andCÓRTESE,
R.(1987). Arecombinant apoA-I-proteinAhybrid reproduces the binding parameters of HDL to its receptor. EMBO J. 6,
3253-3260.
MOTT, J.E., GRANT, R., HO, Y.Z., and PLATT, T. (1985).
Maximizing gene expression from plasmid vectors containing
the
PL
promoter: Strategies for overproducing transcriptiontermination factor P. Proc. Nati. Acad. Sei. USA82, 88-92.
PIRAN, V.,andMORIN,R.J.(1979).Arapid radioassay proce-dure forplasmalecithimcholesterolacyltransferasebycovalent
chromatography. J. Lipid Res.20, 1040-1043.
ROSS, S.E., and CASRSON, S.D. (1985). Rapid
Chromato-graphie purificationofapolipoproteinsA-IandA-II from
hu-manplasma.Anal. Biochem. 149,166-168.
SANGER, F., NICKLEN, S., and COULSON, A.R. (1977). DNAsequencingwith chainterminatinginhibitors. Proc. Nati. Acad. Sei. USA74,5463-5467.
SEILHAMER, J.J., PROTTER, A.A., FROSSARD, P., and
LEVY-WILSON, B. (1984). Isolation and DNA sequence of
full-lengthcDNA and of the entiregenefor human
apolipopro-teinA-I. Discoveryofa newgenetic polymorphismin theapo A-Igene. DNA3,309-317.
SHAPIRO, D., BALLENTYNE, F.C., and SHEPHERD, J.
(1980).Comparisonofimmunonephelometryand electroimmu-noassay for estimation of plasma apolipoprotein A-I. Clin. Chim. Acta 103,7-13.
WU, A.L.,andWINDMUELLER, H.G.(1979). Relative contri-butionsbyliver and intestinetoindividual plasma
apolipopro-teins in therat. J. Biol. Chem. 254,7316-7322.
ZANNIS, V.l., KARATHANASIS, S.K., KEUTMANN, T., GOLDBERGER, G., and BRESLOW, J.L. (1983). Intracellu-lar and extracelluIntracellu-lar processingof human apolipoprotein A-I: SecretedapolipoproteinA-Iisoprotein2 isapropeptide.Proc.
NatiAcad. Sei.USA80,2574-2578.
Address
reprint
requests to:Dr. A. Bollen Service de
Génétique
Appliquée
U.L.B.ruede l'Industrie24 B-1400
Nivelles,
Belgium
Received forpublicationDecember27, 1988,and in revised form