Abstract
Objective: To identify clinical and laboratory data which differentiate Klinefelter syndrome (KS) patients according to age group.
Methods: The study included all cases of hypogonadism, gynecomastia and/or infertility whose karyotype was performed at a university hospital from January 1989 to December 2011, in a total of 105 subjects. The following data were retrospectively analyzed: age at irst visit, ratio of arm span to height, pubic hair, gynecomastia, testicular volume, luteinizing hormone (LH), follicle stimulating hormone (FSH), total testosterone (T), and sperm analysis.
Results: During the study period, 33 patients were diagnosed with Klinefelter syndrome (KS+) and 72 were not (KS-). Out of all KS cases, only seven (21.2%) were diagnosed before 20 years old and two (6.1%) before 10 years old. Age at irst consultation (in years) was similar in both groups (KS+ = 31.3±12.9 and KS- = 27.6±12.1), as were ratio of arm span to height and frequency of gynecomastia. However, in KS+ patients, pubic hair was less developed, testicular volume was smaller and testosterone levels were lower, while LH and FSH levels and frequency of azoospermia were higher.
Conclusions: Klinefelter syndrome is both an under and late diagnosed condition. The most important data for diagnosis are testicular volume, hormone levels and presence of azoospermia in sperm analysis, especially in puberty and adult life.
J Pediatr (Rio J). 2012;88(4):323-7: Klinefelter syndrome; male infertility; delayed puberty; gynecomastia.
O
riginala
rticleCopyright © by Sociedade Brasileira de Pediatria
323
Introduction
Klinefelter syndrome (KS) is the most common sex chromosome abnormality in men, with an estimated prevalence of about one in 600 newborn males.1 It is characterized cytogenetically by the presence of an extra X chromosome (47,XXY), which occurs in around 90% of cases; however, KS variants, such as mosaicism (46,XY/47,XXY) and other much less frequent aneuploidies (48,XXXY, 48,XXYY, 49,XXXXY) have already been described.2
The main clinical indings, present in almost all individuals with KS, are small testes, azoospermia and increased gonadotropins, especially follicle stimulating hormone (FSH). However, other indings, such as gynecomastia, delayed puberty, sparse body and pubic hair, micropenis, tall stature, increased arm span to height ratio, learning disabilities, psychiatric illnesses, peripheral venous disease, abdominal obesity, metabolic syndrome, higher risk of
Klinefelter syndrome:
an unusual diagnosis in pediatric patients
Bruna J. Tincani,1,2 Bruno R. Mascagni,3 Roberto D. P. Pinto,3 Guilherme Guaragna-Filho,1,2
Carla C. T. S. Castro,1,2 Letícia E. Sewaybricker,1,2 Nilma L. Viguetti-Campos,2,4
Antonia P. Marques-de-Faria,2,5 Andréa T. Maciel-Guerra,2,6 Gil Guerra-Júnior2,7
1. Resident, Endocrinologia Pediátrica. Departamento de Pediatria, Faculdade de Ciências Médicas (FCM), Universidade Estadual de Campinas (UNICAMP), Campinas, SP, Brazil.
2. Grupo Interdisciplinar de Estudos da Determinação e Diferenciação do Sexo (GIEDDS), FCM, UNICAMP, Campinas, SP, Brazil. 3. Undergraduate student, Medicina. FCM, UNICAMP, Campinas, SP, Brazil.
4. Biologist. Departamento de Genética Médica, FCM, UNICAMP, Campinas, SP, Brazil.
5. Clinical geneticist. Tenured professor. Departamento de Genética Médica, FCM, UNICAMP, Campinas, SP, Brazil. 6. Clinical geneticist. Full professor. Departamento de Genética Médica, FCM, UNICAMP, Campinas, SP, Brazil. 7. Pediatric endocrinologist. Full professor. Departamento de Pediatria, FCM, UNICAMP, Campinas, SP, Brazil.
No conflicts of interest declared concerning the publication of this article.
Suggested citation: Tincani BJ, Mascagni BR, Pinto RD, Guaragna-Filho G, Castro CC, Sewaybricker LE, et al. Klinefelter syndrome: an unusual diagnosis in pediatric patients. J Pediatr (Rio J). 2012;88(4):323-7.
autoimmune diseases and cancer, may be observed with different frequencies according to population, age group and karyotype.1,3-10
Although irst described 70 years ago,11 KS remains as an under diagnosed disease, because patients do not seek doctors very often, and doctors not always recognize the diagnosis.8 Therefore, only about 25% of all adult patients with KS are diagnosed; most during the investigation of infertility and/or hypogonadism; and less than 10% of cases are diagnosed before puberty.1,12
Therefore, the aim of this study was to identify clinical and laboratory data that differentiate KS cases according to age group.
Methods
This is a retrospective clinical study including all individuals who sought care at the Outpatient Care of the Interdisciplinary Group for the Study of Sex Determination and Differentiation at the Hospital de Clínicas (HC) da Faculdade de Ciências Médicas (FCM) da Universidade Estadual de Campinas (UNICAMP), Brazil, in the period of January, 1989 to December, 2011, to investigate KS. It should be noted that the consultation in this outpatient care is the only possibility of performing the karyotype at the HC-UNICAMP when the suspicion is KS.
All patients were evaluated according to age at irst consultation, main clinical complaint (infertility, gynecomastia, intellectual disability and/or neuromotor delay, evidence of hypogonadism, including delayed puberty, incomplete development of secondary sexual characteristics, microorchidism and micropenis), ratio of arm span to height (A/H), pubic hair by classiication according to Marshall & Tanner,13 presence of gynecomastia, testicular volume (measured by Prader orchidometer), luteinizing hormone (LH), FSH, total testosterone (T), sperm analysis and karyotype. Testicular volume was calculated by the arithmetic mean between the volumes of both testicles of each patient.
All laboratory assessments (LH, FSH, T and sperm analysis) were performed at the Laboratory of Physiology of HC-UNICAMP. In relation to sperm analysis, results were categorized into normal, oligospermia or azoospermia. The examination of the karyotype was performed from culture of peripheral blood leukocytes with counting of at least 32 metaphases at the Cytogenetic Laboratory, Department of Medical Genetics of the FCM-UNICAMP. From the karyotype test results, patients were divided in carriers (KS+) and non-carriers (KS-) of KS.
Data evaluated were compared between the cases of KS+ and KS- by chi-square test or Fisher exact for categorical variables (pubic hairiness, gynecomastia and sperm analysis) and Mann-Whitney test for continuous
variables (age, A/H, testicular size, LH, FSH and T) with p < 0.05. The program used in the statistical analysis was the Statistical Package for the Social Sciences (SPSS, Chicago, IL, EUA) version 16.0.
Results
In total, 105 cases were included in the study, 72 with normal karyotype (46,XY), 31 with 47,XXY karyotype and two with 46,XY/47,XXY, totaling, therefore, 72 cases (68.6%) without KS (KS-) and 33 (31.4%) with KS (KS+).
Age at irst consultation, in the KS- group, ranged from 1.5 to 67.5 years (27.6±12.1 years), and in the KS+ group, from 5.3 to 59.7 years (31.3±12.9 years), with no statistical difference between the groups (p = 0.13). Out of the 105 cases, 30 (28.6%) were evaluated before 20 years old: seven KS+ and 23 KS-; and only three before 10 years old: two KS+ and one KS-. Therefore, among the 33 cases of KS+, seven (21.2%) were diagnosed before 20 years old, and two (6.1%), before 10 years.
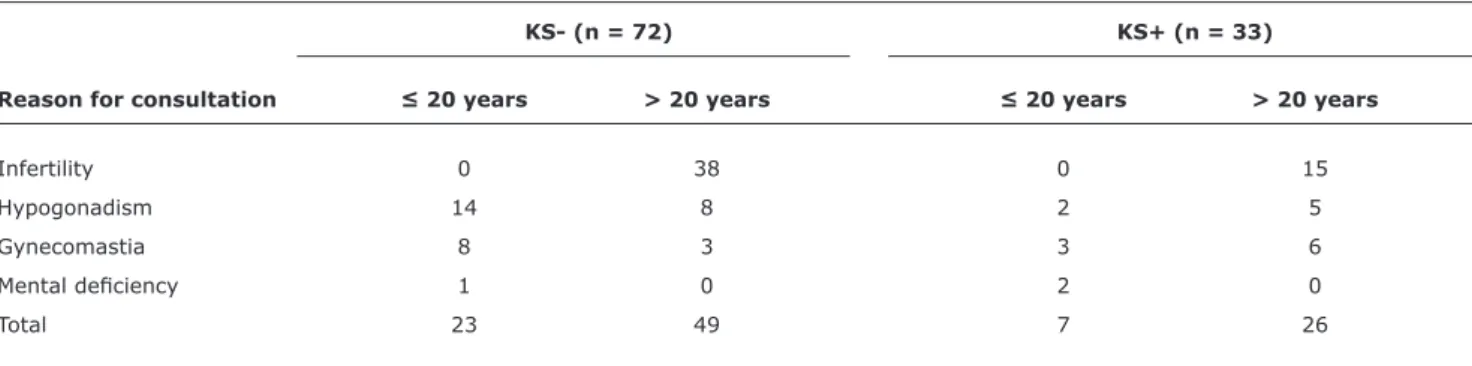
Table 1 shows the main complaint in the irst consultation in relation to age (≤ and > 20 years) and KS+ and KS- diagnosis. Regardless of diagnosis, the main complaint above 20 years was infertility (77.5% in the KS- group and 57.7% in the KS+ group), while in patients under 20, hypogonadism and gynecomastia were the main complaints (91.3% in the KS- group and 71.4% in the KS+ group). The complaint of mental deiciency or neuromotor delay occurred only in patients below 20 years, regardless of diagnosis of KS; and all patients were under 10 years old. There was no statistical difference between the SK+ and SK- groups regarding main complaint when separated by age group (≤ 20 years: chi-square(2) = 4.29; p = 0.12; and > 20 years: chi-square(2) = 5.10; p = 0.08).
Table 2 presents data on pubic hair staging and presence of gynecomastia only among patients above 10 years old (n = 102; 31 KS+ and 71 KS-), and sperm analysis in patients above 18 years (n = 79; 29 KS+ and 50 KS-). There was a statistically signiicant difference between the groups in pubic hair staging (chi-square = 26.22; p < 0.0001), with KS+ group presenting lower pubic hair. There was also a statistically signiicant difference in the outcome of sperm analysis (chi-square(2) = 25.16; p < 0.0001), with the KS+ group presenting only azoospermia. However, there was no statistically signiicant difference in the presence of gynecomastia (chi-square = 2.68; p = 0.10) between the two groups.
KS- (n = 72) KS+ (n = 33)
Reason for consultation ≤ 20 years > 20 years ≤ 20 years > 20 years
Infertility 0 38 0 15
Hypogonadism 14 8 2 5
Gynecomastia 8 3 3 6
Mental deiciency 1 0 2 0
Total 23 49 7 26
Table 1 - Reason for consultation in relation to age and diagnosis (KS- or KS+)
KS- = without Klinefelter syndrome; KS+ = with Klinefelter syndrome.
Variable KS- KS+ p
Pubic hair < 0.0001
1 or 2 5 16
3 to 5 66 15
Gynecomastia 0.10
+ 31 19
– 40 12
Sperm analysis < 0.0001
Normal 7 0
Oligospermia 21 0
Azoospermia 22 29
Table 2 - Pubic hair, gynecomastia and sperm analysis in relation to diagnosis (KS- or KS+)
KS- = without Klinefelter syndrome; KS+ = with Klinefelter syndrome. 1 to 5 = staging according to Marshall & Tanner13; pubic hair and gynecomastia
were assessed only in patients above 10 years, and sperm analysis only in those above 18 years.
KS- KS+
Variable (M±DP) (M±DP) p
Arm span/height 1.02±0.03 1.01±0.03 0.98
Mean testicular volume (mL) 9.5±6.5 3.5±2.5 0.001
LH (U/L) 7.5±8.4 19.6±8.8 0.001
FSH (U/L) 14.9±7.4 43.8±25.4 0.001
T (ng/mL) 8.7±7.3 4.3±2.9 0.01
FSH = follicle stimulating hormone; KS- = without Klinefelter syndrome; KS+ = with Klinefelter syndrome; LH = luteinizing hormone; M±DP = mean ± standard deviation; T = total testosterone.
Table 3 - Ratio of arm span/height, mean testicular volume, luteinizing hormone, follicle stimulating hormone, and total testosterone in relation to diagnosis (KS- or KS+)
(p = 0.01), with lower values for mean testicular volume and T, and higher values for FSH and LH in the KS+ group.
Discussion
Data from this study show that KS is still little and late diagnosed in our area. The HC-UNICAMP serves the population of a region with more than 5 million inhabitants, and for such an amount of patients we should have veriied many more than 33 diagnoses of KS in 23 years (about three cases diagnosed every 2 years), indicating that either the patients do not seek medical help very often or the doctors think too little about this diagnosis. Although apparently small, the sample of this study is similar to that of recent studies,1,3,8,14 where the highest sample was of 98 cases.10
Regarding age at diagnosis, out of 105 cases included in the study, only 30 (28.6%) were assessed before age 20, seven of these (23.3%) with diagnosis of KS and only three (2.9%) were assessed below 10 years old, two of them diagnosed with KS. Therefore, data from this study conirm that the diagnosis of KS is rarely done before 20 years of age, especially before 10 years. Out of the 75 cases evaluated after age 20, the diagnosis of KS occurred more frequently, in the ratio of 1:2, that is, in every three cases, one had the diagnosis of KS. Similar results were found by Abramsky & Chapple,12 when they veriied that 26% of the expected number of cases of KS in North Thames, United Kingdom, between 1990 and 1993, were diagnosed after birth, 28% of those diagnosed before age 20 and only 4% before 10 years old; similar data to those found by Bojesen et al.1 and by the present study.
References
1. Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. J Clin Endocrinol Metab. 2003;88:622-6.
2. Lanfranco F, Kamischke A, Zitzmann M, Nieschlag E. Klinefelter’s syndrome. Lancet. 2004;364:273-83.
3. Kamischke A, Baumgardt A, Horst J, Nieschlag E. Clinical and diagnostic features of patients with suspected Klinefelter syndrome.
J Androl. 2003;24:41-8.
4. Swerdlow AJ, Higgins CD, Schoemaker MJ, Wright AF, Jacobs PA; United Kingdom Clinical Cytogenetics Group. Mortality in patients with Klinefelter syndrome in Britain: a cohort study. J Clin Endocrinol Metab. 2005;90:6516-22.
(mild delay in motor development and language), KS has been identiied in 0.4% of boys in special education programs and in 1.2% of patients with intellectual retardation of undetermined cause16,17; in general, referred initially for the investigation of fragile X syndrome,18 the leading inherited cause of mental retardation, most common in males and with little expressive manifestations before puberty, except for intellectual impairment and behavioral changes. In puberty and adult life, the main clinical characteristic that differentiates KS syndrome from X fragile syndrome is testicular volume, which is small in the irst and big in the latter.
Above age 20, the main complaint in the suspicion of diagnosis was infertility, while in patients below 20 years, though already at the stage of puberty, over 10 years old, hypogonadism and gynecomastia were the main complaints. Reduced pubic hair and lower mean testicular volume were signiicantly more frequent in the KS+ group, which did not occur with gynecomastia and predominance of arm span in relation to height. Gynecomastia is a frequent inding in KS (50 to 88%) and in normal male puberty (60 a 70%), so it is a inding of little signiicance in the diagnosis of SK in puberty,19 once there is an overlap of diagnosis. Reduction of pubic hair and increased ratio of A/H are related to hypogonadism, which is often not suspected in puberty, once most patients with KS begins puberty at the normal stage.19
Regarding laboratory tests, LH, FSH, T and the frequency of azoospermia in sperm analysis were signiicantly higher in the KS+ group. From the hormonal standpoint, patients with KS presented normal prepubertal values of T, LH, FSH, inhibin B and anti-müllerian hormone, with normal serum testosterone responses to human chorionic gonadotropin stimulation.9,10,20,21 During puberty, after a normal increase of T levels, they may, in some cases, remain within the low-normal range for the stage of sexual maturation, though suficient to allow puberty progression and determine the satisfactory development of secondary sexual characteristics.9,10,20,21 From midpuberty onwards, in general, patients with KS already start to present a gradual increase in FSH (relecting the dysfunction of the seminiferous tubules and the Sertoli cells) and LH (relecting the Leydig cell dysfunction), and the increase in FSH generally precedes (about 1 year before) and is more intense than that of LH; such data may, from this moment on, assist in the suspicion of diagnosis, as veriied in the present study. However, the main clinical data on the puberty age group to justify the laboratory determination of LH and FSH and, if increased, the performance of the karyotype, is the disproportion of testicular volume for the stage of sexual maturation. Kamischke et al.3 showed that the mean measure of the testicular volume was the parameter with better sensitivity for the diagnosis of KS and that the mean volume of both testicles by ultrasound was 4.7 mL in cases
of KS+ and 13.7 mL in cases of KS-, similar to our indings through clinical evaluation.
In adulthood, the main data that motivate the investigation of KS are hypogonadism (clinical) and hypergonadotropic (laboratory) and infertility.19 KS is the most frequent genetic cause of infertility, occurring in 11% of azoospermic men and 4% of infertile men.22 In the present study, these data are conirmed, and infertility and hypogonadism are the main complaints after age 20, and all KS+ patients above 18 years old were azoospermic.
Most clinical and laboratory indings present in KS occurs due to the testicular degenerative process present throughout life. In fetuses and neonates, there is a decrease in the number of germ cells and an increased proportion of seminiferous tubules devoid of germ cells, progressing with the reduction of the seminiferous tubules, a greater decrease in the number of spermatogonia in the child and an acceleration of all this process in puberty, ending in adulthood with extensive ibrosis and hyalinization of the seminiferous tubules, absence of spermatogonia, predominance of Sertoli immature cells and hyperplasia of Leydig cells and interstitium.23
It is noteworthy that among the 33 individuals with KS in the present study, 31 presented 47,XXY karyotype and two 46,XY/47,XXY, as, in general, patients with mosaicism presented a less severe phenotype,24,25 but without difference in morbidity and mortality,14 while the patients with 48,XXXY and 49,XXXXY karyotypes presented a more severely affected phenotype, in particular regarding intellectual impairment and association with malformations,26 although also without apparent increase in mortality.5,14
5. Swerdlow AJ, Schoemaker MJ, Higgins CD, Wright AF, Jacobs PA; UK Clinical Cytogenetics Group. Cancer incidence and mortality in men with Klinefelter syndrome: a cohort study. J Natl Cancer Inst. 2005;97:1204-10.
6. Bojesen A, Juul S, Birkebaek NH, Gravholt CH. Morbidity in Klinefelter syndrome: a Danish register study based on hospital discharge diagnoses. J Clin Endocrinol Metab. 2006;91:1254-60.
7. Bojesen A, Gravholt CH. Klinefelter syndrome in clinical practice.
Nat Clin Pract Urol. 2007;4:192-204.
8. Bojesen A, Gravholt CH. Morbidity and mortality in Klinefelter syndrome (47,XXY). Acta Paediatr. 2011;100:807-13.
9. Wikström AM, Dunkel L. Klinefelter syndrome. Best Pract Res Clin Endocrinol Metab. 2011;25:239-50.
10. Pacenza N, Pasqualini T, Gottlieb S, Knoblovits P, Costanzo PR, Stewart Usher J, et al. Clinical presentation of Klinefelter’s syndrome: differences according to age. Int J Endocrinol. 2012;2012:324835.
11. Klinefelter HF Jr, Reifenstein EC Jr, Albright F. Syndrome characterized by gynaecomastia, aspermatogenesis without a-Leydigism, and increase secretion of follicule-stimulating hormone. J Clin Endocrinol Metab. 1942;2:615-27.
12. Abramsky L, Chapple J. 47,XXY (Klinefelter syndrome) and 47,XYY: estimated rates of and indication for postnatal diagnosis with implications for prenatal counselling. Prenat Diagn. 1997;17:363-8.
13. Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child. 1970;45:13-23.
14. Bojesen A, Juul S, Birkebaek N, Gravholt CH. Increased mortality in Klinefelter syndrome. J Clin Endocrinol Metab. 2004;89:3830-4.
15. Wattendorf DJ, Muenke M. Klinefelter syndrome. Am Fam Physician. 2005;72:2259-62.
16. Fales CL, Knowlton BJ, Holyoak KJ, Geschwind DH, Swerdloff RS, Gonzalo IG. Working memory and relational reasoning in Klinefelter syndrome. J Int Neuropsychol Soc. 2003;9:839-46. 17. Khalifa MM, Struthers JL. Klinefelter syndrome is a common cause
for mental retardation of unknown etiology among prepubertal males. Clin Genet. 2002;61:49-53.
18. Youings SA, Murray A, Dennis N, Ennis S, Lewis C, McKechnie N, et al. FRAXA and FRAXE: the results of a ive year survey. J Med Genet. 2000;37:415-21.
19. Radicioni AF, Ferlin A, Balercia G, Pasquali D, Vignozzi L, Maggi M, et al. Consensus statement on diagnosis and clinical management of Klinefelter syndrome. J Endocrinol Invest. 2010;33:839-50. 20. Salbenblatt JA, Bender BG, Puck MH, Robinson A, Faiman C, Winter
JS. Pituitary-gonadal function in Klinefelter syndrome before and during puberty. Pediatr Res. 1985;19:82-6.
21. Topper E, Dickerman Z, Prager-Lewin R, Kaufman H, Maimon Z, Laron Z. Puberty in 24 patients with Klinefelter syndrome. Eur J Pediatr. 1982;139:8-12.
22. Van Assche E, Bonduelle M, Tournaye H, Joris H, Verheyen G, Devroey P, et al. Cytogenetics of infertile men. Hum Reprod. 1996;11:1-24; discussion 25-6.
23. Wikström AM, Dunkel L. Testicular function in Klinefelter syndrome. Horm Res. 2008;69:317-26.
24. Sarkar R, Marimuthu KM. Association between the degree of mosaicism and the severity of syndrome in Turner mosaics and Klinefelter mosaics. Clin Genet. 1983;24:420-8.
25. Smyth CM, Bremner WJ. Klinefelter syndrome. Arch Intern Med. 1998;158:1309-14.
26. Visootsak J, Aylstock M, Graham JM Jr. Klinefelter syndrome and its variants: an update and review for the primary pediatrician.
Clin Pediatr (Phila). 2001;40:639-51.
Correspondence: Gil Guerra-Júnior
Departamento de Pediatria - FCM - UNICAMP Cidade Universitária “Zeferino Vaz”, sem número CEP 13081-970 - Campinas, SP - Brazil