REGULAR ARTICLE
EFFECT OF DEOXYNIVALENOL ON SOME HAEMATOLOGICAL, BIOCHEMICAL AND ANTIOXIDANT PARAMETERS OF PORCINE BLOOD
IN VITRO
Katarína Zbyňovská*, Peter Petruška, Marcela Capcarová
Address: Slovak University of Agriculture in Nitra, Faculty of Biotechnology and Food Sciences, Department of Animal Physiology, Tr. A. Hlinku 2, 949 76 Nitra, Slovakia,
Phone number: +421376414287.
*Corresponding author: zbynovska.katarina@gmail.com
ABSTRACT
Keywords: DON, porcine blood, antioxidants
INTRODUCTION
Mycotoxins are secondary metabolites of molds that have adverse effects on humans, animals, and crops that result in illnesses and economic losses (Zain, 2011). Contamination of food and agricultural commodities by various types of toxigenic molds (fungi) is a serious and widely neglected problem (Bhat et al., 2010). This contamination presented a hazard to human and animal health for decades (Gbore and Akele, 2010). The name “trichotecenes” includes almost 200 various compounds synthetized mainly by fungi from the Fusarium family. The most important and the most occurring Fusarium mycotoxin is deoxynivalenol (DON) (Łazicka and Orzechowski, 2010; Klem et al., 2007). It is mainly produced by
Fusarium graminearums and Fusarium culmorum (Kushiro, 2008). DON, also known as
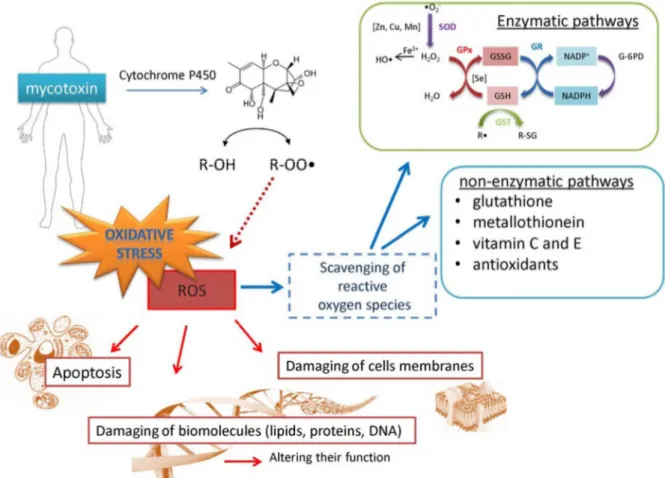
for DON on biochemical pathways (Fig. 1), including those generating reactive oxygen species (ROS) which can damage biomolecules and membranes leading to apoptosis (Desmond et al., 2008). Thus, it is not surprising that the ingestion of mycotoxins activates numerous mechanisms in the body to reduce their toxicity and induce their excretion (Sobrova et al., 2012). Enzymatic or non-enzymatic reactions can scavenge DON-generated ROS, subsequently preventing oxidative stress (Valko et al., 2006).
Figure 1 Scheme of possible way of deoxynivalenol detoxification. The first and one of the most important pathways used for detoxifying DON is cytochrome P450, which serves to catalyse the oxidation of organic substances. This pathway, however, can cleave free hydroxyl groups of DON to produce DON-radical, which can be more dangerous. The DON-radical can be scavenged by enzymatic (glutathione peroxidase (GPx), catalase, superoxide dismutase) or
non-enzymatic ways (reduced glutathione (GSH), metallothionein (MT), and vitamins) (Sobrova et al., 2012)
MATERIAL AND METHODS
Animals and experimental design in vitro
Slovakian White gilts (n=24) at the age of 100-120 days were kept under standard conditions at the Experimental Station of the Animal Production Research Centre Nitra. Conditions of their care, manipulations and use corresponded to the instruction of EC no. 178/2002 and related EC documents, and they were approved by local ethics commission. Animals were slaughtered and blood samples were obtained.
Blood sampling and DON treatment
Blood was collected into EDTA-treated tubes. DON (Romer Labs Division Holding GmbH, Tulln, Austria) was added to blood samples at doses 10, 100 and 1000 ng.ml-1 (Table 1). The blood samples without addition of DON served as control group (C). The blood was incubated for 5 hours at 37oC.
Table 1 Application of DON in to blood in vitro
Group DON (ng.ml-1)
C 0
E1 10
E2 100
E3 1000
n=5 in each group; C- control group, E1 - E3 – experimental groups
with various doses of DON
Analysis of parameters
Austria). Enzymatic activity (SOD – superoxide dismutase and GPx – glutathione peroxidase) were analysed by spectrophotometer Genesys 10 (Thermo Fisher Scientific Inc., USA), using commercial assay kit (Randox, Bratislava). ROS was assayed by a microplate ELISA reader (Multiscan FC, ThermoFisher Scientific, Finland), using commercial assay kit (Biog, Bratislava).
Statistical analysis
Sigma Plot 11.0 (Jandel, Corte Madera, USA) was used to conduct statistical analyses. One-way ANOVA was used to calculate basic statistic characteristics and to determine significant differences among the experimental and the control groups. Data presented are given as mean and standard deviation (SD). Differences were compared for statistical significance at the level P<0.05.
RESULTS
Biochemical parameters of porcine blood
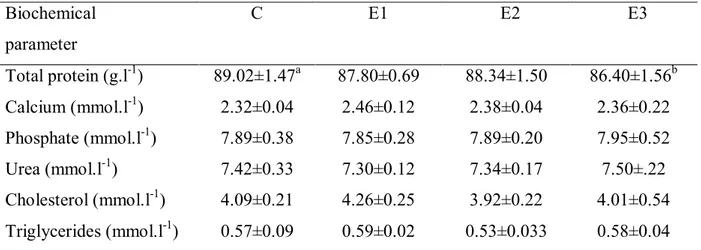
Table 2 Effect of DON on biochemical parameters in porcine blood plasma. Biochemical
parameter
C E1 E2 E3
Total protein (g.l-1) 89.02±1.47a 87.80±0.69 88.34±1.50 86.40±1.56b
Calcium (mmol.l-1) 2.32±0.04 2.46±0.12 2.38±0.04 2.36±0.22
Phosphate (mmol.l-1) 7.89±0.38 7.85±0.28 7.89±0.20 7.95±0.52
Urea (mmol.l-1) 7.42±0.33 7.30±0.12 7.34±0.17 7.50±.22
Cholesterol (mmol.l-1) 4.09±0.21 4.26±0.25 3.92±0.22 4.01±0.54
Triglycerides (mmol.l-1) 0.57±0.09 0.59±0.02 0.53±0.033 0.58±0.04
C - control, E1 – 10 ng.ml-1, E2 100 ng.ml-1, E3 - 1000 ng.ml-1
Values shown as means ± SD (standard deviation),a,b - in row means significant difference (P ‹ 0.05)
significant differences (P > 0.05). No significant differences (P > 0.05) in calcium, phosphorus, urea, cholesterol and triglycerides content of porcine blood were found between the control group and the experimental groups E1, E2 and E3.
Haematological parameters of porcine blood
Results are shown in Table 3. PLT significantly (P < 0.05) decreased in the experimental group E3 when compared with E1 and E2, and the control group. RBC, WBC, HGB and PCV decreased in the all experimental groups when compared with the control group but not significantly (P > 0.05). In the groups E1 and E2 lower values of LYM were measured in comparison with the control group. The highest value of LYM was in E3 group, however without significant differences (P > 0.05) among the groups.
Table 3 Effect of DON on haematological parameters in porcine blood. Haematological
parameter
C E1 E2 E3
RBC (109.l-1) 7.00±0.13 6.93±0.10 6.98±0.09 6.90±0.05
WBC (109.l-1) 19.92±0.32 19.58±0.42 19.60±0.26 19.71±0.52
PLT (109.l-1) 235.40±18.30a 226.20±15.45a 216.40±31.51a 186.20±18.24b
HGB (g.l-1) 130.60±2.30 128.80±2.39 128.20±1.30 129.80±1.30
PCV (%) 44.48±0.81 43.93±0.66 44.00±1.01 43.93±0.36
LYM (109.l-1) 15.38±0.34 15.12±0.37 15.30±0.40 15.77±0.63
C - control, E1 – 10 ng.ml-1, E2 100 ng.ml-1, E3 - 1000 ng.ml-1
RBC - red blood cells, WBC - white blood cells, PLT - plateles, HGB - haemoglobin, PCV - packed cell volume, LYM – lymphocyte, values shown as means ± SD (standard deviation),
a,b - in row means significant difference (P ‹ 0.05)
Anti- and pro-oxidant parameters of porcine blood
measured in comparison with other groups, however differences among the groups remained insignificant (P > 0.05). The higest value of ROS was in E2 group, however without significant differences (P > 0.05) among the groups. In E1 and E3 groups lower concentration of H2O2 when compared with the control group was measured.
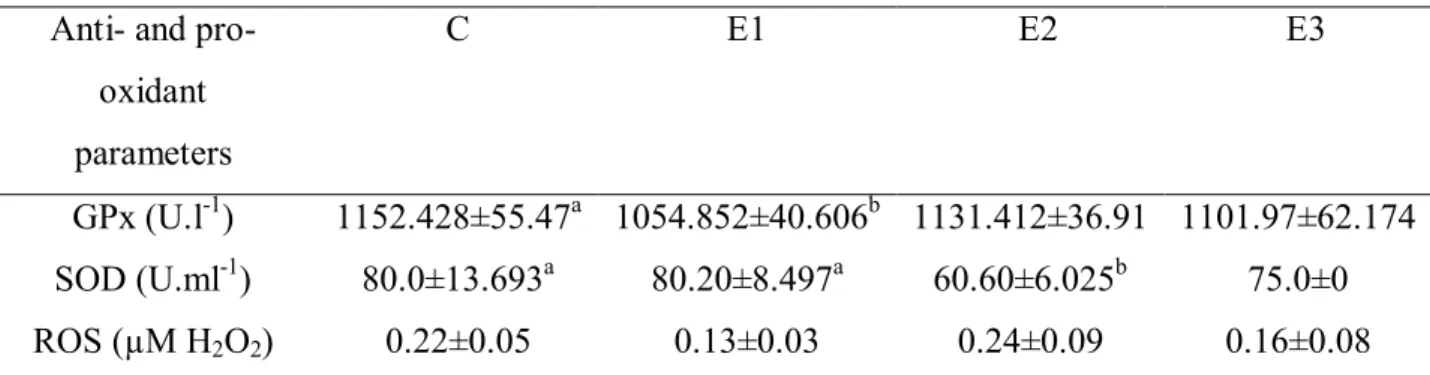
Table 4 Effect of DON on anti- and pro-oxidant parameters in porcine blood. Anti- and pro-
oxidant parameters
C E1 E2 E3
GPx (U.l-1) 1152.428±55.47a 1054.852±40.606b 1131.412±36.91 1101.97±62.174
SOD (U.ml-1) 80.0±13.693a 80.20±8.497a 60.60±6.025b 75.0±0
ROS (µM H2O2) 0.22±0.05 0.13±0.03 0.24±0.09 0.16±0.08
C - control, E1 – 10 ng.ml-1, E2 100 ng.ml-1, E3 - 1000 ng.ml-1
GPx - Glutathione Peroxidase, SOD - superoxide dismutase, ROS - ractive oxygen species.Values shown as means ± SD (standard deviation), a,b - in row means significant difference (P ‹ 0.05)
DISCUSSION
The Fusarium toxin DON is of outstanding importance in pig nutrition because of its frequent occurrence in cereal grains at levels high enough to cause adverse effects such as a decrease in feed intake and impairment of the immune system (Dänicke et al., 2010). Pigs are the most sensitive to DON among livestock. Effects of higher doses of DON on pigs are well known. DON decreased the feed intake (-4.3%.g DON-1.kg feed-1) and growth rate (-7%.g DON-1.kg feed-1) of pigs when the level exceeds 1 mg.kg-1 feed, and only high doses of DON caused vomiting (Eitenne and Waché, 2008).
DON is rapidly absorbed in pigs and oral bioavailability is estimated to be 55% (Rotter et al, 1996). After feeding a diet containing naturally contaminated wheat (4.2 mg.kg -1 feed), the maximum serum DON concentration was found after 4.1 h (Dänicke et al., 2004).
Biochemical parameters of porcine blood
To our knowledge there are not a lot of similar studies on effect of DON in various doses given to the porcine blood in vitro and its effect on biochemical parameters. For this reason, we have compared our results also with results from other in vivo studies.
Calcium ion plays an important role in a variety of biological processes, including gene expression, cell cycle regulation, and cell death (Yet et al., 2005). In our experiment DON insignificantly decreased Ca content in blood plasma. Chaytor et al. (2011) reported no effect of various addition of DON on content of Ca in porcine blood. Côté et al. (1986) found that content of Ca in milk of dairy cattle was not changed after feeding DON contaminated feed. Content of Ca in blood of broiler chicken was not affected by Fusarium mycotoxins (Yegani et al., 2006). In study of Swamy et al. (2002) concentration of DON (3000-5800 µg.kg-1) in feed caused decrease of Ca in porcine blood. Similar results were found by Faixová et al. (2010) in experiment with broiler chickens. Grenier et al. (2011) considered that DON alone or in combination with other mycotoxin (DON 3 mg.kg-1 and fumonisin 6 mg.kg-1) had minimal effect on biochemical parameters of porcine blood. Chronic exposure of mycotoxins may be partly associated with secondary deficiency of vitamin D. Metabolism of calcium and vitamin D was studied in young rats administered with DON at a daily dose of 10 mg.kg-1 perorally within 7 days. The DON treatment caused a moderate hypocalcemia, a decreased absorption of calcium in small intestine as well as decrease of alkaline phosphatase activity in blood and small intestine mucosal membrane. Density and saturation of bone tissue with minerals were not altered (Sergeev et al., 1990).
In our study, content of phosphorus (P) in porcine blood was not affected by any concentration of DON. Studies of Accensi et al. (2006) and Chaytor et al. (2011) confirmed our results. Content of P decreased in chicken blood after DON treatment (Bergsjö et al., 1993; Swamy et al., 2002).
In this study we observed decreased content of total protein in E3 group. The first toxic effect associated with trichothecenes including DON was the inhibition of protein synthesis. Trichothecenes bind to the 60S subunit of eukaryotic ribosomes and interfere with the activity of peptidyltransferase. Based on the induction of emesis, suppose a possible interaction with serotinergic and dopaminergic receptors (Fioramonti et al., 1993). Our results are comparable with results of Faixová et al. (2010). Authors found decrease of total protein in chickens blood after feeding mycotoxins DON and zeralenon (ZEA). Decreased of total protein in porcine blood in vivo after DON inclusion reported Döll et al. (2003), Chen et
al. (2008), Swamy et al. (2002), and Klapáčová et al. (2011). Trichotecenes, such as DON,
interfere with protein synthesis ant the cellular level, and will therefore predominantly damage quickly proliferating cells as found in the immune system (Goyarts et al., 2006). In
in vitro study of De Walle et al. (2010) DON caused decrease content of total protein, what
was associated with decreased incorporation amino acid leucine and this demonstrate inhibition effect of DON in protein synthesis.
In our study cholesterol content was not changed after DON exposure. In chicken blood content of cholesterol increased when added DON in dose 3 mg.kg-1 (Klapáčová et al., 2011). When pigs were feeding combination of DON with aflatoxin content of cholesterol was increased by 74 % when compared with control group (Chaytor et al., 2011).
In our study DON had no effect on triglycerides content in blood plasma of pig. Ghareeb et al. (2012) contend that DON affected metabolism proteins and lipids in broiler chickens. Content of triglycerides was decreased in broiler chickens blood after feeding contaminated grain.
Haematological parameters of porcine blood
transport and concentration of Ca in PLT, so we may also suppose that DON affects to metabolism of Ca.
Acute exposure of DON on proliferation of peripheral LYM in pigs in vitro and inhibition of proliferation of LYM confirmed Goyarts et al. (2006). Pestka and Smolinski (2005) present that ingestion of feed contaminated with DON reduced lymphocyte proliferation upon mitogenic stimulation.
Addition of DON in our study had no significant effect on RBC, WBC, HGB and PVC. Accensi et al. (2006) observed that DON had no effect on biochemical and haematological parameters (in concentration 280 - 840 µg.kg-1) in vivo. Occasionally changes in RBC, WBC and PLT, concentration of HGB and values of PVC observed Etiene and Waché (2008). RBC, PCV and PLT may slightly increase after feed intake decrease (Prelusky et al., 1994). In another study Capcarova et al. (2010) found significant decrease of WBC after pesticide bendiocarbamate additions in rabbits. High doses of toxic substances caused decrease of WBC and low doses could raise the WBC parameter.
DON is also an immunosuppressor Clinical studies revealed cell depletion in thymus, spleen or bursa Fabricius in exposed animals. Sensitivity of B- and T- cells, isolated from spleen, thymus and Peyer’s patches towards DON has been described in many studies. Concomitant factors (lipopolysaccharide of Gram-negative bacteria, viral infections) that modulate transcription of cytokines and chemokines, could explain the differences in clinical responses to low levels of DON in individual herds, due to the strong influence of these factors on DON response. It is important to note that these mechanistic studies have been almost entirely conducted in mice, or rodent and human cell lines. However, the described mechanisms are highly preserved in mammals, including pigs where the response may be even more pronounced due to the renowned susceptibility of pigs to bacterial and viral agents modulating cytokine response (Pizzamiglio, 2008).
Anti- and pro-oxidant parameters of porcine blood
SOD-CAT-GPx catalytic triad is quite ubiquitous and has been found in virtually all cells in prokaryotic and eukaryotic aerobic organisms. Superoxide dismutase catalyzes the dismutation of O2•– to produce H2O2. Although it recycles the superoxide anion free radical, SOD can be considered as a prooxidant because it converts a rather short-life and confined molecule, O2•–, into a quite stable and invasive H2O2. Glutathione peroxidases metabolize various substrates besides H2O2 and are activated even for a small rise in their intracellular or extracellular concentrations, or both, to maintain cell homeostasis. Therefore, GPx activity represents the first protective response for small changes in H2O2 concentrations under normal physiological conditions. In addition, GPx can also metabolize complex organic peroxidized molecules, allowing them to recycle some of the molecules that may have been damaged following H2O2 leakage and, consequently, OH•– production (Mansour and Mossa, 2009; Chabory et al., 2010; Demir et al., 2011).
In our study GPx decreased in all experimental groups in comparison with the control group in E1 significantly. In study of Krishnaswamy et al. (2010) GPx decreased with higher concentration of DON. In chickens, activity of GPx in duodenal mucosa tissues, (SOD) in erythrocytes, in duodenal mucosa were not affected by dietary DON and zearalenon (Borutova et al., 2008). Erdélyi et al. (2011) examined effect of feeding DON contamined diet on glutathione peroxidise activity in blood plasma of broiler chickens after 21 and 39 days. Activity of GPx was decreased. In our study the lowest SOD activity was detected in E2 group with 100 ng.ml-1 DON. In in vivo study, concentration of GPx and SOD decreased after insecticide exposure in rats (Demir et al., 2011). The decrease in the activity of SOD may be attributed to the saturation of SOD during the process of converting O2• to H2O2 (Eraslan et al., 2007). The major function of GPx, which uses glutathione (GSH) as a substrate, is to reduce soluble H2O2 and alkyl peroxides (Bebe and Panemangalore, 2003). GPx also can decompose H2O2 to water (Tian et al., 1998). Inhibition of GPx activity is accompanied by a depletion of GSH, which may result in oxidative stress. Reduced GSH, in conjunction with GPx and glutathione-S-transferase (GST), is responsible for the GSH redox cycles that maintain the redox status of tissues and protect structural and regulatory proteins against ROS-induced damage (Khan and Kour, 2007). In the present study, the decreased GPx activity might reflect cellular oxidative stress due to DON exposure.
group followed by slight decrease of SOD and GPx. Higher concentration of DON propably caused higher activity of antioxidant enzymes, what confirmed by our results of ROS, GPx and SOD in this group. Significantly higher concentration of ROS observed Krishnaswamy et al. (2010) after DON addition in to humans intestinal cells.
CONCLUSION
High doses of DON in porcine blood in vitro influenced content of total protein and count of LYM. Lower doses of DON influenced activity of GPx and SOD. Other studied parameters were not affected by DON. The results from this study determined levels of deoxynivalenol toxicity. Given that DON is the most occuring fumonisine and there are not many studies in vitro about its effects on biochemical, haematological, prooxidant and antioxidant parameters in porcine blood, thus it is neccessary to be examined further.
Acknowledgments: This work was financially supported by VEGA scientific grant 1/0790/11, VEGA 1/0084/12, VEGA 1/0022/13.
REFERENCES
ACCENSI, F. – PINTON, P. – CALLU, P. – ABELLA-BOURGES, N. – GUELFI, J. F. – GROSJEAN, F. – OSWALD, I. P. 2006. Ingestion of low doses of deoxynivalenol does not affect haematological, biochemical, or immune responses of pigletes. In Journal of Animal Science, vol. 84, 2006, no. 7, p. 1935-1942.
BEBE, F. N. – PANEMANGALORE, M. 2003. Exposure to low doses of endosulfan and chlorpyrifos modifies endogenous antioxidants in tissues of rats. In Journal of Environmental Science and Health, vol. 38, 2003, no. 3, p. 349-363.
BERGSJÖ, B. – LANGSETH, W. – NAFSTAD, I. – HÖGSETJANSEN, J. – LARSEN, J. S. 1993. The effect of naturally deoxynivalenol - contamined oats on the clinical condition, blood parameters, performance and carcass composition of growing pigs. In Veterinary Research Communications, vol. 17, 1993, no. 4, p. 283-294.
BORUTOVS, R. – FAIX, S. – PLACHA, I. – COBANOVA, K. – LENG, L. 2008. Effects of deoxynivalenol and zearalenone on oxidative stress and blood phagocytic activity in broilers. In Archives of Animal Nutrition, vol. 62, 2008, no. 4, p. 303-312.
BUKOWSKA, B. 2004. 2,4,5-T and 2,4,5-TCP induce oxidative damage in human erythrocytes: the role of glutathione. In Cell Biology IOnternational, vol. 28, 2004, no. 7, p. 557-563.
CAPCAROVA, M. – PETROVOVA, E. – FLESAROVA, S. – DANKOVA, M. – MASSANYI, P. – DANKO, J. 2010. Bendiocabamate induced alerations in selected parameters of rabbit homeostasis after experimental peroral administration. In Pesticide Biochemistry and Physiology, vol. 98, 2010, p. 213-218.
CHABORY, E. – DAMON, C. – LENOIR, A. – HENRY-BERGER, J. – VERNET, P. – CADET, R. – SAEZ, F. – DREVET, J. R. 2010. Mammalian glutathione peroxidases control acquisition and maintenance of spermatozoa integrity. In Journal of Animal Science, vol. 88, 2010, no. 4, p. 1321-1331.
CHAYTOR, A. – SEE, M. T. – HANSEN, J. A. – SOUZA, A. L. – MIDDLETON, T. K. – KIM, S. W. 2011. Effects of chronic exposure of diets with reduced concentrations of aflatoxin and deoxynivalenol on growth and immune status of pigs. In Journal of Animal Science, vol. 89, 2011, no. 1, p. 124-135.
CHEN, F. – MA, Y. XUE, C. – MA, J. – XIE, Q. – WANG, G. BI, Y. – CAO, Y. 2008. The combination of deoxynivalenol and zearalenone at permitted feed concentrations causes serious physiological effects in young pigs. In Journal of Veterinary Science, vol. 9, 2008, no. 1, p. 39-44.
CÔTÉ, L. M. – DAHLEM, A. M. – YOSHIZAWA, T. – SWANSON, S. P. – BUCK, W. B. 1986. Excretion of deoxynivalenol and its metabolite in milk, urine and feces of lactacing dairy cows. In Journal of Dairy Science, vol. 69, 1986, no. 9, p. 2416-2423.
CREPPY, E. E. 2002. Updateof survey, regulation and toxic effects of mycotoxins in Europe. In Toxicology Letters, vol. 127, 2002, no. 1-3, p. 19-28.
DÄNICKE, S. – VALENTA, H. – DÖLL, S. 2004. On the toxicokinetics and the metabolism of deoxynivalenol (DON) in the pig. In Atchives on animal nutrition, vol. 58, 2004, no. 2, p. 169-180.
DE WALLE, J. V. – SERGENT, T. – PIRONT, N. – TOUSSAINT, O. – SCHNEIDER? Y. J. – LARONDELLE, Y. 2010. Deoxynivalenol effects in vitro intestinal epithelial cell barier integrity through inhibition of protein synthesis. In Toxicology and Applied Pharmacology, vol. 245, 2010, no. 3, p. 291-298.
DEMIR, F. – UZUN, F. G. – DURAK, D. – KALENDER, Y. 2011. Subacute chlorpyrifos-induced oxidative stress in rat erythrocytes and the protective effects of catechin and quercetin. In Pesticide Biochemistry and Physiology, vol. 99, 2011, no. 1, p. 77-81.
DESMOND, O. J. – MANNERS, J. M. – STEPHENS, A. E. – MACLEAN, D. J. – SCHENCK, P. M. – GARDINER, D. M. – MUNN, A. L. – KAZAN, K. 2008. The fusarium mycotoxin deoxynivalenol elicits hydrogenm peroxide production, programmed cell death and defence responses in wheat. In Molecular Plant Pathology, vol. 9, 2008, no. 4, p. 435-445.
DÖLL, S. – DÄNICKE, S. – UEBERSCHÄR, K. H. – VALENTA, H. – SCHUNRRBUSCH, U. – GANTER, M. – KLOBASA, F. – FLACHOWSKY, G. 2003. Effects of graded levels of
Fusarium toxin contamined maize in diets for female weaned piglets. In Archives of Animal
Nutrition, vol. 57, 2003, no. 5, p. 311-334.
DROCHNER, W, - SCHOLLENBERGER, M. – GÖTZ, S. – LAUBER, U. TAFAJ, M. – PIEPHO, H. P. 2006. Subacute effect of moderate feed loads of isolated Fusarium toxin deoxynivalenol on selected parameters of metabolism in weaned growing piglets. In Journal of Animal Physiology and Animal Nutrition, vol. 90, 2006, no. 9-10, p. 421-428.
ERASLAN, G. – SAYGI, S. – ESSIZ, A. – AKSOY, A. – GUL, H. – MACIT, E. 2007. Evaluation of aspect of some oxidative stress parameters using vitamin E, proanthocyanidin and Nacetylcysteine against exposure to cyfluthrin in mice. In Pesticide Biochemistry and Physiology, vol. 88, 2007, no. 1, p. 43-49.
ERDÉLYI, M. – WEBERZ, M. – BALOGH, K. – ANCSIN, Z. – MÉZES, M. 2011. The effect of feeding a diet naturally contaminated with deoxynivalenol on production traits and selected biochemical indicators of broiler chickens. In Acta Veterinaria, vol. 80, 2011, no. 3, p. 287-292.
ETIENNE, M. – WACHÉ, Y. 2008. Biological and physiological effects of deoxynivalenol (DON) in the pig. In Mycotoxins in farm animals. Kerala : Transworld Research Network, 2008. p. 113-130. ISBN 987-81-7895-312-0.
FIORAMONTI, J. – DUPUY, C. DUPUY, J. – BUENO, L. 1993. The mycotoxin, deoxynivalenol, delays gastric emptying through serotonin-3 receptors in rodents. In The
Journal of Pharmacology and Experimental Theraprutics, vol. 266, 1993, no. 3, p.
1255-1260.
GBORE, F. A. – AKELE, O. 2010. Growth performance, haematology and serum biochemistry of female rabbits (Oryctolagus cuniculus) fed dietary fumonisin. In Veterinarski archiv, vol. 80, 2010, no. 3, p. 431-443.
GHAREEB, K. – AWAD, W. A. – BÖHM, J. 2012. Ameliorative effect of a microbial feed additive on infectious bronchitis virus antibody titer and stress index in broiler chicken fed deoxynivalenol. In Poultry Science, vol. 91, 2012, no. 4, p. 800-807.
GOYARDS, T. – DÄNICKE, S. – GROVE, N. – TIEMANN, U. – ROTHKÖTER, H. J. – 2006. Methodical aspects of in vitro proliferation of porcine blood lymphocytes when exposed to deoxynivalenol (DON). In Landbauforschung Völkenrode, vol. 56, 2006, no. 3-4, p. 139-148.
GRENIER, B. – LOUREIRO-Bracarense, A. P. – LUCIOLI, J. – PACHECO, G. D. – COSSALTER, A. M. – MOLL, W. D. – SCHATZMAYR, G. – OSWALD, I. P. 2011. Individual and combined effects of subclinical doses of deoxynivalenol and fumonisins in piglets. In Molecular Nutrition and Food Research, vol. 55, 2011, no. 5, p. 761-771.
HOCHSTEINER, W. – SCHUH, M. – LUGER, K. – BAUMGARTNER, W. 2000. Effect of mycotoxin contamined feed on production parameters of dairy cows. In Berliner und Münchener tierärztliche wochenschrift, vol. 113, 2000, no. 1, p. 14-21.
KHAN, S. M. – KOUR, G. 2007. Subacute oral toxicity of chlorpyriphos and protective effect of green tea extract. In Pesticide Biochemistry and Physiology, vol. 89, 2007, no. 2, p. 118-123.
KLAPÁČPVÁ, K. – FAIXOVÁ, Z. – GREŠÁKOVÁ, Ľ, - FAIX, Š. – MIKLÓSOVÁ, L. – LENG, Ľ. 2011. Effects of feeding wheat naturally contaminated with Fusarium mycotoxins on blood biochemistry and the effectiveness of dietary lignin treatment to alleviate mycotoxin adverse effects in broiler chickens. In Acta veterinaria (Boegrad), vol. 61, 2011, no. 2-3, p. 227-237.
KOUADIO, J. H. – MOBIO, T. A. – BAUDRIMONT, I. – MOUKHA, S. – DANO, S. D. – CREPPY, E. E. 2005. Comparative study of cytotoxicity and oxidative stress induced by deoxynivalenol, zearalenone or fumonisin B1 in human intestinal cell line Caco-2.In Toxicology, vol. 213, 2005, no. 1-2, p. 56-65.
KRISHNASWAMY, R. – DEVARAI, S. N. – PADMA, V. V. 2010. Lutein protects HT-29 cells against Deoxynivalenol-induced oxidative stress and apoptosis: prevention of NF-kappaB nuclear localization and down regulation of NF-NF-kappaB and Cyclo-Oxygenase-2 expression. In Free radical Biology and Medicine, vol. 49, 2010, no. 1, p. 50-60.
KUSHIRO, M. 2008. Effects of milling and cooking processes on the deoxynivalenol content in wheat. In International Journal of Molecular Sciences, vol. 9, 2008, no. 11, p. 2127-2145. ŁAZICKA, K. – ORZECHOVWSKI, S. 2010. The characteristics of the chosen mycotoxins and their toxic influence on the human and animal metabolism. In Natural Science, vol. 2, 2010, no. 6, p. 544-550.
MANSOUR, S. A. – MOSSA, H. A. T. 2009. Lipid peroxidation and oxidative stress in rat erythrocytes induced by chlorpyrifos and the protective effect of zinc. In Pesticide Biochemistry and Physiology, vol. 93, 2009, no. 1, p. 34-39.
MEDVEĎOVÁ, M. – KOLESÁROVÁ, A – CAPCAROVÁ, M. – LABUDA, R. – SIROTKIN, A. V. – KOVÁČIK, J. – BULLA, J. 2011. The effect of deoxynivalenol on the secretion activity, proliferation and apoptosis of porcine ovarian granulose cells in vitro. In Journal of Envirnmental Science and Health, vol. 46, 2011, no. 3, p. 213-219.
PESTKA, J. J. – SMOLINSKI, A. T. 2005. Deoxynivalenol: Toxicology and potential effects on humans. In Journal of Toxicology and Environmental Health, vol. 8, 2005, no. 1. P. 39-69. PIZZAMIGLIO, V. 2008. Nutritional strategies to control mycotoxin damages in swine : doctoral dissertation. Bologna : Universita´di Bologna. 86 p.
PRELUSKY, D. B. – GERDES, R. G. – UNDERHILL, K. L. – ROTTER, B. A. – JUI, P. Y. – TRENHOLM, H. L. 1994. Effects of low-level dietary deoxynivalenol on haematological and clinical parameters of the pig. In Natural Toxins, vol. 2, 1994, no. 3, p. 97-104.
PRELUSKY, D. B. – TRENHOLM, H. L. 1991. Tissue distribution of deoxynivalenol in swine dosed intravenously. In Journal of Agricultural and Food Chemistry, vol. 39, 1991, no. 4, p. 748-751.
SERGEEV, I. N. – PILIIA, N. M. – KUZ´MINA, E. E. – AVREN´EVA, L. I. – KRAVCHENKO, L. V. – SPIRICHEV, V. B. – TUTEL´IAN, V. A. 1990. Calcium and vitamin D metabolism and enzymes of xenobiotic metabolism during chronic action of mycotoxins. In Voprosy pitaniia, 1990, no. 5. P. 25-30.
SOBROVÁ, P. – ADAM, V. – VASATKOVÁ, A. – BELKOVÁ, M. – ZEMAN, L. – KIZEK, R. 2010. Deoxynivalenol and its toxicity. In Interdisciplinary Toxicology, vol. 3, 2010, no. 3, p. 94-99.
SOBROVA, P. – VASATKOVA, A. – SKLADANKA, J. – BEKLOVA, M. – ZEMAN, L. – KIZEK, R. – ADAM, V. 2012. Study of deoxynivalenol effect on metallothionein and glutathione levels, antioxidant capacity, and glutathione-S-transferase and liver enzymes activity in rats. In Chemical Papers, vol. 66, 2012, no. 12, p. 1092-1102.
SPEIJERS, G. J. A. – SPEIJERS, M. H. M. 2004. Combined toxic effects of mycotoxins. In Toxicology Letters, vol. 153, 2004, no. 1, p. 91-98.
SURAI, P. F. – MEZES, M. MELNICHUK, S. D. – FOTINA, T. I. 2008. Mycotoxins and animal health: From oxidative stress to gene expression. In Krmiva, vol. 50, 2008, no. 1, p. 35-43.
SWAMY, H. V. L. N. – SMITH, T. K. – COTTER, P. F. – BOERMANS, H. J. – SEFTON, A. E. 2002. Effect of feeding blends of grains naturally contamined with Fusarium mycotoxins on production and metabolism in broilers. In Poultry Science, vo. 81, 2002, no. 7, p. 966-975.
TIAN, L. – CAI, Q. – WEI, H. 1998 Alterations of antioxidant enzymes and oxidative damage to macromolecules in different organs of rats during aging. In Free radical Biology and Medicine, vol. 24, 1998, no. 9, p. 1477-1484.
VALKO, M. – MONCOL, C. J. – IZAKOVIC, M. – MAZUR, M. 2006. Free radicals, metals and antioxidants in oxidative stress-induced cancer. In Chemico-Biological Interactions, vol. 160, 2006, no. 1, p. 1-40.
WACHÉ, Y. J. – VALAT, C. – POSTOLLEC, G. – BOUGEARD, S. – BUREL, C. – OSWALD, I. P. – FRAVALO, P. 2009. Impact of deoxynivalenol on the intestinal microflora of pigs. In International Journal of Molecular Science, vol. 10, 2009, no. 1, p. 1-17.
YEGANI, M. – SMITH, T. K. – LEE SON, S. – BOERMANS, H. J. 2006. Effect of feeding grains naturally contamined with Fusarium mycotoxins on performance and metabolism of broiler breeders. In Poultry Science, vol. 85, 2006, no. 9, p. 1541-1549.
YET, T. S. – HO, J. D. – YANG, V. W. CH. – TZENG, CH. R. – HSIEH, R. H. 2005. Calcium Stimulates mitochondrial biogenesis in human granulosa cells. In Annals of the New York Academy of Sciences, vol. 1042, 2005, p. 157-162.
ZAIN, M. E. 2011. Impact of mycotoxins on humans and animals. In Journal of Saudi Chemical Society, vol. 15, 2011, no. 12, p. 129-144.