w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Antinociceptive
activity
of
Cistanche
salsa
stolons,
growing
in
the
Republic
of
Kazakhstan
Elmira
B.
Kartbaeva
a,
Graciela
R.
Donald
b,
Zuriyadda
B.
Sakipova
a,
Liliya
N.
Ibragimova
a,
Elmira
N.
Bekbolatova
a,
Inna
I.
Ternynko
c,
Patricia
D.
Fernandes
b,
Fabio
Boylan
d,∗aTheModule
«Pharmacist–Technologist»,AsfendiyarovKazakhNationalMedicalUniversity,Almaty,Kazakhstan
bLaboratóriodeFarmacologiadaDoredaInflamac¸ão,InstitutodeCiênciasBiomédicas,UniversidadeFederaldoRiodeJaneiro,RiodeJaneiro,RJ,Brazil
cTheChairofPharmaceuticalChemistryandPharmacognosy,LuganskStateMedicalUniversity,Rubezhnoe,Stoiteley,Ukraine
dSchoolofPharmacyandPharmaceuticalSciences,TrinityBiomedicalSciencesInstitute,TrinityCollegeDublin,Dublin,Ireland
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received19April2017 Accepted17May2017
Availableonline2September2017
Keywords:
HerbaCistanche
HPLC-UV Echinacoside Acteoside Tubuloside Standardization
a
b
s
t
r
a
c
t
HerbaCistanche(Cistanchespecies)inTraditionalChineseMedicineisusedforthetreatmentofseveral
diseasesandsymptoms,toincludepain.Theobjectiveofthisstudywastoevaluatetheantinociceptive effectofthehydroethanolextractofCistanchesalsa(C.A.Mey.)Beck,Orobanchaceae,stolonsinanimal modelsofpain.ChemicalcompositionofHerbaCistanchewasanalyzedbyHPLC-UV.MiceSwiss Web-ster(25–30g,n=6)wereorallypre-treatedwithHerbaCistanche(10,30or100mg/kg)andevaluatedin theformalintestandinthecapsaicin-orglutamate-inducedlickingresponse.KazakhHerbaCistanche
iscomposedmainlybyphenylpropanoidglycosides,fromwhichechinacoside,acteosideandtubuloside Barethemainconstituents.WhenHerbaCistanchewasadministeredtomiceithadaneffectinboth phasesoftheformalintest(77%activityat30mg/kgforphase1and62%activityat100mg/kgforphase 2)suggestinganalgesicandanti-inflammatoryproperties.KazakhHerbaCistanchewasabletoreduce theanimalslickingtimeafterinjectionofglutamate(81%reductionat30mg/kg)andcapsaicin(81% reductionat100mg/kg).WeconcludethatphenolicspresentinthehydroethanolextractofC.salsacould beresponsibleforitspharmacologicalprofile.Inordertosourceagoodqualityrawmaterialfor Tradi-tionalChineseMedicinewerecommendedthisKazakhspeciestobestandardizedusingechinacoside andacteosideasmarkers.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Cistanche salsa (C.A. Mey) Beck, Orobanchaceae, is a para-siticplantfromtheRepublicofKazakhstanwhere itis usedas industrialfeedstock(Sarsenbayevetal.,2011;Grudzinskayaand Gemedzhieva,2012).ThescientificvalueofthisherbinTraditional Chinese Medicine relates tothe treatment of kidney problems (varyingfrompaintoinsufficiency),impotence,femaleinfertility, morbidleucorrhea,profusemetrorrhagiaandsenileconstipation (Jiangsu New Medical College Dictionaryof TraditionalChinese Drugs,1977;ChineseMedicinalHerbal,1988).Thechemical com-positionofstolonsofotherspeciesofCistanchehasalreadybeen studied in detail by Chinese scientists. The following phenolic compoundswereidentified:echinacoside,tubuloside,acteoside,
∗ Correspondingauthor.
E-mail:Fabio.Boylan@tcd.ie(F.Boylan).
besideslignans,iridoidsandacomplexpolysaccharide(Yongand Tu,2009;Zhangetal.,2003;Xieetal.,2005;Jiangetal.,2009;Sui etal.,2011;Liuetal.,2013;Zhouetal.,2014).
Shimodaetal.(2009)showedthatthisherbpossesses hypoc-holesterolemic effect while Yang et al. (2013) determined its hepatoprotective activity. Nan et al. (2013) reported the anti-inflammatoryactivityforitsextracts.Acomplexpolysaccharide previously isolatedfromthis plantshowedimmunomodulatory effects(Wangetal.,2009).
AlthoughthereisaconsiderableamountofCistanchestolonsin theRepublicofKazakhstan,thereisnopopularuseforthisplant bytheKazakhpopulationalthoughtheneighboursinChina exten-sivelyuseit.Duetothefactthatthereisaconsiderableproblem nowadaysinrelationtothesubstitution/adulterationofmedicinal plantsandtheincreasingneedtostandardizemedicinesfortheuse inTraditionalChineseMedicine,thisstudywasdesignedto ascer-tainthephenolicchemicalcompositionofthisrawplantmaterial growingintheneighbouringKazakhstanaswellastoassessitsuse
http://dx.doi.org/10.1016/j.bjp.2017.05.013
asantinociceptivetopotentiallyeasepain–whichcharacterizes oneofitsmainuseinTraditionalChineseMedicine.
Materialsandmethods
Plantmaterial
Cistanchesalsa(C.A.Mey.)Beck,Orobanchaceae,stolonswere collectedatthedesertofMoinkum,VillageofBakanas,inJuly2014 inAlmaty.TheplantmaterialwasidentifiedbyDr.G.Sitpayeva fromtheInstituteof Botanyand Phytointroduction,Ministryof EducationandSciencesoftheRepublicofKazakhstanwhereitwas depositedunderthenumber01-04/306.
Chemicalanalysis
StolonsofC.salsa(2g)weresubmittedtoamicrowave extrac-tion.Initially thematerial was ground to 0.001–2.000mm and then placed in a hermetic vesseltobe extracted for 10min at 100–1100◦C with ethanol 80% (ratio 1:10). The hydroethanol extract(CSHE)wasanalyzedbyHPLC-MS.Crudeextractwas dis-solvedin methanol(9.6mg/ml) andfilteredthrough a0.45mm Teflonmembraneprior totheanalysis.A liquidchromatograph HP1100 Seriesmodel (company AgilentTechnologies, Inc.,CA, USA),equippedwithaflowingvacuumdegasser,afour-channel low-pressuregradient pump,and anautomaticinjector. Pheno-liccompoundswerechromatographicallyseparatedbyacolumn ZorbaxEclipseXDB-C18,2.1×50mm,filledwithoctadecylsilyl sil-icagelpolymer(1.8).Chromatographicanalysiswascarriedout withamobilephaseflowof0.2ml/min,eluentoperatingpressure of175–200kPa,columnoventemperatureof30◦C,2mlsample volume, gradient eluent feed mode: 0–36min10% A – 90% B; 36min–100%B(eluentA:methanol,eluentB:0.2%formicacid solution).DetectionwasperformedbyUVatthewavelengthsof 254,334,350,410,450and550nm.Comparisonoftheobtained retentiontimes,UVandmassspectrawiththoseofreference com-poundswasusedfortheidentificationofthechemicalcompounds intheextract.Quantitativeanalysiswasperformedbytheuseof standardverbascosideandechinacosideanalyzedunderthesame chromatographicconditions.Theircalibrationcurvesallowedfor thecalculationofthequantityofeachotherphenylpropanoid gly-cosideintheethanolextractofthis plant.Themethodwasnot validated.
Animals
SwissWebstermice(20–25g,twomonthsold)wereusedin this study(donatedby InstitutoVitalBrazil,Niterói, RJ, Brazil). Theanimalswerekeptinstandardconditions(light-darkcycleof 12h,22±2◦Cand70–80%humidity.Foodandwateradlibitum). Animalsreceivedonlywaterinordertoavoidfoodinterference withsubstanceabsorption12hpriortotheonsetof the exper-iments. Acclimatization to the laboratory conditions happened foratleast1hbeforeeachtest.Allprotocolswereconductedin accordancewiththeGuidelinesonEthicalStandardsfor Investiga-tionofExperimentalPaininAnimalsandfollowedtheprinciples andguidelines adoptedbytheNationalCouncil fortheControl ofAnimalExperimentation(CONCEA),approvedbythe Biomedi-calScienceInstitute/UFRJ,EthicalCommitteeforAnimalResearch, and received the number DFBCICB015-04/16. All experimental protocolswereperformed duringthelightphase. Animal num-berspergroupwerekeptataminimumandaccordingtorules fromCONCEA.Attheendofeachexperimentmicewerekilledby ketamine/xylazineoverdose.
Treatments
In this study CSHE was evaluated at 10, 30 and 100mg/kg. The extract wasdissolvedin dimethyl sulfoxide (DMSO, Fisher Biotech)inordertoprepareastock solutionat 100mg/ml.PBS wasusedasdiluentforthepreparationofthedifferentdoses. Solu-tionscontaining10,30and100mg/kgofthehydroethanolextract of C. salsawere prepared.The standard drugs used were mor-phine2.5mg/kg(Merck,dilutedinphosphatebuffersaline(PBS)), acetylsalicylicacid200mg/kg(SigmaAldrich,dissolvedwith5Mof sodiumhydroxide(NaOH)in0.9%saline)andcapsazepine10nMol perpaw. SalineplusDMSO(atthesameconcentrationasinthe highesttreatmentwithextract)wasgiventothenegativecontrol group.Alltreatments(testedextractandstandards)were admin-isteredbyoralroute.Theonlyexceptionwascapsazepinewhich wasadministeredbyintraplantarinjection.
Formalin-inducedacutepain
Asolutionof2.5%formalin(37%formaldehyde)wasinjected (20l)intheplantarregionoftherighthindpawofmice60min aftertreatment(hydroethanolextractofC.salsaoracetylsalicylic acid200mg/kgormorphine2.5mg/kg)(Matheusetal.,2005).The animalswereindividuallyplacedinatransparentglasschamber andthedurationoftimeinsecondsthattheyspentlickingtheirpaw afterinjectionofformalinwasrecordedandanalyzedovertwo sep-arateperiods,earlyphase-neurogenicpain(0–5minafterinjection) andlatephase-inflammatorypain(15–30minafterinjection).
Nociceptioninducedbycapsaicin
ThistestwasbasedonthemethoddescribedbySakuradaetal. withsomemodifications(Sakuradaetal.,1992).Capsaicin(20l) C18H27NO3 (Galena, Campinas, SP) wasinjected in the plantar
regionoftherighthindpawofthemice(1.6g/paw)onehour aftertreatment(hydroethanol extractofC.salsaorcapsazepine 10nMol/paw). The animals wereindividually placed in a glass chamberandpaw-lickingduration(s)wasrecordedbetween0and 5minafterthecapsaicininjectionandthenanalyzed.
Glutamate-inducednociception
InthemethoddescribedbyBeirithetal.(2002),glutamate solu-tioninPBS(20l)(l-glutamicacid,Sigma–Aldrich,3.7ng/paw)was injectedintheplantarregionoftherighthindpawofthemiceone houraftertreatment(hydroethanolextractofC.salsaormorphine 2.5mg/kg).Theanimalswereplacedindividuallyinaglass cham-berandpaw-lickingduration(seconds)wasrecordedbetween0 and15minaftertheglutamateinjectionandthenanalyzed.
Statisticalanalysis
The chemical data is presented as mean±SD of five exper-iments. Allpharmacological experimentalgroups consistedof a minimumofsixmice.Analysisofone-wayvariance(ANOVA) fol-lowed by Dunnett’s test allowed thevisualization of statistical significancebetweengroupsusingGraphPadPrism5.0software.p valueswereconsideredsignificantwhentheywerelessthan0.05 (p<0.05).
Results
0 0.8
0.6
0.4
0.2
0.0
5 10 15 20 25 30 35 Time[min]
Intens
Salsaside cistanoside A
P
oliumoside
isoacteoside
cistanoside C
tub
uloside A
2'
-acetylacteoside
echinacoside
tubuloside B acteoside
Fig.1.ChromatogramoftheCSHE.
Table1
IdentificationofphenylpropanoidglycosidesinHerbaCistanche. Peak Retentiontime(min) Content(mg/g) Compound
1. 10.9 0.65±0.01 Salsaside
2. 15.6 9.44±0.01 Acteoside
3. 17.1 5.68±0.01 Poliumoside
4. 17.7 10.98±0.01 Echinacoside 5. 17.9 0.63±0.01 CistanosideA 6. 18.9 0.08±0.01 Isoacteoside 7. 19.3 2.29±0.01 CistanosideC
8. 19.7 7.94±0.01 TubulosideB
9. 20.5 0.73±0.01 TubulosideA
10. 21.1 1.11±0.01 2′-Acetylacteoside
thephenoliccompoundstogetherwiththeirrespectiveretention timesinthechromatogram.
EffectofCSHEonformalin-inducedacutepain
Intheformalin-inducedacutepaintest,theCSHEat10,30and 100mg/kgwereabletodecreasepaw-lickinginthefirstphaseof thetestknownastheneurogenicpainphase,thesereductionswere 60,77and58%,respectively.Inaddition,theywerealsoeffective inreducinginflammatorypaininducedduringthesecondphase ofthetest,thepercentageinhibitionofinflammatorypainwere 42, 46and 62%,respectively (Fig.2).Morphine(2.5mg/kg)and
acetylsalicylicacid(200mg/kg)presented thefollowingresults: 55%/33%and31%/54%forthefirstandsecondphases,respectively.
EffectofCSHEonglutamate-inducednociception
TheCSHEreducedthelickinginducedbyglutamateatthethree dosestested,10,30and100mg/kgby76,81and53%,respectively (Fig.3).Morphineat2.5%resultedin76%reduction.
EffectofCSHEoncapsaicin-inducednociception
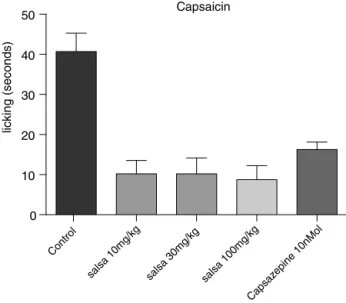
InordertoverifyiftheCSHEwouldaffectnociceptionthrough TRPV1receptors,itwastestedinamodelofpaininducedby cap-saicin.Theantinociceptiveeffectthroughthismodelwasobserved fortheCSHEinthethreetesteddoses,by76,79and81%, respec-tively(Fig.4).Capsazepine10nMol/pawresultedin60%reduction.
Discussion
Atotal oftenphenylpropanoidglucosideswereidentifiedin theC.salsa(growinginKazakhstan)hydroethanolextract. Echi-nacoside (10.98mg/g), acteoside (9.44mg/g) and tubuloside B (7.94mg/g)werethemajorcompoundsidentified.Theobtained datawascorrelatedwiththeavailableliteratureforspeciesof Cis-tanchegrowingindifferentregionsofAsia(Zhouetal.,2014;Xie etal.,2005;Jiangetal.,2009).Echinacosideandacteosidearethe
vehicle F1morphine ASA
salsa 10mgsalsa 30mgsalsa 100mgvehicle F2morphine ASA
salsa 10mgsalsa 30mgsalsa 100mg
0 20 40 60 80
0 50 100 150 200
phase I phase II
*
*
*
*
*
Licking (seconds)
Licking (seconds)
*
*
Glutamate
vehicle morphine
salsa 10mg salsa 30mgsalsa 100mg 0
20 40 60
*
*
Licking (seconds) *
Fig.3.EffectofCSHEonglutamate-inducednociceptioninmice.Animalswere pre-treatedwiththeCSHE10mg/kg,30mg/kgor100mg/kg(p.o.),morphine2.5mg/kg (p.o.)orvehicle(DMSO).Resultsarepresentedasmean±S.D.(n=6).One-way ANOVAwithDunnett’smultiplecomparisonwith vehicle,post-test(*p<0.05). GraphPadPrismversion5.1.
Control
salsa 10mg/kg salsa 30mg/kg salsa 100mg/kg
Capsazepine 10n Mol
0 10 20 30 40
50 Capsaicin
licking (seconds)
Fig.4.EffectofCSHEoncapsaicin-inducednociceptioninmice.Animalswere pre-treatedwithCSHE10mg/kg,30mg/kgor100mg/kg(p.o.)orvehicle(DMSO).Results arepresentedasmean±S.D.(n=6).One-wayANOVAwithDunnett’smultiple com-parisonwithvehicle,post-test(*p<0.05).GraphPadPrismversion5.1.
maincomponents,whichformthebasisforstandardizationofthe officinalspecies C.deserticola and C.tubulosa,listedin the Chi-nesePharmacopoeiain2005.Weproposethatthesecompounds couldalsobeusedforthestandardizationofC.salsastolonsfrom Kazakhstan,astheyarethemajoronesinthestudiedhydroethanol extract.
Accordingtothepharmacologicalresultsofthisstudyitis possi-bletosuggestthattheCSHEisaneffectiveagentagainstneurogenic painand inflammationasobservedin theformalintest. Tothe bestofourknowledgeC.salsahasneverbeentestedinrelation toitsantinociceptiveactions.Astudyfrom2002withC.deserticola (Linetal.,2002)hasshownthatthisplantpresented antinocicep-tiveandanti-inflammatoryactivitieswhenassayedinmodelssuch astotalwrithing,formalinandpawoedema.Althoughtherewas noattempttoinvestigatethemainconstituents intheextracts thatwereactive,theydeemedthebutanolextractandthewater layeras theactive ones.Not surprisingly these solvents are at thescaleofpolaritythatmatchesthephenylpropanoidglycosides foundinC.salsa.Moreover,Linandco-workersestablishedthat
theantinociceptiveactionofC.deserticolaextractswasnotdueto theactionofthecompoundintheopiatereceptororrelatedtothe immunesystem.Inourstudy,thephenylpropanoid-richextract showedactivityinbothphasesoftheformalintest.Theactivity observedwasduein facttothepharmacologicaltarget investi-gatedandnottoanypossiblemotoralterationasevidencedbythe rotarodtest.Formalintestresultsindicatepotentialforneurogenic painaswellaspaininducedbyinflammatorymediators.Initially wedecidedtoinvestigatetheneurogenicpainroute.Because simi-larcompoundsfromotherspeciespreviouslystudieddidnotshow anyopiatereceptoractivity,wedecidedtotestothermodels,such asGlutamateandcapsaicinmodels.
Glutamateisanexcitatoryneurotransmitterthathasan impor-tantroleinmodulatingpainthroughouttheperipheralandcentral nervoussystem.Thisactionismediatedbyligand-gatedionotropic glutamatereceptors(iGluRs)andmetabotropicglutamate recep-tors. The iGluRs can be divided into N-methyl-d-aspartate (NMDA)and␣-amino-3-hydroxy-5-methylisoxazole-4-propionic acid(AMPA)(Kolber,2015).Researchhasshownthatwhen antag-onizingNMDAand AMPAreceptorswithketamineandkainate, respectively,anantinociceptioneffectisobserved.However, antag-oniststargetingthesereceptorssofarhaveinducedasubstantial adverseeffectandforthisreasonanewresearchfocusonmGluRs hopingthatmedicinemediatedthroughthisreceptorwouldcause lesssideeffecthasbeencarriedout(Palazzoetal.,2014).Theresults oftestingtheCSHE showedthatthistreatmentwaseffectivein reducingpaininducedbyglutamate.However,itisstillunknown ifthiseffectismediatedthroughionotropicand/ormetabotropic receptors.Morphineisoneofthedrugsavailablethathasaneffect onglutamatergictransmission(Deyamaetal.,2007).Itisreported intheliteraturethatglutamatereceptoractivationincreasesTRPV1 responses(Szteynetal.,2015).Forthatreason,itispossiblethat theeffectobservedforCSHEcouldbeduetoactivityonTRPV1and notnecessarilythroughadirectresponseonglutamatereceptors.
Thenextstepofthisresearchwastocarryoutapaw-lickingtest usingcapsaicinasthealgesicagent,whichisaTRPV1receptor ago-nist.TheresultsshowedthatindeedCSHEdecreasedpainthrough TRPV1receptor.
InthecaseofCSHE,almostinallthetestedmethodologies,we couldobservethatthedoseof 30mg/kgachievedbetterresults than100mg/kg.Thiscanbeduetothesaturationofthesolution leadingtoprecipitatedcompoundsandeffectivelylessamountof drugsbeingbioavailable.
Confirming the established chemical composition for the
Kazakh extract CSHE was an important step because the
major constituents present in it have already had their
antinociceptive/anti-inflammatory actions somehow confirmed. For example,echinacosidewasestablished asoneof theactive principlesresponsiblefortheantinociceptiveactionofEchinaceae (Hostettmann,2003).Also,apreviousstudybySchapovaland co-workers(Schapovaletal.,1998)pointedoutacteosideasoneofthe mainactiveprinciplesinanethanolextractpreparedwith Stachy-tarphetacayennensis asassessed by pawoedemaand hotplate models.Backhouseandco-workers(Backhouseetal.,2008)also deemedacteosidetobetheactive principleof Buddlejaglobosa usingseveralmodelsofpainassessment,includingformalintest. Newformulationsarenowbeingdevelopedusingthestateofthe artknowledgetoincreasestabilityandprolongtheantinociceptive actionofacteoside(Isacchietal.,2016).
Conclusion
experimentalmodelsperformedherein.Takingintoconsideration thatontheterritoryofKazakhstanthegenusCistancheis repre-sentedmainlybyspeciesC.salsa,wecanrecommenditsharvesting andstandardizationusingechinacosideandacteosideasstandard compounds,tosourceagoodqualityrawmaterialforTraditional ChineseMedicine.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat
nopatientdataappearinthisarticle.
Authors’contributions
EBKexecuted thecollectionof theplant, preparation ofthe extractandanalysisofthechemical resultsaswellasthe writ-ingofthepaper;GRD,executedallthepharmacologicaltests;ZBS, designedthestudyinconjunctionwithFB;LNIandENB, partici-patedinthepharmacopoeialdesignofthestudyfortheinclusion oftheobtainedresultsintheKazakhpharmacopoeia;IIT,executed theHPLCanalyses;PDF,designedthepharmacologicalstudyand FB,organizedtheteamtogetherinordertowritethispaper.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
Theauthors fromBrazilthank Mr.AlanMinho for technical assistanceandtheInstitutoVitalBrazilfordonationsofanimals used.TheyalsowanttoacknowledgethegrantsfromCNPqand Fundac¸ão Carlos Chagas Filho de Apoio à Pesquisa do Estado do Rio de Janeiro. The authors from Irelandwish to acknowl-edgetheHighEducationAuthority’sProgrammeforResearchin Third-LevelInstitutions Cycle 5’sfunding support for TBSI and toreinforcetheimportanceofSFIprogrammeISCA-Brazil(grant no.SFI/13/ISCA/2843)thatcontributedtothecollaborativework betweenBraziland Ireland.The authorsfromKazakhstanwant toacknowledgeKazNMUforthefinancialsupportallowedtothe executionofthiswork.
References
Backhouse,N.,Delporte,C.,Apablaza,C.,Farias,M.,Goïty,L.,Arrau,S.,Negrete, R.,Castro,C.,Miranda,H.,2008.AntinociceptiveactivityofBuddlejaglobosa (matico)inseveralmodelsofpain.J.Ethnopharmacol.119,160–165.
Beirith,A.,Santos,A.R.S.,Calixto,J.B.,2002.Mechanismsunderlyingthenociception andpawoedemacausedbyinjectionofglutamateintothemousepaw.Brain Res.924,219–228.
ChineseAcademyofMedicalSciences,1988.InstituteofMedicinalPlants,Chinese MedicinalHerbal,vol.4.,2nded.People’sMedicalPublishingHouse,Beijing. Deyama,S.,Yamamoto,J.,Machida,T.,Tanimoto,S.,Nakagawa,A.T.,Kaneko,S.,
Satoh,M.,Minami,M.,2007.Inhibitionofglutamatergictransmissionby mor-phineinthebasolateralamygdaloidnucleusreducespain-inducedaversion. Neurosci.Res.59,199–204.
Grudzinskaya,L.M.,Gemedzhieva,N.G.,2012.ListofMedicinalPlantsinKazakhstan. PublishingHouseoftheInstituteofBotanyandPhytointroductionoftheRK, Almaty.
Hostettmann,K.,2003.Historyofaplant:theexampleofEchinacea.ForschKomp. Klas.Nat.Suppl.1,9–12.
Isacchi,B.,Bergonzi,M.C.,Iacopi,R.,Ghelardini,C.,Galeotti,N.,Bilia,A.R.,2016. Liposomalformulationtoincreasestabilityandprolongantineuropathicactivity ofverbascoside.PlantaMed.83,412–419.
Jiang,Y.,Li,S.P.,Wang,Y.T.,Chen,X.J.,Tu,P.F.,2009.DifferentiationofHerba Cis-tanchesbyfingerprintwithhigh-performanceliquidchromatography-diode arraydetection-massspectrometry.J.Chromatogr.A1216,2156–2162. JiangsuNewMedicalCollegeDictionaryofTraditionalChineseDrugs,1977.1sted.,
ShanghaiScientific&TechnologicPublisher,Shanghai.
Kolber,B.J.,2015.mGluRsheadtotoeinpain.Prog.Mol.Biol.Transl.131,281–324. Lin,L.W., Hsieh,M.T.,Tsai, F.H.,Wang,W.H.,Wu,C.R.,2002.Anti-nociceptive and anti-inflammatoryactivity caused by Cistanche deserticolain rodents. J.Ethnopharmacol.83,177–182.
Liu,X.M.,Li,J.,Jiang,Y.,Zhao,M.B.,Tu,P.F.,2013.Chemicalconstituentsfrom Cis-tanchesinensis(Orobanchaceae).Biochem.Syst.Ecol.47,21–24.
Matheus,M.E.,Berrondo,L.F.,Vieitas,E.C.,Menezes,F.S.,Fernandes,P.D.,2005. Eval-uationoftheantinociceptivepropertiesfromBrillantaisiapalisotiiLindaustems extracts.J.Ethnopharmacol.102,377–381.
Nan,Z.D.,Zeng,K.W.,Shi,S.P.,Zhao,M.B.,Jiang,Y.,Tu,P.F.,2013.Phenylethanoid gly-cosideswithanti-inflammatoryactivitiesfromthestemsofCistanchedeserticola culturedinTarimdesert.Fitoterapia89,167–174.
Palazzo,E.,Marabese,I.,deNovellis,V.,Rossi,F.,Maione,S.,2014.Supraspinal metabotropicglutamatereceptors:atargetforpainreliefandbeyond.Eur.J. Neurosci.39,444–454.
Sakurada,T.,Katsumata,K.,Tanno,K.,Sakurada,S.,Kisara,K.,1992.Thecapsaicin testinmiceforevaluatingtachykininantagonistsinthespinal-cord. Neuro-pharmacology31,1279–1285.
Sarsenbayev,K.N.,Isabaev,S.O.,Kolosov,N.G.,2011.Proceedingsofthe Interna-tionalScientificConference“ModernEcologicalStateoftheAralSeaRegion,the ProspectsforSolvingProblems”,Kyzylorda,pp.195–200.
Schapoval,E.E.S.,Vargas,M.R.W.,Chaves,C.G.,Bridi,R.,Zuanazzi,J.A.,Henriques,A.T., 1998.Antiinflammatoryandantinociceptiveactivitiesofextractsandisolated compoundsfromStachytarphetacayennensis.J.Ethnopharmacol.60,53–59. Shimoda,H.,Tanaka,J.,Takahara,Y.,Takemoto,K.,Shan,S.J.,Su,M.H.,2009.The
hypocholesterolemiceffectsofCistanchetubulosaextract,aChinesetraditional crudemedicine,inmice.Am.J.Chin.Med.37,1125–1138.
Sui,Z.F.,Gu,T.M.,Liu,B.,Peng,S.W.,Zhao,Z.L.,Le,L.,Shi,D.F.,Yang,R.Y.,2011. Water-solublecarbohydratecompoundfromthebodiesofHerbaCistanche:isolation anditsscavengingeffectonfreeradicalinskin.Carbohydr.Polym.85,75–79. Szteyn,K.,Rowen,M.P.,Gomez,R.,Du,J.,Carlton,S.M.,Jeske,N.A.,2015.A-kinase
anchoringprotein79/150coordinatesmetabotropicglutamatereceptor sensi-tizationofperipheralsensoryneurons.Pain156,2364–2372.
Wang,X.Y.,Qi,Y.,Cai,R.L.,Li,X.H.,Yang,M.H.,Shi,Y.,2009.TheeffectofCistanche deserticolapolysaccharides(CDPS)onmarcrophagesactivation.Chin. Pharma-col.Bull.25,787–789.
Xie,J.N.,Zhao,M.B.,Wu,F.W.,Tu,P.F.,2005.ChromatographicfingerprintofCistanche deserticolabyHPLC.Chin.Trad.Herb.Drugs36,268–271.
Yang,F.R.,Wen,D.S.,Fang,B.W.,Lou,J.S.,Meng,L.,2013.PreventionofCistanche salsaextractonhepaticfibrosisinducedbycarbontetrachlorideinrats.Chin. Herb.Med.5,199–204.
Yong,J.,Tu,P.F.,2009.AnalysisofchemicalconstituentsinCistanchespecies.J. Chro-matogr.A1216,1970–1979.
Zhang,X.,Li,X.,Rena,K.,Du,N.S.,2003.RP-HPLCdeterminationofechinacoside andacteosideinHerbaCistanchescultivatedondifferentparasiticspeciesand habitats.Chin.J.Pharm.Anal.23,254–256.