REVISTA
BRASILEIRA
DE
ANESTESIOLOGIA
OfficialPublicationoftheBrazilianSocietyofAnesthesiologywww.sba.com.br
SCIENTIFIC
ARTICLE
Hyperalgesic
effect
of
subarachnoid
administration
of
phentolamine
in
mice
夽
Desiré
Carlos
Callegari
a,
João
Antônio
Correa
a,
Oscar
César
Pires
b,∗,
Renan
Batista
Corrêa
Braga
c,
Ana
Flávia
Marques
Gimbo
d,
Adriana
Aparecida
de
Souza
e,
Marta
Helena
Rovani
Pires
f,
Elton
Constantino
b,
Irimar
de
Paula
Posso
baFaculdadedeMedicinadoABC(FMABC),SantoAndré,SP,Brazil
bUniversidadedeTaubaté(Unitau),Taubaté,SP,Brazil
cCursodeMedicinadaUniversidadedeTaubaté(Unitau),Taubaté,SP,Brazil
dCursodeEnfermagemdaUniversidadedeTaubaté(Unitau),Taubaté,SP,Brazil
eCursodeBiologiadaUniversidadedeTaubaté(Unitau),Taubaté,SP,Brazil
fCursodeMedicinadaFaculdadedeMedicinadePetrópolis,Petrópolis,RJ,Brazil
Received12August2013;accepted12September2013 Availableonline26November2014
KEYWORDS
Mice;
Phentolamine; Pain;
Formalintest; vonFrey
Abstract
Backgroundandobjectives: Painful phenomenonis oneofthe mostimportantand complex
experiences. Phentolamineisanon-selective alpha-adrenergic antagonist.The objectiveof
thisstudywas tocomparetheeffectofincreasingdosesofphentolamineintosubarachnoid
spaceinratsinthemodulationofpainfulphenomenon.
Methods:84maleWistarratsweredividedintoformalinandplantarincisiongroups,subdivided
intosixsubgroups(n=7).Controlgroupreceivedonlysaline(10L);activesubgroupsreceived
phentolamine10mg(GF10),20mg(GF20),30mg(GF30),40mg(GF40),and50g(GF50).In
formalingroup,painwasinducedbyinjectionof50Lof2%formalinindorsalregionofright
posteriorpaw.Inplantarincisiongroup,painwasinducedbyplantarincisionandevaluatedusing
vonFreyfilaments.Inductionandmaintenanceofanesthesiawereperformedwith3%halothane
forcatheterplacementintosubarachnoidspaceandplantarincision.Statisticalanalysiswas
performedusingtheJMPprogramfromSASwith5%significancelevel.
Results:Phentolamineatdosesof20and30gincreasedthealgesicresponseintheintermediate
phaseoftheformalintest.Inplantarincisiontest,ithadhyperalgiceffectonfirst,third,fifth,
andseventhdaysatadoseof10gandonfirst,third,andfifthdaysatadoseof20gandon
fifthdayatadoseof30g.
夽
StudyconductedatFaculdadedeMedicinadoABC,SantoAndré,SP,Brazil.
∗Correspondingauthor.
E-mail:ocpires@uol.com.br(O.C.Pires). http://dx.doi.org/10.1016/j.bjane.2013.09.013
Conclusion:Subarachnoidadministrationofphentolamineshowedhyperalgesiceffect,possibly
duetotheinvolvementofdifferentsubclassesofalpha-adrenergicreceptorsinmodulatingpain
pathways.
©2014SociedadeBrasileiradeAnestesiologia.PublishedbyElsevier EditoraLtda.
PALAVRAS-CHAVE
Ratos; Fentolamina; Dor;
Testedaformalina; vonFrey
Efeitohiperálgicodafentolamina,porviasubaracnoidea,emratos
Resumo
Justificativaeobjetivos: Ofenômenodolorosoéumadasmaisimportantesecomplexas
exper-iências.Afentolaminaéantagonistaalfa-adrenérgiconãoseletivo.Oobjetivofoicompararos
efeitosdedosescrescentesdafentolamina,porviasubaracnóidea,emratosnamodulac¸ãodo
fenômenodoloroso.
Método: Foramusados84ratosWistarmachos,divididosnosgruposformalinaeincisãoplantar,
subdivididosem seissubgrupos(n=7).Nosubgrupocontrole(GC)apenas salina(10L),nos
subgrupos ativos, 10g defentolamina(GF10), 20g (GF20),30g (GF30),40g (GF40)e
50g(GF50).Nogrupoformalina,adorfoiinduzidacominjec¸ãode50Ldeformalinaa2%,
naregião dorsal da pata posteriordireita. Nogrupo incisão plantar,ador foi induzidapor
incisãoplantareavaliac¸ãopelosfilamentosdevonFrey.Induc¸ãoemanutenc¸ãoanestésicacom
halotanoa3%paraintroduc¸ãodecateternoespac¸osubaracnóideoefeituradaincisãoplantar.
AnáliseestatísticadosresultadospeloprogramaJMPdoSAScomníveldesignificância5%.
Resultados: Afentolaminanasdosesde20e30gproduziuaumentodarespostaálgicanafase
intermediáriadotestedaformalina.Notestedaincisãoplantar,promoveuefeitohiperálgico
noprimeiro,terceiro,quintoesétimodiasnadosede10g,noprimeiro,terceiroequintodias
nadosede20genoquintodianadosede30g.
Conclusão:Afentolaminaporviasubaracnóideapromoveu efeitohiperálgico,possivelmente
pela participac¸ão de diferentes subclasses de receptores alfa-adrenérgicos nas vias
modu-latóriasdador.
©2014SociedadeBrasileiradeAnestesiologia.PublicadoporElsevierEditoraLtda.
Introduction
The passageof information throughthe posterior horn of thespinalcord(PHSC)towardtherostrallevelsofthe cen-tral nervous system (CNS) undergoes profound excitatory andinhibitoryinfluences.Thepharmacologyelucidationof thesemodulatorysystemshasguidedtheabilityassessment ofspecificreceptors.1
Neurotransmitters,aminoacids,andneuropeptides are
released by primary afferent terminals into PHSC, where
they act in nociceptive transmission modulation. Among
others,the excitatory aminoacids (glutamate and
aspar-tate);neurotransmittersand neuropeptides,includingthe
tachykinins(substanceP[SP],neurokininA[NKA],and
neu-rokininB[NKB]); calcitonin relatedgene peptide (CGRP);
cholecystokinin (CCK); somatostatin; nitric oxide (NO);
prostaglandin (PG); galanin; enkephalins; and endorphins
arehighlighted.2
Phentolamine,analpha-adrenergiccompetitive
antago-nist,whichbelongstothenon-selectiveimidazolinegroup
andhassimilarefficacyonalpha1(␣1)andalpha2(␣2)can
alsoblock5-HTreceptorsandpotassiumchannels (K+)and
causethereleaseofhistaminebymastcells.3
Thisstudy evaluatedthe effectsof escalatingdoses of
phentolamine,administeredinthesubarachnoidspace,on
pain-induced in rats using the modified formalin test and
the incision plantar test to verify if the involvement of
adrenergic pathways in descending pain inhibitorysystem
isphentolaminedose-dependent.
Method
Experimental procedures followed the Ethical Standards
of the International Association for the Study of Pain
(IASP),whichregulatesanimalexperiments(Committeefor
Research and Ethical Issues of the IASP, 1983), and the
project was approved by the Comitê de Ética no Uso de
AnimaisCeua/Unitau,protocol#019/11.Intotal,weused
84maleWistar rats,weighing220---300g,whichwere
indi-vidually placed in transparent glass chamber measuring
15cm×25cm×15cm,withaholeinfrontandbacktoallow
entranceandexitofoxygen,anestheticgases,andcarbon
dioxide. The ratswereanesthetized with3%halothane in
100%oxygen.Whentheratsshowedinabilitytogetaround
the chamber, he was removed and placed in the supine
Este é um artigo Open Access sob a licença de CC BY-NC-ND
positionwiththeabdomenoveraplasticcylinder,withhis
muzzleinamaskthroughwhichhecontinuedtoreceivethe
samehalothaneconcentration.
Subsequently,apolyethylenecatheter(PE10)was
cau-dallyintroducedintothesubarachnoidspace,bypuncture
witha 22G Tuohy needle,at the midlineof the
interver-tebral space above the penultimate lumbar vertebra to
thesubarachnoidspace,whichwasidentifiedbythereflex
movementofthetailoroneofthehindpaws.Thecatheter
wasfixedinthesubcutaneoustissue,theskinsuturedwith
needlednylon4-0,andattheendoftheexperiment,after
theanimal sacrifice withsodiumthiopental,lumbar spine
wassectionedtoconfirmthecatheterpresencein
subarach-noidspace.4
The animals wererandomly allocated intotwogroups:
formalinandplantarincision. Ratsinformalingroupwere
allocatedintosixsubgroups(n=7each);thecontrolgroup
(CG)received10Lsubarachnoidsalineimmediatelyafter
catheterplacement;activegroupsPG10,PG20,PG30,PG40,
andPG50receivedviathesameroute10Lofphentolamine
solutionwiththerespectivedoses10g,20g,30g,40g,
and50g,which,respectively,correspondedto31.5nmol,
63nmol,94nmol,126nmoland157nmolofphentolaminein
sterilesaline.
The formalininducedpain(formalintest modified)was
carriedoutbyadministering50L2%formalinsolutioninto
thedorsalregionoftherighthindpaw,25minaftersaline
orphentolamineadministration.5
All paw withdrawals, which were not related to gait,
wererecordedregardlessofthetimethatitremained
sus-pended. Counting was done continuously for 60min. The
partial number of withdrawals wasrecorded every 5min.
The test was divided into three phases: I, Intermediate,
and II. Phase I included the number of flinches during
the first 5min, the Intermediate Phase from the 6th to
the 20th minute, and Phase II from the 21st to the 60th
minute.
Ratsinplantarincisiongroupwereallocatedintosix
sub-groupsofsevenanimalseach.Controlgroup(CG)received
10Lsubarachnoidsalineimmediatelyaftercatheter
place-ment; active groups PG10, PG20, PG30, PG40, and PG50
received via the same route 10L of phentolamine
solu-tionwiththe respectivedoses 10g,20g,30g, 40g,
and50g,which,respectively,correspondedto31.5nmol,
63nmol,94nmol,126nmoland157nmolofphentolaminein
sterilesaline.
Surgical,longitudinalincisionof1cmextensionwasmade
in the posterior limb of the anesthetized animal, with a
scalpel blade number 11. Skin and fascia of the plantar
region were incised, starting 0.5cm from the calcaneus
edge and extending toward the toes. The plantaris
mus-clewaselevatedandincisedlongitudinallyandtheincision
remained intact. Afterhemostasis withslightpressure on
thesurgicalsite,allplanswereapproximatedandsutured
with two separate points with needled mononylon 4-0.
Hyperalgesia assessment wasdone byapplying von Frey’s
filamentsinthe2ndhourandthe1st,3rd,5th,7th,14th,
and21stdayaftersalineorphentolamineadministration.6
Thesolutionvolume(10L)injectedintothe
subarach-noidspacewasdefinedfrompreviousstudies.4,7,8
Forstatisticalanalysis,weusedtheJMP®Statistical
soft-warebySASInstitute(StatisticalAnalysisSystem) andthe
CG PG10 PG20 PG30 PG40 PG50
7.00 6.00 5.00 4.00 3.00 2.00 1.00 0.00
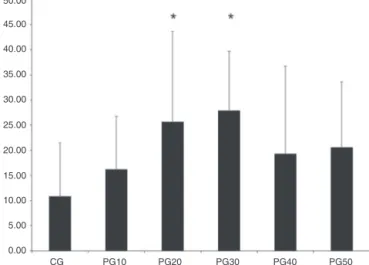
Figure1 Meannumberofanimalflinchesduringthe Interme-diatephaseoftheformalintestinstudygroups.*Statistically significantdifference(p<0.05).
analysisofvariancefollowedbyDunnett’stest,considering
asignificancelevellowerthan5%(p<0.05).
Results
Painintensityassessedbymodifiedphentolamine test
Phentolamine, administered in the subarachnoid space
at doses of 20g and 30g produced increased algesic
responseintheintermediatephaseofthemodifiedformalin
test,astheaverage numberof flinchesduringthe
forma-lintest IntermediatePhase ranged from16.28 inPG10 to
27.95inPG30.ThehighestvalueswerefoundinPG20and
PG30groups, which showedstatisticallysignificant
differ-encescomparedwiththecontrolgroup(Figs.1and2).
The mean number of flinches during the formalin test
PhaseIandPhaseII showednostatisticallysignificant
dif-ferencesbetweengroups.
CG PG10 PG20 PG30 PG40 PG50
0.00 5.00 10.00 15.00 20.00 25.00 30.00 35.00 40.00 45.00 50.00
I INT II Time (min) CN 10 20 30 50 0 10 20 30 40 50 60 70 80 90 100
Number of flinches
Figure3 Responseto vonFrey’sfilaments atthe 2ndhour aftersubarachnoidphentolamine.
CG PG10 PG20 PG30 PG40 PG50
3.00 2.50 2.00 1.50 1.00 0.50 0.00
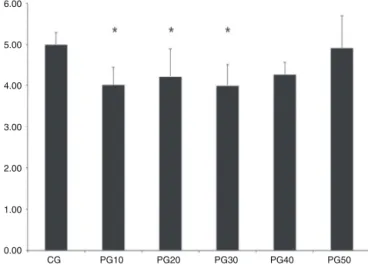
Figure4 Response tovon Frey’sfilaments on thefirst day aftersubarachnoidphentolamine.*Statisticallysignificant dif-ference(p<0.05).
PainintensitymeasuredbyvonFrey’sfilaments,in surgicalincisionplantartest
Subarachnoid phentolamine at a dose of 10g provided
hyperalgic effect on plantar incision-induced pain in the
first,third,fifth, and seventhdays;at adose of 20g,it
providedhyperalgiceffectonthefirst,thirdandfifthdays
of plantar incision-induced pain; and at a dose of 30g,
it provided hyperalgic effect on the fifth day of plantar
incision-inducedpain.
Atthe2ndhourandthefirst,third,andfifthdaysafter
subarachnoidphentolamineapplicationtherewasless
sen-sitivity to von Frey’s filaments in PG10 and PG20, with
statistically significant differences when compared to CG
(Figs.3---6).
Ontheseventhdayofassessment,increasedsensitivity
tovonFrey’sfilamentswasseeninPG10,withastatistically
significantdifferencecomparedtoCG(Fig.7).
Assessmentinthe14thand21stdaysshowedno
statisti-callysignificantdifferencesbetweengroupsinsensitivityto
vonFrey’sfilaments.
CG PG10 PG20 PG30 PG40 PG50
6.00 5.00 4.00 3.00 2.00 1.00 0.00
Figure5 ResponsetovonFrey’sfilamentsonthethirdday aftersubarachnoidphentolamine.*Statisticallysignificant dif-ference(p<0.05).
CG PG10 PG20 PG30 PG40 PG50
6.00 5.00 4.00 3.00 2.00 1.00 0.00
Figure6 Responsetovon Frey’sfilaments onthefifth day aftersubarachnoidphentolamine.*Statisticallysignificant dif-ference(p<0.05).
CG PG10 PG20 PG30 PG40 PG50
6.00 5.00 4.00 3.00 2.00 1.00 0.00
Discussion
The descending pain inhibitory system mainly consists of
four interconnected parts of the CNS: (a) cortical and
diencephalic systems; (b) periaqueductal gray (PAG) and
periventricularmatterrichinenkephalinsandopioid
recep-tors; (c) rostroventralportion of the bulb, especially the
nucleus raphe magnus(NRM) and(d) adjacent nuclei that
receive excitatory impulses from PAG and, in turn, send
noradrenergicandserotonergicfibers,viathedorsolateral
funiculus, which project to the dorsal horn of the spinal
cord and bulb. The fibers of the descending inhibitory
system mainly terminate in laminae I, II, and V where
they inhibit nociceptive neurons, including interneurons
and ascending tracts that project rostrally, among them
thespinothalamic,spinoreticular, andspinomesencephalic
tracts.Anotherimportantfibergroupthathasbeenincluded
in the endogenous paincontrol system formation is locus
coeruleus noradrenergic neurons and central cholinergic
system.2,9---11
Toassesstheeffectsofphentolamineonpainpathways,
multipledosesareadministeredintosubarachnoidspacein
rats. In this study, we used escalating doses, from
previ-ouslyreporteddosesusuallyadministeredbyotherauthors
whohaveshownconflictingresultsandhighlightedtheneed
forstudies withvarying doses,whichmotivatedthis
dose-escalationstudy.4,8,12
Modifiedformalintestiswidelyusedasanimalmodelof
nociceptionbecauseitproducesresponsessimilartothose
occurring in humans.The number of flinches asa device
forquantifyingpainbehaviorinducedbyformalinisclosely
relatedwiththeclassicalformalintest andcardiovascular
changesinresponsetopaincausedbyformalininthepaw,
showing closecorrelation withpainbehavior in conscious
animals.13---16
DuringPhasesIandIIofthemodifiedformalintest,there
wasnodifferencebetweengroups,suggesting no
interfer-encefromthenoradrenergic system.However,during the
Intermediate Phase, proposed as related to central pain
inhibition, we found a significant increase in the number
of flinches, compared withcontrol group, when doses of
20and30gwereadministeredviasubarachnoid,without
statisticallysignificantdifferencecomparedwiththeother
groups.
Studies reporting analgesic effect in the presence of
spinalalpha-2receptorstimulationsuggestthat10gwere
insufficienttohave agonisteffectsonthesereceptorsand
thatdoseshigherthan30gmayalsohaveagonisteffects
onalpha-1receptors,aneffectthatantagonizestheeffects
ofalpha-2receptorstimulation.17
The mousemodel of plantar incisionpain proposedby
Brennanetal.6isveryusefulforunderstandingthe
patho-physiologicalmechanismsofpain.Inclinicalpractice,itis
verysimilartothepainexperiencedbypatientsin
postop-erativeperiod,involuntaryandlessintenseatrest.
Subarachnoidadministrationofphentolamineinratsat
doses of 10 and 20g showed analgesic characteristics
during the second hour after administration. This can be
explainedbythepredominance of thecentralmodulatory
effect on low doses of the drug. These low doses are
unable to antagonize the effect of descending
noradren-ergicrelease,aswiththeincreaseddose,thisantagonism
prevailed, showing noreduction in sensitivity (analgesia).
However,thedose of10gadministeredvia subarachnoid
presentedhyperalgiceffectonthefirst,third,fifth,and
sev-enthdays,aswellasthedoseof20gonthefirst,third,and
fifthdays.The dose of 30gprovidedhyperalgesic effect
onlyonthe fifth day afteradministration, whichsuggests
thatinthisperiodwithoutmodulatoryaction,theblockade
ofadrenergicreceptorswithgreaterselectivity,possiblyon
alpha-2receptors,promotedgreatersensitivityand
hyper-algesia.
Otherstudieshavereportedanalgesiceffectwith
alpha-2 receptor stimulation, located in the spinal cord, by
neuronaldischarge inhibition 17,18. This couldexplain the
resultsofthisexperiment,ashigherdosesofphentolamine
(alpha-adrenergicantagonist)couldalsostimulatealpha-1
receptor,produceantagonisteffecttotheeffectsof
alpha-2receptorstimulation,andpotentiatetheadrenergiceffect
onalpha-1receptors.
Thehypothesisthatlargerdosesoftheantagonist
phen-tolaminecouldstimulatethealpha-1adrenergicreceptors
andproducean antagonisteffecttothe effectofalpha-2
adrenergicreceptorsstimulationandthereforeenhancethe
adrenergic effect on alpha-1 adrenergic receptors should
be elucidated in further studies comparing blockers with
greater selectivity for alpha-1 adrenergic receptors with
highdosesofphentolamine.
Conclusion
Different doses of subarachnoid phentolamine provide
differenteffectsonpainsensitivity,possiblybythe
partici-pationofdifferentsubclassesofalpha-adrenergicreceptors
inmodulatingpainpathways.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.BudaiD.Neurotransmittersandreceptorsinthedorsalhornof thespinalcord.ActaBiolSzegediensis.2000;44:21---38. 2.TermanGW,BonicaJJ.Spinalmechanismsand their
modula-tion. In:Bonica’smanagementofpain. 3rded.Philadelphia: LippincottWilliams&Wilkins;2001.p.73---152.
3.McPhersonGA.Currenttrendsinthestudyofpotassiumchannel openers.GenPharmacol.1993;24:275---81.
4.PiresOC,AshmawiH,ConstantinoE,etal.Antagonistas seroton-inérgicoenoradrenérgicoporviasubaracnóideaaumentama respostaálgicaemratos.RevBrasAnestesiol.2011;61:202---10. 5.D’ArmourFE,SmithD.Amethodfordetermininglossofpain
sensation.JPharmacolExpTher.1941;72:74---9.
6.BrennanTJ,VandermeulenEP,GebhartGF.Characterizationof aratmodelofincisionalpain.Pain.1996;64:493---501. 7.CalejesanAA, Chang HC, ZhuoM.Spinal serotonergic
recep-torsmediateofanociceptivereflexbysubcutaneousformalin injectionintohindpawinrats.BrainRes.1998;798:46---54. 8.LiuRJ,ZhangRX,QiaoJT,etal.Interrelationsofopioideswith
9.VanegasH,SchaibleHG.Descendingcontrolofpersistentpain: inhibitoryorfacilitatory?BrainResRev.2004;46:295---309. 10.MasonP.Deconstructingendogenouspainmodulation.J
Neuro-physiol.2005;94:1659---63.
11.D’MelloR,DickensonAH.Spinalcordmechanismsofpain.BrJ Anaesth.2008;101:8---16.
12.ObataH,SaitoS,SasakiM.Antiallodyniceffectofinthathecally administered5-HT2 agonistsinratswithnerveligation.Pain. 2000;90:173---9.
13.Wheeler-Aceto H,PorrecaF,CowanA. Theratpawformalin test:comparisonofnoxiousagents.Pain.1990;40:229---38. 14.Doak GJ, Sawynok J. Formalin-induced nociceptive
behav-ior and edema: involvement of multiple peripheral 5-hydroxytryptamine receptor subtypes. Neuroscience.1997;3: 939---49.
15.Parada CA, Tambelli CH, Cunha FQ, et al. The major role of peripheral release of histamine and 5-hydroxytryptamine in formalina-induced nociception. Neuroscience. 2001;102: 937---44.
16.AshmawiHA,ChambergoFS,PalmeiraCCA,etal.Theeffects ofpirilamineandcimetidineandnociceptiveflinchingbehavior inrats.AnesthAnalg.2003;97:541---6.
17.AfonsoJ, Reis F. Dexmedetomidina: papelatual em aneste-sia e cuidados intensivos. Rev Bras Anestesiol. 2012;62: 118---33.