w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Analysis
of
flavonoids
in
Rubus
erythrocladus
and
Morus
nigra
leaves
extracts
by
liquid
chromatography
and
capillary
electrophoresis
Luciana
R.
Tallini
a,
Graziele
P.R.
Pedrazza
a,
Sérgio
A.
de
L.
Bordignon
b,
Ana
C.O.
Costa
c,
Martin
Steppe
d,
Alexandre
Fuentefria
e,
José
A.S.
Zuanazzi
a,∗aDepartamentodeProduc¸ãodeMatériaPrima,FaculdadedeFarmácia,UniversidadeFederaldoRioGrandedoSul,PortoAlegre,RS,Brazil
bCentroUniversitárioLaSalle,Canoas,RS,Brazil
cDepartamentodeCiênciaeTecnologiadeAlimentos,UniversidadeFederaldeSantaCatarina,Florianópolis,SC,Brazil
dDepartamentodeProduc¸ãoeControledeMedicamentos,FaculdadedeFarmácia,UniversidadeFederaldoRioGrandedoSul,PortoAlegre,RS,Brazil
eDepartamentodeAnálises,FaculdadedeFarmácia,UniversidadeFederaldoRioGrandedoSul,PortoAlegre,RS,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received15January2015 Accepted30April2015 Availableonline17June2015
Keywords:
HPLC-UV UPLC-DAD/MS CE-DAD
Rubuserythrocladus
Morusnigra
Flavonoids
a
b
s
t
r
a
c
t
Thisstudyuseshighperformanceliquidchromatographyandcapillaryelectrophoresisasanalyticaltools toevaluateflavonoidsinhydrolyzedleavesextractsofRubuserythrocladusMart.,Rosaceae,andMorus nigraL.,Moraceae.Forphytochemicalanalysis,theextractswerepreparedbyacidhydrolysisand ultra-sonicbathandanalyzedbyhighperformanceliquidchromatographyusinganultravioletdetectorand bycapillaryelectrophoresisequippedwithadiode-arraydetector.Quercetinandkaempferolwere iden-tifiedintheseextracts.Theanalyticalmethodsdevelopedwerevalidatedandapplied.Quercetinand kaempferolwerequantifiedinR.erythrocladus,with848.43±66.68gg−1 and304.35±17.29gg−1, respectively,byHPLC-UVand quercetin,836.37±149.43gg−1,byCE-DAD.In M.nigrathe quan-tifications of quercetin and kaempferol were 2323.90±145.35gg−1 and 1446.36±59.00gg−1, respectively,byHPLC-UVand,2552.82±275.30gg−1and1188.67±99.21gg−1,respectively,by CE-DAD.Theextractswerealsoanalyzedbyultra-performanceliquidchromatographycoupledwitha diode-arraydetectorandmassspectrometer(MS),UPLC-DAD/MS.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
ThefruitsofRubus(Rosaceae)andMorus(Moraceae)havebeen extensivelystudiedaroundtheworldbecauseoftheirbeneficial effectsonhealth.Rubus,popularlyknownasthegenusof rasp-berriesandblackberries,iswidelydistributedaroundtheworld, bothaswildandcultivatedspecies(Deightonetal.,2000).Morus isknownasthegenusof mulberry,and isfoundfrom temper-ateregionstosubtropicalregionsofthePlanet(Gundogduetal., 2011).InBrazil,plantsbelongingtothegenusMorusarelistedby thegovernmentasinterestingtoresearchbecauseoftheirusein folkmedicine,andtheirpotentialforuseasphytotherapics(Portal daSaúde,2013).However,RubusandMorusareoftenmistakenin thedifferentiation,duetothesimilarityoftheirfruits.Also,there isalotofinformationaboutthefruitsofthesespecies,butlittle informationabouttheirleaves.
∗ Correspondingauthor.
E-mail:zuanazzi@ufrgs.br(J.A.S.Zuanazzi).
Studies have shown that Rubus leavesdemonstrate antioxi-dant (Venskutoniset al., 2007;Martini etal., 2009), anticancer (Durgo et al., 2012), gastrointestinal (Rojas-Vera et al., 2002), antiangiogenic(Liuetal.,2006),antithrombotic(Hanetal.,2012), hypoglycemic (Jouad et al., 2002), antimicrobial (Panizzi et al., 2002;ThiemandGoslinska,2004;Martinietal.,2009;Ostrosky et al., 2011) and anxiolytic activities (Nogueira et al., 1998; NogueiraandVassilieff,2000).FortheleavesofMorus,thereare studies reporting antioxidant(Katsube et al., 2010; Choiet al., 2013), anticancer(Dat etal.,2010; Skupienet al.,2008), hypo-glycemic(Volpatoetal.,2011;Chungetal.,2013),anti-obesity(Oh etal.,2009;Tsudukietal.,2013),antimicrobial(Omidiranetal., 2012)andvasodilationactivities(Xiaetal.,2008).
Flavonoids derived from quercetin and/or kaempferol were reportedinextractsofRubusleavesanalyzedbyTLC( Tzouwara-Karayanniand Philianos,1982),HPLC(Venskutonisetal.,2007; Martinietal.,2009;Durgoetal.,2012;GudejandTomczyk,2004) andNMR(Panizzietal.,2002;Hanetal.,2012;Gudej,2003).There arenostudiesaboutanalysisofRubusleavesbyCE,andthespecies erythrocladushasneverbeenstudiedbefore.InMorusleaves, sec-ondarymetabolitesderivedfromquercetinand/orkaempferolhave
http://dx.doi.org/10.1016/j.bjp.2015.04.003
beenidentifiedbyHPLC(Katsubeetal.,2010;Thabtietal.,2012; Choietal.,2013),andbyNMR(Datetal.,2010;Katsubeetal.,2010), andtherearetwostudiesonthechemicalcompositionofMorus albabyCE(Suntornsuketal.,2003;Chuetal.,2006).
Highperformanceliquidchromatographyisaclassicmethod usedfortheseparationandquantificationofsecondarymetabolites inplantextractsbutsometimesitcanbeanexpensive analyti-caltool(MerkenandBeecher,2000).Capillaryelectrophoresisis characterizedbyhighseparationefficiencyandgoodcompromise betweenanalysistimeandsatisfactorycharacterizationof pheno-liccompoundsinplants.Thelowoperationalcostandthesmall residuegenerationmakethis techniqueanattractiveoption for thedevelopmentofanalyticalmethodsforphytochemicalanalysis (Carrasco-Pancorboetal.,2006).
TheaimofthisstudywastodevelopHPLC-UVandCE-DAD ana-lyticalmethods for theevaluation ofquercetin and kaempferol inRubus erythrocladus Mart.and MorusnigraL.leavesextracts. Theresultswerestatisticallyanalyzedusingvalidationparameters andthemethodswereappliedtodeterminetheamountofthese flavonoidsinthesesamples.
Experimental
Samples
LeavesofRubuserythrocladusMart.,Rosaceae,werecollectedat Embrapa–TemperateClimate(Pelotas,Brazil)andleavesofMorus nigraL.,Moraceae,werecollectedinPortoAlegre(Brazil).Vouchers havebeendepositedintheHerbarium–UFRGS,FederalUniversity ofRioGrandedoSul(179856and176765,respectively).The sam-plesweredriedatroomtemperaturefortwoweeksandkeptinthe dark.
Chemicalsandmaterials
Quercetin (purity≥98%) and kaempferol (purity≥97%)were obtainedfromSigma(USA)andmethylparabenfromFluka(USA). HPLC-grade acetonitrile and methanol were purchased from Merck (Germany). Throughout the study, deionized and ultra-purewaterwereused.Allotherchemicalswereanalyticalgrade. DichloromethaneandhydrochloricacidwereobtainedfromSynth (Brazil),trifluoroaceticacidfromVetec(Brazil),andethylacetate, sodiumhydroxideandsodiumtetraboratedecahydratefromMerck (Germany).
HPLCconditions
HPLCanalysiswereperformedonaWatersAlliance2695(USA) chromatographusingaUVdetector(UV/VISWaters2487,USA)and aC18reversed-phasecolumn(Phenomenex,150×4.6mm,4m) withguard-column(C18). TheEmpower softwarewasused for thedataacquisition.Aphotodiodearraydetector(DAD)(UV/VIS Waters996,USA)wasalsousedtoevaluatethespecificity.
Flavonoidswereelutedusingagradientsystem.Mobilephase Aconsistedofwaterwith0.01%(v/v)oftrifluoroaceticacidandB consistedofacetonitrilewith0.08%(v/v)oftrifluoroaceticacid.The gradientprofilewas:0min50%ofB,0–2min60%ofB,2–4min80% Band4–7min95%B.Theflow-ratewas0.6mlmin−1anddetection
wasat370nm.
UPLCconditions
AnUltraPerformanceLiquidChromatography(WatersAcquity UPLC, USA) equipped with a diode array detector (DAD) cou-pledwitheletrosprayionizationinthepositiveionmode(ESI(+)) quadrupoletime-of-flight(Q-Tof)massspectrometry(MS)(MS Q-TofMicro-Micromass)wasused.Theseparationofthecompounds
wasperformedonaC18reversephasecolumn(AcquityUPLCbeh, 50.0×2.1mm, 1.7m). The software program Mass Lynx v.4.1 wasusedfortheanalysisanddataacquisitionandtheprogram AcquityUPLCColumnsCalculatorv.1.1.1wasusedtoadjustthe HPLCmethodtotheUPLCmethod.
MobilephaseAconsistedofwaterwith0.1%(v/v)offormicacid andacetonitrileBwith0.1%(v/v)offormicacid.Thegradientwas 0min50%ofB,0–0.09min60%ofB,0.09–0.38min80%ofBand 0.38–0.80min95%ofB.Columntemperaturewasmaintainedat 25◦C,flowratewas0.294mlmin−1,injectionvolumewas1.0l
anddetectionwasbetween200and400nm.
CEconditions
TheCEassays wereconducted ina capillary electrophoresis system(Agilent Technologies, model 7100, Palo Alto,CA, USA) equippedwithadiodearraydetector,temperature-controldevice (maintainedat25◦C)anddataacquisitionandanalysissoftware suppliedbythemanufacturer(HPChemStation®).Beforethefirst
run,thecapillarycolumnwassequentiallyrinsedwith1moll−1
NaOH(30min)andwater(30min).Betweenruns,thecapillarywas flushedfor1minwithbackgroundelectrolyte(BGE).Atthe begin-ningofeachdaythecapillarywasconditionedbyflushingwith 1moll−1 NaOH(20min),followedbya20minflushwith
deion-izedwaterandanelectrolytesolution(20min).Betweenruns,the capillarywasreconditionedwith1moll−1NaOH(2min),deionized
water(1min)andelectrolytesolution(2min).Attheendofeach workingday,thecapillarywasrinsedwith1moll−1NaOH(10min)
andwater(10min).
Separations were conducted in an uncoated fused-silica capillary 48.5cm in length (40cm effective length×50m I.D.×375mO.D.).DirectUVdetectionwassetat210nm,andthe temperaturewasmaintainedat25◦C.Thestandardsandsamples wereintroducedtothecapillarywithahydrodynamicpressureof 50mbar,for3s.Theseparationvoltageappliedwas30kVunder normalpolarity.TheoptimizedBGEusedintheproposedmethod wascomprisedof20mmoll−1(pH9.2)and10%(v/v)acetonitrile,
andmethylparabenwasusedasinternalstandard,dilutedtoobtain afinalconcentrationof45.46gml−1.
Standardpreparationandcalibrationcurves
Thestandardstocksolutionswerepreparedbydissolving1mg ofeachcompoundin1mlofmethanol.Thesesolutionswerestored in dark glass bottles at 4◦C. Working standard solutions were freshly prepared by dissolving a suitable amountof theabove solutions with methanol before injection. Analytical curves for bothquercetinandkaempferolwereprepared,withninepoints between1and100gml−1fortheHPLCanalysisandsevenpoints between15and150gml−1withincrementsofmethylparaben, 45.46gml−1,fortheCEanalysis.
Samplepreparationofextracts
1.00 0.00
0.50
0.45
0.40
0.35
0.30
0.25
0.20
0.15
0.10
0.05
0.00 0.02 0.04 0.06 0.08 0.10 0.12
2.00 3.00 4.00 5.00 6.00 7.00 Minutes
A
QK
Q
K
B
AU
AU
8.00 9.00 10.00 11.00 12.00
1.00 2.00 3.00 4.00 5.00 6.00 7.00 Minutes
8.00 9.00 10.00 11.00 12.00
Fig.1. ChromatogramsofRubuserythrocladus(A)andMorusnigra(B)hydrolyzedleafextractsbyHPLC-UV,370nm.Q=quercetinandK=kaempferol.
Validation
TheHPLCandCEmethodswerevalidatedforthequantitative analysisofquercetinandkaempferolpresentinR.erythrocladusand M.nigraleaves,inaccordancewiththeInternationalConferenceon Harmonizationguidelines,usingtheparametersoflinearity, speci-ficity,precision,accuracy(recoverytest),limitofdetection,limit ofquantificationandrobustness(ICH,2005).ForvalidationinCE, wealwaysusedaninternalstandard(IS)–methylparaben–togive greatercertaintytotheresults.
Specificity was determined by checking the peak purity of the quercetin and kaempferol peaks, using a photodiode-array detector by HPLC and CE. Linearity was analyzed using three standardcurves obtainedonthreedifferentdaysat nine differ-entconcentrationsofquercetinandkaempferol(1–100gml−1) by HPLC and seven different concentrations of quercetin and kaempferol(15–150gml−1)byCE.Forelectrophoresisanalysis, 45.46gml−1 ofmethylparabenwasaddedinallthecalibration curvepoints,asinternalstandard.Linearitywasevaluatedby cal-culatingtheregressionlineusingtheleastsquaresmethod.
Precision was performed in two different levels: intra-day precision and inter-day precision. The intra-day precision was determinedafteranalysesofthree solutionsprepared at differ-entconcentrations,intriplicate,injectedinoneday.Theinter-day precisionwasstudiedby analyzing a solutionof quercetinand
kaempferol, preparedin triplicate,over three consecutivedays. Theaccuracy(assayrecovery)ofthemethodwasassessedby ana-lyzingsamplesofR.erythrocladusandM.nigra,byHPLCandCE, respectively–thesampleswerespikedwithknownamountsof quercetinandkaempferolstandardsolutionsatthreedifferent con-centrations:20,40and60gml−1ofquercetinandkaempferolfor chromatographyanalysisand30,60and90gml−1ofquercetin andkaempferolforelectrophoresisanalysis.Accuracywas deter-minedbythemeanconcentrationrecovered,andtheresultswere expressedasrecoverypercentage.
Detection(LOD)andquantitation(LOQ)limitswerecalculated directlyfromthestandardcurve.LODwascalculatedbythe equa-tion3.3/S,whileforLOQ,theequation10/Swasused,where
istheSDofyinterceptsofregressionlinesandSistheslopeofthe standardcurve.
Robustnesswasavailablefromtheinjectionofaquercetinand kaempferolsolutionbynormalconditionsandbyvaryingthe fol-lowinganalyticalparameters:chromatographiccolumnbatch,pH ofthemobilephase andflowrateforHPLCandtemperatureof cassette,injectiontimeandorganicsolventofelectrolyteforCE.
Resultsanddiscussion
1 0
5 10 15 20 25 30
25
20
15
10
5
0 mAU
mAU
A
B
2 3 4 5 6 7 Min
1 2 3 4 5 6 7 Min
K Q
K Q IS
EOF + #
EOF + # IS
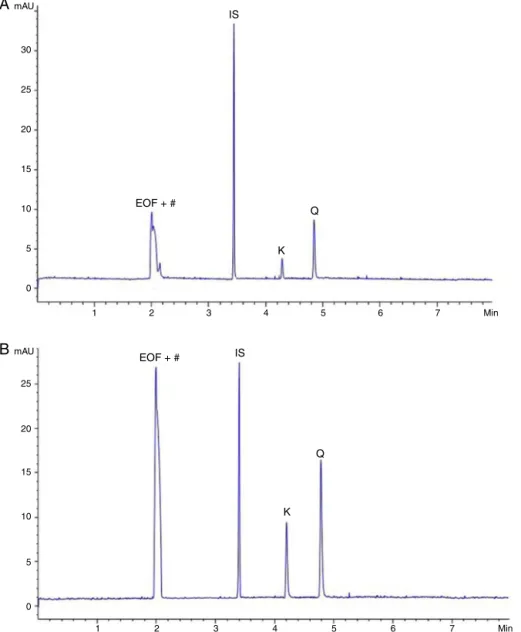
Fig.2.ElecthropherogramsofRubuserythrocladus(A)andMorusnigra(B)hydrolyzedleavesextractsbyCE-DAD,200nm.EOF+#=electroosmoticflow+unidentifiedpeak, IS=internalstandard,K=kaempferolandQ=quercetin.
Table1
Linearequation,linearity,limitofdetection(LOD)andquantification(LOQ),intra-dayandinter-dayprecision(valuesexpressedinRSD%)andrecoveryofquercetinand kaempferolbyHPLC-UVandCE-DAD.
Flavonoid Linearequation R2 LOD(gml−1) LOQ(gml−1) Precisionintraday Precisioninterday Meanrecovery(%)±RSD%
HPLC Quercetin y=83082x+42,829 0.9996 0.9519 2.8844 0.30 1.69 95.35±2.88 Kaempferol y=69225x+56,605 0.9995 1.0519 3.1876 0.23 2.02 95.76±2.77 CE Quercetin y=0.0176x+0.0276 0.9979 5.3043 16.0737 2.48 7.76 104.56±1.77 Kaempferol y=0.0203x+0.005 0.9987 4.1887 12.6931 3.68 8.44 104.31±1.66
Table2
DifferentparametersandresultsofrobustnessofquercetinandkaempferolinHPLC-UVandCE-DADmethods.Valuesexpressedinmean(%)±RSD%.
HPLCparameter Quercetin Kaempferol CEParameter Quercetin Kaempferol
Flow0.55mlmin−1 109.40±0.31 109.40±0.45 25.5◦
C 97.63±0.26 106.27±0.07 Flow0.65mlmin−1 92.35±0.26 92.38±0.02 24.5◦C 98.39
±0.63 106.37±0.71 0.005%TFA 95.89±7.70 93.50±11.58 2s 95.76±1.87 107.73±0.14 0.015%TFA 101.57±0.13 100.14±0.39 4s 97.94±0.25 103.19±4.12 0.04%TFA 102.96±0.61 100.89±0.69 5.0% 99.30±3.71 98.73±2.41 0.12%TFA 104.90±0.16 104.28±0.05 15.0% 98.77±1.43 104.76±0.16
Table3
QuantificationofquercetinandkaempferolinhydrolyzedleavesextractsofRubuserythrocladusandM.nigrabyHPLC-UVandCE-DAD.Valuesexpressedasgg−1ofdried
plant(D.P.).
Flavonoid R.erythrocladusMean(gg−1)±RSD% M.nigraMean(gg−1)±RSD%
HPLC Quercetin 848.43±10.48 2323.90±7.28
Kaempferol 304.35±6.83 1446.36±6.72
CE Quercetin 836.37±9.36 2552.82±7.96
Kaempferol N.Q. 1188.67±9.20
N.Q.=notquantified.
1.9e+1 1.8e+1
1.7e+1
1.6e+1
1.5e+1
1.4e+1 1.3e+1
1.2e+1
1.1e+1
1.0e+1 9.0
AU
8.0
7.0
6.0
5.0 4.0
3.0 2.0
1.0
5.0e+1 4.75e+1 4.5e+1
4.25e+1 4.0e+1
3.75e+1 3.5e+1
3.25e+1 3.0e+1
2.75e+1 2.5e+1
2.25e+1 2.0e+1 1.75e+1 1.5e+1 1.25e+1 1.0e+1
AU
7.5 5.0 2.5 0.0
0.00 0.100.200.30 0.400.50 0.600.700.800.90 1.001.101.201.30 1.401.50 1.601.701.801.902.00 Time
0.0
0.000.100.200.300.400.500.60 0.700.800.901.001.101.201.30 1.401.501.601.701.801.902.00 Time 1.56
1.13 0.89
0.69 0.58
K
0.69
K Q
0.58
Q
A
B
0.85 0.64 0.40
0.47
1.56 0.40
0.47
100
0
50 100 200 300 400 303.0503
A
209.8139
254.8139
209.8139 287.0564
265.8138
365.8138 370.8138
500 550 600650 700750 800850 9009501000 m/z 450
350 250 150
50 100 150 200 250 300 350 400 450 500 550 600 650 700 750 800 850 900 9501000 m/z
%
100
0
B
%
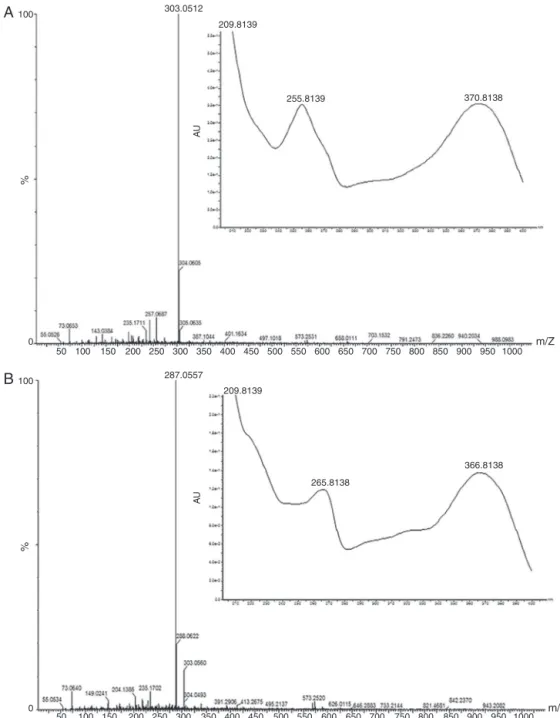
Fig.4. MassandUVspectraofquercetin(A)andkaempferol(B)ofRubuserythrocladushydrolyzedleafextractsbyUPLC-DAD/MS.
chromatography (Fig. 1) and electrophoresis (Fig. 2). The hydrolyzationprocessgivesusclearerchromatogramsthancrude extract and the identification of quercetin and kaempferol in hydrolyzedleavesextractssuggeststhepresenceofquercetinand kaempferolderivativecompoundsintheseleavestoo.
HPLC methods are developed based on the polarity of the molecules.ThisdiffersfromtheCEmethodology,inwhich both flavonoidswereionizedin theelectrolyte usedinthis work,so thattheseparationofquercetinandkaempferolwasdetermined bytheirmolecularweight.Firstweobservedtheelectromigration ofkaempferol(MW=286.24)andthenweobservedthe electromi-grationofquercetin(MW=302.2).
ThepHofthebufferwillinfluencethedegreeofionizationof thesolutes,andhence,theirelectrophoreticmobilities.Giventhat thepKa of quercetinis 7.76and the pKa of kaempferol is7.89
(Tungjaietal.,2008),sodiumtetraborate(pH9.2)waschosenas theelectrolytesystemtoinitiatetheoptimizationprocedure.At
thispH,theelectroosmoticflowisusuallygreaterthanthe elec-trophoreticvelocitiesoftheanions,soquercetinandkaempferol (negatively charged), which are attracted to the positive elec-trode,are carriedtowardthecathode.In thiscase,anionselute ininversesequence totheircharge-to-sizeratios. Initialresults haveshownthatinsufficientresolutionoccurredwithelectrolyte systemcontainingonly tetraborate, sotheuseof anadditional modifierwasrequired.Adramaticimprovementcanbeobtained inselectivity,resolution,andseparationefficiencywhenorganic solvents,suchasmethanol,ethanolandacetonitrileormixtures thereof,areaddedtotheelectrolyte(Baker,1995).The acetoni-trileconcentrationwasstudiedovertherangeof0–15%forafixed amountofsodiumtetraborate,and10%(v/v)wasselectedbecause itrepresentedacompromisebetweenresolutionandanalysistime. Theseparation ofquercetin,kaempferol andI.S. methylparaben under investigation at the optimized conditions is shown in
50
303.0512
209.8139
255.8139 370.8138
100
0
%
AU
A
287.0557
209.8139
265.8138
366.8138 100
0
%
AU
B
100 150 200 250 300 350 400 450 500 550 600 650 700 750 800 850 900 950 1000 m/Z
50 100 150 200 250 300 350 400 450 500 550 600 650 700 750 800 850 900 950 1000 m/Z
Fig.5.MassandUVspectraofquercetin(A)andkaempferol(B)ofMorusnigrahydrolyzedleafextractsbyUPLC-DAD/MS.
Methodsvalidation
Alltheparameterstestedinthevalidationmethodsforthe quan-tificationofquercetinandkaempferolinhydrolyzedleafextracts ofR.erythrocladusandM.nigrashowedsatisfactoryresults,with RSD ofless than 15% (Tables 1 and 2).The purityof the chro-matographic and electrophoretic peaks obtained for quercetin andkaempferolwasevaluatedusingaDADdetector.Theresults showedthatothercompoundsdidnotco-elute/migratewiththe standards.
Sampleanalysis
ItwaspossibletoapplythedevelopedmethodsinRubusand Morussamples(Table3).ByCE,kaempferolcouldnotbe quanti-fiedinR.erythrocladus.Thisresultisduetothelowersensitivity ofthistechnique,asshowninTable1.TheCEsensitivityisrelated tothecapillarydetectionwindow,capillarydiameterandlightUV
dispersion(Li,1996).Lessdilutedsamplescouldhaveprovided bet-terresults.Applyingtheanalyticaltoolt-studentitwasobserved thatnosignificantdifferenceswereobservedinthequantitiesof theseflavonoidsbetweenthetwoformsofanalyses–HPLCandCE (p>0.05)–Table3.
Bothanalyticaltechniquesprovedtobefastandreliable.The HPLC methodcould beapplied as a form of quality control of quercetinand kaempferol inhydrolyzed leafextractsof R. ery-throcladusandM.nigra.EvenwiththelowersensitivityoftheCE method,it ispossibletoobservehigherefficiencyofseparation peaks(Fig.2).Thehighversatility,thelowvolumeofreagentsand sample,andthegoodresultsobtainedforquercetinandkaempferol quantification by CEsuggest that this analytical tool could be appliedasacomplementarytechnicforthequalitycontrolofthese compoundsinnaturalproducts.
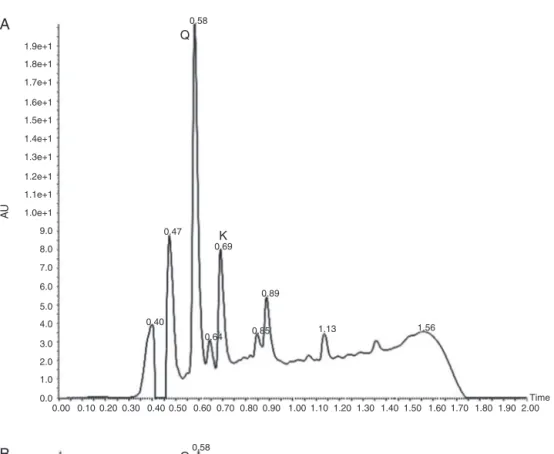
Botanicalandchemicalanalysesofplantsareveryimportantfor theidentificationandqualitycontrolofmedicinalplants(Brazilian Pharmacopoeia,2010).ByUPLC-DAD/MS,adifferencewas iden-tifiedbetweenthechemicalqualitativeprofilesoftheRubusand Morusspecies(Fig.3).
Allpeakswerefragmented,butonlyquercetinandkaempferol (at0.58 and 0.69s, respectively)were identified in hydrolyzed leafextracts of R. erythrocladus and M. nigra by UPLC-DAD/MS (Figs.4and5,respectively).
ThepresenceofkaempferolandquercetininRubusandMorus chromatograms,andsmallunidentifiedpeaksinthechromatogram forR.erythrocladus(Fig.3)byUPLC-DAD/MSsuggestthattheRubus leafextractmaybemorecomplexthantheMorusleafextract.
Conclusions
It was possible to determine the flavonoids present in hydrolyzedleavesextractsofR.erythrocladusandM.nigraandit wasshowedtwofastandreliablemethodstoquantifyquercetin andkaempferolintheseextracts,HPLC-UVandCE-DAD.Thesetwo methodswerevalidatedandcouldbeappliedtothequality con-troloftheseextracts.Moreover,we reportchemical differences betweentheMorusandRubusleavesstudiedbyUPLC-DAD/MSand wealsocontributewithnewinformationabouttheMorusgenus fortheBraziliangovernment.
Authors’contributions
LRTcontributedin samplepreparation, chromatography and electrophoresisanalysis,analysisofdataanddraftof thepaper. GPRPcontributedinsamplepreparationandchromatography anal-ysis.ACOCandMScontributedinelectrophoresisanalysisanddraft ofthepaper.SALBcontributedtoplantscollections.AFandJASZ designedthestudy,leadandadvisedthelaboratoryworkand per-formedtheanalysisofthedataanddraftedthemanuscript.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
WewouldliketothankCAPES,CNPq,PPGCF-UFRGSandDra. Maria do Carmo Bassols Raseira (Embrapa). JASZ acknowledge CNPqforresearcherfellowship.
References
Baker,D.R.,1995.CapillaryElectrophoresis.Wiley,NewYork.
BrazilianPharmacopoeia,2010vol.I.,5thed.Anvisa,Brazil,546p.
Carrasco-Pancorbo,A.,Gómez-Caravaca,A.M.,Cerretani,L.,Bendini,A., Segura-Carretero,A.,Fernández-Gutiérrez,A.,2006.Asimpleandrapidelectrophoretic methodtocharacterizesimplephenols,lignans,complexphenols,phenolic acids,andflavonoidsinextra-virginoliveoil.J.Sep.Sci.29,2221–2233.
Choi,J.,Kang,H.J.,Kim,S.Z.,Kwon,T.O.,Jeong,S.-I.,Jamg,S.-I.,2013.Antioxidant effectofastragalinisolatedfromtheleavesofM.albaL.againstfree radical-inducedoxidativehemolysisofhumanredbloodcells.Arch.PharmacalRes.36, 912–917.
Chu,Q.,Lin,M.,Tian,X.,Ye,J.,2006.Studyoncapillaryelectrophoresis-amperometric detection profilesof differentparts of M. albaL. J. Chromatogr. A1116, 286–290.
Chung,H.I., Kim, J.,Kim, J.Y., Kwon,O., 2013. Acuteintake ofmulberry leaf aqueousextractaffectspostprandialglucoseresponseaftermaltoseloading: randomizeddouble-blindplacebo controlledpilotstudy.J.Funct.Foods5, 1502–1506.
Dat,N.T.,Binh,P.T.X.,Quynh,L.T.P.,Ninh,C.V.,Houng,H.T.,Lee,J.J.,2010.Cytotoxic prenylatedflavonoidsfromM.alba.Fitoterapia81,1224–1227.
Deighton,N.,Brennan,R.,Finn,C.,Davies,H.V.,2000.Antioxidantpropertiesof domesticatedandwildRubusspecies.J.Sci.FoodAgric.80,1307–1313.
Durgo,K.,Belscak-Cvitanovic,A.,Stancic,A.,Franekic,J.,Komes,D.,2012.The bioac-tivepotentialofredraspberry(R.idaeusL.)leavesinexhibitingcytotoxicand cytoprotectiveactivityonhumanlaryngealcarcinomaandcolon adenocarci-noma.J.Med.Food15,258–268.
Gudej,Y.,2003.KaempferolandquercetinglycosidesfromR.idaeusL.leaves.Acta Pol.Pharm.60,313–316.
Gudej,J.,Tomczyk,M.,2004.Determinationofflavonoids,tanninsandellagicacid inleavesfromRubusL.Species.Arch.PharmacalRes.27,1114–1119.
Gundogdu,M.,Muradoglu,F.,GaziogluSensoy,R.I.,Yilmaz,H.,2011.Determination offruitchemicalpropertiesofM.nigraL.,M.albaL.andM.rubraL.byHPLC.Sci. Hortic.132,37–41.
Han,N.,Gu,Y.,Ye,C.,Cao,Y.,Lie,Z.,Yin,J.,2012.Antithromboticactivityoffractions andcomponentsobtainedfromraspberryleaves(R.chingii).FoodChem.132, 181–185.
ICH,2005.Harmonizedtripartiteguideline:validationofanalyticalprocedures:text andmethodologyQ2(R1).
Jouad,H.,Maghrani,M.,Eddouks,M.,2002.HypoglycaemiceffectofR.fructicosisL. andGlobulariaalypumL.innormalandstreptozotocin-induceddiabeticrats.J. Ethnopharmacol.8,351–356.
Katsube,T.,Imawaka,N.,Kawano,Y.,Yamazaki,Y.,Shiwaku,K.,Yamane,Y.,2010.
Antioxidantflavonolglycosidesinmulberry(M.albaL.)leavesisolatedbasedon LDLantioxidantactivity.FoodChem.97,25–31.
Li,S.F.Y.,1996.CapillaryElectrophoresis:Principles,PracticeandApplications. Jour-nalofChromatographyLibrary,Netherlands.
Liu,Z.,Schwimer,J.,Liu,D.,Lewis,J.,Greenway,F.,York,D.A.,Woltering,E.A.,2006.
GallicacidispartiallyresponsiblefortheantiangiogenicactivitiesofRubusleaf extract.Phytother.Res.20,806–813.
Martini,S.,D’addario,C.,Colacevich,A.,Focardi,S.,Borghini,F.,Santucci,A.,Figura, N.,Rossi,C.,2009.AntimicrobialactivityagainstHelicobacterpyloristrainsand antioxidantpropertiesofblackberryleaves(R.ulmifolius)andisolated com-pounds.Int.J.Antimicrob.Agents34,50–59.
Merken,H.M.,Beecher, G.R.,2000. Measurementof foodflavonoids by high-performance liquid chromatography: a review. J. Agric. Food Chem. 48, 577–585.
Nogueira,E.,Rosa,G.J.M.,Haraguchi,M.,Vassilieff,V.S.,1998.AnxiolyticeffectofR. brasilensisinratsandmice.J.Ethnopharmacol.61,111–117.
Nogueira,E.,Vassilieff,V.S.,2000.Hypnotic,anticonvulsantandmusclerelaxant effectsofR.brasiliensis.InvolvementofGABAA-system.J.Ethnopharmacol.70, 275–280.
Oh,K.-S.,Ryu,S.Y.,Lee,S.,Seo,H.W.,Oh,B.K.,Kim,Y.S.,Lee,B.H.,2009.Melanin con-centratinghormone-1receptorantagonismandanti-obesityeffectsofethanolic extractfromM.albaleavesindiet-inducedobesemice.J.Ethnopharmacol.122, 216–220.
Omidiran,M.O.,Baiyewu,R.A.,Ademola,I.T.,Faforede,O.C.,2012.Phytochemical analysis,nutritionalcompositionandantimicrobialactivitiesofwhitemulberry (M.alba).Pak.J.Nutr.11,456–460.
Ostrosky,E.A.,Marcondes,E.M.C.,Nishikawa,S.,de,O.,Lopes,P.S.,Varca,G.H.C., Pinto,T.,de,J.A.,Consiglieri,T.O.,Baby,A.R.,Velasco,M.V.R.,Kaneko,T.M.,2011.
R.rosaefoliusextractasanaturalpreservativecandidateintopicalformulations. AAPSPharmSciTech12,732–737.
Panizzi,L.,Caponi,C.,Catalano,S.,Cioni,P.L.,Morelli,I.,2002.Invitroantimicrobial activityofextractsandisolatedconstituentsofR.ulmifolius.J.Ethnopharmacol. 79,165–168.
Portal da Saúde. http://portalsaude.saude.gov.br/index.php/cidadao/principal/ agencia-saude/noticias-anterioresagencia-saude/3487- (accessed October 2013).
Rojas-Vera,J.,Patel,A.V.,Dacke,C.G.,2002.Relaxantactivityofraspberry(R.idaeus) leafextractinguinea-pigileuminvitro.Phytother.Res.16,665–668.
Skupien, K., Kostrzewa-Nowak, D., Oszmianski, J., Tarasiuk, J., 2008. In Vitro antileukaemicactivityofextractsfromchokeberry(Aroniamelanocarpa[Michx] Elliott)andMulberry(M.albaL.)leavesagainstsensitiveandmultidrugresistant HL60cells.Phytother.Res.22,689–694.
Suntornsuk,L.,Kasemssok,S.,Wongyai,S.,2003.Quantitativeanalysisofaglycone quercetininmulberryleaves(M.albaL.)bycapillaryzoneelectrophoresis. Elec-trophoresis24,1236–1241.
Thabti,I.,Elfalleh,W.,Hannachi,H.,Ferchichi,A.,Campos,M.,da,G.,2012. Identi-ficationandquantificationofphenolicacidsandflavonolglycosidesintunisian MorusspeciesbyHPLC-DADandHPLC–MS.J.Funct.Foods.4,367–374.
Thiem,B.,Goslinska,O.,2004.AntimicrobialactivityofR.chamaemorusleaves. Fitoterapia75,94–95.
Tsuduki,T.,Kikuchi,I.,Kimura,T.,Nakagawa,K.,Miyazawa,T.,2013.Intakeof mul-berry1-deoxynojirimycinpreventsdiet-inducedobesitythroughincreasesin adiponectininmice.FoodChem.139,16–23.
thepredominantspeciesofflavonoidsinphysiologicalbuffer:determinationof solubility,lipophilicityandanticancerefficacy.OpenDrugDeliv.J.2,10–19.
Tzouwara-Karayanni, S.M., Philianos, S.M., 1982. Isolation of quercetin and kaempferolfromR.ulmifoliusandtheirfluorometricassay.Microchem.J.27, 144–161.
Venskutonis,P.R.,Dvaranauskaite,A.,Labokas,J.,2007.Radicalscavenging activ-ityandcompositionofraspberry(R.idaeus)leavesfromdifferentlocationsin Lithuania.Fitoterapia78,162–165.
Volpato,G.T.,Calderon,I.M.P.,Sinzato,S.,Campos,K.E.,Rudge,M.V.C.,Damasceno, D.C.,2011.EffectofM.nigraaqueousextracttreatmentonthematernal–fetal outcome,oxidativestressstatusandlipidprofileofstreptozotocin-induced dia-beticrats.J.Ethnopharmacol.138,691–696.