w w w . s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
The
influence
of
leaf
age
on
methylxanthines,
total
phenolic
content,
and
free
radical
scavenging
capacity
of
Ilex
paraguariensis
aqueous
extracts
Carlos
H.
Blum-Silva
a,
Vitor
C.
Chaves
a,
Eloir
P.
Schenkel
a,
Geraldo
C.
Coelho
b,
Flávio
H.
Reginatto
a,∗aLaboratóriodeFarmacognosia,ProgramadePós-graduac¸ãoemFarmácia,UniversidadeFederaldeSantaCatarina,UFSC,CampusUniversitário,Florianópolis,SC,Brazil bPró-ReitoriadeExtensãoeCultura,UniversidadeFederaldaFronteiraSul,CampusChapecó,SC,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received25April2014 Accepted29January2015 Availableonline12February2015
Keywords:
Mate
Methylxanthines Phenolics Ageing Scavenging
a
b
s
t
r
a
c
t
Yerba-mate(IlexparaguariensisA.St.Hil.,Aquifoliaceae)isaSouthAmericannativespeciesthatiswidely usedforitsindustrialpotentialinthepreparationofdrinks,teasandcosmetics.Itspropertiesaredirectly relatedtothepresenceofitschemicalconstituents,suchassaponins,methylxanthinesandphenolic compounds.Thisstudyaimedtoinvestigatetheinfluenceofleafageonmethylxanthineandtotal phe-noliccontentsbyHighPerformanceLiquidChromatographyandUltravioletSpectroscopy,aswellason freeradicalscavengingcapacity,ofaqueousextractsofI.paraguariensisleaves.Theresultsshowedgreat variabilityinallthemetabolitesmeasured.Leafageingsignificantlyincreasedthemethylxanthine con-tentandtotalphenoliccontentoftheextracts.Freeradicalscavengingcapacitywasalsosignificantly affected(p<0.05)byleafage.Apositivecorrelationwasobserved,betweentheantioxidantactivityand totalphenoliccontent.
©2014SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
IlexparaguariensisA.St.Hil.,Aquifoliaceae,isaSouthAmerican
nativeperennialtreethatispopularlyknownas“yerba-mate”or
“mate”.Itisoneofthemostpopularandwidely-consumed
bever-agesinsouthernBrazil,Argentina,Paraguay,andUruguay,where
itisusedasadecoctionorinfusion.Mateisusedforitscentral
ner-voussystemstimulantproperties,whichareduetothepresence
ofthemethylxanthinescaffeineandtheobromine(Blumenthaland
Brinckmann,2000;Dermarderosian,2001;Filipetal.,1998).
Addi-tionally,yerba-mateisalsoconsideredafunctionalfood,because
ofitsnutritionaland medicinalproperties,suchas
hypocholes-terolemic,hepatoprotective,diuretic, andantioxidantproperties
(Bixbyetal.,2005;Filipetal.,2000;GugliucciandStahl,1995;Heck andDeMejia,2007;Rivellietal.,2007;Valergaetal.,2012),which
can protect against the harmfuleffect of free radicals, thereby
increasingthedefensesystemoftheorganism.It canalsohelp
preventatherosclerosisandcoronaryheartdisease(HeckandDe
Mejia,2007;Mirandaetal.,2008;PuangpraphantanddeMejia, 2009;Boaventuraetal.,2012).
∗ Correspondingauthor.
E-mail:freginatto@pq.cnpq.br(F.H.Reginatto).
These health benefits have been attributed to phenolic
compounds, which are major constituents of I. paraguariensis
(Heck and De Mejia, 2007). The main polyphenols present in
“mate”arecaffeoylderivatives(chlorogenic,3,5-dicaffeoylquinic,
4,5-dicaffeoylquinic and 3,4-dicaffeoylquinic acids), and caffeic
acid.Moreover,yerba-matealsocontainshighmethylxanthines,
saponins,andaminorcontentofflavonoids,suchasquercetin,rutin
andkaempferol(DeSouzaetal.,2011;Coelhoetal.,2010;Filipetal.,
2001;Reginattoetal.,1999;Gosmannetal.,1995).
Itiswidelyknowninnaturalproductchemistrythatthegrowth
conditionsplayaroleintheproductionofphytochemicalsinthe
plant(Gobbo-NetoandLopes,2007;Meyeretal.,2006).Inregardto
ageoftheleaves,therehavebeenfewreportsshowingitsinfluence
onmetabolitecontent.SuchasEsmelindroetal.(2004)showed
thatyoungleavesofI.paraguariensiscontainahighproduction
ofmethylxanthines,andDartoraetal.(2011)reportedno
signif-icantdifferencesbetweenphenolicandmethylxanthinecontents
inleavesat1and6monthsofgrowth.Inaddition,thesereports
suggestthatintrapopulationgeneticconditions,suchasageofthe
leaves, playanimportantrole inthedistributionofthese
com-poundsinI.paraguariensis.Finally,knowledgeaboutthischemical
compositionisimportantforourunderstandingofthechangesin
potentialbiologicalactivitiesofI.paraguariensis.Thepresentwork
thereforeassessestheinfluenceofleafageonthephytochemical
http://dx.doi.org/10.1016/j.bjp.2015.01.002
compositionofI.paraguariensis,andonitsfreeradicalscavenging activity.
Materialsandmethods
Plantmaterial
Theleavesof11treesofI.paraguariensisA.St.Hil.,
Aquifoli-aceae,fromanativepopulationwerecollectedatChapecó,inthe
StateofSantaCatarina,Brazil(27◦08′48′′S;52◦37′01′′W).Theplant
sampleswerecultivated undernatural sunlightconditions. The
plantmaterial(RSPF11074)washarvestedinOctober2010andthe
leaveswereseparatedaccordingtoage,asdefinedby
embranch-ment;leavesatonemonth(firsttothirdleafpairsfromthebranch
tips),attwomonths(forthtoeighthleafpairs)andatsixmonths
(ninthtofifteenthleafpairs).Allthesampleswereimmediately
frozen,lyophilized,crushedseparately,andstoredat−20◦Cuntil
tested.
Extraction
Theextractsofeachsamplewerepreparedbyaqueousinfusion.
Briefly,fivegramsofeachdriedleafsamplewasmixedwith100ml
ofdistilledwater(90±2◦C)for20min.Theextractswerefiltered,
thevolumesadjustedto100mlwithwater,andthesamplesfrozen.
HPLC-DADanalysisofmethylxanthines
Thequantitativeanalysesofcaffeineandtheobromineinthe
extractswerecarriedoutinaPerkinElmerSeries200High
Perfor-manceLiquidChromatography(HPLC)equippedwithaDiodeArray
Detector(DAD),quaternarypump,onlinedegasserand
autosam-pler. Chromera® Workstation software was used for the data
acquisition.Theinjectionvolumewas20landthebaseline
res-olution was obtained at room temperature (24±2◦C). For the
methylxanthineanalysis,separationwasperformedonaPerkin
ElmerBrownleeChoiceC8 column(150mm×4.6mmi.d.;5m)
and a mixture of methanol/ammonium hydroxide 0.2% (20:80
v/v)asthemobilephase,withconstantflowrateat0.9mlmin−1.
Themobile phase wasprepared daily and degassed by
sonica-tionbefore use. The chromatograms wererecorded at 280nm,
whiletheUVspectraweremonitoredoverarangeof200–450nm.
Peakswere characterizedbycomparingtheretention time and
UV spectra with the reference standards, and by co-injection
oftheauthentic samples.Thestandardsolutionswereprepared
in differentranges: 0.625–400gml−1 for the caffeine
(Sigma-Aldrich®
)and 0.3125–75gml−1 for thetheobromine (Fluka®).
Theextractswere analyzedat a concentrationof 2.00mgml−1.
Quantificationofcaffeineandtheobrominewasperformedusing
seven-point regression curves(r2>0.999). Theregression
equa-tionswere“y=16478x+11339”forcaffeineand“y=26525x+1930”
forthetheobromine.Allanalyseswereperformedintriplicate,and
thepeakaverageareasweremeasured.Theresultswereexpressed
asmilligramsofcompoundpergofextract(mgcompoundg−1E).
Totalphenoliccontent
The determination of total phenolic content (TPC)was
per-formed as described by Medina (2011a,b) based on the direct
interactions of polyphenols with Fast Blue BB diazonium salt
(Sigma-Aldrich®). Seven chlorogenic acid (Fluka®) calibration
standard points (r2=0.999) wereprepared within the range of
10–150gml−1 indistilled waterand1.0mlofeachwas
trans-ferredtoaborosilicatetube.A0.1mlaliquotof0.1%FastBlueBB
reagentwasaddedtoallthechlorogenicacidstandardtubes,mixed
for1min,andthen0.1ml5%NaOHwasadded.Thereactionwas
allowedtocompleteatroomtemperature(24±2◦C)for90min
andtheopticaldensitywasmeasuredat420nm.TheTPCoftheI.
paraguariensisextractsweredeterminedasdescribedabove,except
thateachsamplewasanalyzedwithablankcontainingonlythe
sample,tomeasurenaturalnon-phenolicinterferencesat420nm.
Theresultswereexpressedasmilligramsofchlorogenicacid
equiv-alentspergofextract(mgCAg−1E).
Freeradicalscavengingcapacity
Thefreeradicalscavengingcapacitywasdeterminedas
pre-viously described Brandwilliams et al. (1995). Briefly, 0.1ml of
eachsampleextractatfourdifferentconcentrationswasaddedto
3.9mlofa methanolicsolutionof2,2-diphenyl-1-picrylhydrazyl
[DPPH (60M)]. The absorbances were measured at 515nm
(Lambda25UV/Vis,PerkinElmer®).Thepercentageofremaining
DPPH (Sigma-Aldrich®)was calculated and plotted against the
sample concentration, in order to obtainthe EC50, which was
definedastheamountof antioxidantnecessarytodecreasethe
initialDPPHconcentrationby50%.Chlorogenicacidwasusedas
positivecontrol.
ValidationofHPLC-DADanalysisofmethylxanthines
TheanalyticalprocedureswerevalidatedaccordingtoCassand
Degani(2001)andtheICHguidelines(ICH).Thevalidated
param-eterswerespecificity,linearity,accuracy,precision(repeatability
andintermediateprecision),limitofquantification(LOQ)andlimit
ofopticaldetection(LOD).
Dataanalysis
Datawereexpressedasmeanvalues±S.E.M.fromthree
inde-pendent measurements. For the determination of EC50 values,
linear regressionsof concentration–response curves wereused.
DifferencesbetweentreatmentswerecomparedbyANOVA
analy-sisofvariancefollowedbyTukey’stestadopting˛=0.05.
Resultsanddiscussion
ValidationofHPLC-DADanalysisofmethylxanthines
The analytical curves of both authentic standards showed
good linearity (r2>0.999). Linear regression equation for the
calibration curve were “y=16482x+10373” for caffeine and
“y=26495x+2422.7”fortheobromine(Fig.1).
Theobservedvaluesofvalidationparametersaresummarized
inTable1.
TheHPLC-DADquantificationsshowedgoodlinearrelationships
betweenpeakareaandconcentration(r2>0.999)forallstandard
solutionsinbothmethods.Thelimitofquantification(LOQ)and
limitofdetection(LOD)weredefinedbyrelativestandard
devia-tion(RSD<5%)andbyasignal:noiseratioof3:1,respectively.The
precisionwasdeterminedbyrepeatability(intra-dayassay)and
intermediateprecision(inter-dayassay)(CassandDegani,2001;
ICH,2005).Theintra-dayassaywasperformedbytriplicate
anal-ysisofthreedifferentconcentrations ofstandard solutions,and
expressedasrelativestandarddeviation.Goodrepeatabilitywas
obtainedfromlower, mediumandhigher concentrationsofthe
curve,withanRSD≤3.96%forallstandardanalyses.Theinter-day
assaywasdeterminedbytheanalysisofamediumconcentrationin
thecurve,threetimesaday,onthreedifferentdays.Asin
repeat-ability,theintermediateprecisionRSDvaluedidnotexceedthe
limitsrecommendedintheliterature(CassandDegani,2001;ICH,
2005).Inrelationtoaccuracy,goodrecoverywasobservedinthe
8 - 106
6 - 106
4 - 106
2 - 106
0
0 100
A
200 300
Standard concentration (µg. ml–1)
P
eak area
B
P
eak area
400
2.5 - 106
2 - 106
1 - 106
0.5 - 106
0
20
0 40 60
Standard concentration (µg. ml–1)
80 1.5 - 106
Fig.1. CLAE-DADcalibrationcurvesforcaffeine(A)andtheobromine(B).
Table1
ValidatedanalyticalparametersfortheHPLC-DADquantificationofcaffeineandtheobromineinaqueousinfusionsofIlexparaguariensis.
Compound Precisiona Accuracyb(recovery) LOQc(gml−1) LODc(gml−1)
Repeatability Intermediateprecision
Mean(gml−1) R.S.D.(%) Mean(gml−1) R.S.D.(%) Mean(%) R.S.D.(%)
Caffeine
0.625 3.96
100.00 1.52 99.8 1.74 0.10
100.00 0.97 0.625
400.00 1.35
Theobromine
0.3125 4.08
15.00 0.87 101.3 1.33 0.10
15.00 1.26 0.3125
75.00 1.85
aLimit:R.S.D.<5%.
bRecoverywasdeterminedbyinjectionofspikedsamples,intriplicate,withstandardsolution. c LOQ=limitofquantification;LOD=limitofdetection.
Methylxanthinescontent
In the chromatographic analysis of methylxanthines,
theo-bromineandcaffeinepresentedretentiontimesof3.1and9.1min,
respectively.Fig.2showsthemethylxanthinechromatogramof
aqueousextractfromI.paraguariensisleavesofsample2.
Theoph-yllinewasnotdetectedinanysampleanalyzed,afindingthatis
corroboratedbyseveralstudiesintheliterature(Athaydeetal.,
2000;CliffordandRamirez-Martinez,1990;Coelho etal.,2007; Filipetal.,1998;Reginattoetal.,1999).
The data analysis showed that there is great variability in
caffeineandtheobrominecontentsinthispopulationofI.
paraguar-iensis.Thisisclearlyshowedbythevariationincontentsshownin
Table2.
Athayde et al. (2007) also observed significant differences
in methylxanthine content among yerba-mate plants within
thesame population, aswellas betweendifferentpopulations.
In some cases, the caffeine content detected by the authors,
within the same plant population, was more than 100 times
higher.
The statisticalanalysis (Table 3)showedthat leaf agehasa
significantinfluenceoncaffeine,theobromineandtotal
methylx-anthine contents of the extracts(p<0.05). Analyzing themean
caffeinecontentofthetotalsetofsamples,itisevidentthatthere
isatendencyfortotalcaffeineandmethylxanthineproductionto
decreaseovertime.Theobrominedoesnotfollowexactlythesame
pattern,ashigherlevelswerefoundintheleavesatoneandtwo
months.
0 50 100 150 200 250 350
300 400
0 1
2 - A: 280: 10: 400: 10: 1
2 3
1
2
225 250 275 300 325 350 375 0
0.25 0.5 0.75 1.75 1.5
1
4 5 6 7 8 9 10
Time (min)
Absorbance (mA
U)
Table2
VariationincontentsofmethylxanthinesandtotalphenolicsinaqueousextractsofIlexparaguariensisleaves.
Ageofleaves Extentof variation
Caffeine* Theobromine* Methylxanthines* Total phenolics**
1month Minimum
Maximum
46.46± 0.15 133.98± 0.41
3.07± 0.01 24.93± 0.14
50.86± 0.16 137.05 ± 0.42
230.97 ± 7.78 437.81 ± 15.49
2months Minimum
Maximum
38.76± 0.24 90.50±0.12
5.80± 0.13 30.88±0.13
44.73± 0.26 101.80±0.17
172.32 ± 3.95 391.66±0.99
6months Minimum
Maximum
5.68±0.08 81.57±0.18
0.26±0.01 14.25±0.06
6.20±0.09 84.49±0.19
75.27±5.54 257.22±3.43 * Caffeine,theobromineandtotalmethylxanthinescontentsweredeterminedbyCLAE-DADandthedataaremean±S.D.valuesexpressedasmgcompound/gextract (n=11).
** TotalphenoliccontentwasdeterminedbytheFastBlueBBmethodandthedataaremean± S.D.valuesexpressedasmgchlorogenicacid/gextract(n=11).
Thehighercaffeinelevelsinrelationtotheobrominecouldbe
explainedbythebiosynthesispathwayofthesecompounds.
Theo-bromineis the direct precursor of caffeinebiosynthesis by the
methylationofxanthosinebyS-adenosylmethionine(SAM)action
(Katoetal.,2000;Ogawaetal.,2001;Uefujietal.,2003).Although
thisinformationhasbeenobtainedfromcoffee(Coffeaarabica)and
tea(Camellia sinensis), theavailableevidence indicatesthatthe
pathwayisessentiallythesameinotherpurinealkaloid-forming
plants,suchasI.paraguariensis(Ashihara,1993).
Caffeinecatabolismusuallybeginswithitsconversionto
the-ophylline.TheophyllineisdegradedtoCO2farmorerapidlythan
caffeine,indicatingthattheconversionofcaffeinetotheophylline
isthemajorrate-limitingstepofcaffeinecatabolismandthe
rea-sonwhycaffeineaccumulatesinhighconcentrationsintissuesof
C.sinensisandC.arabica(Ashiharaetal.,1996;Itoetal.,1997).
Thereisonlyonepublishedreport,inwhichthepurine
alka-loidbiosynthesisofmatebydifferentagedleaveswasinvestigated.
Youngleaves,butnotmaturedark-greenleaves,incorporatedeach
precursorintotheobromineandcaffeine,andnosignificant
degra-dationofcaffeinewasdetectedbyAshihara(1993).Theseresults
areinagreementwiththedecreaseincaffeinelevelswithageing
detectedbyourresearch.
The influence of age and plant development on secondary
metabolitecontents,andtherelativeproportionsofthese
chem-icalcomponents,hasbeendemonstrated byseveralauthorsfor
differentplantspecies(BowersandStamp,1993;Doanetal.,2004;
Hendriksetal.,1997;Höftetal.,1998).Hartmann(1996)statesthat
youngtissuesgenerallyhavehigherratesofmetabolites’
biosyn-thesis.Althoughtheinfluenceofleafagehasbeendescribedfor
otherspecies,thereisnogeneralconclusionapplicabletoallplant
species.Also, thereareinsufficientstudiesonthis subjectforI.
paraguariensis.Notwithstanding,someauthorshavedemonstrated
theinfluenceofleafageonmate.Mazzafera(1994)foundhigher
caffeinecontentsinyoungerleaves,andEsmelindroetal.(2004)
foundasignificantlyhighercontentofcaffeineandtheobrominein
leavesatsixmonthsthaninolderleaves.Recently,Dartoraetal.
(2011)evaluated themethylxanthinecontent in I.
paraguarien-sissamplesunderdifferentgrowthconditions,treatmentandage.
Theirresultsdidnotshowsignificantdifferencesinmethylxanthine
contentinleavesbetweenoneandsixmonths.Somestudieswith
yerba-mateintheliteraturehaveevaluatedtheinfluenceof
har-vesttimeonmethylxanthinecontent.Schubertetal.(2006)found
highermethylxanthinecontentinthespringandearlysummer.
SimilarresultswereobtainedbyCoelhoetal.(2001)analyzing
sam-plesofI.paraguariensiscollectedintheStateofParaná,Brazil,in
twodifferentperiods.CoelhoandMariath(1996)foundthatthe
mainsproutinginI.paraguariensisoccursinlateSeptemberand
October, andin someplants, sproutingcanalso occurbetween
FebruaryandMarch.TheresultsobtainedbyAthaydeetal.(2000),
Coelhoetal.(2001)andSchubertetal.(2006)canbeexplained
partlybytheageoftheleaves,sinceexperimentalresultsindicate
thatthebiosynthesisofcaffeineinIlexonlyoccursinyoungleaves
(Ashihara,1993).Thus,thehighmethylxanthinecontents
identi-fiedinthesummercanbeattributedtothedevelopmentofthe
youngleaves,whiletheresultsforlatefallandwintermay
indi-cateolder,morematureleaveswithlowbiosyntheticactivity.The
data(Table3)presentedinourstudyconfirmthistheory,since
thecaffeineandtotalmethylxanthinecontentsshoweda
signifi-cantdecreasewithageingoftheleaves.Thus,theageofleavesmay
affectthecharacteristicsoftherawmaterialandtherefore,their
processedproducts.
Totalphenoliccontent
Theobtainedresultsshowedahighconcentrationof
pheno-lic compoundsinthesamplesof yerba-mate, corroboratingthe
numerous literaturedata that reporthigh levelsof these
com-pounds,mainlycaffeoylquinicderivatives.Filipetal.(2001)found
higher levels of these phenolic compounds in I. paraguariensis,
whencomparedtotheothersevenIlexspecies,detectinga
con-centration of 9.6%phenol derivatives ondry extract and 0.06%
flavonoids.MarquesandFarah(2009)describedthatyerba-mate
contains,onaverage,55%caffeoylquinicderivativesingreenleaves
and73%intoastedleaves.Additionally,Bracescoetal.(2003)found
thatphenoliccompoundsarethreetimeshigherthanthecontent
ofthesecompoundsingreentea.Whenevaluatingthemeanofall
thesamples(Table3),itwasnotedthatthereisadecreaseinthe
productionofphenoliccompoundsovertime,sincethecontents
arehigherinleavesatonemonth,intermediateattwomonthsand
loweratsixmonths.Thus,theageoftheleaveshasasignificant
influenceinTPC.However,Dartoraetal.(2011)revealedno
signif-icantdifferencesinlevelsofphenoliccompoundscomparingleaves
atoneandsixmonths.
Table3
Leafageeffectsonthequantitativecontentsofcaffeine,theobromine,methylxanthinesandtotalphenolicsintheaqueousextractsofIlexparaguariensisleaves.
Ageofleaves Caffeine Theobromine Methylxanthines Totalphenolics
1month 75.63± 16.24a 9.22± 3.11a 84.85± 27.49a 286.7± 46.1a
2months 60.19± 13.4a,b 12.4± 4.19a,b 72.6± 20.14a,b 252.6± 50.85a,b
6months 31.94± 14.6c 4.95± 2.85a,c 36.89± 16.52c 166.6± 35.13c
0 50
a
a, b
c
100 150 200
1 2 6
Leaf age (months)
EC
50
(gg DPPH
–1
)
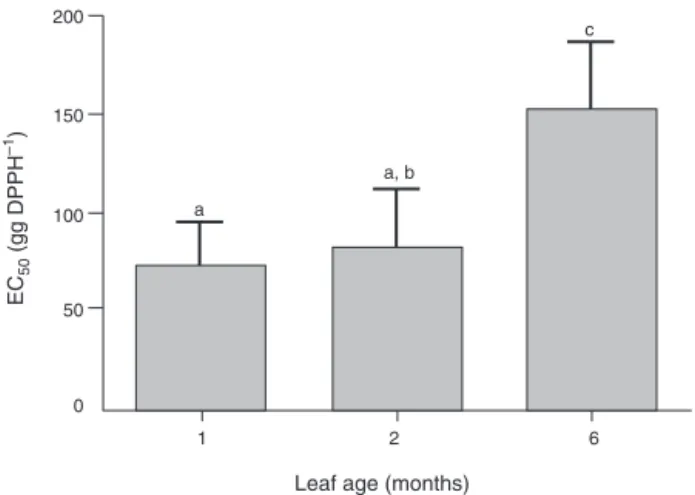
Fig.3. LeafageeffectsonthefreeradicalscavengingcapacityofIlexparaguariensis
aqueousextracts.TheEC50weredeterminedbytheDPPHmethodandthevalues
aremean ± S.D.expressedasgextract/gDPPH(n=11).Differentlettersindicate significantdifferences(ANOVAandTukey’sposttest,p<0.05).
Table4
Correlationanalysis.
Correlation r2value pvalue
TPC*×EC
50**(allsamples)a 0.2720 0.0019
TPC*×EC
50**(byleafagegroups)b 0.9246 0.0022
Caffeine***×EC
50**(allsamples)a 0.2728 0.0018
Caffeine***×EC
50**(byleafagegroups)b 0.8885 0.0048
Caffeine***× TPC*(allsamples)a 0.1978 0.0095
Caffeine***× TPC*(byleafagegroups)b 0.9529 0.0008 aCorrelationofallsamplesindividuallyconsidered.
bCorrelationofthemeanofeachleafagegroup.
*TotalphenoliccontentwasdeterminedbytheFastBlueBBmethod.
**Thefree radicalscavenging capacity(EC50)of Ilex paraguariensisaqueous extractswasdeterminedbytheDPPHmethod.
***CaffeinecontentwasdeterminedbyCLAE-DAD.Moredetails:seeSection “Mate-rialsandmethods”.
Freeradicalscavengingcapacity
Itwasobservedthatleafagesignificantlyaffectstheabilityto
scavengefreeradicals,andthereisasignificantdecreaseinthis
capacityovertime(Fig.3).Itispossibletoobserveaninverse
cor-relationbetweenTPCandEC50values(Table4),becausethehigher
theconcentrationofphenoliccompounds,thelowertheamountof
extractrequiredtoreducetheDPPH.
The potent antioxidant activity of yerba-mate extracts has
beendemonstratedbyseveralauthors,aswellastheircorrelation
withphenoliccompounds.Usingdifferentfreeradicalgenerators,
Schinellaet al. (2000)concluded that theaqueous extract of I. paragariensiswasabletoinhibitlipidperoxidationinenzymatic
andnonenzymaticratlivermicrosomesinadose-dependent
man-ner.Theextractexhibitedradicalscavengingpropertiesinrelation
tothesuperoxideanionandDPPHradical.UsingthesameDPPH
methodology,Bixbyetal.(2005)foundadirectpositivecorrelation
betweenantioxidantactivityandphenolic compounds,withthe
highestactivitybeingobtainedfortheaqueousextractsof
yerba-matecomparedtogreenteaandblacktea(C.sinensis(L.)Kuntze),
marcela(Achyroclinesatureioides(Lam.) DC.)and sometypesof
redandwhitewines.Grujicetal.(2012)alsofoundacorrelation
betweentheDPPHfreeradicalscavengingactivityandthetotal
phenoliccompoundsofmatetea.Likewise,Bassanietal.(2014)
showedthatthetotalcontentofphenoliccompoundswas
signifi-cantlycorrelatedwiththefreeradicalscavengingactivitytowards
DPPHradicals.
Anesinietal.(2012)demonstrated thatchlorogenicand
caf-feicacidsandtheflavonoidrutinpresentinaqueousextractsofI.
paraguariensissamplescontributedirectlytotheantioxidant
activ-itydetected,bypreventinglipidperoxidation. Oxidation oflow
density lipoprotein(LDL)inducedby freeradicals, forexample,
playsanimportantroleinatherosclerosis.Inthiscontext,
aque-ousextractsofI.paraguariensishavedemonstratedtheabilityto
inhibitLDL oxidation,thereby inhibitinglipidperoxidation, and
hencetheoxidationofDNA(Bracescoetal.,2011;Gugliucci,1996;
GugliucciandStahl,1995).Anesinietal.(2012)alsofoundthatthe
methylxanthinecaffeinehasnofreeradicalscavengingactivity,and
induceslipidperoxidationoflinoleicacid,actingasapro-oxidant
compound.
AlthoughDPPHfreeradicalscavengingcapacityofisolated
caf-feinewasnottestedinourstudy,ourdatarevealedasignificantand
directcorrelationbetweenthedetectedcaffeineandthe
antiox-idant activities (Table 4).This correlation can be explained by
TPC,whichisdirectlycorrelatedwiththecaffeinecontents.
There-fore,the detectedactivityis probably associated withphenolic
compoundsandnotwithcaffeine.Thepreventionoflipid
peroxi-dation,inwhichcaffeineisidentifiedasapro-oxidantcompound,
remainsunexplained inourwork,sincetheantioxidantactivity
hasnotbeenevaluatedbytheferricthiocyanatemethod,butby
theDPPHmethod.Thus,we cannotsaywhatistherole of
caf-feinepresentinthesamplesevaluatedinthepreventionoflipid
peroxidation.
Fromthepoint ofviewofchemical ecology,it isinteresting
to evaluatethe individuals in their entirety, in order to better
understandhowplantsindividually respondtothesefactors.In
thisapproach,asignificantcorrelationisobservedinTable4,but
withalowcoefficientoflinearcorrelation.Ontheotherhand,from
thepharmaceuticalandindustrialpointofview,itisnecessaryto
haveanapproachthattakesintoaccounttheconsistencyofthe
raw plantmaterialtobeusedin thepreparation offood
prod-ucts,intermediatepharmaceuticalformsandcosmeticsproperties.
Therefore,whenplantsaregroupedbyleafage,thiscorrelationis
showntobeaseffectiveasthepreviousapproach,butmorelinear
(Table4).
Insummary,leafageshowedasignificantinfluenceon
methylx-anthinesandtotalphenoliccontentsoftheevaluatedextracts,with
adecreaseinthesecontentsovertime.Additionally,thefree
radi-calscavengingcapacitywasalsosignificantlyaffectedbyleafage,
withadirectpositivecorrelationbetweenantioxidantactivityand
totalphenoliccontent.
Authors’contributions
C.H.B.S.(PhDstudent)helpedintherunningofthelaboratory
work,analysisofthedata,anddraftedthepaper.G.C.C.contributed
to thecollection of plants sample and their identification,and
analysisofthedata.F.H.R.andE.P.S. designedthestudy,
super-visedthelaboratorywork,andcontributedtocriticalreadingof
themanuscript.Alltheauthorshavereadthefinalmanuscriptand
approvedthesubmission.
Conflictofinterest
Theauthorsdeclarenoconflictofinterest.
Acknowledgments
We thank CNPq and FAPESC for their financial support
(FAPESC/CNPq–PRONEM2771/2012).Theauthorsarealso
grate-fultoCNPqandCAPESfortheirresearchfellowships,andtoProf.
References
Anesini,C.,Turner,S.,Cogoi,L.,Filip,R.,2012.Studyoftheparticipationofcaffeine andpolyphenolsontheoverallantioxidantactivityofmate(Ilexparaguariensis). LWT–FoodSci.Technol.45,299–304.
Ashihara,H.,1993.Purinemetabolismandthebiosynthesisofcaffeineinmaté leaves.Phytochemistry33,1427–1430.
Ashihara,H.,Monteiro,A.M.,Moritz,T.,Gillies,F.M.,Crozier,A.,1996.Catabolism ofcaffeineandrelatedpurinealkaloidsinleavesofCoffeaarabicaL.Planta198, 334–339.
Athayde,M.L.,Coelho,G.C.,Schenkel,E.P.,2000.Caffeineandtheobrominein epicu-ticularwaxofIlexparaguariensisA.St.Hil.Phytochemistry55,853–857. Athayde,M.L.,Coelho,G.C.,Schenkel,E.P.,2007.Populationaldiversityon
methylx-anthinescontentofmate(IlexparaguariensisA.St.Hil.,Aquifoliaceae).Lat.Am. J.Pharm.26,275–279.
Bassani,D.C.,Nunes,D.S.,Granato,D.,2014.Optimizationofphenolicsandflavonoids extractionconditionsandantioxidantactivityofroastedyerba-mateleaves(Ilex paraguariensisA.St.Hil.,Aquifoliaceae)usingresponsesurfacemethodology. AnaisAcad.Bras.Cienc.86,923–934.
Bixby,M.,Spieler,L.,Menini,T.,Gugliucci,A.,2005.Ilexparaguariensisextractsare potentinhibitorsofnitrosativestress:acomparativestudywithgreenteaand winesusingaproteinnitrationmodelandmammaliancellcytotoxicity.LifeSci. 77,345–358.
Blumenthal,M.,Goldberg,A.,Brinckmann,J.,2000.HerbalMedicine:Expanded ComissionandMonographs.AmericanBotanicalCouncil.
Boaventura,B.C.B.,Di Pietro,P.F.,Stefanuto,A.,Klein,G.A.,deMorais,E.C.,de Andrade,F.,Wazlawik,E.,daSilva,E.L.,2012.Associationofmatetea(Ilex paraguariensis)intakeanddietaryinterventionandeffectsonoxidativestress biomarkersofdyslipidemicsubjects.Nutrition28,657–664.
Bowers,M.D.,Stamp,N.E.,1993.Effectsofplantage,genotypeandherbivoryon Plantagoperformanceandchemistry.Ecology74,1778–1791.
Bracesco,N.,Dell,M.,Rocha,A.,Behtash,S.,Menini,T.,Gugliucci,A.,Nunes,E.,2003. AntioxidantactivityofabotanicalextractpreparationofIlexparaguariensis: pre-ventionofDNAdouble-strandbreaksinSaccharomycescerevisiaeandhuman low-densitylipoproteinoxidation.J.Alt.Complement.Med.9,379–387. Bracesco,N.,Sanchez,A.G.,Contreras,V.,Menini,T.,Gugliucci,A.,2011.Recent
advancesonIlexparaguariensisresearch:minireview.J.Ethnopharmacol.136, 378–384.
Brandwilliams,W.,Cuvelier,M.E.,Berset,C.,1995.Useofafree-radicalmethodto evaluateantioxidantactivity.FoodSci.Technol.Leb.28,25–30.
Cass,Q.B.,Degani,A.L.G.,2001.DesenvolvimentodemétodosporHPLC: fundamen-tos,estratégiasevalidac¸ão.EdUFSCar,SãoCarlos.
Clifford,M.N.,Ramirez-Martinez,J.R.,1990.Chlorogenicacidsandpurinealkaloids contentsofMaté(Ilexparaguariensis)leafandbeverage.FoodChem.35,13–21. Coelho,G.C.,Mariath,J.E.A.,1996.InflorescencesmorphologyofIlexL.
(Aquifoli-aceae)speciesfromRioGrandedoSul,Brazil.FeddesRepert.107,19–30. Coelho,G.C.,Athayde,M.L.,Schenkel,E.P.,2001.MethylxanthinesofIlex
paraguar-iensisA.St.Hil.var.vestitaLoes.andvar.paraguariensis.Braz.J.Pharm.Sci.37, 153–158.
Coelho,G.C.,Rachwal,M.F.G.,Dedecek,R.A.,Curcio,G.R.,Nietsche,K.,Schenkel,E.P., 2007.EffectoflightintensityonmethylxanthinecontentsofIlexparaguariensis A.St.Hil.Biochem.Syst.Ecol.35,75–80.
Coelho,G.C.,Gnoatto, S.B.,Bassani,V.L.,Schenkel,E.P.,2010. Quantificationof saponinsinextractivesolutionofmateleaves(IlexparaguariensisA.St.Hil.). J.Med.Food13,439–443.
Dartora,N.,deSouza,L.M.,Santana,A.P.,Iacomini,M.,Valduga,A.T.,Gorin,P.A.J., Sas-saki,G.L.,2011.UPLC-PDA-MSevaluationofbioactivecompoundsfromleaves ofIlexparaguariensiswithdifferentgrowthconditions,treatmentsandageing. FoodChem.129,1453–1461.
DeSouza,L.M.,Dartora,N.,Scoparo,C.T.,Cipriani,T.R.,Gorin,P.A.J.,Iacomini,M., Sassaki,G.L.,2011.Comprehensiveanalysisofmaté(Ilexparaguariensis) com-pounds:developmentofchemicalstrategiesformatesaponinanalysisbymass spectrometry.J.Chromatogr.A1218,7307–7315.
Dermarderosian,A.,2001.TheReviewofNaturalProducts.Facts&Comparisons,St. Louis.
Doan,A.T.,Ervin,G.,Felton,G.,2004.Temporaleffectsonjasmonateinductionof anti-herbivoredefenseinPhysalisangulata:seasonalandontogeneticgradients. Biochem.Syst.Ecol.32,117–126.
Esmelindro,A.A.,Girardi,J.D.,Mossi,A.,Jacques,R.A.,Dariva,C.,2004.Influenceof agronomicvariablesonthecompositionofmatetealeaves(Ilexparaguariensis) extractsobtainedfromCO2extractionat30◦Cand175bar.J.Agric.FoodChem. 52,1990–1995.
Filip,R.,Lopez,P.,Coussio,J.,Ferraro,G.,1998.Matesubstitutesoradulterants:study ofxanthinecontent.Phytother.Res.12,129–131.
Filip,R.,Lotito,S.B.,Ferraro,G.,Fraga,C.G.,2000.AntioxidantactivityofIlex paraguar-iensisandrelatedspecies.Nutr.Res.20,1437–1446.
Filip,R.,López,P.,Giberti,G.,Coussio,J.,Ferraro,G.,2001.Phenoliccompoundsin sevenSouthAmericanIlexspecies.Fitoterapia72,774–778.
Gobbo-Neto,L.,Lopes,N.P.,2007.Plantasmedicinais:fatoresdeinfluênciano con-teúdodemetabólitossecundários.Quím.Nova30,374–381.
Gosmann, G.,Guillaume, D., Taketa, A.T.C., Schenkel, E.P., 1995. Triterpenoid saponinsfromIlexparaguariensis.J.Nat.Prod.58,438–441.
Grujic,N.,Lepojevic,Z.,Srdjenovic,B.,Vladic,J.,Sudji,J.,2012.Effectsofdifferent extractionmethodsandconditionsonthephenoliccompositionofmatetea extracts.Molecules17,2518–2528.
Gugliucci,A.,1996.AntioxidanteffectsofIlexparaguariensis:inductionofdecreased oxidabilityofhumanLDLinvivo.Biochem.Biophys.Res.Commun.224,338–344. Gugliucci,A.,Stahl,A.J.C.,1995.Lowdensitylipoproteinoxidationisinhibitedby
extractsofIlexparaguariensis.Biochem.Mol.Biol.Int.35,47–56.
Hartmann,T.,1996.Diversityandvariabilityofplantsecondarymetabolism:a mechanisticview.Entomol.Exp.Appl.80,177–188.
Heck,C.I.,DeMejia,E.G.,2007.Yerbamatetea(Ilexparaguariensis):acomprehensive reviewonchemistry,healthimplications,andtechnologicalconsiderations.J. FoodSci.72,R138–R151.
Hendriks,H.,AndersonWildeboer,Y.,Engels,G.,Bos,R.,Woerdenbag,H.J.,1997.The contentofparthenolideanditsyieldperplantduringthegrowthofTanacetum parthenium.PlantaMed.63,356–359.
Höft,M.,Verpoorte,R.,Beck,E.,1998.LeafalkaloidcontentsofTabernaemontana pachysiphonasinfluencedbyendogenousandenvironmentalfactorsinthe naturalhabitat.PlantaMed.64,148–152.
ICH,I.C.o.H.,2005.ValidationofAnalyticalProcedures:TextandMethodology– Q2(R1).ICH,I.C.o.H.,London.
Ito,E.,Crozier,A.,Ashihara,H.,1997.Theophyllinemetabolisminhigherplants.BBA –Gen.Subj.1336,323–330.
Kato,M.,Mizuno,K.,Crozier,A.,Fujimura,T.,Ashihara,H.,2000.Plantbiotechnology: caffeinesynthasegenefromtealeaves.Nature406,956–957.
Marques,V.,Farah,A.,2009.Chlorogenicacidsandrelatedcompoundsinmedicinal plantsandinfusions.FoodChem.113,1370–1376.
Mazzafera,P.,1994.Caffeine,theobromineandtheophyllinedistributioninIlex paraguariensis.Rev.Bras.Fisiol.Veg.6,149–151.
Medina,M.B.,2011a.Determinationofthetotalphenolicsinjuicesandsuperfruits byanovelchemicalmethod.J.Funct.Foods3,79–87.
Medina,M.B.,2011b.Simpleandrapidmethodfortheanalysisofphenolic com-poundsinbeveragesandgrains.J.Agric.FoodChem.59,1565–1571. Meyer,S.,Cerovic,Z.G.,Goulas,Y.,Montpied,P.,Demotes-Mainard,S.,Bidel,L.P.R.,
Moya,I.,Dreyer,E.,2006.Relationshipsbetweenopticallyassessed polyphe-nolsandchlorophyllcontents,andleafmassperarearatioinwoodyplants:a signatureofthecarbon–nitrogenbalancewithinleaves?PlantCellEnviron.29, 1338–1348.
Miranda,D.D.C.,Arc¸ari,D.P.,Pedrazzoli,J.,Carvalho,P.d.O.,Cerutti,S.M.,Bastos, D.H.M.,Ribeiro,M.L.,2008.Protectiveeffectsofmatetea(Ilexparaguariensis)on H2O2-inducedDNAdamageandDNArepairinmice.Mutagenesis23,261–265. Ogawa,M.,Herai,Y.,Koizumi,N.,Kusano,T.,Sano,H.,2001.7-Methylxanthine methyltransferaseofcoffeeplants:geneisolationandenzymaticproperties.J. Biol.Chem.276,8213–8218.
Puangpraphant,S.,deMejia,E.G.,2009.Saponinsinyerbamatetea(Ilex paraguar-iensis A.St. Hil) and quercetinsynergistically inhibit iNOSand COX-2in lipopolysaccharide-inducedmacrophagesthroughNFBpathways.J. Agric. FoodChem.57,8873–8883.
Reginatto,F.H.,Athayde,M.L.,Gosmann,G.,Schenkel,E.P.,1999.Methylxanthines accumulationinIlexspecies–caffeineandtheobromineinerva-mate(Ilex paraguariensis)andotherIlexspecies.J.Braz.Chem.Soc.10,443–446. Rivelli,D.P.,Silva,V.V.d.,Ropke,C.D.,Miranda,D.V.,Almeida,R.L.,Sawada,T.C.H.,
Barros,S.B.d.M.,2007.Simultaneousdeterminationofchlorogenicacid,caffeic acidandcaffeineinhydroalcoholicandaqueousextractsofIlex paraguarien-sisbyHPLCandcorrelationwithantioxidantcapacityoftheextractsbyDPPH· reduction.Rev.Bras.Cienc.Farm.43,215–222.
Schinella,G.R.,Troiani,G.,Davila,V.,deBuschiazzo,P.M.,Tournier,H.A.,2000. AntioxidanteffectsofanaqueousextractofIlexparaguariensis.Biochem. Bio-phys.Res.Commun.269,357–360.
Schubert,A.,Zanin,F.F.,Pereira,D.F.,Athayde,M.L.,2006.Variac¸ãoanualde metilx-antinastotaisemamostrasdeIlexparaguariensisA.St.Hil.(erva-mate)emIjuí eSantaMaria.EstadodoRioGrandedoSul.Quím.Nova29,1233–1236. Uefuji,H.,Ogita,S.,Yamaguchi,Y.,Koizumi,N.,Sano,H.,2003.Molecularcloningand
functionalcharacterizationofthreedistinctN-methyltransferasesinvolvedin thecaffeinebiosyntheticpathwayincoffeeplants.PlantPhysiol.132,372–380. Valerga,J.,Reta,M.,Lanari,M.C.,2012.Polyphenolinputtotheantioxidantactivity