Superoxide dismutase, catalase, glutathione peroxidase and gluthatione
S-transferases M1 and T1 gene polymorphisms in three Brazilian population
groups
Cássia de Oliveira Hiragi
1, Ana Luisa Miranda-Vilela
1*, Dulce Maria Sucena Rocha
2,
Silviene Fabiana de Oliveira
1, Ana Hatagima
3and Maria de Nazaré Klautau-Guimarães
1 1Departamento de Genética e Morfologia, Instituto de Ciências Biológicas, Universidade de Brasília,
Brasília, DF, Brazil.
2
Campus de Planaltina, Universidade de Brasília. Brasília, DF, Brazil.
3
Departamento de Genética, Instituto Oswaldo Cruz, Pavilhão Leônidas Deane, Rio de Janeiro, RJ, Brazil.
Abstract
Antioxidants such as superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX1) reduce the oxidation rates in the organism. Gluthatione S-transferases (GSTs) play a vital role in phase 2 of biotransformation of many substances. Variation in the expression of these enzymes suggests individual differences for the degree of an-tioxidant protection and geographical differences in the distribution of these variants. We described the distribution frequency of CAT (21A/T), SOD2 (Ala9Val), GPX1 (Pro198Leu), GSTM1 and GSTT1 polymorphisms in three Brazil-ian population groups: Kayabi AmerindBrazil-ians (n = 60), Kalunga Afro-descendants (n = 72), and an urban mixed popu-lation from Federal District (n = 162). Frequencies of the variants observed in Kalunga (18% to 58%) and Federal District (33% to 63%) were similar to those observed in Euro and Afro-descendants, while in Kayabi (3% to 68%), de-pending on the marker, frequencies were similar to the ones found in different ethnic groups. Except for SOD2 in all population groups studied here, and for GPX1 in Kalunga, the genotypic distributions were in accordance with Hardy-Weinberg Equilibrium. These data can clarify the contribution of different ethnicities in the formation of mixed populations, such as that of Brazil. Moreover, outcomes will be valuable resources for future functional studies and for genetic studies in specific populations. If these studies are designed to comprehensively explore the role of these genetic polymorphisms in the etiology of human diseases they may help to prevent inconsistent genotype-phenotype associations in pharmacogenetic studies.
Key words:antioxidants, PCR-RFLP, gene polymorphisms, Brazilian ethnicities, population genetics, pharmacogenetics.
Received: May 20, 2010; Accepted: August 24, 2010.
Introduction
There has been much interest and research on single nucleotide substitutions (SNPs) in order to understand the maintenance of such polymorphisms in human popula-tions. These data are useful for studying human evolution and the mechanisms that maintain genetic variability in hu-man populations, as well as for identifying genes associated with complex diseases (Nachman and Crowell, 2000). Many potentially significant genetic variants related to oxi-dative stress have already been identified (Morgenstern, 2004). Several SNPs have been reported to result in chan-ges in the levels or the activities of antioxidant enzymes, which can lead to reduction in protection against oxidative
stress (Forsberget al., 2001). To gain a better understand-ing of the biological significance of these polymorphisms, studies are required to map their distribution in several eth-nicities, since they vary among ethnic groups.
The known superoxide scavenger in mitochondria, manganese superoxide dismutase (MnSOD or SOD2, EC 1.15.1.1), is encoded by a nuclear gene located on chromo-some 6q25 (Rosenblumet al., 1996). It is synthesised with a mitochondrial targeting sequence (MTS), which drives its mitochondrial import. In the mitochondrial matrix, the MTS is cleaved, and the mature protein assembles into the active tetramer (Akyolet al., 2005). The cytosine to thy-mine substitution at nucleotide 47 provokes a valine to alanine (Val9Ala, ref SNP ID: rs179972) substitution in the SOD2 MTS. This, in turn, induces a conformational change which has been reported to change mitochondrial process-ing efficiency, to affect the transport of SOD2 to the mito-chondria, and to decrease SOD2 efficiency against
oxida-www.sbg.org.br
Send correspondence to Ana Luisa Miranda-Vilela. Departamento de Genética e Morfologia, Instituto de Ciências Biológicas, Uni-versidade de Brasília, 70910-900 Brasília, DF, Brazil. E-mail: mirandavilela@unb.br.
tive stress (Shimoda-Matsubayashiet al., 1996; Akyolet al., 2005). The Ala allele varies among ethnic groups (Zhao
et al., 2005) and has been associated with increased risk of different diseases related to oxidative stress and abnormal free radical defence mechanisms (Shimoda-Matsubayashi
et al., 1996; Mitrunenet al., 2001; Yenet al., 2003; Olson
et al., 2004; Akyolet al., 2005; Choiet al., 2008). Glutathione peroxidase 1 (GPX1, EC 1.11.1.9), ex-pressed mainly in erythrocytes (Brigelius-Flohé, 1999), de-toxifies hydrogen peroxide and organic hydroperoxides using glutathione in its reduced form (GSH) as co-substrate (Zhaoet al., 2005; Ravn-Haren et al., 2006). The GPX1 gene (locus 3p21.3) contains the Pro198Leu SNP (ref SNP ID: rs1050450) whose Leu allele has been implicated in GPX1 activity, which becomes less responsive to stimula-tion (Zhaoet al., 2005). Studies have also associated this variant with increased risk of some kinds of cancer (Ratna-singheet al., 2000; Hu and Diamond, 2003; Zhaoet al., 2005). However, such associations were not consistently observed in all populations studied, since Leu allele fre-quency varies according to ethnic group (Zhaoet al., 2005). Catalase (CAT, EC 1.11.1.6) is an enzyme whose ma-jor role involves controlling H2O2concentrations in human
cells, converting H2O2into H2O and O2(Ahnet al., 2006).
The CAT gene (locus 11p13) presents an apparently neutral polymorphism, CAT -21A/T (ref SNP ID: rs7943316), lo-cated within the promoter region, close to the translational initiation site (Ukkolaet al., 2001; Góth and Vitai, 1997; Góthet al., 2004). For this polymorphism, no effects have been reported on catalase expression, catalase activity, or association with disease/pathological changes (Góthet al., 2004). Considering that T allele frequency varies among ethnic groups (Ukkolaet al., 2001; Younget al., 2006), studies mapping the distribution of this allele’s frequency in several ethnicities can be important to gain a better un-derstanding of its biological significance.
The glutathione S-transferases M1 (GSTM1) and T1 (GSTT1) genes code for the cytosolic enzymes GST-m
(mu) and GST-q(theta), respectively. These enzymes cata-lyze reactions involving the conjugation between reduced glutathione (GSH) and a variety of eletrophilic compounds (Cottonet al., 2000; Choet al., 2005), most of these being xenobiotics or products of oxidative stress (Cottonet al., 2000). The glutathione S-transferase M1 (GSTM1, locus 1p13.3) and T1 (GSTT1, locus 22q11.2) genes may be de-leted (null alleles/null genotypes) and these polymorphisms lead to altered GST activity, contributing to inter-indivi-dual differences (Hayes and Strange, 2000). Indiviinter-indivi-duals with homozygous deletions do not have detectable GSTT1 or GSTM1 enzyme activity (Landi, 2000), and associations between GSTM1 and/or GSTT1 null genotypes with car-diovascular diseases (Kimet al., 2007) and cancer (Garteet al., 2001; Chaet al., 2007; Hatagimaet al., 2008) have been reported. Inter-ethnic differences in the allele frequencies of GST null genotypes have been documented worldwide
and some gradients and intra-ethnic differences have al-ready been reported (Cottonet al., 2000; Landi, 2000; Cho
et al., 2005).
In Brazil there are few studies that describe these anti-oxidant polymorphisms. The Brazilian population as a whole is very mixed and heterogeneous, primarily as a re-sult of five centuries of inter-ethnic crosses among Europe-ans, Africans and Amerindians (Alves-Silvaet al., 2000). Samples from four major regions of Brazil (North, North-east, Southeast and South), verified by genomic compari-son in a panel of population-specific alleles, showed that the African contribution ranged from 4 to 34%, and the Amerindian from 0 to 27% (Parraet al., 2002). It is be-lieved that this miscegenation can influence the distribution of certain polymorphisms.
To perform case-control studies analysing the associ-ation of certain polymorphisms with the risk of developing certain diseases, it is important to know the frequency dis-tribution of these genes in human populations. Thus, this work aims to describe the distribution of frequencies of CAT (21A/T), SOD2 (Ala9Val), GPX1 (Pro198Leu), GSTM1 and GSTT1 gene polymorphisms in three Brazil-ian population groups.
Material and Methods
Samples
IBGE census). Most of the Federal District population ini-tially consisted of migrants from other regions of Brazil (Queiroz, 2006), and currently almost half of the District’s inhabitants are migrants. The sample used here (n = 172) was collected in 2002 and consisted of 71 males and 101 fe-males with a median age of 21.1 years. Based on the sub-jects’ self-declared skin colour, 68.5% were ethnically mixed, 24.7% were white, 1.7% were black, and 5.1% did not declare their colour.
This study was conducted according to the guidelines laid down in the Declaration of Helsinki and all procedures involving human subjects were approved by the Ethics Committee for Health Sciences Faculty Research of the University of Brasília and by the National Commission for Ethics in Research (CONEP). Written informed consent was obtained from all subjects and oral informed consent was obtained in Kayabi village.
Laboratory and statistical procedures
Genomic DNA was isolated from peripheral blood samples collected in Vacutainer tubes containing EDTA using the purification kit GFX (GE Healthcare, Buckin-ghamshire, England). The samples were stored below -20 °C until analysis. The presence or absence of GSTM1 and GSTT1 genes was detected using PCR amplification according to the methods of Fryeret al.(1993) and
Kemp-keset al.(1996). Genotyping of the polymorphisms CAT
21 A/T, SOD2 Val-9Ala (T/C) and GPX1 Pro198Leu (C/T) was done according to Ukkolaet al.(2001), Mitrunemet al.
(2001) and Zhaoet al. (2005), respectively. PCR and re-striction endonuclease products were separated by electro-phoresis in 10% (GPX1) and 6% (Cat and SOD2) non-denaturing polyacrylamide gels and visualised by staining with silver nitrate.
Allele and genotype frequencies were estimated by gene counting. The goodness of fit of the genotype distribu-tion to the Hardy-Weinberg equilibrium was assessed by exact tests using Genepop 3.4 (Raymond and Rousset, 1995). Values of p > 0.05 indicated Hardy-Weinberg equi-librium. Chi-square tests were used to compare the frequen-cies of the mutant alleles and genotypes with published data. Data for genetic diversity was assessed by comparing the observed and expected heterozygosities, and FISand FST
(F-statistics) were calculated by Arlequin 3.1.1 (Excoffier and Schneider, 2005).
Results
Table 1 shows the distribution of the antioxidant en-zymes, GSTM1 and GSTT1 variant allele frequencies in the three Brazilian population groups studied in compari-son with frequencies obtained in other populations. The re-sults revealed differences in the three populations, primarily for allele frequency ofGPX1*T(Kayabi = 3%, Kalunga = 18% and Federal District = 33%).
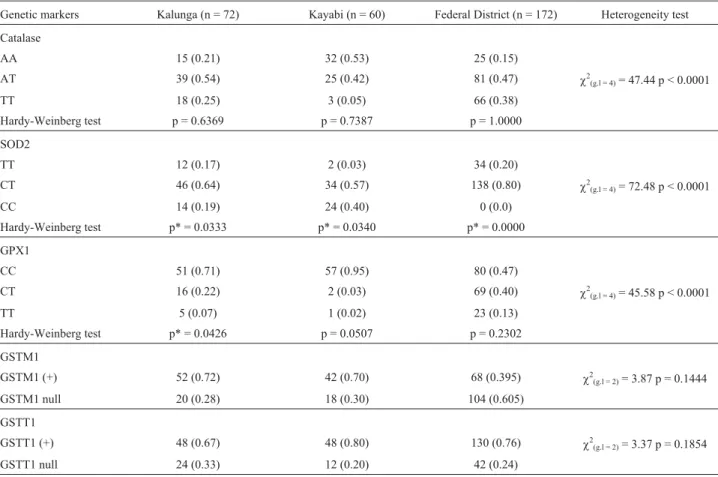
Genotypic frequencies and results of Hardy-Wein-berg Equilibrium analysis, as well as Heterogeneity tests for SOD2, CAT, GPX1 and GST polymorphisms are sum-marised in Table 2. For the SOD2 locus, the results indi-cated a significant deviation from Hardy-Weinberg equilibrium (HWE) in all population groups studied; this was also denoted for the GPX1 locus in Kalunga. For the SOD2 locus, this was due to a heterozygote excess: Fis= -0.27 (Kalunga), Fis= -0.29 (Kayabi) and Fis= -0.67
(Federal District). For GPX1, the results were compatible with a homozygote excess in Kalunga (FIS = 0.26). The
other markers analysed, as well as GPX1 in Kayabi and Federal District, were in accordance with HWE. The distri-butions of CAT, SOD and GPX1 genotypes are signifi-cantly different among the ethnic groups studied (p < 0.0001). Based on the F-statistics, these Brazilian pop-ulation groups showed a moderate degree of genetic differ-entiation (FST= 0.10).
Discussion
Populations of the world vary considerably in their predisposition to diseases and in allele frequencies at phar-macogenetically important loci, probably as a result of ge-netic drift, and also because of adaptation to local selective factors such as climate and available nutrients (Suarez-Kurtz, 2004). Our study describes the frequency distribu-tion of polymorphisms in three different Brazilian groups for SOD2, CAT, GPX1, GSTM1 and GSTT1, these being loci of pharmacological relevance.
TheCATTallele frequency in Kalunga was homoge-neous and similar to that observed in Europeans (Ukkolaet al., 2001), whereasSOD2Callele frequency was homoge-neous to those observed in U.S. and Canadian populations (Ambrosoneet al., 1999; Knightet al., 2004). These values corroborate previous findings suggesting admixture of the Kalunga population with Europeans or Euro-descendants. A strong male contribution from Europeans in forming the Kalunga population was indicated by Y chromosome stud-ies (Ribeiroet al., 2009).
The allele frequency of GPX1Twas lower in Kalunga than in the Federal District. In fact, the frequency observed in Kalunga was neither close to nor homogeneous with any frequency of other studied populations. Even though the GPX1Tfrequency determined in Kalunga was low, it was twice that observed for Asians, an ethnic group that has been reported to have the lowest frequencies for the Pro198Leu GPX1 polymorphism (Bastaki et al., 2006). GSTM1*0frequency in Kalunga was homogenous and in agreement with African-Brazilians from São Paulo (Gattás
al-ready been observed to be of African origin, as seen in a study of classical markers and polymorphisms of Alu inser-tion (Pedrosa and Oliveira, unpublished data).
In the Kayabi group, the frequencies of GSTM1*0
(55%) andGSTT1*0(45%) were homogeneous and similar to Wai Wai Amerindians (52%) and Aché Amerindians (42%), respectively (Gasparet al., 2002). TheSOD2C fre-quency was higher and non-homogeneous compared to that observed in Euro-descendants (41% to 56%) (Knightet al., 2004; Akyolet al., 2005; Bicaet al., 2007). For the other polymorphisms (GPX1 and CAT), the values were similar to those described for Asians, being homogeneous for
CATTallele frequency (Younget al., 2006) and non-homo-geneous for allele frequency of GPX1T (Bastaki et al., 2006). It has been reported that the Kayabi live in an area
which has received intense migration due to gold prospect-ing, and they have consequently become somewhat mixed (Klautau-Guimarãeset al., 2005a). Because this is the first description of SOD2 Val9Ala, GPX1 Pro198Leu and CAT 21A/T polymorphisms in an Amerindian Brazilian group, this recent miscegenation should be taken into account, given that it can influence the distribution of certain poly-morphisms, contributing to deviations from Hardy-Wein-berg Equilibrium.
The Federal District urban population group pre-sentedCATTand GPX1Tallele frequencies that were simi-lar to those described for Europeans (Ukkolaet al., 2001; Hu and Diamond, 2003). For the SOD2 polymorphism, our values were close to other District Federal samples, but it was non-homogeneous with them (Akimotoet al., 2010;
- Distribution of antioxidant enzymes, GSTM1 and GSTT1 variant allele frequencies in the Kalunga, Kayabi and Federal District populations in comparison with the frequencies obtained in other populations.
Genetics markers
Kalunga Kayabi Federal District
Frequencies n References Frequencies n References Frequencies n References
CATT 0.52 72 * 0.26 60 * 0.38 172 *
0.58 244 1 (c22= 2.28; p = 0.3198)
0.31 100 9 (c22= 2.67; p = 0.2631)
0.58 245 1 (c22= 1.02; p = 0.6004)
0.51 72 * 0.68 60 * 0.40 172 *
0.50 110 2 (c2
2= 1.09; p = 0.5798)
0.49 372 3 (c2
2= 7.03; p = 0.0293)
0.41 135 13 (c2
2= 9.12; p = 0.0104)
SOD2C 0.49 372
3 (c22= 3.36; p = 0.1863)
0.56 196 10 (c22= 12.5; p = 0.0019)
0.47 135 14 (c22= 21.53; p = 0.0000)
0.41 370 11 (c22= 8.09; p = 0.0175)
GPX1T 0.18 72 * 0.03 60 0.33 172 *
0.08 122 4 (c2
2= 109.12; p = 0.0000)
0.08 122 4 (c2
2= 148.7; p = 0.0000)
0.33 517 15 (c2
2= 0.12; p = 0.9436)
0.53 72 * 0.55 60 * 0.63 172 *
0.57 137 5 (c21= 0.57; p = 0.4502)
0.52 26 12 (c21= 0.08; p = 0.7732)
0.60 521 16 (c21= 0.81; p = 0.3681)
GSTM1*0 0.58 100 6 (c21= 0.75;
p = 0.3864)
0.61 190 17 (c21= 0.26; p = 0.6101)
0.47 69 7 (c2
1= 0.69; p = 0.4061)
0.58 72 6 (c2
1= 0.56; p = 0.4542)
0.45 60 * 0.49 172 *
0.60 134 8 (c2
1= 0.11; p = 0.7401)
0.42 67 12 (c2
1= 0.09; p = 1.000)
0.49 190 17 (c2
1= 0.002; p = 0.9643)
GSTT1*0 0.49 135
14 (c21= 0; p = 1.000)
0.47 233 5 (c21= 0.24; p = 0.6242)
0.37 135 13 (c21= 5.4; p = 0.0201)
Miranda-Vilelaet al., 2010). Regarding the frequency of the null allele of GSTM1 (63%), this was homogeneous and similar to that observed in Euro-descendants (Santovitoet al., 2008) and African-Brazilians from Curitiba (Macielet al., 2009).
The observedGSTT1*0frequency in the Federal Dis-trict group was similar and homogeneous to that for African Brazilians from Curitiba (Maciel et al., 2009), the other sample from the Federal District (Miranda-Vilela et al., 2010) and European-Brazilians from São Paulo (Gattáset al., 2004). These results denote the participation of African descendants in the formation of the Federal District popula-tion and corroborate the estimate based on autosomal STR analyses indicating a genetic contribution of more than 39% by sub-Saharan Africans (Godinho et al., 2008). Moreover, the distribution of these GST polymorphisms in this sample reflects the history of the creation of the new Capital in the 1960s. It was formed by people from all re-gions of Brazil (Queiroz, 2006) and this very diverse origin suggests that it may be the most representative sam-ple-group of the Brazilian population as a whole.
The considerable range of variation in human popula-tions may reflect, in part, distinct processes of natural selec-tion and adaptaselec-tion to variable environmental condiselec-tions (Barreiroet al., 2008). Deviations from Hardy Weinberg
Equilibrium may be explained by natural selection or recent ethnic admixture. Population growth and positive se-lection increase the proportion of rare alleles (i.e., alleles with low frequency), whereas balancing selection and pop-ulation substructure increases the proportion of intermedi-ate frequency alleles (Serre and Hudson, 2006). Natural selection can act at the level of genes, if particular geno-types allow for increased fitness in specific environments (Barreiroet al., 2008).
Although the cytosine to thymine substitution in the SOD2 gene has been reported to decrease SOD2 efficiency against oxidative stress (Shimoda-Matsubayashi et al., 1996; Akyolet al., 2005), a study conducted with Federal District athletes showed that SOD2 heterozygotes pre-sented less tissue and DNA damages, as well as lower lipid peroxidation indices (Miranda-Vilelaet al., 2009), indicat-ing that SOD2 heterozygosis can favor defense against oxi-dative stress. Furthermore, our results are in accordance with other studies obtained by our research group with other population groups from the Federal District (Akimoto
et al., 2010; Miranda-Vilelaet al., 2009, 2010).
Genes under positive selection are thought to have an important role in human survival and to affect complex phenotypes of medical relevance. Indeed, as reported for negative selection, nonsynonymous SNPs showing signs of
Table 2- Hardy-Weinberg Equilibrium and Heterogeneity tests for Catalase, SOD2, GPX1, GSTM1 and GSTT1 polymorphism in Brazilian groups.
Genetic markers Kalunga (n = 72) Kayabi (n = 60) Federal District (n = 172) Heterogeneity test
Catalase
AA 15 (0.21) 32 (0.53) 25 (0.15)
AT 39 (0.54) 25 (0.42) 81 (0.47) c2(g.l = 4)= 47.44 p < 0.0001
TT 18 (0.25) 3 (0.05) 66 (0.38)
Hardy-Weinberg test p = 0.6369 p = 0.7387 p = 1.0000
SOD2
TT 12 (0.17) 2 (0.03) 34 (0.20)
CT 46 (0.64) 34 (0.57) 138 (0.80) c2(g.l = 4)= 72.48 p < 0.0001
CC 14 (0.19) 24 (0.40) 0 (0.0)
Hardy-Weinberg test p* = 0.0333 p* = 0.0340 p* = 0.0000
GPX1
CC 51 (0.71) 57 (0.95) 80 (0.47)
CT 16 (0.22) 2 (0.03) 69 (0.40) c2
(g.l = 4)= 45.58 p < 0.0001
TT 5 (0.07) 1 (0.02) 23 (0.13)
Hardy-Weinberg test p* = 0.0426 p = 0.0507 p = 0.2302
GSTM1
GSTM1 (+) 52 (0.72) 42 (0.70) 68 (0.395) c2
(g.l = 2)= 3.87 p = 0.1444
GSTM1 null 20 (0.28) 18 (0.30) 104 (0.605)
GSTT1
GSTT1 (+) 48 (0.67) 48 (0.80) 130 (0.76) c2(g.l = 2)= 3.37 p = 0.1854
GSTT1 null 24 (0.33) 12 (0.20) 42 (0.24)
positive selection are observed more frequently than ex-pected in genes involved in disease (Barreiroet al., 2008). Many indigenous people in Latin America still live in iso-lated environments where conditions are harsh. Contact with workers in mining and exploration projects affects in-digenous people’s health (Montenegro and Stephens, 2006). Tuberculosis constitutes a major health problem among the indigenous people of the upper Rio Negro in Brazil (Buchillet and Gazin, 1998) and a pattern of moder-ate endemism with a prevalence of previous HBV (Hepati-tis B virus) infection of 55.7% and 49.5% was observed for two indigenous groups of Pará, Brazil (Nuneset al., 2007).
Similarly, the Kalunga population lives in very poor conditions in remote settlements in the mountains on both sides of the Paraná River. The majority of the individuals live at low socioeconomic and education levels, with poor hygiene and crowded conditions. Also, the majority of them live basically on subsistence agriculture or cattle-raising, and their houses have no sewage system or tap wa-ter service. Rates of 80%, 30% and 0.5% were found for HAV (Hepatitis A virus), HBV (Hepatitis B virus) and HCV (Hepatitis C virus) infections, respectively (Matoset al., 2009). In the presented contexts, it is likely that in the Kayabi and Kalunga population groups, heterozygotes have a selective advantage in the global aspect of diseases, thus increasing their frequency in these populations. Neverthelesss, selection in another area of the SOD2 gene or in another unknown gene located in the close vicinity of the SOD2 gene should also be taken into account.
Deviation from Hardy-Weinberg Equilibrium was also detected for the GPX1 polymorphism in Kalunga, which showed excess of CC homozygotes. This was also observed in a study on Asians/Pacific Islanders (Bastakiet al., 2006). As the Leu allele (GPX1T) has been implicated in effects on GPX1 activity, which becomes less responsive to stimulation (Zhaoet al., 2005), these results are expected, mainly because this population lives in precarious condi-tions.
To conclude, we think that the SNPs described in this report will be valuable resources for future functional stud-ies and for specific population genetic studstud-ies designed to comprehensively explore the role of these genetic poly-morphisms in the etiology of human diseases. It is neces-sary to characterize genetic variation among different pop-ulation groups when assessing disease risk. The differences in allelic frequencies observed among samples emphasize the importance of being careful in planning epidemiologi-cal studies.
Acknowledgments
This work was supported by the Universidade de Brasília, the Conselho Nacional de Desenvolvimento Cien-tífico e Tecnológico (CNPq) and by the Fundação de Em-preendimentos Científicos e Tecnológicos (FINATEC).
References
Ahn J, Ambrosone CB, Kanetsky PA, Tian C, Lehman TA, Kropp S, Helmbold I, Fournier DV, Haase W,et al.(2006) Poly-morphisms in genes related to oxidative Stress (CAT, MnSOD, MPO, and eNOS) and acute toxicities from radia-tion therapy following lumpectomy for breast cancer. Clin Cancer Res 12:7063-7070.
Akimoto AK, Miranda-Vilela AL, Alves PCZ, Pereira LCS, Lor-delo GS, Hiragi CO, Silva ICR, Grisolia CK and Klautau-Guimarães MN (2010) Evaluation of gene polymorphisms in exercise-induced oxidative stress and damage. Free Rad Res 44:322-331.
Akyol O, Yanik M, Elyas H, Namli M, Canatan H, Akin H, Yuce H, Yilmaz HR, Tutkun H, Sogut S,et al.(2005) Association between Ala-9-Val polymorphism of Mn-SOD gene and schizophrenia. Prog Neuropsychopharmacol Biol Psychia-try 29:123-131.
Alves-Silva J, Santos MS, Guimarães PEM, Ferreira ACS, Ban-delt HJ, Pena SDJ and Prado VF (2000) The ancestry of Bra-zilian mtDNA lineages. Am J Hum Genet 67:444-461. Ambrosone CB, Freudenheim JL, Thompson PA, Bowman E,
Vena JE, Marshall JR, Graham S, Laughlin R, Nemoto T and Shields PG (1999) Manganese superoxide dismutase (MnSOD) genetic polymorphisms, dietary antioxidants, and risk of breast cancer. Cancer Res 59:602-626.
Barreiro LB, Laval G, Quach H, Patin E and Quintana-Murci L (2008) Natural selection has driven population differentia-tion in modern humans. Nat Genet 40:340-345.
Bastaki M, Huen K, Manzanillo P, Chande N, Chen C, Balmes JR, Tager IB and Holland N (2006) Genotype-activity relation-ship for Mn-superoxide dismutase, glutathione peroxidase 1 and catalase in humans. Pharmacogenet Genom 16:279-286. Bica CG, Cruz IBM, Silva LLM, Toscani NV, Zettler CG and Graudenz MS (2007) Association of manganese superoxide dismutase gene polymorphism (Ala-9Val) and breast cancer in males and females. J Bras Patol Med Lab 43:219-225. Brigelius-Flohé R (1999) Tissue-specific functions of individual
glutathione peroxidases. Free Radic Biol Med 27:951-965. Buchillet D and Gazin P (1998) A situação da tuberculose na
população indígena do alto rio Negro (Estado do Amazonas, Brasil). Cad Saúde Públ 14:181-185 (Abstract in English). Cha IH, Park JY, Chung WY, Choi MA, Kim HJ and Park KK
(2007) Polymorphisms of CYP1A1 and GSTM genes and susceptibility to oral cancer. Yonsei Med J 48:233-239. Cho H-J, Lee S-Y, Ki C-S and Kim J-W (2005) GSTM1, GSTT1
and GSTP1 polymorphisms in the Korean population. J Ko-rean Med Sci 20:1089-1092.
Choi JY, Neuhouser ML, Barnett MJ, Hong CC, Kristal AR, Thornquist MD, King IB, Goodman GE and Ambrosone CB (2008) Iron intake, oxidative stress-related genes (MnSOD and MPO) and prostate cancer risk in CARET cohort. Car-cinogenesis 29:964-970.
Cotton SC, Sharp L, Little J and Brackton N (2000) Glutathione S-transferase polymorphisms and colorectal cancer: A HuGE review. Am J Epidemiol 151:7-32.
Excoffier LGL and Schneider S (2005) Arlequin v. 3.0: An inte-grated software package for population genetics data analy-sis. Evol Bioinform Online 1:47-50.
Fryer AA, Zhao L, Alldersea J, Pearson WR and Strange RC (1993) Use of site-directed mutagenesis of allele-specific PCR primers to identify the GSTM1A, GSTM1B, GSTM1AB, and GSTM1 null polymorphisms at the gluta-thione S-transferase, GSTM1 locus. Biochem J 295:313-315.
Fujihara J, Yasuda T, Iida R, Takatsuka H, Fujii H and Takeshita H (2009) Cytochrome P450 1A1, glutathione S-transferases M1 and T1 polymorphisms in Ovambos and Mongolians. Leg Med 11:S408-S410.
Garte S, Gaspari L, Alexandrie AK, Ambrosone C, Autrup H, Autrup JL, Baranova H, Bathum L, Benhamou S, BoVetta P,
et al.(2001) Metabolic gene polymorphism frequencies in control populations. Cancer Epidemiol Biomarkers Prev 10:1239-1248.
Gaspar PA, Hutz MH, Salzano FM, Hill K, Hurtado M, Petzl-Erler ML, Tsuneto T and Weimer TA (2002) Polymor-phisms of CYP1a1, CYP2e1, GSTM1, GSTT1, and TP53 genes in Amerindians. Am J Phys Anthropol 19:249-256. Gattás GJ, Kato M, Soares-Vieira JA, Siraque MS, Kohler P,
Gomes L, Rego MAV and Bydlowski SP (2004) Ethnicity and glutathione S-transferase (GSTM1/GSTT1) polymor-phisms in a Brazilian population. Braz J Med Biol Res 37:451-458.
Godinho NMO, Gontijo CC, Diniz MECG, Falcão-Alencar G, Dalton GC, Amorim CEG, Barcelos RSS, Klautau-Gui-marães MN and Oliveira SF (2008) Regional patterns of ge-netic admixture in South America. FSI Genet 1:329-330. Góth L, Rass P and Páy A (2004) Catalase enzyme mutations and
their association with diseases. Mol Diagn 8:141-149. Góth L and Vitai M (1997) Polymorphism of 5’ of the catalase
gene in Hungarian acatalasemia and hypocatalasemia. Elec-trophoresis 18:1105-1108.
Hatagima A, Costa E, Marques C, Koifman R, Boffetta P and Koifman S (2008) Glutathione S-transferase polymor-phisms and oral cancer: A case-control study in Rio de Ja-neiro, Brazil. Oral Oncol 44:200-207.
Hayes DJ and Strange RC (2000) Glutathione S-transferase poly-morphisms and their biological consequences. Pharmacol-ogy 61:154-166.
Hu YJ and Diamond AM (2003) Role of glutathione peroxidase 1 in breast cancer: Loss of heterozygosity and allelic differ-ences in the response to selenium. Cancer Res 63:3347-3351.
Kempkes M, Golka K, Reich S, Reckwitz T and Bolt HM (1996) Glutathione S-transferase GSTM1 and GSTT1 null geno-types as potential risk factors for urothelial cancer of the bladder. Arch Toxicol 71:123-126.
Kim S-J, Kim M-G, Kim K-S, Song J-S, Yim S-V and Chung J-H (2007) Impact of glutathione S-transferase M1 and T1 gene polymorphisms on the smoking-related coronary artery dis-ease. J Korean Med Sci 23:365-372.
Klautau-Guimarães MN, D’Ascenção R, Caldart FA, Grisolia CK, Souza JR, Barbosa AC, Cordeiro CMT and Ferrari I (2005a) Analysis of genetic susceptibility to mercury con-tamination evaluated through molecular biomarkers in at-risk Amazon Amerindian populations. Genet Mol Biol 28:827-832.
Klautau-Guimarães MN, Hiragi CO, D’Ascenção RF, Oliveira SF, Grisolia CK, Hatagima AH and Ferrari I (2005b) Distri-bution of glutathione S-transferase GSTM1 and GSTT1 null
phenotypes in Brazilian Amerindians. Genet Mol Biol 28:32-35.
Knight JA, Onay UV, Wells S, Li H, Shi EJQ, Andrulis IL and Ozcelik H (2004) Genetic variants of GPX1 and SOD2 and breast cancer risk at the Ontario site of the Breast Cancer Family Registry. Cancer Epidemiol Biomarkers Prev 13:146-149.
Kvitko K, Gaspar PA, Torres MR and Hutz MH (2006) CYP1A1, GSTM1, GSTT1 and GSTP1 polymorphism in a Afro-bra-zilian population. Genet Mol Biol 29:613-616.
Landi S (2000) Mammalian class theta GST and diVerential sus-ceptibility to carcinogenesis: A review. Mutat Res 463:247-283.
Maciel ME, Oliveira FK, Propst GB, Bicalho MG, Cavalli IJ and Ribeiro EMSF (2009) Population analysis of xenobiotic me-tabolizing genes in South Brazilian Euro and Afro-des-cendants. Genet Mol Biol 32:723-728.
Matos MAD, Reis NRS, Kozlowski AG, Teles AS, Motta-Castro ARC, Mello FCA, Gomes AS and Martins RMB (2009) Epi-demiological study of hepatitis A, B and C in the largest Afro-Brazilian isolated community. Trans R Soc Trop Med Hyg 103:899-905.
Miranda-Vilela AL, Akimoto AK, Alves PCZ, Pereira LCS, Gon-çalves CA, Klautau-Guimarães MN and Grisolia CK (2009) Dietary carotenoid-rich pequi oil reduces plasma lipid pero-xidation and DNA damage in runners and evidence for an association with MnSOD genetic variant - Val9Ala. Genet Mol Res 8:1481-1495.
Miranda-Vilela AL, Alves PCZ, Akimoto AK, Lordelo GS, Gon-çalves CA, Grisolia CK and Klautau-Guimarães MN (2010) Gene polymorphisms against DNA damage induced by hy-drogen peroxide in leukocytes of healthy humans through comet assay: A quasi-experimental study. Environ Health 9:21.
Mitrunen K, Sillanpaa P, Kataja V, Eskelinen M, Kosma VM, Benhamou S, Uusitupa M and Hirvonen A (2001) Associa-tion between manganese superoxide dismutase (MnSOD) gene polymorphism and breast cancer risk. Carcinogenesis 22:827-829.
Montenegro RA and Stephens C (2006) Indigenous health in Latin America and the Caribbean. Lancet 367:1859-1869. Morgenstern R (2004) Oxidative stress and human genetic
varia-tion. J Nutr 134:3173S-3174S.
Nachman MW and Crowell SL (2000) Estimate of the mutation rate per nucleotide in humans. Genetics 156:297-304. Nunes HM, Monteiro MRCC and Soares MCP (2007) Prevalência
dos marcadores sorológicos dos vírus das hepatites B e D na área indígena Apyterewa, do grupo Parakanã, Pará, Brasil. Cad Saúde Pública 23:2756-2766.
Oliveira SF, Pedrosa MAF, Souza SMB, Mingronineto R, Sandes KA, Ferrari I, Barbosa AAL, Auricchio MTBM and Klau-tau-Guimarães MN (2002) Heterogeneous distribution on HbS and HbC alleles in Afro-derived Brazilian populations. Int J Hum Genet 2:153-160.
Olson SH, Carlson MDA, Ostrer H, Harlap S, Stone A, Winters M and Ambrosone CB (2004) Genetic variants in SOD2, MPO, and NQO1, and risk of ovarian cancer. Gynecol Oncol 93:615-620.
Ratnasinghe D, Tangrea JA, Andersen MR, Barrett MJ, Virtamo J, Taylor PR and Albanes D (2000) Glutathione peroxidase codon 198 polymorphism variant increases lung cancer risk. Cancer Res 60:6381-6383.
Ravn-Haren G, Olsen A, Tjonneland A, Dragsted LO, Nexo BA, Wallin H, Overvad K, Raaschou-Nielsen O and Vogel U (2006) Associations between GPX1 Pro198Leu polymor-phism, erythrocyte GPX activity, alcohol consumption and breast cancer risk in a prospective cohort study. Carci-nogenesis 27:820-825.
Raymond M and Rousset F (1995) Genepop v. 3.3: A population genetics software for exact tests and ecumenicism. J Hered 86:248-249.
Rebbeck TR (1997) Molecular epidemiology of the human gluta-thione S-transferase genotypes GSTM1 and GSTT1 in can-cer susceptibility. Cancan-cer Epidemiol Biomarkers Prevent 6:733-743.
Ribeiro GGBL, De Lima RR, Wiezel CEV, Ferreira LB, Sousa SMB, Rocha DMS, Canas MCT, Nardelli-Costa J, Klautau-Guimarães MN, Simões AL,et al.(2009) Afro-derived Bra-zilian populations: Male genetic constitution estimated by Y-chromosomes STRs and YAP element polymorphisms. Am J Hum Biol 21:354-356.
Rodrigues AD (1994) Línguas Brasileiras: Para o Conhecimento das Línguas Indígenas. Loyola, São Paulo, 136 pp. Rodrigues P, Barbosa AC, Ferrari I and Souza JR (2002)
Avalia-ção da contaminaAvalia-ção por mercúrio na terra indígena dos Mundurukus do Pará. In: Gramkow MM (ed) Demarcando Terras Indígenas II Experiências e Desafios de um Projeto de Parceria. FUNAI/PPTAL/GTZ, Brasília, pp 123-148. Rosenblum JS, Gilula NB and Lerner RA (1996) On signal
se-quence polymorphisms and diseases of distribution. Proc Natl Acad Sci USA 93:4471-4473.
Santovito A, Cervella P, Burgarello C, Bigatti MP, Sella G and DelPero M (2008) Analysis of glutathione S-transferase M1 and glutathione S-transferase T1 gene polymorphisms sug-gests age-related relationships in a northern Italian popula-tion. Arch Toxicol 82:903-907.
Serre D and Hudson TJ (2006) Resources for genetic variation studies. Annu Rev Genom Hum Genet 7:443-457.
Shimoda-Matsubayashi S, Matsumine H, Kobayashi T, Naka-gawa-Hattori Y, Shimizu Y and Mizuno Y (1996) Structural
dimorphism in the mitochondrial targeting sequence in the human manganese superoxide dismutase gene. A predictive evidence for conformational change to influence mitochon-drial transport and a study of allelic association in Parkin-son’s disease. Biochem Biophys Res Commun 226:561-565.
Suarez-Kurtz G (2004) Pharmacogenomics in admixed popula-tions: The Brazilian pharmacogenetics/pharmacogenomics network-REFARGEN. Pharmacogenomics J 4:347-348. Ukkola O, Erkkilä PH, Savolainen MJ and Kesäniemi YA (2001)
Lack of association between polymorphisms of catalase, copper/zinc superoxide dismutase (SOD), extracellular SOD and endothelial nitric oxide synthase genes and macro-angiopathy in patients with type 2 diabetes mellitus. J Intern Med 249:451-459.
Yen J-H, Chen C-J, Tsai W-C, Lin C-H, Ou T-T, Hu C-J and Liu H-W (2003) Manganese superoxide dismutase and cyto-chrome P450 1A1 gene polymorphisms in rheumatoid ar-thritis in Taiwan. Hum Immunol 64:366-373.
Young RP, Hopkins R, Black PN, Eddy C, Wu L, Gamble D, Mills GD, Garrett JE, Eaton TE and Rees MI (2006) Func-tional variants of antioxidant genes in smokers with COPD and in those with normal lung function. Thorax 61:394-399. Zhao H, Liang D, Grossman HB and Wu X (2005) Glutathione
peroxidase 1 gene polymorphism and risk of recurrence in patients with superficial bladder cancer. Urology 66:769-774.
Internet Resources
Queiroz EP (2006) A migração intrametropolitana no Distrito Federal e Entorno: O consequente fluxo pendular e o uso dos equipamentos urbanos de saúde e educação. http://www.abep.nepo.unicamp.br/encontro2006/docspdf/ ABEP2006_724.pdf (October, 2010).
Information about current population of the Federal District: http://www.distritofederal.df.gov.br.
Associate Editor: Francisco Mauro Salzano