The equine caecum-colon environment: influence of energy and
nitrogen on microbial yield
Ana Sofia Gonçalves Santos
Supervisors
Prof. Miguel António Machado Rodrigues Departamento de Zootecnia Universidade de Trás-os-Montes e Alto Douro Prof. Rui José Branquinho de Bessa Departamento de Produção Animal Faculdade de Medicina Veterinária Universidade Técnica Lisboa
Thesis presented to Universidade de Trás-os-Montes and Alto Douro to obtain the PhD Degree in
Animal Science
Universidade de Trás-os-Montes e Alto Douro
Vila Real, 2011
Aos detentores do meu coração e da minha alegria:
Meus filhos João e Maria
Meu irmão Nuno Miguel
Minha avó Amélia
"Eu pretendo tocar no céu e não dizer a ninguém que consegui!!"
by: U-XeD - Mike, JP, Ventura e Pigmeu
The research described in this thesis was financially supported by the Portuguese
Foundation for Science and Technology (Doctoral grant SFRH/BD/31294/2006)
vii
Although the characterization of the microbial populations in the equine hindgut has been often studied, very little is known on hindgut ecosystem activity in the horse and information concerning the metabolism of this microbial population and its nutritional requirements is lacking. If we consider the type of substrate that in normal conditions arrives to the hindgut, we can expect it to provide limited nitrogen based substrates for microbial fermentation. In this way, this work was conducted to provide additional information regarding the nitrogen requirements of the equine caecal microbial population. In addition, caecal and colon contents of horses where characterized in terms of purine bases (PB) and odd- and branched chain fatty acid (OBCFA) profile.
This work was divided into three parts: the first part meant to gather existing information on the equine digestive tract and nutritional strategies, with a specific focus on the hindgut environment and its functioning, microbial population, energy and protein metabolism. The second part consisted in the characterization of equine hindgut contents in PB and OBCFA, and assessing their potential use as microbial markers in equine metabolism studies in order to use these techniques in future studies. Finally, in the third part we studied in vitro fermentation responses of equine caecal contents to different nitrogen and energy availability.
Information compiled in Chapter 2 provides a general overview of the equine digestive tract, with an emphasis on the hindgut role and importance to the nutrition of the horse, speculating on the possible adaptation of the hindgut microbial population to an environment where nitrogen is limiting microbial growth.
Characterization of caecum and colon contents obtained in Chapter 3 indicated that the bacterial populations isolated from these contents were different in the PB and OBCFA profile when compared to rumen PB and OBCFA profile. Differences obtained may reflect different growth stages or nutrition of microbial population as well as different metabolic activities of these microbial populations. Results obtained also indicated that PB can be use as microbial markers in subsequent studies, namely in Chapters 4 and 5, and that OBCFA profile might be used in non invasive techniques such as faecal characterization.
In Chapter 4, different microbial yields and fermentation profiles were observed when caecal contents were provided with energy and increasing amounts of either casein or urea as nitrogen sources. Results obtained showed higher microbial growth efficiencies at lower nitrogen levels, possibly indicating that the microbial population is adapted to an environment where nitrogen availability is low.
viii
Results indicate that maximization of the fermentative activity appears to be achieved when urea, together with soluble carbohydrates, are provided to equine caecal inoculums. Results observed when energy was available in excess and no nitrogen source was provided where unexpected, since there was no clear indication that this treatment lead to an energy spilling situation. This observation needs further studies to be clarified.
The data obtained in this work suggest that hindgut microbial population will respond to nitrogen (casein and urea) with an increase in VFA production. However, microbial growth efficiency will be higher with urea. This indicates that, under our study conditions, the caecal microbial population mainly used non protein nitrogen for growth.
ix
A população microbiana do ceco-colon do cavalo tem sido bastante estudada em termos de tipos de populações e estirpes bacterianas. No entanto, pouco se sabe sobre a actividade fermentativa no ecossistema ceco-cólico do cavalo, nomeadamente sobre o seu metabolismo e necessidades nutricionais. A localização pós-gástrica do local de actividade fermentativa implica que a disponibilidade de substrato seja condicionada pela digestibilidade pré-cecal da dieta. Assim sendo, será de esperar que o substrato que chega ao ceco-colon seja deficiente em azoto, o que poderá limitar o crescimento microbiano. Desta forma, este trabalho foi desenvolvido com o objectivo de aprofundar o conhecimento relacionado com o ecossistema ceco-cólico, nomeadamente estudar as necessidades azotadas desta população microbiana. Adicionalmente, a caracterização em perfil de purinas (PB) e ácidos gordos ímpares e ramificados (OBCFA) foi efectuada em biomassa microbiana recolhida de conteúdos do ceco e do cólon de cavalos.
Este trabalho foi dividido em três partes: a primeira teve como objectivo reunir informação sobre o sistema digestivo do cavalo e estratégias nutricionais, com particular ênfase no ceco-colon, nomeadamente sua população microbiana, parâmetros fermentativos e metabolismo da fermentação; a segunda parte deste trabalho isolou e caracterizou bactérias do ceco e do cólon de cavalos em termos de PB e OBCFA, analisando a utilização destas substâncias como marcadores microbianos em estudos subsequentes; a terceira parte deste trabalho teve como objectivos estudar as respostas fermentativas in vitro de conteúdos cecais mediante a alteração da disponibilidade de energia e de azoto (proteico ou não proteico).
A informação recolhida no Capítulo 2 fornece uma perspectiva geral do sistema digestivo do cavalo, com especial ênfase ao ecossistema ceco-cólico, especulando sobre a possibilidade da população microbiana estar adaptada a um ambiente onde a falta de azoto limita o crescimento microbiano, sem prejudicar a fermentação.
A caracterização dos conteúdos do ceco e cólon obtida no Capitulo 3 indicou diferenças acentuadas entre a população bacteriana nestes conteúdos e a população bacteriana do rúmen. Estes resultados foram de encontro às ideias levantadas no Capitulo 2, indicando populações microbianas adaptadas ao ambiente ceco-cólico. Os resultados obtidos permitiram também a utilização das PB como marcadores microbianos nos trabalhos seguintes.
x
diferentes. Os resultados obtidos revelaram uma eficiência de crescimento da população microbiana superior nos níveis inferiores de azoto, indicando uma possível adaptação da população microbiana a níveis baixo de azoto.
A resposta microbiana em situações onde a energia ou azoto (caseína ou ureia) eram limitantes no meio de cultura, ou em situações onde nem a energia nem o azoto (caseína ou ureia) limitavam o crescimento e actividade microbiana, foi estudada no Capitulo 5. Os resultados obtidos indicaram que a maximização da actividade fermentativa parece ser obtida quando ureia, juntamente com hidratos de carbono solúveis, são fornecidos à população microbiana. Os resultados obtidos em situações em que a energia (na forma de hidratos de carbono solúveis) estava disponível em excesso e sem fonte azotada revelaram-se surpreendentes, uma vez que a resposta microbiana não foi no revelaram-sentido claro de iniciar mecanismos de ―energy spilling‖ como seria de esperar. Estes resultados necessitam de ser aprofundados.
Os resultados obtidos neste trabalho permitem-nos afirmar que, embora a população microbiana do ceco do cavalo responda à adição de azoto (caseína e ureia) com um aumento da actividade fermentativa, esta resposta é superior em termos de eficiência de crescimento quando o azoto é não proteico (ureia). Estes resultados indicam que, nas nossas condições de estudo, a população microbiana do ceco utiliza maioritariamente azoto não proteico para o seu crescimento e actividade.
xi
Papers published in peer-reviewed journals
Santos, A.S., M.A.M. Rodrigues, R.J.B. Bessa, L.M. Ferreira and W.
Martin-Rosset. 2011. Understanding the equine cecum-colon environment:
current knowledge and future perspectives. Animal, 5:1, 48-56
Santos, A.S., Ferreira, L.M.M., Martin-Rosset, W., Cotovio, M., Silva, F.,
Bennett, R.N., Cone, J.W., Bessa, R.J.B. and Rodrigues, M.A.M.. 2011.The
influence of casein and urea as nitrogen sources on in vitro equine caecal
fermentation. Animal (In press).
Papers submitted to peer-reviewed journals
Santos, A.S., E. Jerónimo, Ferreira, L.M., Bennet, R.N., M.A.M. Rodrigues
and R.J.B. Bessa. 2011. Technical note: Fatty acids and purine profile of
equine cecum and colon contents and their use as microbial markers in
equine microbial metabolism studies. (Submitted)
Santos, A.S., Ferreira, L.M.M, Martin-Rosset, W, Cone, J.W., Bessa, R.J.B
and Rodrigues, M.A.M. 2011. Effect of nitrogen and carbohydrate sources
on in vitro fermentation profiles and microbial yield using equine caecal
content. (Submitted).
Publications in peer-reviewed books
Santos, A. S., Ferreira, L. M. M., Cotovio, M., Guedes, C.V.M., Cone,
J.W., Bessa, R.J.B.., Rodrigues, M.A.M., 2010. In vitro equine caecal
fermentation of different casein levels. The impact of nutrition on the
health and welfare of horses. A.D. Ellis, A.C. Longland, M. Coenen and N.
xii
Publications in conference proceedings
Santos, A.S., Ferreira, L.M.M., Guedes, C.M., Cotovio, M., Silva, F.,
Bessa, R.J.B., Rodrigues, M.A.M., (2010). In vitro fermentation parameters
of equine cecal contents in a nitrogen deficient environment. In Book of
abstracts of the 61st Annual Meeting of the European Association of
Animal Production, 23 a 27 de Agosto, Heraklion, Greece, pp. 298.
Santos, A.S., E. Jerónimo, L.M. Ferreira, M.A.M. Rodrigues and R.J.B.
Bessa. 2008. Fatty acid composition of liquid and solid associated bacteria
in the cecum and colon of horses. In Book of abstracts n.º 14 of the 59th
Annual Meeting of the European Association of Animal Production,
Vilnius, Littuania. Pp. 221.
Santos, A.S., Jerónimo, E., Ferreira, L.M., Rodrigues, M.A.M. Bessa,
R.J.B., 2007. Chemical composition of liquid and solid associated bacteria
in the cecum and colon of horses. Book of Abstracts of the 58th Annual
reunion of the EAAP, Dublin, August, 2007.
xiii
Having reached the final part of this stage I would like to express my deepest recognition and gratitude to all those who helped to make this thesis a reality.
To the Portuguese Foundation for Science and Technology (FCT) for its financial support.
To the University of Trás-os-Montes and Alto Douro for giving me permission to perform this research work using the animals, facilities, and nutrition laboratory of the Animal Science Department. To the Research Center of Animal and Veterinary Science (CECAV-UTAD) for all the support given throughout this research and the National Zootechnic Station for all the help in handling and analyzing samples.
To my supervisors, Professor Miguel Rodrigues and Professor Rui Bessa, for accepting me as their PhD student. It was a privilege. You are both exceptional and great minds. Thank you for your support, patience, constant encouragement, for trying to keep my feet on solid ground throughout the entire thesis and for your friendship.
To Professor William Martin-Rosset, you have been an inspiration. Thank you for all you great support throughout these years, for believing in me, and for giving me the chance, it is a privilege to work with you.
To Professor Luis Ferreira for his constant support, systematic and thorough review of documents, for always being in a good mood and for trying to keep me also in a good mood. You are a close friend.
To Mário Cotovio and Filipe Silva for the support with the surgeries and for their friendship. A close thank to Ana Jacinta and Patricia and all the rest of the interns at the Veterinary Hospital of UTAD.
To Eliana Jerónimo for her precious help in the chemical and fatty acid analysis of contents. To Nita, my lifeguard on the laboratory, thank you for keeping all data in a good port, and for you companionship throughout all this time.
To Eng.º Paulo Fontes and all the Animal Production Unit staff, specially to D. Maria, Arsénio, Paulo and Eurico for all the help with ―my‖ horses.
To my dearest friends Elisabete, Belita and Rui, for keeping me distracted when needed, for all the companionship, support and patience when listening to my complaints, for all the precious help, for always being there. To Maria João Fradinho, close friend and ―companion in fight‖, for listening, for all the strength and constant encouragement. To Teresa Mateus, my soul mate: thank you for keeping my soul and mind where they belong: around.
xiv
gratitude and how honored I am for having you in my life! Thank you! To my brothers Pedro and Miguel: we three are unstoppable and eternal! NO MATTER WHAT!!
I would like to express my deepest love to my kids, João and Maria, and to Filipe. For all the times I was not there, for all the times I was late, for all the times I did not had enough patience. Without you I could not have done it. You are one of the reasons I get up in the morning!
xv
To Miguel.
My dearest kid brother touched by God.
Never-ending source of happiness, bliss and light. You always were and
continue to be my pride and joy. Always in my heart and mind, every second of every
hour of every day.
Forever with me until the day we meet again...
Thank you for making my life special!!!
This is my ―music‖ to you…
xvii
Abstract ... vii
Resumo ... ix
Publications ... xi
Acknowledgments ... xiii
List of Figures ... xxi
List of Tables ... xxi
Abbreviations and nomenclature ... xxiii
Chapter one General State of the art ... 1
1.1. Introduction ... 3
1.2. Hindgut microbial environment ... 3
1.3. Microbial protein synthesis ... 4
1.3.1. Techniques used to estimate microbial protein synthesis ... 6
1.3.2. Total purines as microbial marker ... 7
1.3.3. Odd and branched chain fatty acids as microbial markers ... 10
1.4. In vitro gas production and microbial mass measurement ... 11
1.5. Aims of the thesis ... 13
1.6. References ... 14
Chapter Two Understanding the cecum-colon ecosystem: current knowledge and future perspectives ... 21 2.1. Implications ... 23 2.2. Abstract ... 23 2.3. Introduction ... 24 2.4. Pre-cecal digestion... 25 2.4.1. Stomach ... 26 2.4.2. Small intestine ... 26 2.5. Hindgut environment ... 27
xviii
2.5.3. Microbiological environment ... 29
2.5.4. Fermentation parameters ... 33
2.5.5. Nitrogen metabolism in the hindgut ... 34
2.6. Final considerations ... 36
2.7. References ... 39
Chapter Three Fatty acids and purine profile of liquid and solid associated bacteria as indicators of the equine microbial metabolism ... 47
3.1. Abstract... 49
3.2. Introduction ... 49
3.3. Materials and methods ... 50
3.4. Results and Discussion ... 51
3.5. References ... 55
Chapter Four The influence of casein and urea as nitrogen sources on in vitro equine caecal fermentation ... 57
4.1. Implications ... 59
4.2. Abstracts ... 59
4.3. Introduction ... 60
4.4. Materials and methods ... 61
4.4.1. Gas production incubations ... 61
4.4.2. Chemical analysis ... 62 4.4.3. Calculations ... 63 4.4.4. Statistical analysis ... 64 4.5. Results... 64 4.5.1. Fermentation parameters ... 64 4.5.2. Gas production ... 67
xix
4.8. References ... 73
Chapter Five Effect of nitrogen and carbohydrate sources on in vitro fermentation profiles and microbial yield using equine caecal contents ... 77
5.1. Abstract ... 79
5.2. Introduction ... 79
5.3. Materials and methods ... 80
5.3.1. Gas production incubations ... 80
5.3.2. Chemical analysis ... 81 5.3.3. Calculations ... 82 5.3.4. Statistical analysis ... 83 5.4. Results ... 83 5.5. Discussion ... 86 5.6. Conclusions ... 90 5.7. References ... 91 Chapter Six General discussion, conclusions and perspectives... 95
6.1. General discussion ... 97
6.2. Conclusions and perspectives ... 100
xxi
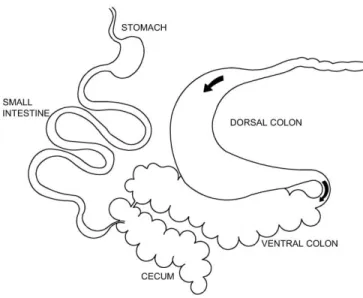
Figure 1.1 Localization of main selective retention sites in the hindgut of horses. ...28
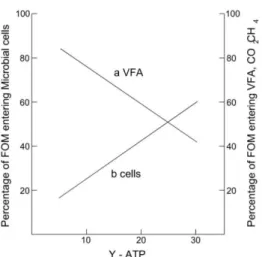
Figure 1.2 Relationship between microbial growth efficiency (Y-ATP) and the percentage of fermentable organic matter that is partitioned into VFA‘s and gases (methane and carbon dioxide) and that entering into microbial cells (adapted from Preston and Leng, 1987) ....37
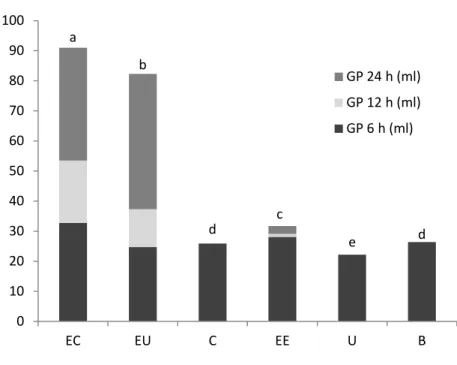
Figure 5.1 Cumulative gas production for the different substrates studied: carbohydrates plus casein (EC), carbohydrates plus urea (EU) casein (C), carbohydrates (EE), urea (U) and no substrate (B). Different letters represent significant differences between substrates. ...85
Figure 6.1 YATP variation with increasing N levels after 24h incubation with caecal fluid estimated by the acrylate - YATP(A) and the succinate – YATP(S) pathways. ...99
List of Tables
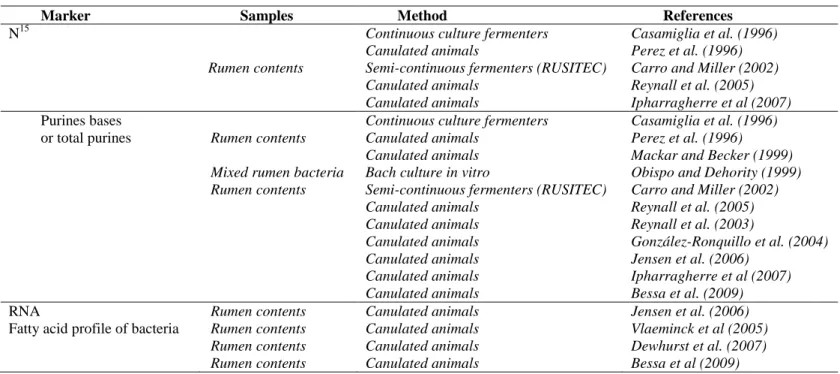
Table 1.1 Microbial markers commonly used to estimate microbial protein yield. ...8
Table 2.1 Variation of the molar composition in the large intestine with the crude fiber content of feeds (Vermorel and Martin-Rosset, 1997). ...34
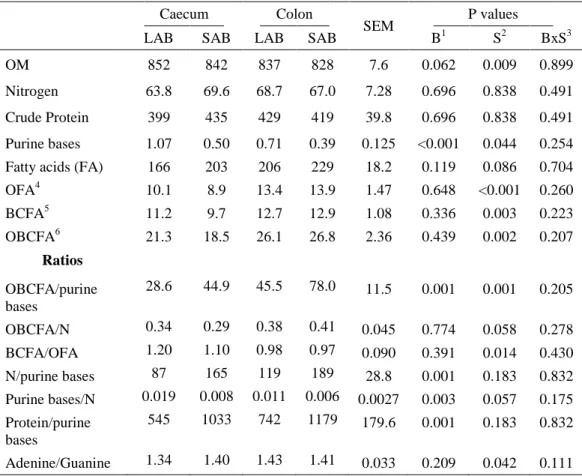
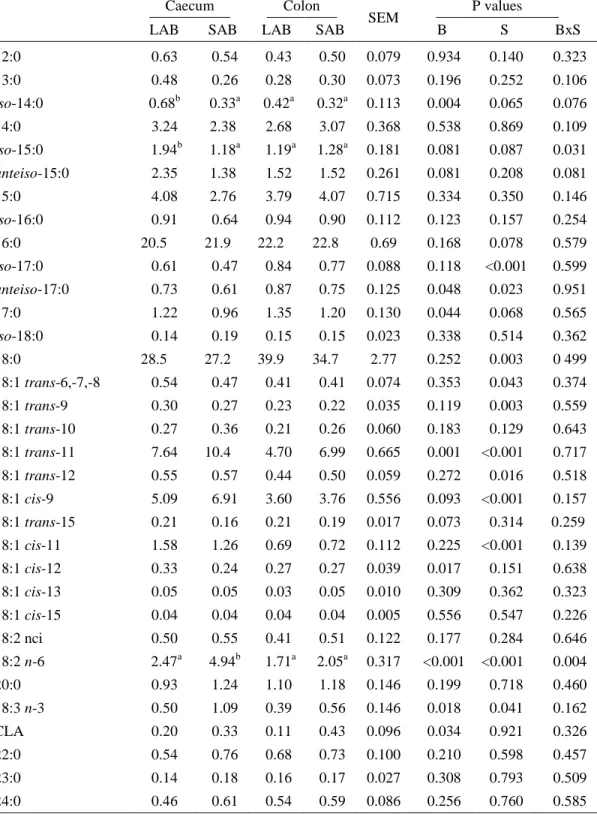
Table 3.1 Organic matter (mg/g DM) and purine bases, nitrogen, crude protein and fatty acid concentration (mg/g organic matter, OM) of fractions (LAB vs SAB) collected from caecal and colon contents. ...52
Table 3.2 Fatty acid profile (g/100 g total fatty acids) of bacterial fractions (LAB vs SAB) collected from caecal and colon contents. ...54
Table 4.1 Fermentation parameters measured after a 24h fermentation period. ...65
xxii
Table 5.1 Fermentation parameters measured after a 24h fermentation period. ... 84
Table 5.2 Microbial biomass (mg), estimated ATP yield (mmol), YATP and efficiency of microbial growth related to gas production (Egas) after a 24hour fermentation period. .... 87
xxiii
AA – Amino acidsADP – Adenosine diphosphate ATP – Adenosine triphosphate BCFA – Branched chain fatty acids c.f.u. – Cell free units
C2 – Acetate C3 – Propionate C4 – Butyrate CH4 – Methane
CLA – Conjugated linoleic acids CO2 – Carbon dioxide
CP – Crude protein
DNA – Deoxyribonucleic acid DOM – Digestible organic matter dOM – Organic matter digestibility
Egas – Microbial growth efficiency related to gas production FA – Fatty acids
FAME – Fatty acids methyl esthers GIT – Gastro intestinal tract LAB – Liquid associated bacteria MATP – Maintenance ATP MP – Microbial protein MRT – Mean retention time N – Nitrogen
NAN – Non-ammonia nitrogen NH3-N – Ammonia nitrogen
OBCFA – Odd and branched chain fatty acids OFA – Odd fatty acids
OM – Organic matter PB – Purine basis
PROC MIXED – LSD – Least square means PUFA – Polyunsaturated fatty acids
xxiv
TCL – total caecal and colon contents TP – Total purinesTT – total transit
VFA – Volatile fatty acids
YATP – Microbial growth efficiency NRC – Nacional research council
INRA – Institut National de recherché agronomique RVC – Right ventral cólon
LVC – Left ventral cólon RDC – Right dorsal colon LDC – Left dorsal colon
Chapter one
3
1.1. IntroductionThe dominant characteristic of the digestive tract of herbivores is the existence of enlarged locations that provide conditions to the development of a dense microbial population. This population is essential for an efficient digestion process, especially fiber degradation, allowing the survival on forage based diets. In equines, the major sites for fermentation are the caecum and colon, were the microbial population maintains, under normal feeding conditions, a balance with its host, keeping the integrity of the ecosystem (Julliand, 1998).
Regardless of its importance for the nutritional status of the host, the significance of hindgut fermentation has not yet been fully understood, and few reports have focused deeply on the contribution of the microbial population of the hindgut to the nitrogen and energy requirements of the host animal. Although characterization of the microbial populations in the equine hindgut has been studied by several authors (Fomtebelle et al., 2003; Julliand 1998), limited information is known on how these microbial populations interact and their nutrient requirements (Santos et al., 2011).
1.2. Hindgut microbial environment
In ruminant animals, the efficiency of the microbial population is well studied, since maximization of microbial protein synthesis is a nutritional objective (Preston and Leng, 1987). One of the major factors that can affect microbial growth in the rumen is the availability of precursors (glucose, AA, nucleic acids, peptides, ammonia and minerals) hence the coupling of energy and protein availability should be achieved to optimize the synthesis of bacterial protein to promote maximum volatile fatty acids (VFA) production (Russel and Cook, 1995, Russell, 2007). In equines this may not be the case. Considering post-gastric placement of the hindgut, it is expected that cytoplasmic protein and soluble sugars will not reach the hindgut, or do so in low amounts. Nevertheless, the high majority of cell wall carbohydrates and linked nitrogen will reach the hindgut, and therefore we can expect it to be a nitrogen limiting environment (Martin-Rosset and Tisserand, 2004). If this is to be the case, the hindgut microbial population would be adapted to an environment quite different from that of the rumen.
4
The extent to which hindgut bacteria utilize protein and non-protein nitrogen (N) is not well understood. Caecal bacteria show proteolytic activity, and although caecal isolates have been shown to use ammonia and urea as nitrogen sources for microbial growth, many caecal bacteria can use other nitrogen sources other than ammonia or urea for growth (Maczulack et al., 1985). Nitrogen balance studies have shown that urea is utilized in the equine gastrointestinal tract (Slade et al., 1970, Houpt and Houpt, 1971, Prior et al., 1974). Prior et al (1974) reported that in ponies 60-65 percent of urea was released in the intestine, and 50 percent of it would be recycled. Urease activity in caecal fluid has also been previously reported (Hintz et al., 1972). However, according to these authors it was only 17 to 25% of that reported in bovine rumen fluid. Nevertheless, latter studies (Cheng and Wallace, 1979) have demonstrated that a large amount of the urease activity can be associated with bacteria adherent to the rumen epithelium, this can also be the case in equines and therefore urease activity values can be higher.
An upgrade of the existent concepts and the intensification of studies focusing on microbial protein synthesis and its efficiency in the hindgut, as well as in the ammonia production and absorption should be undertaken. These processes will depend on residual crude protein (CP) content and composition of dietary CP after enzymatic digestion in the small intestine, degradability of residual CP content of ileal digesta, amount and composition of fermentable organic matter available and on energy availability.
1.3. Microbial protein synthesis
Factors that can affect the microbial ecosystem are as numerous and complex as the microorganisms that inhabit it (Hespell and Bryant, 1979). The rumen ecosystem is probably one of the most studied since microbial processes of the rumen confer the ability to convert fibrous feeds and low-quality protein, even non-protein-nitrogen, into valuable nutrients for the ruminant animal. A large amount of the amino acids absorbed by ruminants derive from microbial protein. Several authors have looked on the description of theoretical and mathematical issues concerning microbial growth (Tempest and Neijssel, 1984; Russell and Wallace, 1997), and many studies have
5
accessed microbial growth using pure or mixed cultures of rumen microbes (Russell and Baldwin, 1979; Hespell and Bryant, 1979; Cotta and Russell, 1982; Bates et al., 1985).
The yield of microbial biomass is related to the amount of substrate available (concentrations in the rumen fluid of precursors eg. glucose, nucleic acids, amino acids, peptides, ammonia and minerals) and to the maintenance requirements of the microbes (Preston and Leng, 1987, Dewhurst et al., 2000). In this way, YATP is defined as weight (g) of microbial cells that is produced per mole of available ATP. The available ATP is estimated from the knowledge of the reactions in the fermentative pathways. MATP is defined as the ATP that is directed from growth to other processes (motility, cellular turnover, active transport) (Preston and Leng, 1987; Dewhurst,et al., 2000). Maintenance energy requirements are dependent on the bacterial growth rate with the slower growth rates requiring proportionally more maintenance energy than faster growth rates (Russell and Cook, 1995, Dewhurst et al., 2000). When bacteria are depleted of exogenous substrates, cells often use endogenous materials as an energy source. In this way, ―maintenance‖ and ―endogenous metabolism‖ should not be considered as synonymous (Russell and Cook, 1995). Maintenance should be used to define growth when most of the cells in the population are capable of growing. Endogenous metabolism should be defined as a state when no net growth is possible (Russel and Cook, 1995).
These theoretical concepts obtained in laboratory cultures are often not relevant in the natural ecosystem (rumen or hindgut). In laboratory cultures, feed sources are supplied to exactly match microbial requirements and environmental conditions are kept close to the optimum for growth (if that is the objective of study). For example, under laboratory conditions, it is possible to alter microbial growth rates by manipulating the supply of nitrogen. However, when these manipulations are used in vivo, their effects are likely to be influenced by several other factors, namely those relating to interactions between rumen microorganisms (Dewhurst et al., 2000). In this way, information concerning microbial protein synthesis in the rumen is confusing and often contradictory, both as a result of the several and complex factors involved as well as the difficulties of measuring it.
In relation to ruminal activity, very little is known on the activity of the hindgut ecosystem in the horse, and comparison between these digestive compartments (rumen and hindgut), despite their anatomical and placement differences, can be discussed (Santos et al., 2011).
6
1.3.1. Techniques used to estimate microbial protein synthesis
Microbial protein (MP) yield and/or microbial growth efficiency estimation is very complex. Concerning the rumen there are two distinct problems, estimating how much material leaves the rumen and assessing what proportion of that material is microbial protein. Any detailed analysis of the process is prevented by the technical problems of obtaining meaningful measurements of bacterial and protozoal synthesis, and by the complexity of a system in which the microbial population, culture conditions and supply of energy and other nutrients can fluctuate widely with time. Therefore, the determination of microbial protein in the presence of partially degraded dietary protein is a major technical issue. These problems are also present when estimating MP in vitro. Nevertheless, in vitro systems have the advantage that representative sampling can be assured and, if using a continuous culture system, the outflow of both particulate and liquid phases can be accurately controlled (Stern et al., 1978; Merry et al., 1987). A criticism of both batch and continuous culture models is that compared to the rumen where absorption occurs, substrate levels must be reduced and/or outflow rates raised to maintain physiological conditions (Dewhurst et al., 2000). In this way, in vitro systems can be used to test hypotheses and examine mechanisms, but in vivo studies are essential to confirm results.
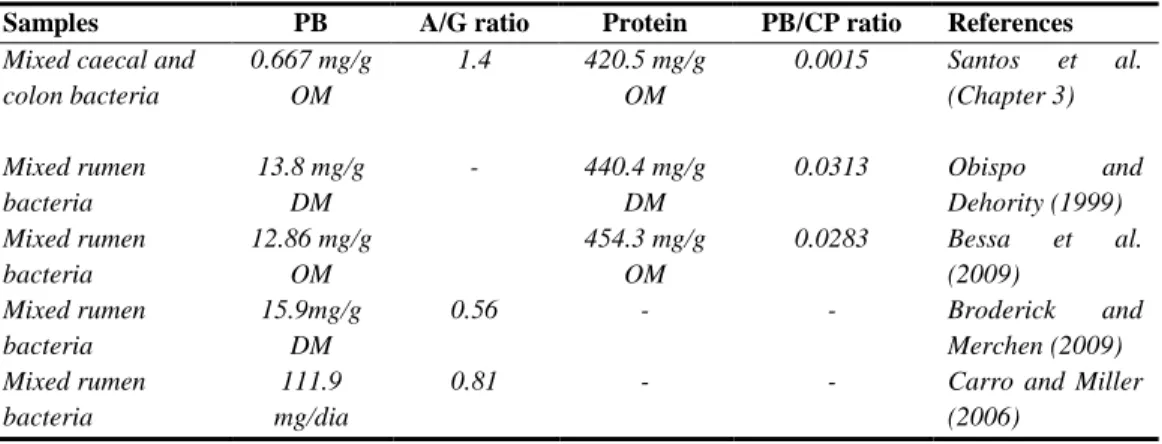
Several approaches have been made to identify MP in rumen contents (both in vivo and in vitro). Microbial markers have been most popular. According to Obispo and Dehority (1999): ―The ideal microbial marker should 1) not be present in the feed; 2) not be absorbed; 3) be biologically stable; 4) have a relatively simple assay procedure; 5) occur in a similar percentage between the various types of microbes (i.e., bacteria, protozoa, and fungi); 6) be a constant percentage of the microbial cell in all stages of growth; and 7) all forms should flow at a similar rate (i.e., free and bound).‖
Microbial markers can be classified as internal markers (present in the microorganisms e.g. purines or fatty acids) or external markers (added to the medium to label microorganisms, e.g., 15N).
Internal microbial markers have been used to predict the contribution of microbial crude protein (CP) to duodenal non-ammonia N (NAN) flows (Broderick and Merchen, 1992) and to study the distribution of microbes within the rumen ecosystem (Legay-Carmier and Bauchart, 1989). Several substances have been proposed as internal markers, including diaminopimelic acid, D-alanine, 2-aminoethylphosphonic acid,
7
chitin, adenylate energy charge, purines and ribonucleic acid. Table 1 summarizes the most commonly used microbial markers for quantifying microbial protein in ruminants.
1.3.2. Total purines as microbial marker
The high concentrations of DNA and especially RNA in unicellular organisms led to the recognition of their potential use as markers for ruminal microbial protein (bacterial and protozoa). Microbial protein yields have been estimated using RNA, total nucleic acids or individual basis.
Concerns when using nucleic acids as microbial markers are related to the possible presence of nucleic acids in dietary ingredients and, more important, to different nucleic acid:protein ratios in bacterial and/or protozoa and also among the fluid and particle phases within the rumen (Broderick and Merchen (1992). McAllan and Smith (1970) studied degradation of exogenous purines to conclude that abomasal and intestinal passage of dietary nucleic acids, was insignificant. After reviewing studies on this matter, Broderick and Merchen (1992) refer that most of the common feedstuffs are lower in purines compared to ruminal microbes (Broderick and Merchen, 1992). Nevertheless, Perez et al. (1996) working with sheep referred that dietary contribution of purine basis should be considered in order to increase prediction efficiency of purines as microbial marker.
Studies carried out with nucleic acids and their component bases reveals its variation in marker content in bacteria, and a wide range of values have been reported for purine N:total N in several in vivo and in vitro experiments (from 0.08 to 0.12; Dewhurst, 2000). Ruminant species and time of sampling have also been reported to influence these ratios. Smith and McAllan (1974) observed that RNA:protein ratios in pure cultures of rumen bacteria increased with the rise in their specific growth rates, and that mixed bacteria from sheep fed a high concentrate diet showed a higher RNA:protein ratio in free floating bacteria also referred to as liquid associated bacteria (LAB). Latter studies have confirmed differences related to time of sampling and animal species (Bates et al., 1985; Craig et al., 1987).
8
Table 1.1 Microbial markers commonly used to estimate microbial protein yield
Marker Samples Method References
N15
Rumen contents
Continuous culture fermenters Canulated animals
Semi-continuous fermenters (RUSITEC) Canulated animals
Canulated animals
Casamiglia et al. (1996) Perez et al. (1996) Carro and Miller (2002) Reynall et al. (2005) Ipharragherre et al (2007) Purines bases
or total purines Rumen contents
Mixed rumen bacteria Rumen contents
Continuous culture fermenters Canulated animals
Canulated animals Bach culture in vitro
Semi-continuous fermenters (RUSITEC) Canulated animals Canulated animals Canulated animals Canulated animals Canulated animals Canulated animals Casamiglia et al. (1996) Perez et al. (1996)
Mackar and Becker (1999) Obispo and Dehority (1999) Carro and Miller (2002) Reynall et al. (2005) Reynall et al. (2003) González-Ronquillo et al. (2004) Jensen et al. (2006) Ipharragherre et al (2007) Bessa et al. (2009)
RNA Rumen contents Canulated animals Jensen et al. (2006)
Fatty acid profile of bacteria Rumen contents Rumen contents Rumen contents Canulated animals Canulated animals Canulated animals Vlaeminck et al (2005) Dewhurst et al. (2007) Bessa et al (2009)
9
Cecava et al. (1990) concluded that solid associated bacteria (SAB) or mixed ruminal bacteria, isolated from digesta obtained over the entire feeding cycle, were the most appropriate for determining the TP:N ratio of bacteria leaving the rumen, these authors took into account earlier findings of Craig et al. (1987) that showed typically 20% of total ruminal microbial biomass was associated with the LAB fraction.
TP:protein or TP:N ratios have been reported to differ between bacteria associated with fluid and particle phases. The content of purine bases (usually expressed in relation to N content) tends to be greater in the LAB (Merry and McAllan, 1983; Legay-Carmier and Bauchart, 1989; Craig et al., 1987; Bessa et al., 2009). By using the TP analysis method of Zinn and Owens (1986), Cecava et al. (1990) assessed the relative TP:N ratios of liquid associated bacteria (LAB), of solid associated bacteria (SAB) and mixed ruminal bacteria, observing different ratios among these three sources of bacterial biomass, particularly between LAB and SAB.
Employing only the bacterial purine:N ratios may underestimate MP yields. Protozoa passing to the abomasum or duodenum contribute to total purine flow, but protozoal purine:N ratios typically are about half those of ruminal bacteria (Broderick and Merchen, 1992). Nevertheless, as stated earlier, it is not expected protozoa to play a major role in microbial outflow from the rumen.
According to several authors (Broderick and Merchen, 1992; Stern et al., 1994) the total purine method (Zinn and Owens, 1986) has been suggested as the best procedure to quantify microbial protein yields (Obispo and Dehority, 1999). Low recoveries of TP have been reported when using the method of Zinn and Owens (1986; Makkar and Becker, 1999; Obispo and Dehority, 1999). Nevertheless, recent modifications of this method (Makkar and Becker, 1999; Reynal et al., 2003; Reynall and Broderick, 2009) have improved purine recovery. Also, direct quantification and complete recovery of adenine (A) and guanine (G) using an HPLC method resulted in microbial protein flows that were highly correlated (R = 0.94) to those estimated with the modified Zinn and Owens method (Reynal et al., 2003).
These purine bases are commonly used as markers to quantify microbial protein and organic matter flow from the rumen. In chapters 3, 4 and 5 of this thesis we use this markers to quantify the amount of bacterial biomass formed during in vitro incubations of caecal contents in different availabilities of nutrients.
10
1.3.3. Odd and branched chain fatty acids as microbial marker
Recent research in ruminants has studied the potential of odd and branched chain fatty acids (OBCFA) as bacterial internal markers (Bessa et al., 2009, Vlaeminck et al., 2005; 2006a; 2006b). These fatty acids are mainly present in bacterial membrane lipids (Kaneda, 1991; Mackie et al., 1991).
The OBCFA of rumen bacteria seem largely determined by the fatty acid synthetase of the microorganisms and by physiological and culture conditions (Vlaeminck et al., 2006a), suggesting that variations in the profile of OBCFA from the rumen can be considered a reflection of changes in the relative abundance of bacterial populations in the rumen (Vlaeminck et al., 2006a).
The fatty acid composition of rumen bacteria is characterized by a large proportion of OBCFA in their membrane lipids (C15:0, iso C15:0, iso C15:0, C17:0, iso C17:0, ante-iso C17:0 and C17:1) (Kaneda, 1991).
Fatty acid content and composition of LAB and SAB are different. Total fatty acid content is generally higher in SAB than in LAB. Vlaeminck et al. (2006) reported that fatty acid content in rumen SAB were 1.6 to 2.8 times higher than that in LAB, and decreased with increasing forage:concentrate (F:C) ratio. This trend for FA to be higher in SAB than in LAB has also been observed by other authors (Bauchart et al., 1990; Bessa et al., 2009). Differences between LAB and SAB in chemical composition and in enzyme activity demonstrate that the distribution of bacterial species is different in the liquid and solid phases of the rumen (Michalet-Doreau et al. 2001). This is in agreement with the current analysis of OBCFA, which suggest that the species composition of the adherent population differs from that of the liquid phase.
Relations between LAB and SAB bacterial populations and F:C ratio is also reported in the literature. Increasing the F:C ratio is usually beneficial for the pool of SAB because more cellulolytic bacteria attach to forage particles (Weimer et al., 1990). On the other hand, results reported by several authors suggest that LAB are enriched in amylolitic bacteria (Dehority and Orpin, 1998, Vlaeminck et al., 2006).
Cellulolytic bacteria contain high amounts of iso-fatty acids with Ruminococcus flavefaciens enriched in odd-chain fatty acids and Ruminococcus albus in even-chain iso-fatty acids. In general, the starch using bacteria show low levels of branched-chain iso-fatty acids and are relatively enriched in linear odd-chain fatty acids. In conclusion, large differences in the OBCFA profile observed among rumen bacteria suggest that they could be useful in
11
assessment of the composition of, or shifts, in the rumen microbial population (Bessa et al., 2009, Vlaeminck et al., 2005; 2006).
By using these techniques to caecum and colon contents, Santos et al. (2007; 2008) found a similarity in chemical and fatty acid composition of caecum bacteria to solid associated bacteria (SAB) of the rumen, indicating a preponderant cellulolytic activity. Concerning colon bacteria, results suggest that they have a chemical and fatty acid profile close to that of rumen LAB, mainly of the starch utilizing bacteria. These preliminary data indicate that these techniques can also be useful in characterizing caecum and colon bacteria populations (Santos et al., 2007, 2008).
On chapter three fatty acid composition of both caecal and colon contents is characterized, and the potential use of fatty acids as internal markers for bacterial populations of the hindgut of the horse are discussed.
1.4. In vitro gas production and microbial mass measurement
The gas production technique was developed to determine fermentation characteristics of organic matter (OM) (Steingass, 1983). Originally developed for ruminants (Menke et al., 1979), the cumulative gas production technique has been adapted to allow its utilization in horses (Janssen et al., 2007, Lowman et al., 1996).
When a feed is incubated with buffered rumen/caecal fluid in vitro, carbohydrates are fermented into VFA, gases (mainly CO2 and CH4) and microbial cells. Gas production is mainly the result of carbohydrates fermentation to acetate, propionate and butyrate (Wolin, 1960; Blummel and Ørskov, 1993; Getachew et al., 1998) since gas produced from protein fermentation is relatively small as compared to carbohydrate fermentation (Wolin, 1960). Gas measured is the sum of the direct gas produced as a result of fermentation to CO2 and CH4, and the indirect gas produced from the buffering of VFA (CO2 released from the bicarbonate buffer) (Getachew et al., 1998).
The most commonly used source of microbial inoculum in this method is obtained from fistulated cows and sheep with cannulas in the rumen. However, its use in simple stomach animals is also referred. In horses, caecal and colon fluid can be collected via right ventral cannulas and used as microbial inoculum. Lowman et al. (1996) used the gas production technique to compare in vitro fermentation of equine caecal or faecal inoculums. Also Jansen et al. (2007) compared cellulose digestibility of caecal, colonic and faecal inoculums of horses
12
using the gas production technique. More recently, this technique has been used to study potential using of equine faecal inoculum (Murray et al., 2008, 2009 and 2010, Santos et al., 2008, 2010).
When combined with measures of degradation, the gas production technique can provide a measure of the proportion of substrate that is fermented as opposed to that which is partitioned to microbial growth since the other nutritionally important fermentation product is microbial biomass. Although VFA and microbial biomass are linked by ATP production, it is well known that different amounts of microbial biomass can be produced per unit of ATP (YATP) (Hespell and Bryant, 1979; Harrison and McAllan, 1980; Demeyer, 1991). This can impose an inverse relationship upon the production of short-chain fatty acids and microbial biomass yield (Preston and Leng, 1987). It has been shown that this relationship also applies to in vitro gas production and microbial biomass yield when both variables are related to a unit of substrate fermented (Blümmel et al., 1997). Rymer and Givens (1999) have shown that feeds that produce large amounts of gas and VFA, yield small amounts of microbial mass per unit of feed truly degraded.
An aspect of the anaerobic system is that stoichiometric laws of fermentation balance can be applied since fermentation products must be derived from the substrate incubated. High correlations between recorded and stoichiometrically calculated gas values have been reported by Beuvink and Spoelstra (1992), Blümmel and Ørskov (1993) and Opatpatanakit et al. (1994). The stoichiometric balance allows the theoretical calculation of the products (VFA and gases) (Van Soest, 1994). If the molar proportion and amount of VFA are known, the theoretical amounts of CO2 and CH4 expected from the rumen fermentation can be calculated.
Blümmel and Bullerdick (1997) suggested complementing the in vitro gas production with residue determination in evaluation of the nutritive value of feeds. In this approach, the residue determination reveals how much substrate is used in the fermentation and the gas measurement reflects how much of this fermented substrate is converted into the VFA and gases. The ratio of substrate truly degraded to gas volume produced, defined as ‗partitioning factor‘(PF) will vary depending on molar proportions of VFA (acetate to propionate ratio) and with YATP (Blümmel and Bullerdick, 1997; Getachew et al. 1998). Alternatively, gas measurements can be combined with microbial mass determination using internal (e.g., purines) markers (Getachew, 1998).
Recently, Morgan et al. (2004) and Mould et al. (2004) using gas production incubated rumen fluid in an N-free medium with maize starch to study the utilization of amino acids (Morgan et al., 2004) and urea (Mould et al., 2004) by the ruminal microbial population.
13
Latter, Cone et al. (2005, 2009) adapted the gas production technique to describe the protein fermentation characteristics of different feedstuffs. To achieve this, incubations were carried out with an excess of rapidly fermenting carbohydrates. Incubations performed in an N-free environment makes N the limiting factor for microbial growth. Thus, microbial growth will depend on the availability of N from the samples that are incubated (Cone et al., 2005). The use of specific substrates allows the metabolic activity of the inoculums to be examined directly and the impact of dietary manipulations on a know microflora can be assessed (Mould et al. (2005).
In chapters four and five we use this technique to obtain fermentation patterns and to estimate microbial protein synthesis of equine caecal inoculums, provided with protein and non protein nitrogen sources.
1.5. Aims of the thesis
Considering the previous remarks, our initial hypotheses is that, due to nitrogen limitation, the hindgut environment would aim to favor VFA‘s production by promoting microbial activity with low microbial growth. If this is the case, then the hindgut environment is adapted to a permanent uncoupling situation, with fiber degradation being maximized but not microbial growth.
In order to test this hypothesis, the main aims of this thesis were defined: 1) gather current knowledge on the equine digestive tract and nutritional strategies, with a specific focus on the hindgut environment and its functioning, microbial population, energy and protein metabolism; 2) characterize caecum and colon bacterial composition in terms of fatty acids and purine bases, and to access the use of these substances as internal markers for microbial protein quantification in the horse; 3) access in vitro fermentation characteristics and bacterial metabolic response of caecal fluid to different levels and of protein (casein) and non-protein (urea) nitrogen; 4) study in vitro fermentation characteristics of equine caecal fluid in response to the availability of energy and nitrogen, and to measure microbial biomass yield.
14
1.6. ReferencesBates, B.B., Gillett, J.A., Barao, S.A. and Werner, G.B., 1985. The effect of specific growth on nucleic acid-protein values of pure cultures and mixed ruminal bacteria. Journal of Animal Science, 61:713-724.
Bauchart, D., LegayCarmier, F., Doreau, M. and Gaillard, B., 1990. Lipid metabolism in liquid associated and solidadherent bactéria in rumen contents of dairy cows offered lipid supplements diets. British Journal of Nutrition 63: 563–578.
Bessa, R.J.B., Maia, M.R.G., Jerónimo, E., Belo, A.T., Cabrita, A.R.J., Dewhurst, R.J. and Fonseca, A.J.M., 2009. Using microbial fatty acids to improve understanding of the contribution of solid associated bacteria to microbial mass in the rumen. Animal Feed Science and Technology, 150: 197-206.
Beuvink, J.M.W. and Spoelstra, S.F., 1992. Interactions between substrate, fermentation end-products, buffering systems and gas production upon fermentation of different carbohydrates by mixed rumen microorganisms in vitro. Applied Microbiology Biotechnology, 37: 505–509.
Blümmel, M., Steingass, H. and Becker, K., 1997. The relationship between in vitro gas production, in vitro microbial biomass yield and N15 incorporation and its implications for the prediction of voluntary feed intake of roughages. British Journal of Nutrition, 77: 911-921.
Blümmel, M. and Bullerdick, P., 1997. The need to complement in vitro gas measurements with residue determination from in sacco degradabilities to improve the prediction of voluntary intake of hays. Animal Science, 64, 71–75.
Blümmel, M. and Ørskov, E.R., 1993. Comparison of gas production and nylon bag degradability of roughages in predicting feed intake in cattle. Animal Feed Science and Technology, 40: 109–119.
Broderick, G.A. and Merchen, N.R., 1992. Markers for quantifying microbial protein synthesis in the rumen. Journal of Animal Science. 75: 2618–2632.
Calsamiglia, S., Stern, M.D. and Firkins, J.L., 1996. Comparison of nitrogen-15 and purines as microbial markers in continuous culture. Journal of Animal Science, 74:1375-1381. Carro, M.D. and Miller, E.L., 2002. Comparison of microbial biomarkers (N15 and purine
bases) and bacterial isolates for the estimation of rumen microbial protein synthesis. Animal Science, 75: 315-321
15
Cecava, M.J., Merchen, N.R., Gay, L.C. and Berger, L.L., 1990. Composition of ruminal bacteria harvested from steers as influenced by dietary energy level, feeding frequency and isolation techniques. Journal of Dairy Science, 73: 2480-2488.
Cheng, K.F. and Wallace, R.J., 1979. The mechanism of passage of endogenous urea through the rumen wall and the role of ureolytic epithelial bacterial in the urea flux. British Journal of Nutrition, 42: 553-557.
Cone, J.W., Jongbloed, A.W., Van Gelder, A.H. and De Lange, L., 2005. Estimation of protein fermentation in the large intestine of pigs with a gas production technique. Animal Feed Science and Technology, 123-124: 463–472.
Cone, J.W., Rodrigues, M.A.M., Guedes, C.M. and Blok, M.C., 2009. Comparison of protein fermentation characteristics in rumen fluid determined with the gas production technique and the nylon bag technique. Animal Feed Science and Technology, 153: 28-38.
Cotta, M.A. and Russell, J.B., 1982. Effect of peptides and amino acids on efficiency of rumen bacterial protein synthesis in continuous culture. Journal of Dairy Science, 65: 226-234.
Craig, W.M., G.A. Broderick and Ricker, D.B., 1987. Quantitation of microorganisms associated with the particulate phase of ruminal digesta Journal of Nutrition 117:56. Dehority, B.A. and Orpin, C.G., 1988. Development of and natural fluctuations in, rumen
microbial populations. In: The rumen microbial ecosystem 2nd edition (eds P.N. Hobson and C.S. Stewart), pp 151–183. Blackie Academic and Professional, London, England.
Demeyer, D.I., 1991. Quantitative aspects of microbial metabolism in the rumen and hindgut. In: Rumen microbial metabolism and ruminant digestion (ed. J.P. Jouany), pp. 217– 237. INRA Publications, Paris.
Dewhurst, R.J., Davies, D.R. and Merry, R.J., 2000. Microbial protein supply from the rumen. Animal Feed Science and Technology, 85:1-21
Getachew, G., Blümmel, M., Makkar, H.P.S. and Becker, K., 1998. In vitro gas measuring techniques for assessment of nutritional quality of feeds: a review. Animal Feed Science and Technology, 72: 261–281
Gonzaléz-Ronquilho, M., Balcells, J., Belenguer, J., Castrillo, C. and Mota, M., 2004. A comparison of purine derivatives excretion with conventional methods as indices of microbial yield in dairy cows. Journal of Dairy Science, 87: 2211–2221.
16
Harrison, D.G. and McAllen, A.B., 1980. Factors affecting microbial growth yields in the reticulo-rumen. In: Digestive Physiology and Metabolism in Ruminants (eds. Y. Ruckebusch and P. Thivend), pp. 205-226. AVI Publishing Co., Inc., Westport, CT. Hespell, R.B. and Bryant, M.P., 1979. Efficiency of Rumen Microbial Growth: Influence of
some Theoretical and Experimental Factors on YATP. Journal of Animal Science, 46: 1640-1659.
Hintz, H.F. and Schryver, H.F., 1972. Nitrogen Utilization in Ponies. Journal of Animal Science, 34: 592-595.
Houpt, T.R. and Houpt, K.A., 1971. Nitrogen conservation by ponies fed a low-protein ration. American Journal of Veterinary Research, 32: 579-588.
Ipharraguerre, I.R., Reynal, S.M., Lineiro, M., Broderick, G.A. and Clark, J.H., 2006. A comparison of sampling sites, digesta and microbial markers, and microbial references for assessing the postruminal supply of nutrients in dairy cows. Journal of Dairy Science, 90:1904–1919
Jansen, W.L., Cone, J.W., Geelen, S.N.J., Sloet van Oldruitenborgh-Oosterbaan, M.M., Van Gelder, A.H., Oude Elferink, S.J.W.H. and Beynen, A.C., 2007. High fat intake by ponies reduces both apparent digestibility of dietary cellulose and cellulose fermentation by faeces and isolated caecal and colonic contents. Animal Feed and Science Technology, 133: 298-308.
Jensen, C., Weisbjerg, M.R. and Hvelplund, T., 2006. Evaluation of methods for estimating the amino acid supply to the duodenum of microbial, endogenous and undegraded feed protein on maize silage diets fed to dairy cows. Animal Feed Science and Technology, 131:1–24
Julliand, V., 1998. Ecologie microbienne du système digestif des équidés: nouvelles approches: conséquenses pratiques. 24ème Journée Recherche Equine, Haras Nationaux ed., Paris, pp. 105-113.
Kaneda, T., 1991. Iso and anteiso-fatty acids in bacteria: biosynthesis, function, and taxonomic significance. Microbiology and Molecular Biology Reviews, 55: 288–302. Legay-Carmier, F. and Bauchart, D., 1989. Distribution of bacteria in the rumen contents of
dairy cows given a diet supplemented with soyabean oil. British Journal of Nutrition, 61: 725–740.
Lowman, R.S., Theodorou, M.K., Longland, A.C. and Cuddeford, D., 1996. A comparison of equine faeces or caecal digesta as sources of inoculum for in vitro fermentation studies using the pressure transducer technique. Animal Science, 62: 683A.
17
Mackie, R.I., White, B.A. and Bryant, M.P., 1991. Lipid metabolism in anaerobic ecosystems. Critical Review on Microbiology, 17: 449–479.
Maczulack, A.E., Dawson, K.A. and Baker, J.P., 1985. Nitrogen utilization in bacterial isolates from the equine cecum. Applied and Environmental Microbiology, 50: 1439-1443.
Makkar, H.P.S. and Becker, K., 1999. Purine quantification in digesta from ruminants by spectrophotometric and HPLC methods. British Journal of Nutrition, 81: 107–112. Martin-Rosset, W. and Tisserand, J.L., 2004. Evaluation and expression of protein allowances
and protein value of feeds in the MADC system for the performance horse. In: Julliand, V. and Martin-Rosset, W. (Eds.). Nutrition of the performance horse: which system in Europe for evaluating the nutritional requirements? EAAP no 111. Wageningen Academic Publishers, Wageningen, The Netherlands, pp. 103–140
McAllan, A.B., and Smith, R.H., 1969. Nucleic acid metabolism in the ruminant. Determination of nucleic acids in digesta. British Journal of Nutrition, 23:671.
Menke, K.H., Raab, L., Salewski, A., Steingass, H., Fritz, D. and Schneider, W., 1979. The estimation of the digestibility and metabolizable energy content of ruminant feedingstuffs from the gas production when they are incubated with rumen liquor in vitro. Journal of Agriculture Science, 93: 217-222.
Merry, R.J. and McAllan, A.B., 1983. A comparison of the chemical composition of mixed bacteria harvested from the liquid and solid fractions of rumen digesta. British Journal of Nutrition, 50: 701-709.
Merry, R.J., Smith, R.H., McAllan, A.B., 1987. Studies of rumen function in an in vitro continuous culture system. Archives of Animal Nutrition, 37: 475-488.
Michalet-Doreau, B., Fernandeza, I., Peyrona, C., Millet, L. and Fonty, G., 2001. Fibrolytic activities and cellulolytic bacterial community structure in the solid and liquid phases of rumen contents. Reproduction Nutrition Development, 41: 187-194.
Morgan, R., Kliem, K. and Mould, F.L., 2004. The use of a nitrogen free medium for in vitro fermentation studies. Proceedings of the British Society of Animal Science, 232. Mould, F.L., Morgan, R. and Kliem, K., 2004.Ruminal degradation of amino acids assessed
using a complement in vitro technique. Journal of Animal Science (suppl. 1) 163. Murray, J.M.D., Bice, R.K.T. and Moore-Colyer, M.J.S., 2010. The effect of particle size on
the in vitro fermentation of different ratios of high-temperature dried Lucerne and sugar beet pulp incubated with equine faecal inocula. Animal Feed Science Technology, 162: 47-57.
18
Murray, J.M.D., Longland, A. and Dunnet, C., 2008. Effect of yeast supplementation on the in vitro fermentation of high-temperature dried Lucerne incubated with an equine faecal inoculums. Animal Feed Science and Technology, 146: 149-159.
Murray, J.M.D., Scott, B. and Hastie, P.M., 2009. Fermentative capacity of equine faecal inocula obtained from clinically normal horses and those predisposed to laminitis. Animal Feed Science and Technology, 151: 306-311.
Obispo, N.E. and Dehority, B.A., 1999. Feasibility of using total purines as a marker for ruminal bacteria. Journal of Animal Science, 77: 3084-3095.
Perez, J.F., Balcells, J., Guada, J.A. and Castrillo, C., 1996. Determination of rumen microbial-nitrogen production in sheep: a comparison of urinary purine excretion with methods using N15 and purine bases as markers of microbial-nitrogen entering the duodenum.British Journal of Nutrition, 75: 699-709.
Pérez, J. F., C. A. Rodríguez, J. González, J. Balcells, and J. A. Guada., 1996. Contribution of dietary purine bases to duodenal digesta in sheep. In situ studies of purine degradability corrected for microbial contamination. Animal Feed Science and Technology, 62:251– 262.
Preston, T.R. and Leng, R.A., 1987. Digestive physiology of ruminants. In: Matching ruminant production systems with available resources in the tropics and subtropics (ed. Penambul Books), pp. 21–48. Penambul Books Ltd, Armidale, NSW, Australia. Prior, R.L., Hintz, H.F., Lowe, J.E. and Visek, W.J., 1974. Urea Recycling and Metabolism of
Ponies. Journal of Animal Science, 38: 565-571.
Reynal, S.M., Broderick, G.A. and Bearzi, C., 2005. Comparison Of Four Markers For Quantifying Microbial Protein Flow From The Rumen Of Lactating Dairy Cows. Journal of Dairy Science, 88:4065–4082.
Russell, J.B., 2007. The energy spilling reactions of bacteria and other organisms. Journal of Molecular Microbiology and Biotechnology, 13: 1–11.
Russell, J.B. and Cook, M.C., 1995. Energetics of bacterial growth: balance of anabolic and catabolic reactions. Microbiological Reviews, 59: 48–62.
Russell, J.B. and Wallace, R.J., 1997. Energy-yielding and energy-consuming reactions. In: The rumen microbial ecosystem 2nd edition (eds P.N. Hobson and C.S. Stewart), pp 246-282. Blackie Academic and Professional, London, England.
Russell, J.B. and Baldwin, R.L., 1979a. Comparison of substrate affinities among several tureen bacteria: a possible determinant of rumen bacterial competition. Applied Environmental Microbiology, 37:537—543
19
Ryenall, S.M. and Broderick, G.A., 2009. Technical note: A new high-performance liquid chromatography purine assay for quantifying microbial flow. Journal of Dairy Science, 92: 1177-1181.
Santos, A. S., Ferreira, L. M. M., Cotovio, M., Guedes, C.V.M., Cone, J.W., Bessa, R.J.B. and Rodrigues, M.A.M., 2010. In vitro equine caecal fermentation of different casein levels. In: The impact of nutrition on the health and welfare of horses (eds. A.D. Ellis, A.C. Longland, M. Coenen and N. Miraglia), pp. 199-202. EAAP publication n.º 128. Wageningen Academic Press, Netherlands.
Santos, A. S., Guedes, C.V.M., Ferreira, L. M. M., Evangelista, L., Gaspar, P. H., Bertin, G. and Rodrigues, M.A.M., 2008. The effect of Saccharomyces cerevisiae CBS 493.94 level on in vitro caecal fermentation using faecal inoculum from horses fed diets with different fibre concentrations. In: Nutrition of the exercising horse. (eds. M.T. Saastamoinen and W. Martin-Rosset ), pp. 361-364. EAAP publication n.º 125.Wageningen Academic Press, Netherlands.
Santos, A.S., Jerónimo, E., Ferreira, L.M., Rodrigues, M.A.M. and Bessa, R.J.B., 2007. Chemical composition of liquid and solid associated bacteria in the cecum and colon of horses, In: 58th Annual Meeting of the European Association of Animal Production, Wageningen Academic press, Dublin, Ireland, p. 293.
Santos, A.S., Jerónimo, E., Ferreira, L.M., Rodrigues, M.A.M. and Bessa, R.J.B., 2008. Fatty acid composition of liquid and solid associated bacteria in the cecum and colon of horses, 59th Annual Meeting of the EAAP, Wageningen Academic Vilnius, Lituania, p. 221.
Santos, A.S., Rodrigues, M.A.M., Bessa, R.J.B., Ferreira, L.M. and Martin-Rosset, W., 2011. Understanding the equine cecum-colon ecosystem: current knowledge and future perspectives. Animal, 5: 48–56.
Slade, L.M., Robinson, D.W. and Casey, K.E., 1970. Nitrogen Metabolism in Nonruminant Herbivores. I. The Influence of Nonprotein Nitrogen and Protein Quality on the Nitrogen Retention of Adult Mares. Journal of Animal Science, 30: 753-760.
Smith, R.H. and McAllan, A.B., 1974. Some factors influencing the chemical composition of mixed rumen bacteria. British Journal of Nutrition, 31: 27-34.
Steingass, H., 1983. Bestimmung des energetischen Futterwertes von wirtschaftseigenen Futtermitteln aus der Gasbildung bei der Pansenfermentation in vitro. Ph.D. Thesis, University of Hohenheim, Germany.
20
Stern, M.D., Hoover, W.H., Sniffen, C.J., Crooker, B.A. and Knowlton, P.H., 1978. Effects of nonstructural carbohydrate, urea and soluble protein levels on microbial protein synthesis in continuous culture of rumen contents. Journal of Animal science, 47: 944. Stern, M.D., Varga, G.A., Clark, J.H., Firkins, J.L., Huber, J.T. and Palmquist, D.L., 1994.
Evaluation of chemical and physical properties of feeds that affect protein metabolism in the rumen. Journal of Dairy Science, 77: 2762-2786
Tempest, D.W. and Neijssel, O.M., 1984. The staus of YATP and maintenance as biologically interpretable phenomena. Annual Review of Microbiology, 38:459-486
Vlaeminck, B., Dufour, C., van Vuuren, A.M., Cabrita, A.R.J., Dewhurst, R.J., Demeyer, D. And Fievez, V., 2005. Use of Odd and Branched-Chain Fatty Acids in Rumen Contents and Milk as a Potential Microbial Marker. Journal of Dairy Science, 88: 1031-1042. Vlaeminck, B., Fievez, V., Cabrita, A.R.J., Fonseca, A.J.M. and Dewhurst, R.J., 2006a.
Factors affecting odd- and branched-chain fatty acids in milk: A review. Animal Feed Science and Technology, 131: 389-417.
Vlaeminck, B., Fievez, V., Demeyer, D. and Dewhurst, R.J., 2006b. Effect of Forage:Concentrate Ratio on Fatty Acid Composition of Rumen Bacteria Isolated From Ruminal and Duodenal Digesta. Journal of Dairy Science, 89 : 2668-2678.
Weimer, P.J., Waghorn, G.C., Odt, C.L. and Mertens, D.R., 1999. Effect of diet on population of three species of ruminal cellulolytic bacteria in lactating dairy cows. Journal of Dairy Science, 82: 122–134.
Wolin, M.J., 1960. A theoretical rumen fermentation balance. Journal of Dairy Science, 43: 1452–1459.
Zinn, R.A. and Owens, F.N., 1986. A rapid procedure for purine measurement and its use for estimating net ruminal protein synthesis. Canadian Journal of Animal Science, 66: 157-166.
Chapter Two
Understanding the equine cecum-colon ecosystem: current
knowledge and future perspectives
____________________________________________________________________________________ Published as: A.S. Santos, M.A.M. Rodrigues, R.J.B. Bessa, L.M. Ferreira and W. Martin-Rosset. 2011. Understanding the equine cecum-colon environment: current knowledge and future perspectives. Animal, 5:1, 48-56
23
2.1. ImplicationsThe process of understanding and describing degradation mechanisms in the equine digestive ecosystem in general and in the hindgut in particular, is essential to provide information for proper feeding practices to be implemented. Regardless of its importance for the nutritional status of the host, the significance of hindgut fermentation has not yet been fully understood, and few reports have focused deeply on the contribution of the hindgut microbial population to the nitrogen and energy requirements of the horse. In this review paper, we intend to gather existing information on the equine ecosystem and to provide future perspectives of research.
2.2. Abstract
Having evolved as grazing animals, horse‘s digestive physiology is characterized by a rapid gastric transit, a rapid but intense enzymatic digestion along the small intestine, and a long and intense microbial fermentation in the large intestine. Understanding and describing feed degradation mechanisms in the equine digestive system in general, and in the hindgut ecosystem in particular, is essential. Regardless of its importance for the nutritional status of the host, the significance of the cecum-colon ecosystem has not yet been fully understood, and few reports have focused deeply on the contribution of the hindgut microbial population to the nitrogen and energy requirements of the horse. Compared to ruminal activity, very little is known on hindgut ecosystem activity in the horse, information concerning the metabolism of this microbial population, and its requirements is lacking. The use of internal bacterial markers for quantifying microbial outflow in ruminants is widely reported, these techniques can be applied to cecum-colon microbial quantification, contributing to a better characterization of this ecosystem.
It is likely wrong to believe that the optimization strategy in the hindgut is similar to what happens in the rumen - that is to maximize microbial growth and, therefore, fermentation. If we consider the type of substrate that, in normal conditions, arrives to the hindgut, we can expect it to be nitrogen limiting, providing limited nitrogen based substrates for microbial fermentation.
In this review paper, we intend to gather existing information on the equine ecosystem and to provide future perspectives of research.