´
Relatório Final de Estágio
Mestrado Integrado em Medicina Veterinária
SMALL ANIMAL MEDICINE AND SURGERY Yvette Charlotte Klerx
Orientador
Dr. Miguel Augusto Soucasaux Marques Faria Co-orientador
Dr. Alfred Legendre (John & Ann Tickle Small Animal Teaching Hospital, University of Tennessee)
Relatório Final de Estágio
Mestrado Integrado em Medicina Veterinária
SMALL ANIMAL MEDICINE AND SURGERY Yvette Charlotte Klerx
Orientador
Dr. Miguel Augusto Soucasaux Marques Faria Co-orientador
Dr. Alfred Legendre (John & Ann Tickle Small Animal Teaching Hospital, University of Tennessee)
III
Abstract
This report aims to conclude Integrated Master in Veterinary Medicine. It consists of a brief
description and discussion of five clinical cases that I have followed during my externship at the
John and Ann Tickle Small Animal Hospital of the University of Tennessee, for a total of 16
weeks, with a main focus on the area of Small Animal Medicine and Surgery.
During this period, I had the opportunity to join and participate in specialized areas, going
through different rotations, such as integrative medicine, cardiology, orthopedic rehabilitation,
internal medicine, soft tissue surgery, ophthalmology, oncology and dermatology. My
contribution consisted of performing appointments and physical examinations, presenting
diagnostic and therapeutic plans, assisting or participating in procedures, updating medical
records as well as communicating with clients. Alongside this, I presented clinical cases during
rounds, performed research, discussed recent articles and prepared various presentations.
The realization of this externship was of great relevance to expand and ameliorate my clinical
skills, autonomy as well as self-confidence in execution of duties. The main objectives were
fulfilled by employing maximum focus on all assigned responsibilities, being a team player,
learning how to manage bureaucratic aspect and by interacting with the patient’s owners.
Overall, this externship was an extremely educational and unique experience which has not
only been very significant for my development but will also have a positive impact on my future
IV
Acknowledgements
My sincerest thanks to my mentor, Dr. Miguel Faria, for the availability and useful
suggestions provided through this final stage and entire course. To Dr. Alfred Legendre for
endowing this unique opportunity for many years, for the 24/7 availability and the wonderful
conversations in the Dutch language that made me feel at home. It was a pleasure to meet you.
My mother and father, Anneke and Hans Klerx, to whom this is dedicated. Thank you for
making this possible and supporting me through all my decisions. For believing in me and
encouraging me. For being there and for giving strength when everything seems to be going
wrong. For teaching that hard workers achieve success and that optimism is an important key
for life.
To my one and only sister, Michèle Klerx, for being my role model and best friend. For being
proud of me and matching my level of craziness. For being there even when different countries
separate us from being together. For remaining the same silly sister and for caring about me,
even if we don’t see each other that often. You know I'm proud of you as well.
To the sweetest grandmother, Jet Klerx, my guardian angel.
To Riet Van Riel, my other lovely grandmother for receiving me for one entire month, we had
a fantastic time together.
To Donny and Peer for sharing the same passion for animals, for following me through all
those years and being such a great support.
To Annemiek Van Riel, for the honesty and constructive critic. I appreciate your dedication
and willingness to be involved.
To Joana Portugal for your friendship and all the never-ending conversations we had through
those years. For the adventures and hilarious stories. As I said before, we could write a book!
To Ana Salazar, for the funny emails we exchanged when an Atlantic Ocean was impeding
V
To my Tennessee girls, Daniela Bento, Daniela Martins, Marta Gomes and Sara Dinis. Thank you for being the best roomies in the world. For supporting through ups and downs and for being my lovely American family during those 3 months. To Inês Palhinhas for
understanding that a life without cupcakes and Mexican food is not the same.
To Joana Soares, simply for your enormous support.
To Jessica Miner, the sweetest American girl I met. Thank you for being there for me. And to
all the other amazing people as well as patients, for making this experience unforgettable.
To Franky, for the 14 years of pure happiness. For growing up by my side and teaching me
to appreciate the simple things in life.
VI
List of Abbreviations:
%– Percentage
ACTH – Adrenocorticotropic hormone
ALP– Alkaline Phosphatase
ALT – Alanine aminotransaminase
aPTT – Activated partial thromboplastin time
AT – Adrenocortical tumor
ATH– Adrenocortical tumors causing hyperadrenocoriticism
BAL– Bronchoalveolar lavage
bFGF– Basic fibroblast growth factor
BG– Blood glucose
BID– Twice daily
CBC– Complete Blood Count
CK– Creatine Kinase
Cm– Centimeters
COPD– Chronic obstructive pulmonary disease
CO2– Carbon dioxide
cPLI – Canine pancreatic lipase immunoreactivity
CRI – Constant rate infusion
CT– Computerized tomography
cTLI– Canine trypsin-like immunoreactivity
DIC– Disseminated intravascular coagulation
dL– Deciliter
ECG– Electrocardiography
EOD– Every other day
EPI– Exocrine pancreatic insufficiency
FAST– Focused Assessment with Sonography for Trauma
fL– Femtoliters
flair– Fluid-attenuated inversion recovery
Fr – French scale
g– Gram
HDDST– High dose dexamethasone suppression test
HSA– Hemangiosarcoma
ICU– Intensive care unit
IV – Intravenous
IM – Intramuscular
KCS – Keratoconjunctivitis sicca
Kg– Kilogram
VII LDDST– Low dose dexamethasone
suppression test
MAHA– Microangiopathic hemolytic anemia
m2– Square meter
MDR1 – Multidrug resistance protein-1
mEq– Miliequivalents
mg/Kg– Milligram by kilogram
mg/m2– Milligram by meter square
mL– Milliliter
mL/h– Milliliter per hour
mm– Millimeter
mmHg– Millimeter of mercury
mmol– Millimoles
MRI– Magnetic resonance imaging
ng– Nanogram
OS– Left eye
OU– Both eyes
P53– Phosphoprotein p53
PCV– Packed cell volume
PO– Per os
Pg - Picogram
PT– Prothrombin time
QID– Four times daily
Ras– Rat sarcoma
SID– Once daily
T4 - thyroxine
TS– Total solids
Tsc2– Tuberous sclerosis-2
TSH– Thyroid stimulating hormone
UTVMC– University of Tennessee Veterinary medical center
UTI– Urinary tract infection
UV – Ultraviolet light
VEGF– Vascular endothelial growth factor
VPC– Ventricular premature complex
µg– Microgram
µL– Microliter
ºC– Celsius
VIII
Contents
Abstract………..………... III
Acknowledgements………..……….……….... IV
List of Abbreviations……….……… VI
Contents……… VIII
Clinical case Nr. 1: Gastroenterology –Chronic pancreatitis………….………... 1
Clinical case Nr. 2: Pulmonology –Chronic bronchitis ………..… 7
Clinical case Nr. 3: Soft Tissue Surgery –Adrenalectomy ………. 13
Clinical case Nr. 4: Ophthalmology –Descemetocele ………. 19
Clinical case Nr. 5: Oncology –Splenic hemangiosarcoma ………... 25
Appendixes Appendix I ………...… 31
Appendix II ……….. 33
Appendix III ………... 34
Appendix IV ……….... 36
1
Clinical case Nr. 1: Gastroenterology - Chronic pancreatitis
Characterization of patient and reason of appointment: Blackjack is a 9 year old, male castrated, 35 Kg, Labrador Retriever, that was presented to the UTVMC Emergency service
with signs of acute lethargy and vomiting with the duration of one day.
Anamnesis: Blackjack had all vaccines up to date and was dewormed regularly. He lived indoors with private/public outdoor access and had the tendency to go into trash, plants or toxic
agents. The diet was inconstant, ranging from a miscellaneous human diet to a low fat
veterinary prescription diet. According to medical history, three cases of pancreatitis had been
reported within the last year. Although the recurrence, he recovered successfully from all
episodes. During this period, he had three other episodes at home and the owners treated by
withholding food for one day and reintroducing it gradually (Royal Canin GI Low Fat® or a
homemade sweet potato with chicken), and by giving maropitant, tramadol and acepromazine.
This last episode, the vomiting initiated immediately after a walk through a park, and consisted
mainly of bile. In the morning, Blackjack was lethargic, unable to stand, had polydipsia, anorexia
and a mild cough. However, his stools and urination were normal. Physical exam: Blackjack was presented in sternal recumbency, mildly dehydrated (7%) and had tacky mucous
membranes. Femoral pulse was weak but with a normal rate. The respiratory rate was normal.
No crackles or wheezes were ausculted. Additionally, he had occasional non-productive cough
and a rectal temperature of 38.9°C. Gastrointestinal examination: Superficial and deep abdominal palpation did not reveal clear signs of pain or discomfort. Examination of head,
esophagus, abdomen and rectum did not evince significant changes. List of problems: Lethargy, vomiting, cough, anorexia, polydipsia, dehydration, weak pulse. Differential diagnosis: Dietary indiscretion, intolerance or allergy; IBD; lymphangiectasia; gastrointestinal obstruction due foreign body, neoplasia or stricture; pancreatic abscess; EPI; acute/chronic
pancreatitis; aspiration pneumonia; infectious pneumonia (B. bronchiseptica, S. zooepidemicus, P. multocida, P. aeruginosa or K. pneumoniae). Diagnostic tests: FAST (Focused assessment with sonography for trauma) scan - Ascites. Abdominocentesis - Exsudate with moderate
neutrophilic inflammation (Appendix I, table 3). PCV and TS: 56% and 8.0 g/dL. Blood glucose -
114 mg/dL. Lactate - 1.9 mg/dL. Blood pressure - 85 mmHg. Complete blood count - Left shifted
neutrophilia, lymphopenia and mild thrombocytosis (Appendix I, Table 1). Chemistry panel -
Mildly elevated liver enzymes, elevated creatine kinase and hypercholesterolemia (Appendix I,
Table 2). PT / aPTT - 8.2 / 24.9 seconds. Abdominal ultrasound - Moderate abdominal effusion,
hypoechoic pancreas with irregular margins, hyperechoic fat surrounding pancreas and other
organs in cranial abdomen; two hyperechoic splenic nodules. Abdominal and Thoracic
2
inflammation and possible fibrosis; findings consistent with acute pancreatitis. Diagnosis: Chronic pancreatitis with acute flare-up and aspiration pneumonia. Treatment and evolution: Blackjack was hospitalized and remained in the ICU for 6 days. Day 1 - He received a poly-ionic solution (Plasmalyte A®) at 288 mL/h IV, and later a 350 mL IV bolus to correct hypovolemia.
Fentanyl with lidocaine IV at CRI of 1.4-2.8 mL/h was provided as pain management. In order to
control the aspiration pneumonia and prevent from sepsis, 1050 mg of ampicillin/sulbactam
(Unasyn®) IV TID was provided as well. Additionally, 35 mg of maropitant and pantoprazole IV
SID was given to control nausea and hyperacidity, respectively. The primarily given fluid bolus
drastically improved his mentation and blood pressure (120 mmHg), allowing him to stand. He
had no vomiting or diarrhea while hospitalized. Day 2 - A 250 mL fresh frozen plasma transfusion was provided during 4 to 6 hours to correct the hypoalbuminemia. He received 2
mg/kg IM SID of diphenhydramine (Bendadryl®) preceding the plasma transfusion as prevention
for possible anaphylactic reaction. In the afternoon, Blackjack began to have increased
respiratory rate and ptyalism, indicative of severe pain. The pain management was reassessed
and the fentanyl-lidocaine rate increased. Amylopectin (Vetstarch®), a synthetic colloid was
added at 20 mL/h as attempt to reduce fluid retention. Abdominal circumference was
re-evaluated on a daily basis (increased from 83.8 to 88.9 cm). Day 3 and 4 – The abdominal circumference decreased to 61 cm and to 55.9 cm, respectively. At this point, the patient was
kept on the same medication, and an ultrasound guided FNA of the pancreas confirmed the
diagnosis of acute pancreatitis. As anorexia persisted, a nasogastric tube was placed and was
fed with 4 mL/h of a peptide-based nutrition for metabolic stess (Perative®). Day 5 - The nasogastric tube came out after a sneezing episode but the appetite returned gradually. The
fluid therapy was reduced and the pain management adjusted accordingly. Day 6 - Blackjack's blood work showed no abnormalities, so he was sent home with oral omeprazole (40 mg), a
broad spectrum antibiotic (amoxicillin and clavulanic acid 375 mg) and strict instructions for a
consistent low fat diet. Prognosis: Fair if strict low fat diet is followed and recurrences are avoided.
Discussion: Pancreatitis may be classified as acute or chronic and is a condition that consists of pancreatic hyperstimulation caused by premature enzyme activation, leading to
severe inflammation.1,2,3 It’s a very common but under-diagnosed disease, affecting any breed,
age or sex.2,3 The most frequently affected breeds are Miniature Schnauzer, Shetland
Sheepdog, Yorkshire Terrier, Boxer, Spaniels, Terriers and Collie, while the age range is
between 5 to 9 years old.1,2,3 The breed predisposition suggests the existence of a possible
underlying genetic predilection as well as an emerging association in some breeds with
imune-mediated diseases (Cocker Spaniel), especially correlated to keratoconjunctivitis sicca.1
3
50% develop diabetes mellitus, exocrine pancreatic insufficiency, or both.1 However, approximately 90% of the cases are idiopathic with no evident cause, others than a possible
hereditary factor.1 A less frequent cause might be duct obstruction or bile reflux to pancreatic
duct.1 The idiopathic form seems to be particularly common in Spaniels, Collies and Boxers.1,2
According to some studies, many dogs with pancreatitis are obese or have subclinical
endocrinopathies associated, such as hyperadrenocorticism, hypothiroidism and diabetes
mellitus.3,8 Other studies propose a mild increased risk in female dogs and with the use of certain medications such as, potassium bromide, phenobarbital, azathioprine, and
L-asparginase, as well as diet indiscretion.3,8 Although severe hypertriglyceridemia has not yet
been proven to be an exact cause in dogs, others than the Miniature Schnauzer, it is presumed
that it might have impact on the disease in dogs of all breeds.3
The main function of the exocrine pancreas is secreting digestive enzymes, bicarbonate and
intrinsic factor into the proximal duodenum.1 The function of those enzymes is to achieve
primary digestion of the larger food molecules which mostly requires an alkaline pH
environment.1 The pancreas itself, secretes several other enzymes, implicating α-amilase and
lipase as active molecules and proteases, phospholipases, ribonucleases and
deoxyribonucleases as inactive precursors, or also known as zymogens.1,2 However, tryspsin, a
serine protease, is the major inactive zymogen that is activated by zymogen trypsinogen
contained by the pancreatic acini.1 Consequently, trypsinogen is activated by the enzyme
enterokinase localized within the duodenum in response to intraluminal fat and amino acids.1,2
This causes peptide cleavage and results in activation of trypsin, which then leads to activation
of other zymogens within the intestinal lumen.1,4 In addition to this, other factors can contribute
to pancreatic secretion.1,2 Those involve the enteric nervous system, the vagus nerve and the
hormones secretin and cholecystokin.1,2 Pancreatitis is often a self-limiting process but may
progress to pancreatic necrosis due to reduced pancreatic blood flow, leukocyte and platelet
migration, leading to electrolyte and acidbase imbalance.4 While in chronic pancreatitis the most
common findings are low cellularity, lymphocytic or neutrophilic infiltration, with irreversible
fibrosis and atrophy, in the accute form of the disease it is more suggestive to find
hypercellularity, neutrophilic infiltration (intact and degenerated), degenerated pancreatic acinar
cells, pancreatic necrosis, peripancreatic fat necrosis and edema.2,3
The clinical signs may vary from anorexia, vomiting and diarrhea, abdominal pain, lethargy or
weakness but no pathognomonic signs exist for canine pancreatitis.3 A study concluded that the
signalment on presentation was similar in acute and chronic pancreatitis, of which the most
frequent were lethargy, inappetence, vomiting and diarrhea.9 However, other recent reports
proved that pancreatitis can be subclinical, occurring with lethargy and weakness as very mild
4
such as icterus, fever or hypothermia, dehydration, hemorrhage, diathesis, ascites or even
cardiovascular shock, DIC and multiorgan failure.3 Blackjack had evidence of anorexia, vomiting
and lethargy but no apparent abdominal pain or diarrhea.
In terms of diagnostics, a complete blood count, biochemical panel and urinalysis are not
specific but might give an indication of the disease and should always be included to rule out
other differentials.1,3 The clinicopathologic findings are often normal in mild cases, however
anemia or hemoconcentration, leukocytosis or leucopenia, as well as thrombocytopenia can be
some of the abnormalities.3,4 Other than this, electrolyte imbalance, elevated hepatic enzyme,
hyperbilirubinemia or azotemia due dehydration may be appreciated.3,4 Measurement of serum
amylase or lipase activity is often reported on routine biochemical panels. Anyway, there are
several typical pancreatic enzyme assays of which serum cPLI concentration is considered the
most sensitive (64-93%) and specific available, comparing to cTLI (36.4-46.7%), serum amylase
activity (18.2-73.3%), serum lipase activity (13.6-69%) and abdominal ultrasound (67-68%).3 For
Blackjack these testing was not essential regarding the obvious history and diagnostic findings.
Regarding the low sensitivity (24%), the diagnosis can not be based on abdominal radiographs
solely.3 Nonetheless, typical radiographic findings such as Blackjack’s, involve an increased soft
tissue opacity with decreased serosal detail, especially in the right cranial abdomen which is
indicative of localized peritonitis.3,4 Occasionally, it is possible to identify punctiform calcification
in longstanding diseases, which is mainly caused due saponification of mesenteric fat around
the pancreas.4 In case of acute disease, gaseous dilation of bowel loops or displacement of the
stomach and duodenum, abdominal effusion, as well as, intestinal obstruction and false
masslike appearance caused by surrounding fat necrosis may be identified.1,3 Ultrasonography
is considered the most sensitive (68%) technique to obtain accurate visualization of the
pancreas.3 Some of the characteristic ultrasonographic findings consist of hypoechoic and
enlarged pancreas, dilated pancreatic duct, swollen hypomotile duodenum, biliary dilation,
peritoneal fluid and cavitary lesions such as abscess or pseudocyst.4 Performing a correct
measurement of the pancreas might help diagnosing abnormal morphological changes in size,
which seems to be more subtle in chronic pancreatitis and EPI, than in acute pancreatitis.6
Other advanced imaging modalities have not been proven worth cost-effective results in
veterinary medicine, and beside that, have a high complexity level associated and limited
availability.3 Given that no single specific modality is totally reliable, abdominal ultrasonography
in combination with histopathology or cytology are vital for a truthful diagnosis.3,4 In any case of
pancreatitis, histology is highly from all, the gold standard and is ultimate to differentiate
inflammation from possible neoplasia.1,5 According to one study, some dogs with chronic
pancreatitis were erroneously diagnosed with chronic hepatitis on basis of increased serum
5
histologically.5 Chronic pancreatitis appears to be associated with reactive hepatitis but not
chronic hepatitis.5 For Blackjack, a biopsy or a fine needle aspirate were both suggested and
after explaining the advantages and disadvantages of both modalities, the last option was
chosen by the owners. The fine needle aspirate is clearly the less risk-associated method but
not the most diagnostic.3,4,5
Therapeutically, canine pancreatitis consists mainly of symptomatic treatment.3,4
Dehydration, hypovolemia and consequent tissue hypoperfusion with diminished pancreatic
microcirculation might have effect on the development of local and systemic complications and
are so, a reason to perform a proper fluid therapy.3 The treatment of choice is the replacement
with isotonic fluid solutions such as 0.9% NaCl or lactated Ringer’s solution, but it is unclear
which shows the best results.3,4 The fluid therapy consists of a 10 mL/kg bolus with 5
mL/kg/hour rate for the first 8 hours.10 According to response, the bolus can be discontinued
and the rate can be decreased to 2.5 mL/kg/h if responded adequately, or the same bolus and
rate can be repeated if the patient is refractory, monitoring carefully for fluid overload.10 More
severely affected animals may require initial shock rates (90 mL/kg/h for 30 to 60 minutes)
followed by synthetic colloids.1 Synthetic colloids and plasma administration may purpose
benefits due the proteinase inhibitors as component, which is responsible for the correction of
hypoalbuminemia, replacement of α-macroglobulins and coagulation factors, as well as
improvement of systemic inflammation.10 Electrolyte imbalance such as hypokalemia, should be
considered since most of the fluid solutions contain only 4 mEq/L of potassium when most
cases require at least 20mEq/L.1 The correction can be achieved with potassium chloride IV at a
rate of 0.15 to 0.5 mEq/kg/h.1
Analgesia is the next step of the treatment approach, as pancreatitis causes severe
abdominal pain and discomfort which should not be underestimated. Buprenorphine can be an
excellent option for mild to moderate pain, while morphine, hydromorphine, methadone or
fentanyl are best for severe pain.3 Blackjack was submitted to a multimodal pain management,
combining fentanyl with lidocaine, allowing not only to lower dosages, but also to diminish side
effects. Other options for combined pain management are ketamine or morphine.3 The
prophylactic use of antibiotics has no proven benefits and ins regarding to this, only
recommended in infectious complications, such as Blackjack’s aspiration pneumonia.3
Blackjack
received a broad spectrum antibiotic (ampicillin/sulbactam), but metronidazole, ciprofloxacin or
chloramphenicol are other alternatives that have the ability to achieve therapeutic levels in the
pancreas.3 As management of emesis and high susceptibility to gastroduodenal ulcers,
Blackjack received maropitant and pantoprazole, respectively. Other antiemetics as
metoclopramide seem to be very effective but not ideal, since it may increase pancreatic
6
Nutrition management is an essential step that has been changing along the years.1,2,3 While
initially food withholding was recommended, now the opposite is accomplished in practice.1,2,3 It
is recognized that patients should not be withhold of enteral nutritional support for longer than
24 hours, and that parenteral and enteral nutritional support are both better alternatives than
providing none.3,4 In case of absence of vomiting signs, the patient should be fed by mouth and
if anoretic, a feeding tube should be placed.3 A jejunostomy tube should only be considered in
animals with refractory vomiting or severe pancreatitis, enduring exploratory or therapeutic
laparotomy.1,3,4,10 The selection between parenteral or enteral is mainly made according to
toleration of the patient, since the application of the latter is more complex.3
Beside this, a continued fat restriction or a strict and balanced low fat diet is currently the
preferred option for dogs.3,4 Surgical intervention is rarely an indication but may be necessary
regarding the sequels that chronic or recurrent inflammation can cause, involving pancreatic
abscess or pseudocysts, necrotic masses and extrahepatic biliary tract obstruction.3,4
The prognosis for dogs with pancreatitis is difficult to foretell in order to the unpredictable
nature of the disease, depending mainly of the severity on presentation and complications
associated.1,3,4 This way, patients such as Blackjack, with acute flare-ups require exactly the
same intense treatment as those who present with the classical acute form, covering the
equivalent risk of mortality.1
References:
1- Nelson R.W, Couto C.G (2009) “The Exocrine Pancreas” in Small Animal Internal Medicine, 4th edition, Mosby Saunders, 579-596.
2- Steiner J. M (2010) “Canine Pancreatic Disease” in Ettinger S.J, Feldman E.C Textbook of Veterinary Internal Medicine, 7th edition, Saunders Elsevier, vol 2, 1296-1315.
3- Washabau R.J (2012) “Diseases of Gastrointestinal Tract” in Washabau R.J, Day M.J Canine and Feline Gastroenterology, 1st edition, Saunders Elsevier, 799-811.
4- Simpon K. W (2003) “Diseases of the Pancreas” inTams T.R Handbook of Small Animal Gastroenterology, 2nd edition, Saunders Elsevier, 353-365.
5- Watson P.J, Roulois A.J, Scase T.J, Irvine R, Herrtage M.E (2010) “Prevalence of hepatic lesions at post-mortem examination in dogs and association with pancreatitis” in Journal of Small Animal Practice, 51, 566-572.
6- Penninck D.G, Zeyen U, Taeymans O.N, Webster C.R (2012) “Ultrasonographic measurement of the pancreas and pancreatic duct in clinically normal dogs” in American Journal Veterinary Research, Vol 74, No.3, 74, 433-437.
7- Watson P.J, Roulois A.J, Scase T.J, Holloway A, Herrtage M.E (2011) “Characterization of Chronic Pancreatitis in English Cocker Spaniel” in Journal of Veterinary Internal Medicine, 25, 797-804.
8- Watson P.J, Roulois A.J, Scase T.J, Johnston E.J, Thompson H, Herrtage M.E (2007) “Prevalence and breed distribution of chronic pancreatitis at post-mortem examination in first-opinion dogs” in Journal of Small Animal Practice, 48, 609-618. 9- Bostrom B.M, Xenoulis P.G, Newman S.J, Pool R.R, Fosgate G.T, Steiner J.M (2012) “Chronic pancreatitis in dogs: A
retrospective study of clinical, clinicopathological, and histopathological findings in 61 cases” in The Veterinary Journal, 195, 73-79
7
Clinical case Nr. 2: Pulmonology - Chronic bronchitis
Characterization of patient and reason of appointment: Maximus was a 6 year old, male castrated, 25 Kg, English Bulldog, that was presented to the UTVMC Emergency service with a
history of chronic coughing which initiated approximately one month ago.
Anamnesis: Maximus was an indoors dog that was walked in a public environment several times a day. The diet was based on dry food (Purina Light & Healthy®
)
. Recently, Maximus wasvaccinated for distemper, parvovirus, adenovirus type 1, parainfluenza, leptospirosis and rabies,
and was dewormed regularly. The cough initiated approximately one month ago and had
progressively worsened. One month prior to this, he was treated by the referring veterinarian
with trimetoprim/sulphadiazine (Tribrissen®) but did not seem to improve. After this first
treatment attempt, he boarded twice regarding the cough. Concerning to medication, he was
receiving prednisone 20 mg EOD, doxycycline 100 mg BID and milbemycinoxime with lufenuron
(Sentinel®) for heart worm prevention. Physical exam: The lung sounds were mildly decreased on the left thorax. Thorough cardiovascular examination did not reveal any signs of cardiac
dysfunction and rectal temperature was 39.2 ºC. Respiratory exam: Decreased lung sounds, more intense on the left side of the thorax with very mild crackles. Both nares were slightly
stenotic, frontal sinuses sounded normal on percussion, larynx and trachea were normal on
palpation. List of problems: Cough, decreased lung sounds, mild crackles. Differential diagnosis: Brachycephalic airway syndrome, chronic or allergic bronchitis, aspiration pneumonia, dirofiloriasis, carcinoma, lymphoma, granuloma, blastomycosis, histoplasmosis,
bacterial pneumonia or bronchitis caused by B. bronchiseptica, S. zooepidemicus, P. multocida, P. aeruginosa, K. pneumonia, Mycoplasma spp. Diagnostic tests: Complete blood count - No significant changes. Chemistry panel - Mild hyperglobulinemia (3.8 g/dL) and mild elevation in
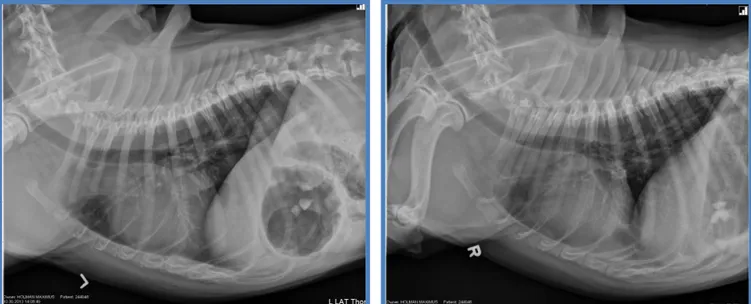
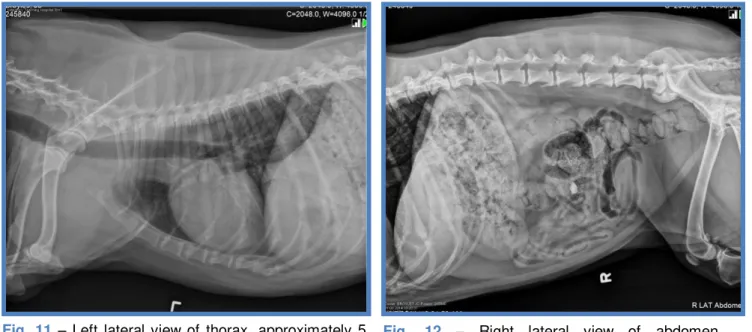
bicarbonate (26mmol/L). Thoracic radiographs - Mild bronchial pattern and bronchial wall
mineralization (Appendix II, Fig. 4, 5 and 6).
Maximus remained in ICU for respiratory watch during the first hours and was released in the
afternoon. Maximus' state was not critical and so, he was discharged in the condition of
returning in the morning fasted. In order to perform a flexible bronchoscopy with
bronchoalveolar lavage for cytology and culture, which is considered the gold standard to
diagnose chronic bronchitis. An elongated soft palate, as well as, mildly thickened bronchial
mucosa, slightly thickened mucus accumulation in bronchioles and hyperemic trachea were
appreciated. During the bronchoscopy, coupage was used to release mucus and promote coughing in contemplation of obtaining samples from the deeper airways. Bronchoalveolar
lavage - Low grade neutrophilic to mixed (few lymphocytes) inflammation and goblet cell
8
Diagnosis: Maximus was diagnosed with sterile chronic bronchitis. Treatment and evolution: He was discharged the same day of the bronchoscopy, but no medication was prescribed.
However, an antihistamine, such as cetirizine hydrochloride (Zyrtec®) at 5 to 10 mg, was
suggested as trial in pursuance of evaluating, whether or not existed an allergic or seasonal
component to the disease. Any irritants within the household or environment were suggested to
be removed or minimized as prevention. Other recommendations were to continue the
prednisone but on a dose of 25 mg for 2 weeks, then decreasing it to the lowest effective dose,
with a 25 to 30% decrease every 2 weeks until reaching an effective maintenance dose.
Alternatively, inhaled corticosteroids were indicated, such as fluticasone at 125 µg BID
associated with a special inhaler for dogs. In addition, theophylline at 10mg/kg PO BID was also
suggested. For the worsening periods of the cough during night time, a cough suppressant was
indicated, as long as the cough continued to be non-productive (hydrocodone 5.5 mg QID-BID
as needed or diphenoxylate 5 to 12.5 mg BID until the cough was under control, and then, as
needed). Prognosis: Good to fair.
Discussion: Canine chronic bronchitis is a longstanding inflammation of the lower airways that can present different etiologies. Those can enclose an infectious, toxic or alergic cause and
it usually emerges with evident signs of coughing of at least two months of duration in one
consecutive year.1,2,3,4 The etiology is often unknown, but the disease itself is a reflection of
repeated inflammatory sequences that leads to mucosal damage, mucus hypersecretion,
obstruction of the airways and thus, disturbed mucociliary clearance.2,4 Consequently,
predisposition to infections can occur due to reduced defense mechanisms.1,4 Other potential
etiologies for increased secretion in the bronchi are atmospheric pollution, such as chronic
exposure to sulfur dioxide, passive smoking in poorly ventilated and confined spaces,
respiratory tract infections, hypersensitivity to certain allergens and genetic or acquired defects
such as α1-antitrypsin deficiency, immunodeficiency and mucociliary defects.1 The breeds
affected are generally middle-aged to older small breed dogs, which can be related to the high
susceptibility to mitral valve insufficiency and tracheal collapse.1,3,4 However, Cocker Spaniels,
Poodles and Terriers appear to be the principal breeds involved.3,4 Several other diseases can
lead to coughing, giving especial emphasis to the cardiac diseases that arise isolated or
simultaneously with a coadjuvant pulmonary condition, such as bronchitis.3,4 The major heart
diseases that can accompany coughing are left atrial dilation due to valvular insufficiency or
generalized cardiomegaly, pulmonary hypertension and congestive heart failure.4 Nonetheless,
in presence of a heart murmur, the chronic cough is more likely to have pulmonary than cardiac
origin.3 Whereas in younger dogs it is important to search for an infectious disease, in older
dogs a neoplasic cause should be considered as well. The most common neoplasias that may
9
sterile chronic bronchitis is mainly made by exclusion.2,3 It is of great relevance to obtain a
detailed history in order to determine a possible environmental or infectious cause, evaluating
the frequency, pattern and development of the cough and differentiating it from another possible
underlying or primary disease.1,2,3,4 Physical examination may reveal normal to increased lung
sounds, with occasional expiratory wheezes or pan-inspiratory crackles.2 Other signs are
expiratory dyspnea and if more severe, exercise intolerance and collapse.2,3 However, most of
the dogs with chronic bronchitis are geriatric and seem to be systemically healthy, presenting
with only one exclusive chief complaint, which is persistent and productive cough.3 This is
similar to Maximus, that was presented with one main clinical sign, chronic coughing, although,
non-productive. Additionally he had diminished lung sounds on the left side of the thorax which
could be due to thickening of the bronchial walls that causes mild obstruction of the airways.4 It
is recognized that longstanding inflammation can lead to bronchiectasy, which is usually
irreversible in this stage of the disease.1,4 Other complications that may occur are COPD
(chronic obstructive pulmonary disease), pulmonary hypertension, tracheobronchomalacia, and
mycoplasmal or other bacterial infections.4 In some way, the canine lower airways appear to
have extensive interconnections between alveoli and bronchioles, which permits collateral
ventilation.1 This means that the disease must be highly developed before the clinical signs and
COPD are evidenced.1
In case the patient is systemically affected, it is important to evaluate the blood parameters
by performing a CBC (complete blood count), a chemistry panel and possibly a urinalysis.1,4 On
Maximus a blood work was performed since he was a middle-aged dog that had no recent
references of testing available. The results were mostly unremarkable, evincing a very mild
hyperglobulinemia with elevated bicarbonate. The hyperglobulinemia was explained by the
chronic inflammation, while the elevation in bicarbonate could be due metabolic alkalosis
comparing to the slight decrease in chloride (107mEq/L). No obvious signs of allergy or parasitic
infection were detected, however, the latter could had been confirmed by performing fecal
examination, even though he was dewormed.
Whereas pulmonary function testing is common to perform in humans due to high specificity
for bronchitis, in veterinary medicine it has added difficulties to execute on a routinely basis
concerning the inaccessible equipment.3 These testing consist of analyzing arterial blood gas,
endtidal CO2, or pulse oximetry to evaluate gas exchange and breathing effort.3 More practical
tests, consist of collecting blood for arterial blood gas analysis which can detect hypoxemia or
increased alveolar-arterial gradient to confirm pulmonary dysfunction.3 Additionally, a 6-minute
walk test is based on measuring the distance a dog is capable to walk within 6 minutes.3 If the
result is less than 400 meter, the patient is considered to have respiratory disease.3 One study
10
pharmacological stimulation, could help characterizing pulmonary function and ventilatory
deficits in dogs with restrictive pulmonary disease, such as obesity.6 However, thoracic
radiography may also be supportive. To accomplish this appropriately, at least three views
should be obtained: left lateral, right lateral and ventrodorsal. This is applicable not only to
identify the lung affected but also to detect possible masses, cardiac disease or foreign bodies,
which is impracticable without a tridimensional view. Radiographs usually reveal bronchial or
interstitial infiltrates, even though they might be subtle.2 Controversially, a study showed that
dogs with bronchitis shared many radiographic findings with healthy dogs, linking interstitial
bronchial calcification or dilatation and interstitial infiltrates.2 The major divergence found was
thickening of the airway walls, revealing that radiography has low sensitivity (~50-65%) and
might as well be unreliable to detect pulmonary dysfunction, particularly in very obese
patients.2,4 Maximus’ radiographs were consistent with mild bronchial pattern and bronchial wall
mineralization which is indicative of chronic bronchitis. No signs of pneumonia were seen,
however, two accidental findings were evidenced: 1) a presumptive herniated and mineralized
intervertebral disc at T13-L1, as well as a 2) mild degenerative joint disease of right
scapulohumeral joint, both appeared to be asymptomatic, and were for this reason, no further
investigated (Appendix II, Fig. 6).
CT scanning seems to be promising since the airway detail is superior comparing to
radiographs, but it is expensive and requires general anesthesia and is so, not commonly
employed.3 Beside this, bronchoalveolar lavage (BAL) is convenient to obtain samples for
cytology and microbiology.3 Cytology typically discloses neutrophilic or mixed inflammation,
mucus, hyperplastic bronchial epithelial cells, ciliated cells, macrophages and goblet cells.1,4
The presence of degenerative neutrophils may indicate bacterial infection, while eosinophilia is
suggestive of allergic component or parasitic disease.1,4 Isolation of Bordetella bronchiseptica, Streptococcus spp., Pasteurella spp., Escherichia coli, Pseudomonas spp., Klebsiella spp., or Mycoplasma spp. are strongly recommended and even aerobic bacteria can be isolated, since the respiratory tract is not totally sterile.1,2 A significant growth and a positive bacterial infection
is considered when greater than 1.7 x 103 colony-forming units/mL is achieved in combination
with a suppurative inflammation on cytology.2 Bronchoscopy combined with BAL is the ideal and
preferred procedure to evaluate the severity of the disease and exclude other differentials.1,2,3,4
This technique has the potential to observe minutely within the deepest airways, and obtaining
this way, representative samples.1 Characteristically, the airways evidence excessive mucus,
irregular and thickened mucosa with hyperemia, fibrosis, epithelial hyperplasia, glandular
hypertrophy, and inflammatory infiltrates.1,2,3,4 According to one study, older dogs (>8 years),
seem to have more irregular bronchial mucosa, prominent mucosal vessels and bronchiectasis,
11
as a higher percentage of lymphocytes comparatively to middle-aged dogs (5-8 years).7
Patients that during this procedure experience bronchial collapse as regards to passive
expiration seem to have a worse prognosis.1,3
The treatment is generally managed symptomatically, starting by eliminating possible
environmental factors, such as tobacco, cleaning products, humidity and by replacing air filters,
as well as controlling obesity or anything that is recognized to have influence on the disease.3,4
However, obesity is the most significant factor and should be adequately managed, promoting
exercise according to the ability of the patient and by increasing it gradually.1,3,4 Due the
increased tracheal sensitivity for cough, it is recommended to use a harness instead of the
regular collars.1,3 As soon as bronchopulmonary infection is excluded, a treatment with
glucocorticoids should be initiated in order to reduce inflammation and secretion of mucus,
improving the cough.1,2 More specifically, prednisone (0.5 to 1 mg/kg) as oral medication is very
effective.2,3 It should be tapered as soon as improvement is seen (~10-14 days), reducing 25%
every 2 to 3 weeks until the lowest dose effective is achieved.2,3 According to a study, patients
that experience excessive side effects from glucocorticoids or those with coexisting medical
conditions, seem to respond positively to inhalation therapy with fluticasone proprionate (125 µg
BID).2,3 The medication should be inhaled approximately 6 to 8 times, however the ideal dosage
is uncertain because of undefined quantity that achieves the lungs.2,3 An adequate starting dose
is approximately 10 to 20 µg/kg twice daily.3 Controversially, endocrine effects evaluated by a
study, revealed cortisol suppression particularly by oral prednisolone and inhaled fluticasone,
considering inhaled budesonide a safer choice.5 Other medications that can contribute to the
treatment plan are bronchodilators, of which theophylline, a methylxanthine, is the most
commonly used. Theophylline has unproved and unspecific potential to decrease diaphragmatic
fatigue and increase mucociliary clearance, making this a preferred option.3,4 A long-acting form
of theophyline at a dose of 10 mg/kg BID or 15mg/kg SID, might also improve the expiratory
airflow and enclose synergic effects with glucocorticoids.3,4 However, most of the dogs with
chronic bronchitis don’t cover reversible bronchodilation which makes the use of this medication
controversial or unideal.1 Other bronchodilators used are aminophyline and oxtriphyline.4
Comparatively, sympathomimetics such as terbutaline or albuterol, do not achieve the same
efficiency and may cause anxiety and restlessness.3,4 Antibiotherapy is limited to patients with a
positive culture.3 In high suspicion of a non-confirmed bacterial infection, doxycycline or
azithromycin may be initiated since both have satisfying anti-inflammatory and antimicrobial
effects.3 On presentation, Maximus was on doxycycline and did not improve. Afterwards, the
results of cytology and culture confirmed the absence of an infectious cause. Other antibiotics
as fluorquinolones have elevated risk of bacterial resistance as well as theophylline toxicity, and
12
In association with the treatment, nebulization followed by coupage should be recommended to fluidize the secretions and to promote clearance of the airways.2 Chronic bronchitis is
generally an irreversible condition that is managed by reducing inflammation and ameliorating
quality of life, not only because the disease itself attends to be incurable, but also due to the
uncertain outcome.3 Concerning to this, before a therapeutic plan is stipulated, it is best to
initiate one medication at a time, in order to determine the ideal pharmacological combination
and adapting it over time, because relapse of the coughing may occur.3,4
References:
1- Kuehn N. F (2003) “Chronic Bronchitis in dogs” in King L.G Textbook of Respiratory Diseases in Cats and Dogs, Saunders Elsevier, 1st edition, 379-387.
2- Johnson L. R, Mckiernan B. C (2010) “Canine tracheobronchial disease” in Fuentes V.L, Johnson L.R, Dennis S Bsava Canine and Feline Cardiorespiratory Medicine, BSAVA British Small Animal Veterinary Association, 2nd edition, 274-279.
3- Rozanski E (2014) “Canine Chronic Bronchitis” in Veterinary Clinics of North America: Small Animal Practice, 44, 107-116. 4- Nelson R.W, Couto C.G (2009) “Disorder of the Trachea and Bronchi” in Small Animal Internal Medicine, 4th edition, Mosby
Saunders, 285-291.
5- Melamies M, et al. (2012) “Endocrine effect of inhaled budosenide compared with inhaled fluticasone propionate and oral prednisolone in healthy Beagle dogs” in The Veterinary Journal, 194, 349-353.
6- Manens J, et al. (2011) “Effects of obesity on lung function and airway reactivity in healthy dogs” in The Veterinary Journal, 193, 217-221.
13
Clinical case Nr.3: Soft Tissue Surgery – Adrenalectomy
Characterization of patient and reason of appointment: Katie was a 7 to 9 year old, female spayed, 21 Kg, Australian Shepherd, that was presented to the UTVMC Soft Tissue
Surgery service for a right adrenalectomy.
Anamnesis: Katie was adopted one year ago, had vaccinations as well as deworming up to date, and was fed with a dry Premium quality diet. She had a history of controlled discoid lupus erythematosus, however, Katie's main problem was the recurrent UTI that she had been
experiencing every month, for 11 consecutive months. Ultrasound confirmed the diagnosis of
emphysematous cystitis as well as right adrenomegaly (0.90 cm). Amoxicillin was added to
enrofloxacin, which was extended to 4-6 weeks. Due to the slow hair growth and marked
lethargy, hypothyroidism was suspected which was confirmed later with a TSH/T4 level test.
Physical exam: The skin changes (alopecia, seborrhea, crustae and erythema) were related to the previous diagnosis of calcinosis cutis. No murmurs or crackles were ausculted and the rate was normal. Abdominal palpation revealed a mild hepatomegaly and the rectal temperature was
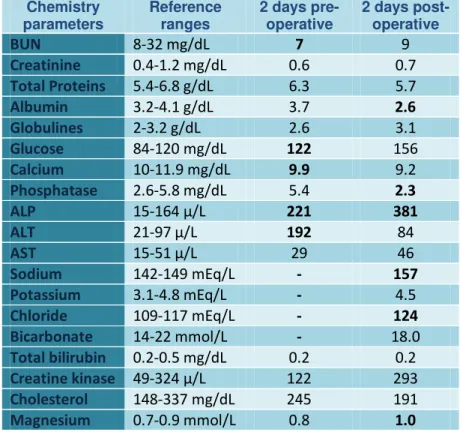
38.8ºC. Diagnostic tests: Crossmatch test- receptive to all blood donors. Chemistry panel - mild decrease in BUN, moderate elevation in ALP and ALT (Appendix III, table 5). CT scan -
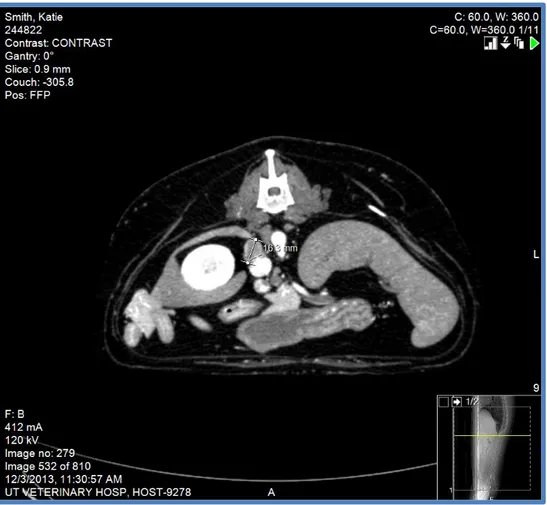
confirmed increased size of right adrenal mass of 1.63 cm (Appendix III, Fig. 7). PCV/TS – 57%
/ 8.0 mg/L. On re-evaluation, an ultrasound revealed an increase in size of the right adrenal
gland with invasion of the vena cava. Beside this, thrombus formation in caudal vena cava with
mineralization and a mild hepatomegaly were also appreciated, while a skin biopsy returned as
calcinosis cutis. Katie was experiencing exacerbated lethargy, polyphagia as well as polyuria and polydipsia. An ACTH stimulation test was inconclusive, nonetheless, a LDDS (Low Dose
Dexamethasone Suppression) test, as well as a HDDS (High Dose Dexamethasone
Suppression) test made it possible to confirm the presence of adrenal-dependent Cushing's
disease. Pre-operative treatment: Prednisone 1 mg/kg PO SID, administered one day previous to surgery and received last dose on the morning of surgery. Fluid therapy with
lactated Ringer’s solution at 100 mL/h. Anesthesia: Pre-medication – Fentanyl 5 µg/kg IV and lidocaine 2mg/kg IV; Induction – Ketamine 5 mg/kg IV, midazolam 0.5 mg/kg IV, propofol 2
mg/kg IV; Maintenance – Propofol 2 mg/kg IV given twice, ephedrine sulfate 0.1 mg/kg after
induction due hypotension, dopamine 1 mL/h at CRI, ketamine 2 mg/kg IV, sevoflurane 2% with
oxygen, fentanyl-lidocaine at CRI; dexamethasone diluted at CRI during surgery; synthetic
colloid 120 mL IV and fluid bolus of isotonic solution 10 mg/kg during surgery; Antibiotic –
Cefazolin 20 mg/kg IV at anesthetic induction. Surgery: After trichotomy and antisepsis, Katie was placed in dorsal recumbency. The midline incision was made from the xyphoid to just
14
subcutaneous tissues were cut with Metzenbaum scissors and thebodywall was cut a with
curved Mayo scissor. The falciform fat was broken down digitally and tied off with 2-0
Monocryl®, and was then removed. A Balfour retractor was placed in the cranial abdomen and
the abdominal cavity was explored. After this, the mesoduodenum was retracted carefully,
without damaging the pancreas, to allow visualization of the right adrenal gland. During the
exploratory laparotomy, it was noticed that the right kidney's surface looked irregular. A red
rubber catheter (18 Fr) was cut in four pieces to create a Rumel tourniquet. The retroperitoneum
was bluntly dissected using curved hemostats for placement of the umbilical tape with the
Rumel tourniquets. The caudal torniquet was placed around the caudal vena cava and renal
vein (the left phrenicoabdominal vein was not included to allow blood flow). Cranial Rumel
tourniquet was placed over the vena cava cranial to the right adrenal gland. The tourniquets
were just placed but not actually tightened down. The right adrenal gland was dissected from
right kidney using curved hemostats and hemostasis was maintained with bipolar. The right
adrenal gland had a round egg shaped tumor with a stalk. Two malleable retractors were placed
to allow more visualization of the abdomen. A third malleable retractor was placed, as blunt
dissection of the fascia of the vena cava from the tumor continued. Medium hemoclips were
placed across the phrenicoabdominal vein and the vessel was transected with Metzenbaum
scissors. Fat was stripped next to the tumor using cotton tipped applications. A lymphatic
branch was transected in dissection. Dissection from vena cava continued carefully and
thrombus was manipulated out of the vena cava and into the phrenicoabdominal vein. Three
large hemoclips were placed across the stalk of the tumor. The tumor stalk (phrenicoabdominal
vein) was transected using curved Metzenbaum scissors. No hemorrhage was seen. Finally the
body wall was closed using 0 PDS® in a simple continuous pattern, 2-0 Monocryl® in
subcutaneous tissue in a simple continuous pattern and 3-0 in skin with a ford interlocking
suture. Post-operative complementary tests:PCV/TS – 45% / 5.1 mg/L. Blood Glucose – 137 mg/dL. Histopathology – confirmed adrenocortical carcinoma with vena cava and multifocal
vascular invasion. Complete blood count – moderate neutrophilia, monocytosis (Appendix III,
table 4). Chemistry panel – mild hypoalbuminemia, mild hyperglycemia, mild hypocalcemia, mild
hypophosphatemia, moderate elevation of ALP, mild hypernatremia and mild hyperchloremia
(Appendix III, table 5). Post-operative treatment: Katie recovered from surgery in the ICU, where she remained during the night having received a poly-ionic and isotonic solution
(Plasmalyte A®) at 54 mL/h IV, and fentanyl + lidocaine IV at CRI of 1.6-6.5 mL/h. The
temperature, pulse and respiratory rate were re-evaluated every 6 hours. PCV, TS and blood
pressure were repeated twice daily. Katie was also on Soloxine® 0.3 mg PO daily and
amoxicillin 400 mg every 8 hours. During surgery Katie received 1.1 mg of dexamethasone IV
15
prednisone on a dose of 10mg PO BID, and 50 mg of tramadol and 200 mg of gabapentin were
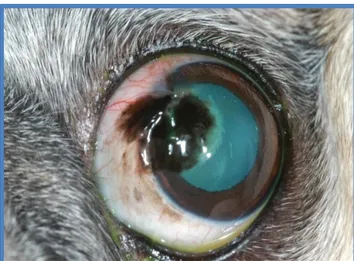
administered orally, every 8 hours. During physical examination, a corneal ulcer was noted on
the right eye so adequate treatment was provided. The following day, she was transferred to the
regular wards since her medical condition was not considered critical anymore. At this point,
blood work was repeated, as well as an ACTH stimulation test, in order to obtain a baseline of
cortisol levels posterior to surgery (even while on exogenous steroids). Previously to the ACTH
stimulation, cortisol levels were 31.5 ng/L and post stimulation, cortisol levels decreased to 29.6
ng/L (Appendix III, table 6). Katie stayed stable and was sent home the next day with 50 mg of
tramadol, 100 mg of gabapentin, 20 mg of prednisone, 0.2 mg of Soloxine®. Prognosis: Fair regarding metastatic disease.
Discussion: Adrenocortical tumors (AT) occur in approximately 15 to 20% of dogs with hyperadrenocorticism, which corresponds to less than 1% of all canine neoplasms.4,5 The most
commonly affected patients are middle-aged to older dogs without evident sex predilection,
although, a slight greater risk in females has been reported.3 The adrenal tumors that are most
frequently seen are mainly adenoma and carcinoma that account with equal incidence.4
Concerning to localization, the medullary tumors secrete catecholamines that tend to origin
severe hypertension, the adrenocortical secrete excessive glucocorticoids causing Cushing’s
syndrome. Nevertheless, in carcinomas, multiple hormone secretions, such as glucocorticoids,
mineralocorticoids and sex hormones, have been reported, even if very unusual.3,5 One study
reported that, approximately 50% of the dogs with adrenocortical tumors causing
hyperadrenocortisim (ATH), weigh more than 20 kg.4
Histopathology seems to be the only modality to distinguish the type of AT, however, it is
acknowledged that carcinomas tend to be larger in size, are more likely to invade other
structures and metastasize specially to liver, spleen, lungs and tricuspid valve.4,5 Thus, imaging
modalities likewise to abdominal ultrasound, radiography and CT scan might support
differentiation of ATs, and according to presence or absence of metastatic disease, the
malignancy might be predicted as well.3 Even though metastatic lesions represent no more than
26.7% of the canine adrenal tumors, it should always be considered.5 The exact pathogenesis
remains uncertain, but in humans, it is recognized that abnormal biosynthetic pathways result in
lack of adrenal steroidogenic enzymes (21β-hydroxilase and 11β-hydroxilase), leading to
hypersecretion of precursor steroids.3 Another factor for increased steroid precursors might be
invasion of the adrenal cortex by malignant cells that could possibly interfere with the normal
enzymatic trial.3 Some steroid intermediates, such as progestins, might disconnect cortisol from
its serum-binding protein, achieving increased concentrations of cortisol in the active form,
which will enhance the typical hyperadrenocorticism signs.3 Those progestins might as well act
16
cortisol is produced by the adrenal tumor which causes pituitary suppression, with consequent
decrease in ACTH concentration and atrophy of contralateral adrenal gland.4
The characteristic clinical signs consist of polyuria, polydipsia, polyphagia, panting,
pendulous abdomen, skin atrophy, symmetric alopecia, bruising, muscle atrophy and lethargy.3
Diagnostic wise, increased serum alkaline phosphatase activity, hypercholesterolemia and
hyposthenuric urine might suggest hyperadrenocorticism, making clinical pathology as well as
urianalysis with culture of enormous relevance.4 Approximately 85% of dogs with
hyperadrenocorticism have ALP activity higher than 150 IU/L.4
Katie’s recurrent UTI’s were explained by the endogenous glucocorticoid-induced immunosuppression, as well as polydipsia, that turned not only the urine diluted, but made Katie
more predisposed to infection, causing interference with the identification of the infection.4
However, the definitive diagnosis can only be made by combining abdominal ultrasound with
respective measurement of the adrenal glands and a LDDS/ HDDS/ Endogenous ACTH tests.4
Radiographs are not efficient to identify adrenal glands or adrenal tumors, but might identify
calcification, hepatomegaly, distension of the urinary bladder and create suspicion of Cushing’s
disease.3,4 Approximately 50% of ATH are calcified, which is equally distributed between
adenoma and carcinoma.4 On ultrasound, adrenomegaly is considered when the maximum
width is greater than 0.8 cm, and adrenal atrophy at maximum width less than 0.3 cm.4
Nevertheless, AT can occur bilaterally and even if uncommon, it should not be confused with
bilateral macronodular hyperplasia, which consist of multiple nodules of different sizes within the
adrenal cortex and is mainly thought to be a variant of pituitary-dependent hyperadrenocorticism
(PDH).4 CT or MRI should be considered to evaluate size and extension of the tumor prior to
adrenalectomy.2,4
Therapeutically, ATH’s treatment of choice is adrenalectomy, as long as no severe
metastatic disease is appreciated.1 To minimize the high risks associated post-operatively,
especially during the following 72 hours, it is vital to manage a preoperatively plan to guarantee
the patient is stabilized. It is recognized that the larger the size of the tumor, the greater the
complications which makes the diagnostic testing previous to surgery crucial as well.4 Despite
other underlying diseases, Katie was considered a good candidate for surgery, given that the
right adrenal mass presented a size of approximately 1.63 cm. Many times those tumors can
extend from 6 to 8 cm of diameter which escalates the surgery complexity.4
The removal of the adrenal gland leads to abrupt decrease of glucocorticoids and to
signalment such as depression, inappetence, lethargy and collapse.1,2 Along mineralocorticoid
suppression, other electrolyte and acidbase imbalance may be induced, involving
hyponatremia, hyperkalemia, acidosis and azotemia.1 As prevention, essentially in surgery,
17
Katie initiated the supplementation prior and during surgery regarding the fact that some
animals seem to have a delayed response to exogenous steroids.1 The healing process of the
suture line might be slower due catabolism and often protein depletion, requiring special care.1,2
The most worrying complications are hypertension with consequent cardiovascular disease and
pulmonary thromboembolism.1,2,4 To anticipate it, measurement of blood pressure is necessary
and a low-dose of aspirin can be provided along suspected hypercoabulability.1,4 For patients
that experience respiratory distress, oxygen as well as anticoagulant and thrombolytic agents
should be considered.1 Regarding to this Katie was maintained in cage rest, constant
respiratory watch and her position was changed every 4 hours, post-operatively.
The surgical approach can be performed by a ventral midline, as Katie, or paracostal
incision.1,2 The ventral midline incision allowed to execute a complete exploration of the
abdomen to detect possible metastasis and could had been a more useful choice in cases with
bilateral adrenalectomy.1,2 The second surgical approach gives better access to the adrenal
gland, although just unilaterally and does not cover the benefit to able a complete evaluation of
the abdomen.1,2 However, on ventral midline approach it is possible to extend the incision
paracostally on the side of the affected adrenal gland in case additional exposure is necessary.1
For Katie this was not required since the use of a Balfour retractor and three self-retaining
malleable retractors were efficient to obtain accurate visualization of the operating field.
Moistened sponges could had been used to cover the retractors, minimizing trauma to
surrounding organs and absorbing possible bleeding, although, its adequate removal from the
abdominal cavity must be certified.1 After dissection, an exploratory laparotomy was performed,
revealing irregular kidney surface of the right kidney and very mild hepatomegaly. Renal
damage could be consequence of chronic and recurrent UTI while hepatomegaly is explained
by hyperadrenocorticism. For temporary occlusion of the vena cava, a Rumel tourniquet was
created cutting a red rubber catheter and by using two parts of it for introduction of the umbilical
tape. In Katie, a double ligation was required due to invasion of the tumor within the vena
cava.1,2 The creation of a temporary occlusion of the major vein was crucial and critical at the
same time, since it avoids severe blood loss but also compromises blood supply, encourages
clotting and increases the risk of potential thromboembolism. However, the methodology with
these materials creates a better control on clamping since it tightens and unfastens easily, and
causes as well, a milder trauma to the vessels. The methods of hemostasis were achieved with
bipolar electrocautery for smaller vessels and hemoclips for phrenicoabdominal vein and tumor
stalk. Hand ligatures are difficult to perform around this area because vessels are small and
space area is limited.1,2 The hemoclips were used in larger vessels due to the rich blood supply
to the adrenal gland, as well as to assist with the removal of the tumor, for the reason that it
18
cavity. Adequate dissection can be challenging since the adrenal capsule must remain intact.1,2
In aggressive invasions, vascular surgery might be required.1,2 Around 25% of adrenal
neoplasms seem to invade the vena cava, phrenicoabdominal veins or renal veins,
nevertheless, it appears more frequently in pheochromocytomas.1
Regarding the delayed healing process, those patients require a tough suture with adequate
tensile strength.1,2 For Katie, polydioxanone was used, a slowly absorbable suture for the body
wall and Monocryl®, absorbable as well, for subcutaneous tissue and skin.
Postoperatively, the patient requires monitoring for dehydration, electrolyte imbalance,
thromboembolism, hemorrhage, infection and hypoadrenocortical collapse.1 An unilateral
adrenalectomy causes temporary adrenal insufficiency which means the patient requires
temporary glucocorticoid supplementation until the remaining adrenal gland responds, produces
sufficient endogenous steroids and homeostasis is achieved.1,2,4 This occurs progressively,
particularly because the function of the contralateral adrenal gland was suppressed by the AT.1
The main difficulty is prognosticating the exact moment when homeostasis is achieved to
perform ACTH stimulation test and decrease glucocorticoid supplementation. Along bilateral
adrenalectomy, a life-long glucocorticoid and/or mineralocorticoid replacement is imperative.1
Katie’s treatment plan consisted of initiating dexamethasone post and operatively, and by changing it for prednisolone the following day. This dose was continued for one week and then
tapered down gradually in 10 weeks. However, the medication adjustment could had been
made the same day, following surgery.1
According to one study, dogs with an adrenal gland tumor with maximum width of 5 cm, that
reported metastatic disease or vasculature invasion had poorer prognosis.6 Metastatic disease
seemed to be more frequent in dogs with adenocarcinoma and vein thrombosis.6 The median
survival time was 492 days as reported by a different study, which makes the prognosis for
Katie fair.7
References:
1- Fossum T.W (2012) “Adrenalectomy” in Small Animal Surgery, 4th edition, Mosby Elsevier, 633-646.
2- Adin C. A, Nelson R. W (2011) “Adrenal Glands”in Tobias K, Johnston S. Veterinary surgery Small Animal, 1st edition, Elsevier Saunders, 2033-2041.
3- Hill K. (2013) “Primary Functioning Adrenal Tumors Producing Signs Similar to Hyperadrenocorticism Including Atypical Syndromes in Dogs” in Rand J, Behrend E, Gunn-Moore D, Campbell-Ward M. Clinical Endocrinology of Companion Animals, 1st edition, Wiley-Blackwell, 65-69.
4- Nelson R.W, Couto C.G (2009) “Disorders of the Adrenal Gland” in Small Animal Internal Medicine, 4th edition, Mosby Saunders, 810-846.
5- Frankot J. L, Behrend E. N, Sebestyen P, Powers B. E (2012) “Adrenocortical Carcinoma in a Dog with Incomplete Excision Managed Long-term with Metastasectomy Alone” in Journal of the American Animal Hospital Association, 48, 417-423. 6- Massari F, Nicoli S, Romanelli G, Buracco P, Zini E (2011) “Adrenalectomy in dogs with adrenal gland tumors: 52 cases
(2002–2008)” in Journal of the American Veterinary Medical Association, 239, 216-221.