Braz. J. of Develop.,Curitiba, v. 6, n.5, p.23242-23252 may. 2020. ISSN 2525-8761
Study of the effect of high hydrostatic pressure in the decrease of listeria
monocytogenes in pasteurized mixed cheese
Estudo do efeito da alta pressão hidrostática na diminuição de listeria
monocytogenes em queijo misturado pasteurizado
DOI:10.34117/bjdv6n5-032
Recebimento dos originais:15/04/2020 Aceitação para publicação:04/05/2020
Alda Cristiane de Oliveira Alves
MSc Food Quality, Development and Innovation of Universidad de Valladolid Secretary of State for Science, Technology and Vocational and Technological Education
Address: Arcipreste Manoel Teodoro, 1020, Belém, Brazil E-mail: alda.alves@sectet.pa.gov.br
Marta Hernández Pérez
PhD Institute of Molecular Biology of Barcelona-CSIC
Agricultural and Technological Institute of Castilla y Leon ( ITACyL ) Address: Burgos, 47071 Valladolid, Spain
E-mail: ita-herperma@itacyl.es
ABSTRACT
This study evaluated the effect of high pressure treatment on the inactivation of different strains of Listeria monocytogenes in pasteurized mixed cheese. Twelve strains were inoculated separately into the cheese, which was, then, submitted to processing under the pressure of 600 MPa during 1, 3, 5 and 10 minutes. Inactivation varied between 1.25 and 6.49 Log CFU / g. From the results, the four most resistant strains (LBMM1175, LBMM1178, LBMM 1291 and 1289) were identified as well as the processing parameters (600MPa for 5 minutes) for effective inactivation of L. monocytogenes in the studied product.
Keywords: high hydrostatic pressure; Listeria monocytogenes; cheese; food safety.
RESUMO
Este estudo avaliou o efeito do tratamento de alta pressão na inativação de diferentes linhagens de Listeria monocytogenes em queijo misto pasteurizado. Doze cepas foram inoculadas separadamente no queijo, que foi submetido ao processamento sob pressão de 600 MPa por 1, 3, 5 e 10 minutos. A inativação variou entre 1,25 e 6,49 Log UFC / g. A partir dos resultados, foram identificadas as quatro cepas mais resistentes (LBMM1175, LBMM1178, LBMM 1291 e 1289), bem como os parâmetros de processamento (600MPa por 5 minutos) para inativação efetiva de L. monocytogenes no produto estudado.
Braz. J. of Develop.,Curitiba, v. 6, n.5, p.23242-23252 may. 2020. ISSN 2525-8761
1. INTRODUCTION
Listeria is a gram-positive bacillus that causes, amongst others, a disease known as listeriosis, a major foodborne disease with one of the highest rates of mortality (30%) (EFSA, 2015). Frequently present in the environment, soil, plants and animal faeces, listeria presents a greater capacity to grow in a refrigerated environment when compared to most other microorganisms, a feature that turns L. monocytogenes into an important challenge in food production, especially in those ready for consumption (CDC, 2015).
Some researches propose the grouping of strains of L. monocytogenes into three distinct strains related to outbreaks of foodborne diseases (1 / 2b, 3b, 4b, 4d and 4e) that should be considered in terms of public health (WIEDMANN et al., 1997). However, in countries that have an active health surveillance against listeria, all the strains are considered equally pathogenic, since genotypic and phenotypic markers for the determination of its virulence are still missing (Jacquet, et al., 2004).
Cheeses are dairy products traditionally associated with listeriosis outbreaks, such as the one that occurred in the European Union in 2001, in which 11 cases of listeriosis resulted in 4 deaths. A homemade cheese was identified as the cause of this outbreak. (EFSA / ECDC, 2013). The microbiological criterion for the product category under study allows a maximum limit of 100 CFU / g of L. monocytogenes in Spain (Regulation (EC) No 1441/2007) and preconizes its absence in products ready for consumption in the United States and in cheeses with average moisture content in Brazil (Brazil, 2001).
One of the alternative technologies for obtaining safe food is the high pressure processing (HPP), which is capable of denaturing enzymes and destroying pathogenic microorganisms without causing significant nutritional and / or sensory loss in food (Barykow et al., 2009; DARYAEI & BALASUBRAMANIAN, 2012, TAO et al., 2014). Although the structures of molecules with high molecular weight, such as proteins and carbohydrates, can be altered, smaller molecules such as volatile compounds, pigments, vitamin and other nutritional related compounds are less affected. This technique receives great attention from the food industry today (MUJICA -PAZ et al., 2011), although this process had been studied for the first time at the end of the nineteenth century in the United States (HITE, 1899). Regarding its effects on microorganisms, there are records in the literature showing that 50 MPa of pressure can inhibit protein synthesis and reduce the number of ribosomes, 100 MPa may induce partial denaturation of the protein, and 200 MPa causes damage to the cell membrane and internal cellular structure, whereas irreversible denaturation of enzymes and
Braz. J. of Develop.,Curitiba, v. 6, n.5, p.23242-23252 may. 2020. ISSN 2525-8761 proteins occurs at 300 MPa, which causes rupture of the cell membrane and the excretion of internal substances, that is, bacterial death (ABE, 2007).
2. MATERIAL AND METHODS
The research was carried out during 6 trials, aiming at establishing the efficacy of HPP in the artificial contamination of cheese.
2.1 STRAINS
12 strains of Listeria monocytogenes (Table 1) provided by the Agricultural Technology Institute of Castilla y León (ITACyL) were used. These strains are part of the collection of isolated microorganisms from the Laboratory of Molecular Biology and Microbiology and, due to confidentiality, their origin will not be mentioned. Stock cultures were stored at -80°C in a glycerol solution.
Table 1 - Strains of L. monocytogenes.
Strain Sorogroup LBMM 1109 1 / 2c LBMM 1163 1 / 2a LBMM 1172 4b LBMM 1175 1 / 2a LBMM 1178 4b LBMM 1181 4b LBMM 1187 4b LBMM 1289 LBMM 1290 LBMM 1291 LBMM 1294 LBMM 1371 1/ 2b 2.2 CHEESE
A pasteurized mixed cheese from a single manufacturer was used. For reasons of confidentiality, the manufacturer will not be revealed.
2.3 INOCULUM PREPARATION
The inoculums of each strain were prepared from the stock cultures. Each strain was
isolated with the use of 10 μl loop on plates with Chromogenic Agar Listeria -ALOA (OXOID) and incubated for 48 to 72 hours at 37 ºC. The isolated colonies were then transferred with sterile loops of the ALOA plates into tubes with 5 ml
Braz. J. of Develop.,Curitiba, v. 6, n.5, p.23242-23252 may. 2020. ISSN 2525-8761 of Brain Heart Infusion -BHI (BD BIOSCIENCES) and cultured for 16 to 18 hours at 37ºC, and then stored at 4ºC until its use.
2.4 INOCULUM COUNT
An initial inoculum concentration count was performed 24 hours prior to the test, from the sowing of 0.1 ml of 10-5, 10 -6, 10-7 dilutions of the inoculum on Brain Infusion Heart Agar - BHIA, incubated at 37 ° C. The concentration of the inoculum was adjusted to 2.5 × 108 CFU / ml (for 25 g of contaminated cheese with 0.1 ml of inoculum).
2.5 TEST PLAN
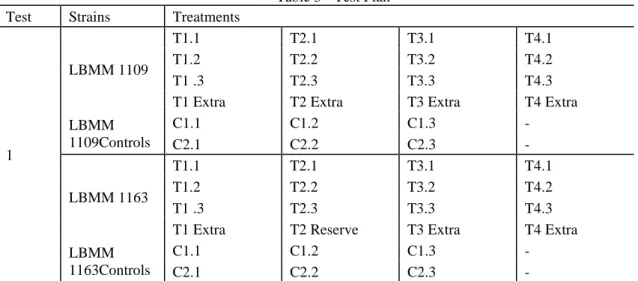
The assays were designed to perform the study of two strains at a time. The samples were prepared to be submitted to the four defined treatments (Table 2), with three replicates (plus an extra one) and two controls. Example: Test 1 (Table 4) with the strains LBMM1109 and LBMM1163. Each strain must have T1, T2, T3 and T4 treatments, each with its replicates plus an extra replicate. Controls C1 and C2, each with 3 replicates and an extra one (extra replicate was reserved to possible problems in some of the replicates).
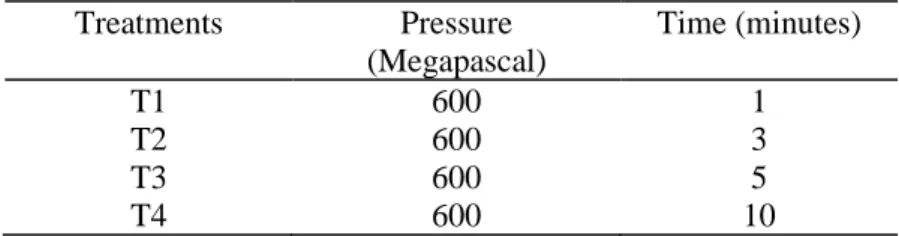
Table 2 - The treatment parameters of the tests.
Treatments Pressure (Megapascal) Time (minutes) T1 600 1 T2 600 3 T3 600 5 T4 600 10
Braz. J. of Develop.,Curitiba, v. 6, n.5, p.23242-23252 may. 2020. ISSN 2525-8761
Table 3 - Test Plan Test Strains Treatments
1
LBMM 1109
T1.1 T2.1 T3.1 T4.1
T1.2 T2.2 T3.2 T4.2
T1 .3 T2.3 T3.3 T4.3
T1 Extra T2 Extra T3 Extra T4 Extra LBMM 1109Controls C1.1 C1.2 C1.3 - C2.1 C2.2 C2.3 - LBMM 1163 T1.1 T2.1 T3.1 T4.1 T1.2 T2.2 T3.2 T4.2 T1 .3 T2.3 T3.3 T4.3
T1 Extra T2 Reserve T3 Extra T4 Extra LBMM
1163Controls
C1.1 C1.2 C1.3 -
C2.1 C2.2 C2.3 -
C1: Inoculated cheese portions non-submitted to treatment. Control to determine the actual count, taking into account the matrix - cheese (matrix/inoculum control) analyzed on the day of HHP treatment.
C2: Inoculated cheese portions non-submitted to treatment. Control for determining the actual count, which is analyzed at the time of samples count, one day after HHP treatment.
2.6 PREPARATION OF SAMPLES: ARTIFICIAL CONTAMINATION, INOCULATION AND PACKAGING
The cheese was cut in at least 2 slices (25g total) and put in plastic sacks. For the artificial contamination, 0.1 ml of the prepared inoculum was placed between the two slices of cheese, leaving the samples at rest for about of 5 minutes before proceeding to the vacuum container.
Samples were placed in polypropylenes sacks with triple protection (to ensure that the product was not contaminated if the disruption of some package occurred). The primary package contained the contaminated slices. The second package contained the sacks with the three replicates. The third package contained the sacks that would suffer the same treatment. In the end, there were 4 packages, each for each treatment.
2.7 HIGH PRESSURE PROCESSING (HPP)
The high pressure processing was performed immediately after inoculation (artificial contamination) using Hiperbaric 135 equipment (Hiperbaric SA, Burgos) adjusted to 10°C processing temperature under pressure of 600 MPa at different times (1, 3, 5 and 10 minutes). Each package concerning each treatment was individually placed in the equipment. Cycle time and pressure were determined for each treatment.
Braz. J. of Develop.,Curitiba, v. 6, n.5, p.23242-23252 may. 2020. ISSN 2525-8761 After treatment, the samples were stored at 4°C until the time of detection analysis of surviving L. monocytogenes cells within a maximum period of 24 hours after treatment. During high pressure processing, there was a temperature variation during the periods of compression (heating to ± 3 ° C per 100 MPa) and decompression (cooling) of the equipment in the fluid and processed product, a phenomenon known as adiabatic heating or cooling (Knorr 1993). However, this small temperature variation is usually not considered an influencing variable. Therefore, it was not taken into account in the present study.
2.8 MICROBIOLOGICAL ANALYSIS - LISTERIA MONOCYTOGENES COUNT
Each sample that was vacuum packed was opened after the cleaning of its surface with ethanol. Each sample (about 25 g) was diluted in 250 ml (1/10 dilution) of peptone water (Merck), followed by analysis performance, according to the UNE-EN ISO 11290-1: 1997 / A1: 2005 horizontal method for the detection and counting of Listeria
monocytogenes. Part 1: detection method. Modification 1: Modification of isolation medium
and hemolysis test and inclusion of precision data (ISO, 2004).
For the microbiological analysis of plate count, dilutions of 10 -1, 10 -2 and 10 -3 (1 mL divided into 2 plates) were sowed for each sample, ie : one strain, 4 treatments , 3
replicates , 3 dilutions and 2 plates for each dilution , totalizing 72 plates of treated samples and two controls with their respective dilutions and replicates, totalizing more 36 control plates. The study of each strain resulted in a total of 108 plates. All plates were incubated at 37 ° C for 48 h and counts of typical colonies of L. monocytogenes was performed manually. To express the counts as logs of colony forming units (log UFC / g), the following formula (1) was used:
(1)
N: number of microorganisms per milliliter or grams.
Σc: Sum of microorganisms colonies of all plates selected for counting. V: Volume of inoculum applied to each plate.
n1: number of plates in the first selected dilution. n2: number of plates in the second selected dilution. d: dilution rate corresponding to the first selected dilution.
Braz. J. of Develop.,Curitiba, v. 6, n.5, p.23242-23252 may. 2020. ISSN 2525-8761 2.9 STATISTICAL ANALYSIS
The experiments were performed with three repetitions. The means and standard deviations were calculated using Excel 2016. Some of the results were below the detection limit, counting <10 CFU / g, or less than 1 log, preventing the calculation of Averages (M) and standard deviations (SD) at the first moment.
To perform the statistical analysis, a log U FC / g value of 0.5 was considered for the counts that resulted in <10 CFU / g. The results were statistically evaluated in the XVII STATGRAFICS Centurion software, using an analysis of variance (treatment and strain) for the results of listeria logarithmic counts.
In the step of verification of the effect of different times, microorganisms under the same pressure, the strains and the different treatment times were considered as independent variables, while the dependent variable refers to the log values CFU listeria.
Tests and graphs were performed to check whether the factors have a statistically significant effect on listeria counts, and to evaluate the level of significance of interactions between factors.
3. RESULTS AND DISCUSSION
This study was conducted to determine the optimum combination of the parameters time and pressure to inactivate an initial concentration of 6 to 7 log CFU / g of L.
monocytogenes in pasteurized mixed cheese. The working pressure was 600 MPa and the
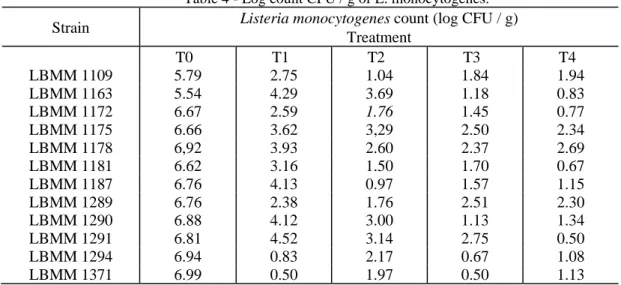
times were 1, 3, 5 and 10 minutes. The results demonstrated an effective reduction (5 to 6 log CFU / g of L. monocytogenes) at least in one of four treatments, results that are similar to those obtained in earlier studies, performed in different type of foods, such as guacamole, sauces, juices fruit, meat, seafood and cheese (SANDRA et al., 2004, RASTOGI et al., 2007). In this study, the most resistant strains had a reduction of at least 4.3 log CFU / g of L. monocytogenes. Table 4 presents the average values of L. monocytogenes counts in the cheese samples inoculated with each of 12 strains without treatment (T0) and treated at 600 MPa for 1 minute (T1), 3- (T2), 5 (T3) and 10 (T4) minutes.
Braz. J. of Develop.,Curitiba, v. 6, n.5, p.23242-23252 may. 2020. ISSN 2525-8761
Table 4 - Log count CFU / g of L. monocytogenes.
Strain Listeria monocytogenes count (log CFU / g) Treatment T0 T1 T2 T3 T4 LBMM 1109 5.79 2.75 1.04 1.84 1.94 LBMM 1163 5.54 4.29 3.69 1.18 0.83 LBMM 1172 6.67 2.59 1.76 1.45 0.77 LBMM 1175 6.66 3.62 3,29 2.50 2.34 LBMM 1178 6,92 3.93 2.60 2.37 2.69 LBMM 1181 6.62 3.16 1.50 1.70 0.67 LBMM 1187 6.76 4.13 0.97 1.57 1.15 LBMM 1289 6.76 2.38 1.76 2.51 2.30 LBMM 1290 6.88 4.12 3.00 1.13 1.34 LBMM 1291 6.81 4.52 3.14 2.75 0.50 LBMM 1294 6.94 0.83 2.17 0.67 1.08 LBMM 1371 6.99 0.50 1.97 0.50 1.13
The s results demonstrate that, with the exception of strains LBMM 1175 and LBMM 1178, all the others were sensitive to the treatment of high pressure, as they reached a count of less than 2 log CFU / g in at least one of the treatments. LMBB 1175 and LBMM 1178 are the strains that presented greater resistance to treatments.
With these values, the graph representing the effect of the pressure on the logarithmic reduction of listeria, shown in Figure 1, was constructed.
Figure 1 - Effect of 600 MPa pressure on the logarithmic reduction of Listeria
monocytogenes at 1, 3, 5 and 10 minutes and constant temperature of 10 ° C.
The graph of Figure 1 shows that, generally, the count of microorganisms tends to decrease with increasing treatment time, with the exception of strains LBMM 1109, LBMM 1187, LBMM 1294 and LBMM 1371. It is observed that the behavior of each strain for each treatment varies and that not always the treatment with greater time is the one that results in a greater reduction of the microbial count. This diversity of behaviors justifies the importance of the strain on the effect of the treatment by high pressure, ie, there are more resistant strains than others. Therefore, it is important to identify and characterize the strains in the product of interest to, then, define the best parameters of treatment by high pressure. It is not safe to extrapolate the results obtained for a given product and use the same treatment in another distinct product.
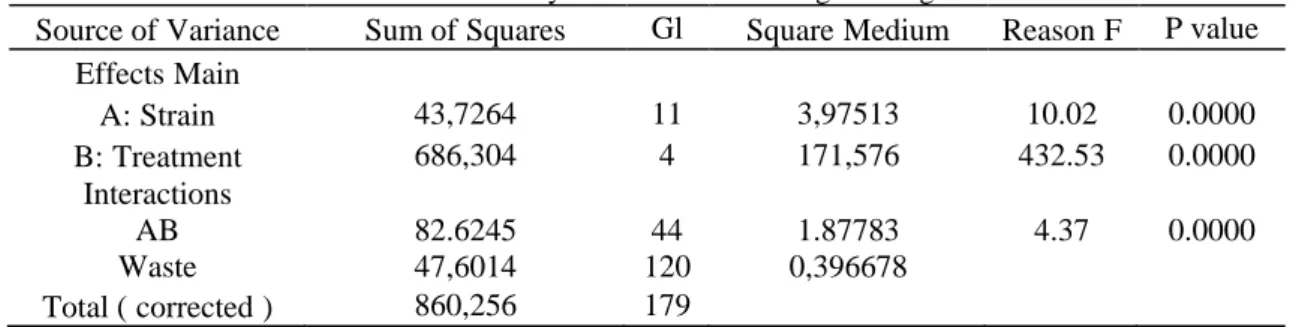
The analysis of variance shown in Table 5 shows that there was a statistically significant difference between the log CFU / g values for the factors (strain and treatment) and their interactions. The Fisher's Test (LSD) shows that there was a significant minimum
Braz. J. of Develop.,Curitiba, v. 6, n.5, p.23242-23252 may. 2020. ISSN 2525-8761 difference among the averages with a 95% confidence level among all treatments, except for treatments T3, and T4, as it is seen in the graph of Figure 2.
Table 5 - Analysis of Variance for log UFC / g values.
Source ofVariance Sum ofSquares Gl SquareMedium ReasonF P value EffectsMain A:Strain 43,7264 11 3,97513 10.02 0.0000 B:Treatment 686,304 4 171,576 432.53 0.0000 Interactions AB 82.6245 44 1.87783 4.37 0.0000 Waste 47,6014 120 0,396678 Total (corrected) 860,256 179
Figure 2 - Means of treatment results.
A significant difference between treatments was expected. However, the fact that there was surprisingly significant difference between T3 and T4, with times of 5 and 10 minutes, respectively, would suggest that under a pressure of 600 MPa at a temperature of 10 ° C, increasing treatment time beyond 5 minutes would not result in an increase in the effectiveness of the treatment. It would be interesting to investigate changes in working temperature, as reported by TOMASULA et al. (2014), that used the pressure of 600 MPa at temperatures of 20°C and 40°C and achieved logarithmic reductions of 4.6 and 3.5 (respectively) of L.
monocytogenes in fresh cheese, reaching the minimum level of detection in only 5 minutes of
treatment.
It should be noted that, considering working with the treatment T3, in addition to LBMM 1175 and LBMM 1178 strains, LBMM 1289 and LBMM 1291 strains should also be considered as the toughest, since all of them presented a top score of 2 log UFC / g for this treatment. Considering such results, the use of these four more resistant strains can be advised as a reference for the validation of the efficacy of the treatment using a pressure of 600 MPa for 5 minutes. The treatment will be considered valid and effective if microbial reduction is proven within the amount recommended by law.
4. CONCLUSIONS
There is a great variability regarding the behavior of the studied strains in relation to
Braz. J. of Develop.,Curitiba, v. 6, n.5, p.23242-23252 may. 2020. ISSN 2525-8761 as well as its parameters, depend on the product of interest and the strains that are present in such product.
T3 and T4 were the most effective treatments. Since there was no significant difference between these, T3 treatment (600 MPa, 5 minutes) was chosen as the best, once it was carried out in a shorter time, thus resulting in lower process costs and lower influence in the sensory characteristics of the product.
Although the treatment of 600 MPa, for 5 minutes has not reached a final score lower than 2 log CFU / g for the most resistant strains (LBMM 1175, LBMM 1178, LBMM 1289 and LBMM 1291) it must be taken into account that the industries work with good manufacturing practices and hazard analysis and critical control points, being highly unlikely that the final product presents contamination in the range of 6 to 7 log Listeria
monocytogenes as the studied case.
Therefore, this study confirms the efficacy of the treatment by high pressure in pasteurized mixed cheese in the reduction of L. monocytogenes contamination, as other studies carried out with other food products have shown.
REFERÊNCIAS
Araújo, M.S.R.; Deodato, J.N.V.; Martins, W.F.; Silva, G.A.S.; Lima, F.F.; Araujo, A.S. (2011). Qualidade microbiológica das maioneses servidas nas lanchonetes de Pombal-PB. Brasil (2010). Manual integrado de vigilância, prevenção e controle de doenças transmitidas por alimentos. Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica. 158 p.
Brasil (2013). Ministério da Saúde. Política nacional de alimentação e nutrição. 83 p. Brasil (2017). Ministério da Saúde. Surtos de Doenças Transmitidas por Alimentos. 17 p. Brasil (2001). Ministério da Saúde. Agência Nacional de Vigilância Sanitária. Resolução RDC n° 12, de 2 janeiro de 2001. Diário Oficial da União.
Carvalho, S.; Campos, W. (2016). Estatística básica simplificada. São Paulo: Jus Podivm. Cordeiro, P.M.D.; Vandesmet, V.C.S. (2014). Análise microbiológica de maioneses caseiras temperadas produzidas e consumidas em lanchonetes da cidade do Juazeiro do Norte- Ceará. In: IV Semana de Iniciação Científica, Juazeiro do Norte. Anais...
Braz. J. of Develop.,Curitiba, v. 6, n.5, p.23242-23252 may. 2020. ISSN 2525-8761 Casemiro, L.P.; Martins, A.L.O. (2016). Prevalência de contaminação microbiológica e parasitológica de maioneses caseiras comercializadas em carrinhos de cachorro-quente. RBAC. 48(4):394-9.
Elias, S.O. (2014). Modelagem dos parâmetros cinéticos de multiplicação de Salmonella Enteritidis SE86 em maionese caseira e práticas de preparo, estocagem e consumo desse alimento no Rio Grande do Sul. Universidade Federal do Rio Grande do Sul (Dissertação - Mestrado Microbiologia Agrícola e do Ambiente).
Forsythe, S.J. (2013). Microbiologia da segurança dos alimentos. Porto Alegre: Artmed. Franco, B. (2014). Análise microbiológica de alimentos: importância do plano de amostragem. Food Safety Brazil.
Franco, B.D.G.M.; Landgraf, M. (2008). Microbiologia dos alimentos. São Paulo: Atheneu. Machado, M.R.G.; Antunes, A.C.N.; Silva, C.S.J.; Oliveira, J.S.; Souza, V.M.; Vilanova, L.B. (2015). Qualidade microbiológica de maioneses industrializadas adquiridas em Pelotas, RS em Bento Gonçalves, Rio Grande do Sul. In: Simpósio de Segurança: Alimentação e Saúde, 5, 2015, Bento Gonçalves. Anais.
Maia, A.G.; Souza, M.L.; Furtado, C.M. (2010). Avaliação microbiológica de maioneses produzidas e consumidas em lanchonetes e lanches ambulantes.
Rodrigues, M.L.C. (2009). Alimentação e nutrição no Brasil. Brasília: Universidade de Brasília, 92 p.
Rocha, J.A. (2015). A Química da Maionese: um Tema Estruturador para o Ensino de Coloides. Scientia Plena. 11(6):1-9.
Vanderzant, C.; Splittstoesser, D.F. (2001) Compendium of methods for the microbiological examination of foods. Washington: APHA - American Public Health Association (Cap.8, p.69-82).