w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Punica
granatum
suppresses
colon
cancer
through
downregulation
of
Wnt/

-Catenin
in
rat
model
Hanaa
H.
Ahmed
a,
Hanan
S.
El-Abhar
b,
Elsayed
Abdul
Khalik
Hassanin
c,
Noha
F.
Abdelkader
b,
Mohamed
B.
Shalaby
c,∗aDepartmentofHormones,MedicalResearchDivision,NationalResearchCentre,Cairo,Egypt bDepartmentofPharmacologyandToxicology,FacultyofPharmacy,CairoUniversity,Cairo,Egypt
cNationalNutritionInstitute,GeneralOrganizationforTeachingHospitalsandInstitutes,MinistryofHealth,Cairo,Egypt
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received17March2017 Accepted17May2017 Availableonline15August2017
Keywords:
Coloncancer Pomegranate Wnt/-Catenin Inflammation Proliferation Apoptosis
a
b
s
t
r
a
c
t
ThisstudyaimstoelucidatethebeneficialeffectofPunicagranatumL.,Lythraceae(pomegranate)peel extractinthemanagementofcoloncancerinducedintrarectallywithN-methylnitrosourea.Adultmale Sprague-DawleyratswereadministeredN-methylnitrosourea(2mgin0.5mlwater/rat)intrarectally threetimes/week forfiveweekstoinducecolorectalcancer,followedbytreatmentwitheither 5-fluorouracil(12.5mg/kg,i.p.)orPunicapeelextract(2.25or4.5g/kg,p.o.).Developedtumorelevated plasmaTGF-,andBcl2,serumepidermalgrowthfactor,carcinoembryonicantigen,coloncancerspecific antigens,andmatrixmetalloproteinase-7.Besides,immune-histochemicalstudiesrevealedanincrease inCOX-2,cyclinD1andsurvivincontent,aswellasupregulationoftheexpressionofcolonic-Catenin, K-rasandC-mycgenes.Theseresultswerefurthersupportedbythehistologicalfindings.Punicapeel extract-treatedrats,particularlythosetreatedwithahighdose,exhibitedamarkedreductioninthe aforementionedparametersandimprovedthehistologicalorganizationofthecolontissue.These alter-ationswereconsistentwiththosemediatedthrough5-fluorouracil.Thepresentstudyencouragesthe useofP.granatumL.againstcoloncancer.BecausePunicapeelextractpromotesapoptosis,mitigates inflammationandsuppressestumorcellproliferationinvivo,thepotentialmechanismunderlyingthese activitiesmightdependontheinhibitionoftheWnt/-Cateninsignalingpathway.
©2017PublishedbyElsevierEditoraLtda.onbehalfofSociedadeBrasileiradeFarmacognosia.Thisis anopenaccessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/
4.0/).
Introduction
Colorectalcancer(CRC)istheleadingcauseofcancer-related mortalityworldwideandisthethirdmostcommonlydiagnosed cancerin menand thesecondmost commonlydiagnosed can-cerinwomenintermsofincidence(Zhaoetal.,2014).Theonset andprogressionofCRCinvolvesunregulatedepithelialcell pro-liferationreflectingaccumulatedgeneticmutations(Zhao etal., 2014).Recentevidencehasshownthattheprolongedsurvivalof geneticallyunstablecolorectalepithelialcells,eventuallyleading tomalignanttransformation is accompaniedbytheprogressive suppressionofapoptosis(Zhaoetal.,2014).
Themajority of sporadicforms of CRCharbor genetic alter-ationsin keyelementsoftheWnt/-Cateninsignalingcascade, particularlyinAdenomatouspolyposiscoli(APC)and-Catenin,
∗ Correspondingauthor.
E-mail:mohamed.shalaby@std.pharma.cu.edu.eg(M.B.Shalaby).
therebyincreasingthetranscriptionalactivityofthelatter(Kundu et al., 2006). -Catenin target genes play an ultimate role in tissue homeostasis, and the initiation and progression of CRC throughtheregulationofvariouscellularprocesses,including pro-liferation,stemcell fate,survival,differentiation,migration and angiogenesis(Srimuangwongetal.,2012).Particularly,thegenes involvedinproliferationandmigrationwereoverexpressedinCRC (Srimuangwongetal.,2012).
Interestingly,theingestionofaphytochemical-richdiet, includ-ingfruitsand vegetables,hasbeenassociated witha decreased riskofCRCincidence(Sharmaetal.,2010).Amongfoods,small fruitsandberrieshaveattractedmuchattention,andthe associ-ationbetweentheirbioactivecomponentsandcancerprevention hasbecomethefocusofkeenscientificinterest.
The fruit of Punica granatum L., Lythraceae (pomegranate), possesses many medicinal properties, reflecting anti-oxidant and anti-inflammatory potentials (Adhami et al., 2009). The compoundspresentinP.granatum,whichhavebeenlargely inves-tigated for cancer preventive properties, include polyphenols,
http://dx.doi.org/10.1016/j.bjp.2017.05.010
particularlyellagitannins (ET), punicalagins, flavonoids and the 3-glucosides/3,5-diglucosides of the anthocyanins delphinidins, cyanidinsandpelargonidins(Middhaetal.,2013).Ellagitanninsare metabolizedintotwoactivecompounds,viz.,ellagicacid(Sharma etal.,2010)andurolithin A(UA),generated throughtheaction ofgutmicrobiotaonET(Sharmaetal.,2010).Urolithinssuppress theproliferationofcoloncancercells,stimulatecellcyclearrest, amelioratekey cellular processes associated with colon cancer development,suchas mitogen-activatedprotein kinase(MAPK) signaling(González-Sarrías et al., 2009); they alsoreduced the colonicmucosainflammatory progressionin a ratcolitis model (Larrosaetal.,2009).Furthermore,rutin(aflavonolglycoside) pro-motesapoptosisandcellcyclearrestinhumancoloncancer(Vijay etal.,2016).Noteworthy,thepeel,whichisthenon-ediblepartof fruits,containslargerquantitiesofthesepolyphenolsthanthe edi-bleparts,accompaniedbyhighercanceranti-proliferativepotential (Orgiletal.,2014).ArecentstudyshowedthatP.granatumpeel extract(PPE)reducescellproliferationandinducesapoptosisin MCF-7humanbreastcancercells viaanti-oxidantandapoptotic activities(Shirodeetal.,2014).Accordingly,thepresentstudywas delineatedtoexploretheunderlyingmechanism(s)infavorofthe antitumoractivityofP.granatumagainstcoloncancerinducedin theexperimentalanimals.
Materialsandmethods
Herbalextract
Punicagranatum L., Lythraceae, peel extract (PPE) was sup-pliedbyUnitedGroupPharmaCo.(Badercity,Cairo,Egypt).Test plantswereauthenticatedbyProf.IbrahimEl-Garf, Department of Botany, Faculty of Science, Cairo University, Egypt. Voucher specimen(number20170402M)waskeptintheherbariumof Phar-macognosyDepartment,FacultyofPharmacy,CairoUniversity.
PreparationofPunicagranatumpeelextract(PPE)
FourkilogramsofpeelwereseparatedfromP.granatumfruits inAugust2014(5–6kg).Thepeelswerecutintosmallpiecesand blendedwith4lofmethanol(70%)usinganelectricblender, fol-lowedbyincubationfor10–12h.Theextractwasfilteredthrough filterpaperandthesolventwasevaporatedusingarotary evap-orator(BüchiLabortechnikAG,Flawil,Switzerland).Theresulting extractwasdehydratedinanovenat50◦Cfor24h(El-Toumyand Rauwald,2002).
Chemicals
Gallic acid, protocatechuic acid, catechin, rutin, ellagic acid, puanicalaginwereobtainedfromSigma–AldrichChemical Com-pany(St.Louis,USA).
DeterminationofpolyphenolsbyHPLC-DAD
TheHPLCsystemwasanAgilent1100equippedwitha quater-narypump,onlinedegasser,autosampleranddiode-arraydetector (DAD).Datacollectionandanalyseswereperformedusing Chem-stationsoftware.Chromatographicseparationsoftheextractswere carriedoutonaZorbaxC18column(250×4.6mm,particlesize 5m,Agilent)usingwater/aceticacid(98:2,v/v)(SolventA)and methanol(SolventB)asthemobilephasesataflowrateof1ml/min. Theelutionprogramusedwasasfollows:5%Bfor5min,5–70%B for25min,and70–5%Bfor10min.Thecolumntemperaturewas maintainedat35◦Candthedetectionwasmonitoredat254,280, and360nm.UVspectraofthecomponentsweretaken continu-ouslybetween200and 400nmthroughouttheelutioninorder
todeterminecomponentidentityandpeakpurity.Theinjection volumeforstandardsandsampleswas10l.
Animalsandethicsstatement
Adult male Wistar rats, weighing 150–170g, were obtained fromtheAnimalFacilityBreedingColonyoftheNationalResearch Center,Cairo,Egypt.Theanimalswereacclimatizedforoneweek inaspecificpathogen-freebarrier areaatconstanttemperature (25±1◦C),humidity(55%),anda12hlight/darkcycle.Ratswere housedwithastandardlaboratorydietrecommendedbythe Amer-icanInstituteofNutrition(Reevesetal.,1993)andhadfreeaccess tofoodandwaterandtheirbodyweightwasassesseddaily.The animalsweremanagedaccordingtotheGuidefortheCareand UseofLaboratoryAnimalspublishedbytheUSNationalInstitutes ofHealth(NIHPublicationNo.85-23,revised1996)andthestudy protocolwasapprovedbytheEthicalCommitteeforAnimal Exper-imentationattheFacultyofPharmacy,CairoUniversity(Permit Number:PT664).
Experimentaldesign
Forty adult male Sprague-Dawley rats, weighing 150–170g, wererandomlyallocatedintofivegroups(8rats/eachgroup); ani-malsofthefirstgroupreceivedvehicle(1mlDMSO(5%),p.o.)and servedasthenormalcontrolgroup.Ratsintheotherfourgroups wererectallyadministeredN-MNU(2mgin0.5mlwater/rat)three times/weekforfiveweekstoinduceCRC(Ahmedetal.,2013)a modelthatwasreportedtomimichistopathologicallyhumancolon tumors(NarisawaandFukaura,2003).CRCanimalsweredivided intogroupII,whichreceivedthevehicleandservedastheCRC untreatedgroup,whiletheremainingthreegroupsweretreated throughoutthefourmonthsexperimentalperiodwiththe follow-ingtreatments.IngroupIII,ratsweretreatedwith5-fluorouracil (5-FU;12.5mg/kg, i.p. [equivalent to2mg/kg for humans(Shin etal.,2010)])ondays1,3and5,withthecyclerepeatedevery fourweeks(Watsonetal.,1998).AnimalsingroupsIVandVwere treateddailywithPPEforfourmonthsattwodoselevels(2.25and 4.5g/kginDMSO(5%),p.o.).TheselecteddosesofPPE;viz.,4.5g/kg b.wt[equivalentto0.729g/kgforhumans(Shinetal.,2010)]and itshalfdose2.25g/kgb.wt[equivalentto0.365g/kgforhumans (Shinetal.,2010)]wereadministereddailyfor6months.Afteran overnightfast,thefinalbodyweightsweremeasuredandtherats wereeuthanizedusingCO2.Bloodwascollectedthroughcardiac
puncture,andthebloodsamplesweredividedintotwoaliquotsfor theseparationofplasmaandserum.Subsequently,theplasmaand serumsampleswereacquiredthroughcentrifugationat2555×g for30minat4◦C.Thecolontissueswererapidlyexcised,cleaned andwashedinice-coldsaline,blotteddryandequallydividedinto twolongitudinalportions.Thefirstportionwaspreservedin forma-linsalineforhistologicalandimmunohistochemicalexamination, respectively,whilethesecondportionwascollectedinliquid nitro-genandstoredat-80◦Cforsubsequentbiochemicalandmolecular geneticanalyses.
Biochemicalmeasurements
Semi-quantitativereal-timePCR(sqRT-PCR)detectionof ˇ-Catenin,K-rasandC-mycgeneexpressions
IsolationoftotalRNA
Total RNA was extracted from the colon tissue of rats using TRIzol® reagent (Cat#15596-026, Invitrogen, Darmstadt, Germany) according to the manufacturer’s instructions with minor modifications. The tissue samples(50mg) were homog-enized in 1ml of TRIzol® reagent and the RNA was dissolved in diethylpyrocarbonate (DEPC)-treated water. Total RNA was treated with 1U of RQ1 RNAse-freeDNAse (Invitrogen, Darm-stadt, Germany) to digest DNA residues and re-suspended in DEPC-treatedwater.ThepurityoftotalRNAwasassessed accord-ing to the 260/280nm ratio (between 1.8 and 2.1), and the integritywasassessedthroughethidiumbromidestaininganalysis of28Sand18Sbandsafterformaldehyde-containingagarosegel electrophoresis.
Reversetranscription(RT)reaction
Thecomplete poly(A)+ RNA,isolated fromeachcolon tissue,
was reverse transcribed into cDNA in a total volume of 20l using the Revert AidTM First Strand cDNA Synthesis Kit (MBI,
Opelstrasse,Germany)accordingtomanufacturer’sinstructions. TheRTreactionwasperformedat25◦Cfor10min,followedby 1hat42◦C,andcompletedwithadenaturationstepat99◦Cfor
5min.Subsequently,thereactiontubescontainingRTpreparations were flash-cooledin an ice chamber until furtherusefor DNA amplificationthroughsqRT-PCR.
Semi-quantitativereal-time-polymerasechainreaction
AniQ5-BIO-RADCycler(Cepheid,CA,USA)wasusedto deter-minethecDNAcopynumber.PCRreactionsweresetupin25l reactionmixtures containing12.5l1×SYBR® PremixExTaqTM
(Takara, Biotech Co. Ltd., Saint-Germain-en-Laye, France), with 0.5l of sense primers (0.2M), 0.5l of antisense primer (0.2M),6.5lofdistilledwater,and5lofcDNAtemplate.The sequencesof theprimersaredescribed inBox 1.At theend of eachsqRT-PCRameltingcurveanalysiswasperformedat95◦Cto assessthequalityoftheusedprimers.Therelativequantification ofthetargetgeneswasdeterminedthroughtheCTmethodusing -actinasareferencegene.
Immuno-histochemicalandhistologicalexamination
Afterthecolontissueswerefixedinformalinsalinefor24h, thespecimens wereprocessed forparaffinembeddingand two setsof4msectionswereprepared.Inthefirstset,thesections werepreparedforimmune-histochemicalexamination.The sec-tionswerecollectedontoglass-positiveslidesandwerefixedin
mi
0 5 10 15 20
mAU
0 200 400 600 800
DAD1 B, Sig=254,16 Ref=off (TRA\NOV00514.D)
Galli
c
Pr
otocatechuic
Ru
tin
E
llagic
Cateachin
puanicalagin
mi
0 5 10 15 20
mAU
0 200 400 600 800 1000 1200 1400 1600
DAD1 B, Sig=254,16 Ref=off (TRA\NOV00513.D)
Gallic protocatechuic
Cateachin
Rutin
Ellagic
Puanicalagin
A
B
Box1:PrimersequencesusedforsqRT-PCR
Gene Primersequence(5′–3′) Sequencereferences
-Catenin F:CAATGGGTCATATCACAGATTC TT
R:TCTCTTTTCTTCACCACAACATTT
Austinatetal.(2008)
K-ras F:AGTACGACCCTACGATAGAGG ACTCCT
R:CAATCTGTACTGTCGGATCTC TCTCACC
Fuentes-Calvoetal.(2010)
C-myc F:TGACGAGACCTTCGTGAAGA R:ATTGATGTTATTTACACTTAA GGGT
Taoetal.(2002)
-Actin F:CCCCATCGAGCACGGTATTG R:ATGGCGGGGGTGTTGAAGGTC
Eshaketal.(2010)
a65◦Covenfor1h.Subsequently,theslidesweredeparaffinized andthesampleswereblockedforendogenousperoxidase activ-ityafterimmersingtheslidesin3%hydrogenperoxidefor10min. Next,thesectionswerewashedwithTrisbufferedsaline,and sim-ilarlytreatedaccordingtotheimmune-histochemicalprocedure describedabove.ThePower-StainTM-1.0PolyHRPDABKit(Cat#
54-0017,GenemedBiotechnologies,SanFrancisco,CA,USA)was usedtovisualizeanyantigen-antibodyreactiononthetissues.The slidesweresubsequentlyincubatedwithrabbitprimarypolyclonal
antibody cyclooxygenase-2 (COX-2;Cat# RB-9072-R7, Thermo-scientific,Waltham,MA,USA),cyclinD1(Cat#RB-9041-R7, Ther-moscientific,Waltham,MA,USA)orsurvivin(Cat#RB-9245-R7, Thermoscientific,Waltham,MA,USA)overnightat4◦Cina humid-itychamber.Henceforward,poly-horse-radishperoxidaseenzyme conjugatewasappliedfor20min,and3,3′-diaminobenzidine chro-mogen was prepared and applied for 2min. Subsequently, the slideswererinsed,counterstainedwithMayer’shematoxylin, fol-lowedbycover-slippingasthefinalsteppriortoexaminingthe slidesunderthelightmicroscope.ImageJSoftware(NIH,version v1.45e,Bethesda,MD,USA)wascalibratedforimageanalysis.Inthe secondset,thesectionswerecollectedontoglassslides, deparaf-finizedand stainedwithhematoxylin andeosin (H&E)for light microscopicexamination(NikonMicroscopeSE,Nippon Kogaku KK,Tokyo,Japan)at40×and64×magnificationsofthehistological changes.
Statisticalanalysis
The study results were analyzed using GraphPad Prism 5 (GraphPad Software, Inc, La Jolla, CA, USA). The results are expressed as the means±SD; a probability level of less than 0.05 was accepted as statistically significant. The results from eachexperimentalgroupwerecomparedusingone-wayANOVA
Table1 Bodyweight.
Groups Normalgroup Tumorgroup 5-FU PPE2.25 PPE4.5
Initialbodyweight 171.4±4.0 181.1±3.3 175.3±2.3 173.5±2.4 173.8±2.5
Finalbodyweight 199.0±6.5 150.8±4.1a 184.5±4.8b 178.6±4.2b 181.1±6.2b
Valuesaremeans±SD.StatisticalanalysiswascarriedoutusingonewayANOVAfollowedbyTukey’smultiplecomparisontest.Ascomparedwithcontrol(a)and tumor/N-MNU(b)controlgroups(p<0.05).PPE:P.granatumpeelextract.
analysisofvariancefollowedbyTukey’sposthoctest.Differences
in mean values among the groups were tested using Tukey’s
test.
Results
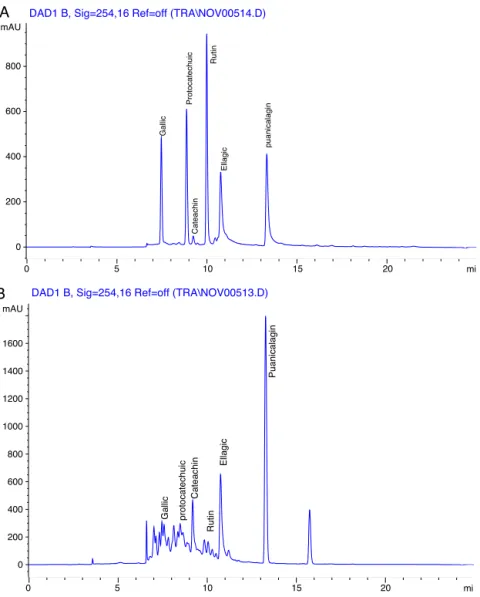
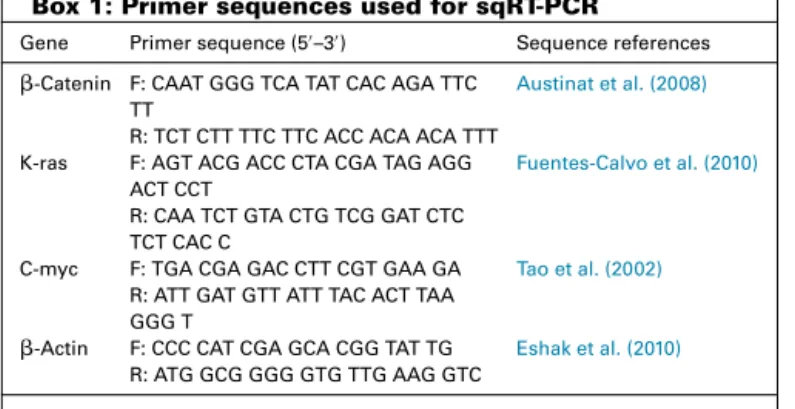
AnalysisoftheextractbyHPLC
HPLC chromatograms have affirm six marker components
existent in ethanol extract of P. granatum peel as shown in
Fig.1.Thesephenoliccomponentshavebeenidentifiedasgallic acid(Rt:7.4min;1.366mg/ml),protocatechuicacid(Rt:8.8min; 0.047mg/ml), cateachin (Rt: 9.2min; 0.377mg/ml), rutin (Rt: 9.9min;0.136mg/ml),ellagicacid(Rt:10.7min;4.643mg/ml),and punicalagin(Rt:13.2min,1.910mg/ml)bytheirretentiontimeand UVabsorbanceofpurifiedstandards.
Effectofdifferenttreatmentsonbodyweightandfoodintake
Theinitialbodyweightwasnotsignificantlydifferentamongthe studiedgroups.However,thefinalbodyweightwassignificantly decreasedintheN-MNUgroup(tumorgroup)comparedwiththe negativecontrolgroup(p<0.05).Treatmentwith5-FUandthetwo dosesofPPEsignificantlyincreasedthebodyweightvstheN-MNU group(p<0.05)(Table1).
EffectofPPEand5-FUonplasma/serumlevelsofTGF-ˇ,Bcl2,EGF, CEA,CCSA-4andMMP-7inN-MNU-inducedCRCinrats
AsillustratedinFig.2,N-MNU-inducedCRCresultedina 1.5-foldelevationofplasma(A)TGF-and(B)Bcl2,aswellasserum (C)EGF,(D)CEA,(E)CCSA-4and(F)MMP-7comparedwiththe negativecontrolgroup.Inaddition,treatmentwithPPEreverted suchincrementsinadose-dependentmannerandthehighdose effectswereconsistentwiththoseof5-FU.
EffectofPPEand5-FUoncolontissuemRNAlevelsofˇ-Catenin, K-ras,andC-mycgenesinN-MNU-inducedcoloncancerinrats
N-MNUmarkedlyup-regulatedtheexpressionof-Catenin, K-ras,andC-mycgenesincolontissuesatthemRNAlevelalmost4-, 2.8-,and3.2-folds,respectively,versusthenegativecontrolgroup (Fig.3).Treatmentwith5-FUamendedthesealterationsinthegene expressionlevels71.85%,58.95%,and64.66%,respectively,versus theN-MNUgroup.Similarly,PPEsignificantlydownregulatedthe expressionlevelofthetestedgenesinadose-dependentmanner relativetotheN-MNUgroup.
EffectofPPEand5-FUonimmune-histochemicalparametersin N-MNU-inducedcoloncancerinrats
AsshowninFig.4,immune-histochemicalstainingofthecolon tissuein(A)negativecontrolratsusingaCOX-2antibodyexhibited
amildintracellularpositivereaction,while(B)N-MNUand(C)5-FU treatedratsrevealedasevereintracellularpositivereaction.In con-trast,PPEsignificantlyalleviatedtheaforementioneddisruptions inadosedependentmanner.(D)Amoderateintracellularpositive reactionwasobservedfollowingtreatmentatalowdose,while(E) amildintracellularpositivereactionwasshowninthehigh dose-treatedgroup.Immuno-histochemicalexaminationofcyclinD1in colontissueof(F)negativecontrolratsrevealedmildintracellular positivereaction,whereas(G)apronouncedintracellularpositive reactionwasobservedinthecancergroup.Treatmentwith(H) 5-FUshowedmoderateintracellularpositivereaction,whileeither (I)lowor(J)highdosesofPPErevealedmildintracellularpositive reactions.Similarly,immuno-histochemicalreactionsofsurvivin in(K)negativecontrolratsrevealedamildintracellularpositive reaction,whereas(L)adistinctintracellularpositivereactionwas
observed in thecolon tissue of thecancergroup. Management with(M)5-FUor(O)ahighdoseofPPEshowedmildintracellular positivereactions,while(N)thelowdoseofPPEshowedmoderate intracellularpositivereactions.
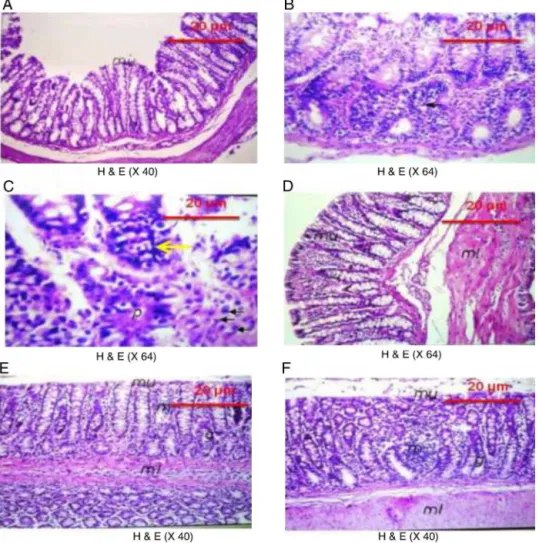
EffectofPPEand5-FUonhistopathologicalalterationsin N-MNU-inducedcoloncancerinrats
Asdepicted in Fig. 5, photomicrographsof the (A) negative control section of colon tissue showed the normal histologi-cal features of the mucosa, submucosa and muscularis layers. However, the section (B) of N-MNU untreated group shows dysplasia and anaplasia in the epithelial cells lining the glan-dular structure and its (C) magnification shows the mitotic activity of the nuclei (black arrows) and hyperchromasia
Fig.4. (continued)
(yellow arrow). (D) Section of the 5-FU treated group shows milddysplasia,withoutanaplasia,while(E)thetissuesectionof low-dosePPEtreatedgroupshowsinflammatorycellinfiltration in thelamina propria of themucosal layerand the underlying musculature,withintactmucosalepithelium.(F)Sectionoftissue treatedwithhigh-dosePPEshowsinflammatorycellinfiltration inthelaminapropriaofthemucosallayer,withintactmucosal epithelium.
Discussionandconclusion
Thepresentstudyhasprovidedcompellingevidencefavoring thechemopreventiveeffectofP.granatumL.againstcoloncancer intheexperimentalmodelandvalidatedthesuggested hypothe-sis.Inthepresentwork,PPE-treatedrats,particularlyatthehigh dose,exhibitedamarkedreductionincoloncancerasdocumented bythereductioninthecoloncancermarkers,viz.,CEAandCCSA-4, throughananti-proliferativeeffect(TGF-,EGF,C-mycandcyclin D1),pro-apoptoticpotential(survivin andBcl2),anti-metastasis
(MMP-7),and anti-inflammatoryaction(COX-2),andthe down-regulationof-CateninandK-rasgenes.
TheWnt/-Cateninsignalingpathwayplaysapivotalrolein thetranscriptionalregulation process thatimpacts cell growth, development,and differentiationinmany malignancies, includ-ing CRC (Ashihara et al., 2015). Wnt proteins dysregulated through the activation of -Catenin, a downstream activa-tor of the Wnt signaling pathway, have been implicated in many cancers (Ashihara et al., 2015). -Catenin targets genes that regulate differentcellularprocesses involvingproliferation (e.g., C-myc, cyclin D1), survival/anti-apoptosis (survivin), dif-ferentiation,migration(MMP7),andangiogenesis(Herbstetal., 2014). While -Catenin plays a key role in embryonic devel-opmentand tissue homeostasis, this proteinalso participatesin the initiation and progression of colon cancer. In particular, the deregulation of genes involved in proliferation and migra-tion has been frequently observed in colorectal carcinomas (Herbstetal.,2014).
Fig.5. RepresentativephotomicrographsofcolontissuewithorwithoutN-MNU-inducedCRC.(A)Normalcontrolsectionshowingthenormalhistologicalfeaturesofthe mucosa,submucosaandmuscularislayers,while(B)N-MNUuntreatedsectionshowsdysplasiaandanaplasiaintheepithelialcellsliningtheglandularstructureandits(C) magnificationshowsthemitoticactivityofthenuclei(blackarrows)andhyperchromasia(yellowarrow).(D)5-FUtreatedsectionshowsmilddysplasia,withoutanaplasia, while(E)sectiontreatedwithlow-dosePPEshowsinflammatorycellinfiltrationinthelaminapropriaofthemucosallayerandtheunderlyingmusculature,withintact mucosalepithelium.(F)High-dosePPEsectionshowsinflammatorycellinfiltrationinthelaminapropriaofthemucosallayer,withintactmucosalepithelium.
theattenuationof theWntsignalingpathwayin hepatocellular carcinoma(Bhatiaet al.,2013).PPEactive constituents,suchas ellagicacid(EA), havealsobeendemonstratedtomodulatethe Wntsignalingpathwaythroughtheinhibitionofcaseinkinase,a positiveregulatoroftheWntsignalingpathway;EAalsofunctions asamodulator oftheinteractionbetween-Cateninand mem-bersofthe-Catenindestructioncomplex(Sharmaetal.,2010). RutinhasantitumoreffectthroughinducingG2/Mcellcyclearrest andpromotingapoptosisanddecreasingBCL2 expression(Chen etal., 2013).Moreover,PPE accumulatesin theG2/M phase of thecellcycleassociatedwiththesignificantdown-regulationof theC-mycgene(Adhamietal.,2009).Furthermore,puanicalagin, oneoftheactiveanti-cancercomponentsofPPE,inhibitshuman coloncancergrowthassociatedwiththeinhibitionofcyclinD1and survivinexpressionthroughtheWnt/-Cateninsignalingcascade (Tangetal.,2016).Thesuppressionof-Catenintranslocationand thesubsequentexpressionofthetargetgenesthroughPPEwere reflectedinthehistologicalexaminationofcolontissuesthrough PPE-mediatedprotectionagainstN-MNU-inducedcolon adenocar-cinoma.In addition,PPEmediatedapoptosis byenhancingBcl2 cleavage,aneffectpresumablyresultingfromtheactionof this treatmentontheoncogenicNotchandWntpathwaysandtheir downstreamtargets,viz.,-Catenin,C-myc,cyclinD1,cyclinB1, pERK,MMP-7,MMP-9andEGF(Taoetal.,2002;Middhaetal.,2013; Herbstet al.,2014).TreatmentwithPPEextractalsodecreased
C-mycandCOX-2,whichareregulatedthrough-Catenin(Patel etal.,2008).Moreover,EAdown-regulatestheexpressionlevelsof K-ras(González-Sarríasetal.,2009).
Inthepresentstudy,thegeneexpressionlevelsof-Catenin, K-ras and C-myc in the colon tissues of the 5-FU treated rats inthecancergroupweredown-regulatedincomparedwiththe untreated cancer group. In addition, 5-FU substantially abated thelevelsofCEA,CCSA-4,TGF-,EGF, cyclinD1, MMP-7, COX-2, Bcl2 and survivin. The activity of 5-FU primarily depends on intracellular delivery to the active metabolite, 5-fluoro-2′ -deoxyuridine-5′-monophosphate, which inhibits DNA synthesis throughtheformationofastablecomplexwiththymidylate syn-thase(TS)inthepresenceoffolates,followedbytheinitiationof cell-cyclearrestorcelldeath(Kikuchietal.,2009).TSdecreased Bcl-2expression(Longleyetal.,2003),confirmingtheresultsof thepresentandpreviousstudies.5-FUtriggersapoptosisin DN-HIF-transfectedA549cellsviathereductionofsicyclinD1(cyclin D1-specificinterferenceRNA)andthedownregulationofC-myc mRNAexpression,phosphorylatedC-mycinhumancoloncancer KM12Ccells and survivinmRNAexpression(Wen et al.,2010). Moreover,5-FUsignificantlydownregulatedtheexpressionof -CateninproteinandsuppressedtheWntcanonicalpathway(Refaat etal.,2015).
bydecreasingcolon cancermarkers,viz.,CEAand CCSA-4.PPE, whichisrichinmultiplebioactivenaturalconstituents,mediated itseffectpossiblybyitsanti-proliferativeeffect(TGF-,EGF,C-myc andcyclinD1),pro-apoptoticpotential(survivinandBcl2), anti-metastasis(MMP-7),andanti-inflammatoryaction(COX-2),and thedown-regulationof-CateninandK-rasgenes.Theseeffects involvethemodulationoftheWnt/-Cateninsignalingpathway. Thestudy,hence,nominatestheuseofPPEasanadditiveontherapy tobestudiedinclinicaltrials.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethattheyhave fol-lowed theprotocolsof theirworkcenter onthe publicationof patientdata.
Right to privacy and informed consent. The authors have
obtainedthewritteninformedconsentofthepatientsorsubjects mentionedinthearticle.Thecorrespondingauthorisinpossession ofthisdocument.
Authorscontributions
MBS(PhDstudent)contributedincollectingandrunningthe laboratorywork.HHAsupervisedthelaboratorywork.HHA,HSE andNFAcontributedinwritingthemanuscript.HHAandHSE con-tributedindesigningthestudy,criticalanalysisofdata,supervised thelaboratorywork.EAKHcontributedtomolecularandHPLC anal-ysis.Alltheauthorshavereadthefinalmanuscriptandapproved thesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
TheauthorsexpresssincereappreciationtoProf.AdelBakeer Kholoussy,FacultyofVeterinaryMedicine,CairoUniversity;Prof. IbrahimEl-Garf,DepartmentofBotany,FacultyofScience,Cairo UniversityandUnitedGroupPharmaCo.(Badercity,Cairo,Egypt).
References
Adhami, V.M., Khan, N., Mukhtar, H., 2009. Cancer chemoprevention by pomegranate:laboratoryandclinicalevidence.Nutr.Cancer61,811–815. Ahmed,H.H.,Abdel-Rahman,M.,Salem,F.E.H.,Shalby,A.B.,Lokman,M.S.,2013.
AntitumorefficacyofBoswellaserrataextractinmanagementofcoloncancer inducedinexperimentalanimal.Int.J.Pharm.Pharmaceut.Sci.5,379–389. Ashihara,E.,Takada,T.,Maekawa,T.,2015.TargetingthecanonicalWnt/-Catenin
pathwayinhematologicalmalignancies.CancerSci.106,665–671.
Austinat, M., Dunsch, R., Wittekind, C., Tannapfel, A., Gebhardt, R., Gau-nitz,F.,2008.Correlationbetweenbeta-Catenin mutationsand expression of Wnt-signaling target genes in hepatocellular carcinoma. Mol. Cancer, http://dx.doi.org/10.1186/1476-4598-7-21.
Bhatia, D.,Thoppil, R.J.,Mandal, A.,Samtani, K.A.,Darvesh,A.S., Bishayee,A., 2013.Pomegranatebioactiveconstituentssuppresscellproliferationandinduce apoptosisin an experimental model of hepatocellularcarcinoma: roleof Wnt/-Cateninsignalingpathway.EvidBasedComplement.Alternat.Med., http://dx.doi.org/10.1155/2013/371813.
Chen,H., Miao, Q., Geng, M.,Liu, J., Hu,Y., Tian, L.,Pan, J.,Yang, Y., 2013. Anti-tumor effect of rutin on human neuroblastoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Sci. World J., http://dx.doi.org/10.1155/2013/269165.
El-Toumy,S.A.,Rauwald,H.W.,2002.TwoellagitanninsfromPunicagranatum heart-wood.Phytochemistry61,971–974.
Eshak,M.G.,Ghaly,I.S.,Khalil,W.K.B.,Farag,I.M.,Ghanem,K.Z.,2010.Genetic alter-ationsinducedbytoxiceffectofthermallyoxidizedoilandprotectiveroleof tomatoesandcarrotsinmice.J.Am.Sci.6,175–188.
Fuentes-Calvo,I.,Blázquez-Medela,A.M.,Santos,E.,López-Novoa,J.M., Martínez-Salgado, C.,2010. Analysis ofk-ras nuclear expression infibroblasts and mesangialcells.PLoSOne14,e8703.
González-Sarrías,A.,Espín,J.C.,Tomás-Barberán,F.A.,García-Conesa,M.T.,2009. Geneexpression,cellcyclearrestandMAPKsignallingregulationinCaco-2cells exposedtoellagicacidanditsmetabolites,urolithins.Mol.Nutr.FoodRes.53, 686–688.
Herbst,A.,Jurinovic,V.,Krebs,S.,Thieme,S.E.,Blum,H.,Göke,B.,Kolligs,F.T., 2014.Comprehensiveanalysisof-Catenintargetgenesincolorectal carci-nomacelllineswithderegulatedWnt/-Cateninsignaling.BMCGenomics, http://dx.doi.org/10.1186/1471-2164-15-74.
Kikuchi,M.,Mikami,T.,Sato,T.,Tokuyama,W.,Araki,K.,Watanabe,M.,Saigenji, K.,Okayasu,I.,2009.HighKi67,Bax,andthymidylatesynthaseexpression wellcorrelateswithresponsetochemoradiationtherapyinlocallyadvanced rectalcancers:proposalofalogisticmodelforprediction.Br.J.Cancer101, 116–123.
Kundu,J.K.,Choi,K.Y.,Surh,Y.J.,2006.beta-Catenin-mediatedsignaling:anovel molecular target forchemoprevention with anti-inflammatorysubstances. Biochim.Biophys.Acta1765,14–24.
Larrosa,M.,Ya ˜néz-Gascón,M.J.,Selma,M.V.,González-Sarrías,A.,Toti,S.,Cerón, J.J.,Tomás-Barberán,F.,Dolara,P.,Espín,J.C.,2009.Effectofalowdoseof dietaryresveratroloncolonmicrobiota,inflammationandtissuedamageina DSS-inducedcolitisratmodel.J.Agric.FoodChem.57,2211–2220.
Longley,D.B.,Latif,T.,Boyer,J.,Allen,W.L.,Maxwell,P.J.,Johnston,P.G.,2003.The interactionofthymidylatesynthaseexpressionwithp53-regulatedsignaling pathwaysintumorcells.Semin.Oncol.30,3–9.
Middha,S.K.,Usha,T.,Pande,V.,2013.Areviewonantihyperglycemicand antihep-atoprotectiveactivityofeco-friendlyPunicagranatumpeelwaste.EvidBased Complement.Alternat.Med.,http://dx.doi.org/10.1155/2013/656172. Narisawa,T.,Fukaura,Y.,2003.Preventionbyintrarectal5-aminosalicylicacidof
N-methylnitrosourea-inducedcoloncancerinF344rats.Dis.ColonRectum46, 900–903.
Orgil,O.,Schwartz,E.,Baruch,L.,Matityahu,I.,Mahajna,J.,Amir,R.,2014.The antiox-idativeandanti-proliferativepotentialofnon-edibleorgansofthepomegranate fruitandtree.LWT-FoodSci.Technol.58,571–577.
Patel, R.,Ingle, A., Maru,G.B., 2008. Polymericblack tea polyphenols inhibit 1,2-dimethylhydrazine inducedcolorectal carcinogenesisby inhibitingcell proliferationviaWnt/beta-Cateninpathway.Toxicol. Appl.Pharmacol.227, 136–146.
Reeves,P.G.,Nielsen,F.H.,Fahey,G.C.,1993.AIN-93purifieddietsforlaboratory rodents:finalreportoftheAmericanInstituteof365Nutritionadhoc writ-ingcommitteeonthereformulationoftheAIN-76Arodentdiet.J.Nutr.123, 1939–1951.
Refaat,B.,El-Shemi,A.G.,Kensara,O.A.,Mohamed,A.M.,Idris,S.,Ahmad,J.,Khojah, A.,2015.VitaminD3enhancesthetumouricidaleffectsof5-fluorouracilthrough multipathwaymechanismsinazoxymethaneratmodelofcoloncancer.J.Exp. Clin.CancerRes.34,71.
Sharma,M.,Li,L.,Celver,J.,Killian,C.,Kovoor,A.,Seeram,N.P.,2010.Effectsoffruit ellagitanninextracts,ellagicacid,andtheircolonicmetabolite,urolithinA,on Wntsignaling.J.Agric.FoodChem.58,3965–3969.
Shin,J.W.,Seol,I.C.,Son,C.G.,2010.Interpretationofanimaldoseandhuman equiv-alentdosefordrugdevelopment.J.KoreanOrientalMed.31,1–7.
Shirode,A.B.,Kovvuru,P.,Chittur,S.V.,Henning,S.M.,Heber,D.,Reliene,R.,2014. AntiproliferativeeffectsofpomegranateextractinMCF-7breastcancercellsare associatedwithreducedDNArepairgeneexpressionandinductionofdouble strandbreaks.Mol.Carcinog.53,458–470.
Srimuangwong, K., Tocharus, C., Tocharus, J., Suksamrarn, A., Chintana, P.Y., 2012.Effectsofhexahydrocurcuminincombinationwith5-fluorouracilon dimethylhydrazine-inducedcoloncancerinrats.WorldJ.Gastroenterol.18, 6951–6959.
Tang,J.M.,Min,J.,Li,B.S.,Hong,S.S.,Liu,C.,Hu,M.,Li,Y.,Yang,J.,Hong,L.,2016. Therapeuticeffectsofpunicalaginagainstovariancarcinomacellsinassociation with-Cateninsignalinginhibition.Int.J.Gynecol.Cancer26,1557–1563. Tao,L.,Kramer,P.M.,Wang,W.,Yang,S.,Lubet,R.A.,Steele,V.E.,Pereira,M.A.,2002.
Alteredexpressionofc-myc,p16andp27inratcolontumorsandits rever-salbyshort-termtreatmentwithchemopreventiveagents.Carcinogenesis23, 1447–1454.
Vijay,M.,Sivagami,G.,Thayalan,K.,Nalini,N.,2016.Radiosensitizingpotentialof rutinagainsthumancolonadenocarcinomaHT-29cells.Bratisl.Lek.Listy.117, 171–178.
Watson,S.A.,Michael,D.,Justin,T.A.,Grimes,S.,Morris,T.M.,Robinson,G.,Clarke, P.A.,Hardcastle,J.D.,1998.Pre-clinicalevaluationofthegastrimmune immuno-genaloneandincombinationwith5-fluorouracil/leucovorininaratcolorectal cancermodel.Int.J.Cancer75,873–877.
Wen,W.,Ding,J.,Sun,W.,Wu,K.,Ning,B.,Gong,W.,He,G.,Huang,S.,Ding,X., Yin,P.,Chen,L.,Liu,Q.,Xie,W.,Wang,H.,2010.SuppressionofcyclinD1by hypoxia-induciblefactor-1viadirectmechanisminhibitstheproliferationand 5-fluorouracil-inducedapoptosisofA549cells.CancerRes.70,2010–2019. Zhao,Y.,Miao,G.,Li,Y.,Isaji,T.,Gu,J.,Li,J.,Qi,R.,2014.Microrna130bsuppresses