w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Dendranthema
grandiflorum
,
a
hybrid
ornamental
plant,
is
a
source
of
larvicidal
compounds
against
Aedes
aegypti
larvae
Kassia
C.V.W.
Spindola
a,
Naomi
K.
Simas
b,
Celso
E.
dos
Santos
a,
Ary
G.
da
Silva
c,
Wanderson
Romão
d,
Gabriela
Vanini
d,
Samantha
R.C.
da
Silva
d,
Grazielle
R.
Borges
e,
Fernando
G.
de
Souza
e,
Ricardo
M.
Kuster
a,d,∗aInstitutodePesquisasdeProdutosNaturais,CentrodeCiênciasdaSaúde,UniversidadeFederaldoRiodeJaneiro,RiodeJaneiro,RJ,Brazil
bFaculdadedeFarmácia,CentrodeCiênciasdaSaúde,UniversidadeFederaldoRiodeJaneiro,RiodeJaneiro,RJ,Brazil
cFunctionalEcologyLaboratory,UniversidadedeVilaVelha,BoaVista,ES,Brazil
dNúcleodeCompetênciaemQuímicadoPetróleo,DepartamentodeQuímica,UniversidadeFederaldoEspíritoSanto,Vitória,ES,Brazil
eBiopolymersandSensorsLaboratory,InstitutodeMacromoléculas,UniversidadeFederaldoRiodeJaneiro,RiodeJaneiro,RJ,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received15October2015 Accepted21January2016 Availableonline9February2016
Keywords:
Aedesaegypti
Caffeoylquinicacids Agribusiness Fattyacids Larvicidalactivity Triterpenoids
a
b
s
t
r
a
c
t
Inhybridcultivatedform,Dendranthemagrandiflorum(Ramat.)Kitam.,Asteraceae,flowers( Chrysan-themummorifoliumRamat.)wereutilizedintheproductionofextracts,whichwereanalyzedforlarvicidal activityagainstAedesaegyptithirdinstarlarvae.MethanolanddichloromethaneextractsshowedLC50
valuesof5.02and5.93ppm,respectively.UsingGC–MS,phytochemicalanalysesofthedichloromethane extractshowedthepresenceoftriterpenoidsandfattyacids,whileflavonoidsandcaffeoylquinicacids wereshowntooccurinthemethanolextractbyESIFourierTransformIonCyclotronResonanceMass Spectrometry(ESI-FT-ICR-MS).Triterpenoidsandfattyacidsarewellknowninsecticidalcompounds. Fromthisstudy,itcanbeconcludedthatD.grandiflorumgrownforfloriculture,asanagribusiness,can haveadditionalapplicationsasrawmaterialfortheproductionofinsecticidalproducts.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Knownforover2000years,thechrysanthemumisan
ornamen-talplantnativetoChina.ItbelongstothegenusDendranthema, Asteraceae,andnumerouscultivarshavebeenproduced(Kimetal.,
2015).Thecutchrysanthemumisoneofthemostcommercially
successfulflowersbythediversityofitsinflorescence(Brackmann etal.,2005).Floricultureis arepresentativeactivityin Brazilian agribusinessand, assuch,it hasmadeanoutstandingeconomic and technological contribution. In particular, the
chrysanthe-mumculture (Dendranthema grandiflorum (Ramat.) Kitam.) has
greatimportanceinBrazilianfloriculture,anditscultivationhas increasedthroughoutthecountry(Polanczyketal.,2008).Climate isanimportantfactorthatfavorstheexpansionoffloriculture,as anagribusiness,inBrazil.Specifically,itallowsforthecultivationof temperateandtropicalflowerswithlowcostandhighproduction allyearlong(DaSilvaJunioretal.,2011).
∗ Correspondingauthor.
E-mail:ricardo.m.kuster@ufes.br(R.M.Kuster).
DengueisamajorpublichealthprobleminBrazil.The trans-missionofthedenguevirustohumansoccursthroughthebiteof themosquitoAedes aegypti.Thediseasecanbetemporarily dis-abling,butinitshemorrhagicform,itcanresultindeath.Although publicpoliciesexist formosquitocontrol,resistanceto conven-tionalinsecticidesisconcerningtohealthauthorities(Liu,2015). Therefore,this paperdescribesa naturalproduct,extractsof D. grandiflorum,whichareobtainedfromthecultivar“yellowsheena”, asasourceofcompoundswithlarvicidalactivityagainstA.aegypti
thirdinstarlarvae.Thestudiedplantisorganicallycultivatedinthe StateofRiodeJaneiro,NovaFriburgoCity.Inviewofitslarvicidal potential,thecutflowershouldalsosupporttheestablishmentof floricultureasanimportantagribusinessinBrazil.
Materialsandmethods
Plantmaterial
Dendranthema grandiflorum (Ramat.)Kitam., Asteraceae, yel-lowsheena,ahybridunderorganiccultivation,wasobtainedfrom farmersinNovaFriburgo,StateofRiodeJaneiro,inSeptember2013 andbotanicallyidentifiedbyDr.MarianaMachadoSaavedrafrom
http://dx.doi.org/10.1016/j.bjp.2016.01.003
theRiodeJaneiroBotanicalGarden(voucherspecimendeposited attheherbariumProf.JorgePedroPereiraCarautaundernumber
HUNI3531).
Preparationofextracts
Theflowersweredriedinanovenat50◦Cfor7days(223g).
Afterthat, they weresubmitted tomethanol extractionover a
periodof15days.Theextractwasfilteredandthesolventremoved byrotativeevaporationundervacuumtoobtainthefirstproduct,a dryMeOHextract(80g).Seventygramsweresuspendedinwater, andaftersolventremoval,thesuspensionwaspartitioned with dichloromethanetoyieldthesecondproduct,adryCH2Cl2extract (29g).
Phytochemicalanalysis
Electron Impact Ionization/Mass Spectrometry (EI/MS) was
obtainedusing a ShimadzuQP5050 GC–MSon a DB-5 column
(30m×0.25mm and film thicknessof 0.25m, J&W Scientific) withanionizingenergyof70eV.Programmingoftheoven temper-aturestartedat80◦C,whichwasmaintainedfor2min.Then,the temperaturewasraisedto260◦Catarateof15◦C/minandagain increasedto320◦Cat5◦C/min.Anisothermduring10minwas performedatthisfinaltemperature.Thetemperaturesofinjector andionsourcewerekeptat250◦Cand280◦C,respectively.Helium wasusedasthecarriergaswithaconstantflowof1.1mlmin−1. Onemicroliterofthesamplewasinjectedwithasplitratioof70:1.
MassspectraofextractcompoundswerecomparedwiththeNIST
MassSpectralDatabase(MSSearch2.0)andwithliteraturedata (AssimoupoulouandPapageorgiou,2005).
Themethanolicextractof D. grandiflorumwasanalyzedin a
mass spectrometer(Model9.4 T Solarix, BrukerDaltonics,
Bre-men,Germany),whichwassettooperateinnegativeionmode,
ESI(-),overamassrangeofm/z200–1300.Theparametersofthe ESI(-)sourcewereasfollows:nebulizergaspressureof0.5–1.0bar, capillaryvoltageof3–3.5kV,andtransfercapillarytemperatureof 250◦C.ThemassspectrumwasprocessedusingtheCompassData Analysissoftwarepackage(BrukerDaltonics,Bremen,Germany). Aresolvingpower,m/m50%=∼500,000,inwhichm50%isthefull peakwidthathalf-maximumpeakheightofm/z∼=400andamass accuracyof<1ppm,providedtheunambiguousmolecularformula assignmentsforsinglychargedmolecularions.Elemental compo-sitionsofthecompoundsweredeterminedbymeasuringthem/z
values.Theunsaturationlevelofeachmoleculecouldbededuced directlyfromitsdoublebondequivalent(DBE),followingthe equa-tionDBE=c−h/2+n/2+1,wherec,h,andnarethenumbersof carbon,hydrogen,andnitrogenatoms,respectively(Ferreiraetal., 2014;Costaetal.,2014;DeSáetal.,2015;Nascimentoetal.,2015). Molecularformula,measuredm/zvalues,DBE,andmasserrorare showninTable1.Tandemmassspectrometry(MS2)experiments
were also performedon a quadrupole analyzer coupledto the
FT-ICRmassspectrometer,andquadrupoleFouriertransformion
cyclotronresonancemassspectrometry(Q-FT-ICRMS)was
per-formedforionsofm/z353,431,447,449and515.Thefragments produced (m/zvalues) fromESI(-)-MS/MSspectraare shownin Table3.
Larvicidalactivity
A. aegypti larvae that originatedfrom theNPPN strain were rearedinthelaboratoryundercontrolledphotoperiod(12hlight and12hdark)at27◦Cand80±10%rel.humidityintraysfilled withdechlorinatedtapwaterandcaninefood.
Larvicidalactivitywasconductedfollowingthemethodadapted fromWHO(1970).Foreachtreatmentandcontrol,fivelarvaeof thirdstageinstars,orearlyfourthstageinstars,weretransferred into20mlglassesin14.9mlof distilledwater. Thesolutionsof crudeextracts,orfractions,werepreparedinethanolandadded (0.1ml)tothetreatmentglasseswithapipettetogivethefinal
assay concentrations. Controls received aqueous solution with
0.1mlofethanolonly.After24h,thenumberofdeadlarvaeineach glasswascounted.Alltreatmentswerereplicatedthreetimes.The 50%lethalconcentration(LC50)wascalculatedwitha95% confi-dencelimitbyProbitanalysis.
Resultsanddiscussion
Phytochemicalanalysis
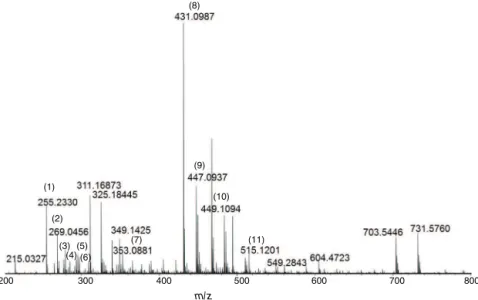
As shown in Fig. 1, methanol extract of D. grandiflorum by
ESI(-)FT-ICRMSyielded 11compoundsidentified asfattyacids, phenylpropanoidsandflavonoids(Table1).Allcompoundswere detected as deprotonated molecules, i.e., adduct ion, as repre-sentedby[M−H]− ion,formedbytheinteractionofa molecule withaproton,orhydrogen(Table1).Amongfattyacids,palmitic
acid, m/z 255.2330, was the major compound found in the
crudeextract.Phenylpropanoids,suchasmonocaffeoylquinicacids,
(8)
(9)
(10)
(11) (1)
(2)
(3) (5) (7)
(4)
200 300 400 500 600 700 800
(6)
m/z
Table1
Fattyacids,flavonoidsandcaffeicacidderivativesofmethanolextractofDendranthemagrandiflorum,asidentifiedfromESI(-)-FT-ICRMS.
Identification m/zmeasured Identifiedcompounds [M−H]− FragmentsMS/MS DBE Error(ppm)
1 255.2330 Palmiticacid C16H31O2 – 1 0.05
2 269.0456 Apigenin C15H9O5 – 11 0.26
3 279.2330 Linoleicacid C18H31O2 – 3 0.32
4 285.0406 Luteolin C15H9O6 – 11 0.31
5 293.2123 Linolenicacidmethylester C18H29O3 – 4 0.30
6 295.2280 Linoleicacidmethylester C18H31O3 – 3 0.45
7 353.0881 Monocaffeoylquinicacid C16H17O9 191 8 0.77
8 431.0987 Apigenin7-O-glucoside C21H19O10 269 12 0.65
9 447.0937 Luteolin7-O-glucoside C21H19O11 285 12 0.90
10 449.1094 Eriodictyol7-O-glucoside C21H19O11 431,413,387,311,and287 11 1.01
11 515.1201 Dicaffeoylquinicacid C25H23O12 353 14 1.18
Table2
Compositionoffattyacids,steroidsandtriterpenoidsinthedichloromethanepartitionofDendranthemagrandiflorum.
Retentiontime–RT(min) MajorfragmentsfromMSdata Identifiedcompounds Relativeconcentration(%)
12.95 270(M+),227,143,99,87,74(100%),55 Methylpalmitate 2.98
13.20 256(M+),213,185,171,157,143,129,115,83,73,57(100%) Palmiticacid 3.97 13.40 284(M+),256,241,227,213,157,101,88(100%),83,73,55 Ethylpalmitate 0.23
14.15 294(M+),263,95,81,67(100%),55 Linoleicacidmethylester 0.15
14.20 292(M+),264,149,135,122,108,93,79(100%),67,55 Linolenicacidmethylester 0.17
14.36 298(M+),255,199,143,87,74(100%),55 Methylstearate 0.12
Totalrelativeconcentrationoffattyacids 8.42
32.75 412(M+),55(100%) Stigmasterol 3.68
33.79 414(M+),55(100%) Sitosterol 13.47
34.45 426(M+),218(100%),203(49%),189 -Amyrin 13.53
35.20 426(M+),218(100%),203(18%),189 ␣-Amyrin 22.03
35.79 468(M+),218(100%),207(61%),203(40%),189(20%) -Amyrinacetate 1.68 36.50 468(M+),218(100%),207(67%),203(21%),189(40%) ␣-Amyrinacetate 4.78 36.68 426(M+),207,189(100%),175,147,135,121,109,95,81,68,55 Lupeol 5.06
Totalrelativeconcentrationoftriterpenoidsandsteroids 64.23%
m/z 353.0878, and dicaffeoylquinic acids, m/z 515.1201, were detectedasminorcompounds.Amongtheflavonoidsidentified, apigenin7-O-glucoside,m/z431.0987,eriodictyol7-O-glucoside,
m/z449.1093,andluteolin7-O-glucoside,m/z447.0933,werethe maincompoundsoftheMeOHextract.PurificationandNMR iden-tificationoftheflavonoidsweredescribedbySpindola(2015),and allthecompoundsweredescribedtooccurinD.grandiflorum(Lin andHarnly,2010;Ueharaetal.,2012).Table1showstheidentified compoundsforfattyandcaffeicacids,aswellasflavonoidsofD. grandiflorummethanolextract,asidentifiedfromESI(-)-FT-ICRMS data.
GC–MSanalysesofCH2Cl2extractshowsabundantpeaksintwo differentregionsinthechromatogram:(i)from6to18minand(ii) from30to38min.Ingeneral,fourteencompoundswere identi-fied,andtheirproposedstructures,molecularionsandfragments, retentiontime,andabundancerelativevaluesaresummarizedin Table2.
Inthefirstregion(approximatelybetween13.0and15.0min), themajor fatty acids were as follows: methyl palmitate, ethyl palmitate,palmiticacid,stearicacid,ethylstearate,linoleicacid methyl ester and linolenic acid methyl ester (Table 2). How-ever,in thesecond region(32–38min), steroids(sitosterol and
stigmasterol) and triterpenoids (-amyrin, ␣-amyrin, -amyrin acetate,␣-amyrinacetateandlupeol)canbeseenaslessvolatile substancesofthechromatogram.Theywerepreviouslyidentified inChrysanthemummorifolium(TakahashiandSato,1979).The rel-ativeabundancesofthetwomainclassesofidentifiedcompounds were8.42%forfattyacidsand64.23%fortriterpenoidsandsteroids. AsshowninTable2,triterpenoidsandsteroidsarethemajor compoundsinthelipophilicextractofD.grandiflorum.Theywere alsodescribedtooccurinotherChrysanthemumspecies,suchasC. indicum,C.macrocarpumaswellasC.morifolium(D.grandiflorum) cultivars(TakahashiandSato,1979;Akihisaetal.,2005;Kumar etal.,2005).
Larvicidalactivity
LC50valuesforMeOHandCH2Cl2extractswere5.02ppmand 5.93ppm,respectively(Table3).
Attheconcentration1g/ml,temephos(TradeName:Abate®), aspositivecontrol,killedallthetestedlarvae.SincetheCH2Cl2 extractwasobtainedasthesecondproductfromthecrudeMeOH extract,itisreasonabletothinkthatthelarvicidalactivityofthe flowersisconcentratedinthemorelipophiliccompoundsofthe
Table3
LarvicidalefficacyofextractsfromDendranthemagrandiflorumflowersagainstAedesaegypti.
Samples LC50(ppm)(LCL/UCL) LC95(ppm)(LCL/UCL) LC99(ppm)(LCL/UCL)
Dichloromethaneextract 5.9322
(5.1476/6.7862)
12.5650 (11.0297/14.9183)
15.3131 (13.3070/18.4474)
Methanolextract 5.0220
(3.6004/6.3780)
14.5585 (12.1309/18.8137)
18.5096 (15.2542/24.3771)
Temephos nd nd 1.0
plant.Infact,fromotherstudiedplants,ourgrouphasidentified larvicidalcompoundswiththatnonpolarcharacteristic,including terpenesandalkamides(Simasetal.,2004,2007,2013),although more polarcompounds,like saponins(Amin et al.,2011), have alsobeendescribedaslarvicides.Lipophiliccompoundsaremore active larvicidesbased ontheir easiertransport through insect cellwallandcytoplasmicmembrane(MannandKaufman,2012). FromtheCH2Cl2extractofD.grandiflorum,severallipophilic com-poundswerefound,includingfattyacids,steroidsandtriterpenes. Palmiticacidwasthemajorfattyacididentified.Rahumanetal. (2000)testedpalmiticacidasalarvicide againstCulex quinque-fasciatus,Aedes stephensi and A. aegypti.The LC50 values found were129,79and57ppm,respectively.Thesteroidssitosteroland stigmasterol werealso identified.Sitosterol, isolated from Abu-tilonindicum(L.)Sweet,wasactiveatLC5011.5,3.5and26.7ppm, respectively,againstA.aegypti,A.stephensiandC. quinquefascia-tuslarvae(Ghosh,2013;Rahumanetal.,2008).Triterpenesseem tobethemostactiveofallthecompoundsidentified.Oneofthe triterpenesinD.grandiflorum,␣-amyrinacetate,showedeffective activityagainstallfourlarvalgrowthstages(LC500.01–0.02ppm) andpupa(LC500.005ppm)of themosquitoAnophelesstephensi (Kuppusamyetal.,2009).Cholesterolisoneofthemostimportant nutrientsforA.aegyptilarvaldevelopment.TheproteinAeSCP-2 isusedbymosquitoesforthetransportof cholesterol. Inactiva-tionofitkillsthelarvae.Kumaretal.(2010)biocomputationally evaluatedtheabilityoftriterpenestoinactivatethisprotein,and
␣-amyrinwasstructurallyconsideredtobethemosteffective larvi-cidewiththisparticularmechanismofaction.Themostabundant compoundsintheCH2Cl2 extractoftheflowerswere␣-amyrin (22%),alongwith-amyrin(13.5%)andsitosterol(13.47%).
Khanetal.(2014)comparedthelarvicidalactivityofextracts of C. morifolium leaves(LC50 1.5ppm) withextracts of Parthe-niumhysterophorusL.leaves(LC50 1.02ppm)and foundslightly betterefficiencyofthelatteragainstAedesalbopictusthirdinstar mosquitolarvae.Unfortunately,theydidnotdescribethe chemi-calcompositionofthecomparedplants.Bothplantsbelongtothe Asteraceaefamily;therefore,someclassesofcompoundsare simi-lar.P.hysterophorusisasourceofsesquiterpenelactones(Dattaand Saxena,2001),compoundswithhighinsecticidal,cytotoxic, antitu-mor,allergenic,antifeedantandphytotoxicactivity,andtheplant isconsidereda noxiousweedthatthreatensbiodiversity(Patel, 2011).Littleevidencesupportsthepresenceofsesquiterpene lac-tonesinC.morifolium,thechrysanthemumofflorists(Schulzetal., 1975),althoughalantolactonewasdescribedtooccurinthisspecies (Sertoli et al., 1985). Also no environmentaldamage hasbeen reportedforC.morifolium.Pyrethrinesterscompriseanother com-poundclasswithhighinsecticidalactivity.Thesecompoundsare formedbycombiningtwoacids(chrysanthemicacidandpyrethric acid)withthreealcohols(pyrethrolone,cineroloneandjasmolone). Theyare,however,restrictedtoChrysanthemumcinerariaefolium
Vis. (Nagar et al., 2015).Using the techniques described, both sesquiterpenelactonesandpyrethrinswereinvestigated,butnot found,inD.grandiflorum.
InaNuclearMagneticResonance(NMR)-basedmetabolomics
study,Leissetal.(2009)wereabletocomparethemetabolome ofthrips-resistantandthrips-susceptiblechrysanthemums.They concludedthattheresistancewasassociatedwithhigheramounts ofchlorogenicacid,amonocaffeoylquinicacid,inthrips-resistant species.Phenylpropanoidsareknownfortheirinhibitoryeffecton herbivoresandpathogens.Thepossibletoxicityofchlorogenicacid toinsectsresultsfromitsoxidationproduct,chloroquine(Felton etal.,1991).Tunónetal.(1994)testedanethanolextractof Achil-lea millefolium L. for antifeedant activity against the mosquito
A.aegypti.Afterfractionationoftheextract,chlorogenicacidat 1.2mg/cm2wasoneofthemostactivecompoundsasamosquito repellent,causing100%larvalmortality.Flavonoidsandfattyacids,
atthesameconcentration,wereactive,butwithlower percent-ageofmortality.ThegreaterlarvicidalactivityofMeOHextractof
D.grandiflorum,whencomparedCH2Cl2extract,whichoriginates fromit,could,therefore,resultfromthepresenceofcaffeoylquinic acidsandotherphenolics,apartfromthetriterpenoids,steroids and fattyacids,aspreviously discussed.Basedontheresultsof thepresentstudy,itmaybespeculatedthatthecompounds,when
combined,mightactasapotentphytocomplexwithsynergistic
effect,butthisneedstobeconfirmedinfuturestudies.
D.grandiflorumhybridcanbeasourceoflarvicidalcompounds easilyextractedwithmethanol.Ornamentalplantsareabundantly cultivatedinBrazilianhighlands;however,afteraging,theyare dis-carded.Yet,theresultsofthepresentstudydemonstratethatsuch discardedplantscouldberecycledfortheirmethanolicextractsand usedtoproposeinnovativeproducts,as,forexample,larvicides, thushelpingtodevelopagribusinessinBrazil.
Authors’contributions
KCVWS(PhDstudent)collectedandidentifiedplantsamples,
ranthelaboratorywork,analyzeddata,anddraftedthepaper.NKS, CESandGRBcarriedoutbiologicalstudies.WRandGVperformed massspectrometryanalysisandSRCStochromatographicanalysis. FGSperformedacriticalreadingofthemanuscript.AGSconducted statisticalanalysis.RMKdesignedthestudy,supervisedthe labo-ratoryworkandofferedcriticalcommentsonthemanuscript.All authorshavereadthefinalmanuscriptandapprovedthe submis-sion.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
TheauthorsthankCAPESandCNPqforfinancialsupportand
Prof. Sandra Zorat Cordeiro, UNIRIO-RJ, for the plant specimen depositattheherbarium(Prof.JorgePedroPereiraCarauta).
References
Akihisa,T.,Franzblau,S.G.,Ukiya,M.,Okuda,H.,Zhang,F.,Yasukawa,K.,Suzuki, T.,Kimura,Y.,2005.AntitubercularactivityoftriterpenoidsfromAsteraceae flowers.Biol.Pharm.Bull.28,158–160.
Amin,E.,El-Hawary,S.S.,Fathy,M.M.,Mohammed,R.,Ali,Z.,Tabanca,N.,Wedge, D.E.,Becnel,J.J.,Khan,I.A.,2011.Triterpenoidalaaponins:bioactivesecondary metabolitesfromZygophyllumcoccineum.PlantaMed.77,488–491.
Assimoupoulou,A.N.,Papageorgiou,V.P.,2005.GC–MSanalysisofpenta-and tetra-cyclictriterpenesfromresinsofPistaciaspecies.PartI.Pistacialentiscusvar.Chia. Biomed.Chromatogr.19,285–311.
Brackmann,A.,Bellé,R.A.,deFreitas,S.T.,deMello,A.M.,2005.Vaselifeof chrysan-themum(Dendranthemagrandiflorum)ingibberellicacidsolutions.Cienc.Rural 35,1451–1455.
Costa,H.B.,Souza,L.M.,Soprani,L.C.,Oliveira,B.G.,Ogawa,E.M.,Korres,A.M.N., Ven-tura,J.Á.,2014.Monitoringthephysicochemicaldegradationofcoconutwater usingESI-FT-ICRMS.FoodChem.174,139–146.
DaSilvaJunior,A.G.,Perez,R.,Amin,D.,Ferreira,M.A.S.,2011.Designand implemen-tationofflowerexportconsortiuminCearástate,Brazil.In:2011International EuropeanForum,Innsbruck-Igls,Austria.
Datta,S.,Saxena,D.B.,2001.Pesticidalpropertiesofparthenin(fromParthenium hysterophorus)andrelatedcompounds.PestManage.Sci.57,95–101. DeSá,L.Z.C.M.,Castro,O.S.,Lino,F.M.A.,Bernardes,M.J.C.,Viegas,J.C.J.,Dinis,T.C.P.,
Santana,M.J.,Romão,W.,Lião,L.M.,Ghedini,P.C.,Rocha,M.L.,2015.Antioxidant potentialandvasodilatoryactivityoffermentedbeveragesofjabuticababerry (Myrciariajaboticaba).J.Funct.Foods8,169–179.
Felton,G.W.,Donato,K.K.,Broadway,R.M.,Duffey,S.S.,1991.Impactofoxidized plantphenolicsonthenutritionalqualityofdietaryproteintoanoctuid herbi-vore,Spodopteraexigua.J.InsectPhysiol.38,277–285.
Ghosh,A.,2013.Efficacyofphytosterolasmosquitolarvicide.AsianPac.J.Trop.Dis. 3,252.
Khan,G.Z.,Khan,I.A.,Khan,I.,2014.ExploitingthelarvicidalpropertiesofParthenium hysterophorusL.forcontrolofdenguevector,Aedesalbopictus.Pak.J.WeedSci. Res.20,431–438.
Kim,Y.S.,Kim,S.H.,Sung,S.Y.,Kim,D.S.,Kim,J.B.,Jo,Y.D.,Kang,S.Y.,2015.Genetic relationshipsamongdiversespray-andstandard-typechrysanthemum vari-etiesandtheirderivedradio-mutantsdeterminedusingAFLPs.Hortic.Environ. Biotechnol.56,498–550.
Kumar,A.,Singh,S.P.,Bhakuni,R.S.,2005.SecondarymetabolitesofChrysanthemum
genusandtheirbiologicalactivities.Curr.Sci.89,1489–1501.
Kumar,R.B.,Shanmugapriya,B.,Thiyagesan,K.,Kumar,S.R.,Xavier,S.M.,2010. Asearchformosquitolarvicidalcompoundsbyblockingthesterolcarrying protein,AeSCP-2,throughcomputationalscreeninganddockingstrategies. Pharmacogn.Res.2,247–253.
Kuppusamy,C.,Murugan,K.,Arul,N.,Yasodha,P.,2009.Larvicidalandinsectgrowth regulatoreffectof␣-amyrinacetatefromCatharanthusroseusL.againstthe malariavectorAnophelesstephensiListon(Diptera:Culicidae).Entomol.Res.39, 78–83.
Lin,L.-Z.,Harnly,J.M.,2010.Identificationofthephenolicscomponentsof chrysan-themumflower(ChrysanthemummorifoliumRamat).FoodChem.120,319–326. Leiss,K.A.,Maltese,F.,Choi,Y.H.,Verpoorte,R.,Klinkhammer,P.G.L.,2009. Identi-ficationofchlorogenicacidasaresistancefactorforthripsinChrysanthemum. PlantPhysiol.150,1567–1575.
Liu, N.,2015. Insecticideresistance in mosquitoes:impact, mechanisms, and researchdirections.Annu.Rev.Entomol.60,537–559.
Mann,R.S.,Kaufman,P.E.,2012.Naturalproductpesticides:theirdevelopment, deliveryanduseagainstinsectvectors.Mini-Rev.Org.Chem.9,185–202. Nagar,A.,Chatterjee,A.,UrRehman,L.,Ahmad,A.,Tandon,S.,2015.Comparative
extractionandenrichmenttechniquesforpyrethrinsfromflowersof Chrysan-themumcinerariaefolium.Ind.CropsProd.76,955–960.
Nascimento,I.R.,Costa,H.B.,Souza,L.M.,Soprani,L.C.,Merlo,B.B.,Romão,W.,2015. Chemicalidentificationofcannabinoidsinstreetmarijuanasamplesusing elec-trosprayionizationFT-ICRmassspectrometry.Anal.Methods7,1415–1424. Patel,S.,2011.HarmfulandbeneficialaspectsofPartheniumhysterophorus:an
update.3Biotech1,1–9.
Polanczyk, R.A., Pratissoli, D., Paye, H.S.,Pereira, V.A., Barros, F.L.S., Oliveira, R.G.S.,Passos,R.R.,MartinsFilho,S.,2008.Induc¸ãoderesistênciaàmosca
minadora em crisântemo usando composto silicatado. Hortic. Bras. 26, 240–243.
Rahuman,A.A.,Gopalakrishnan,G.,Ghouse,B.S.,Arumugam,S.,Himalayan,B.,2000. EffectofFeronialimoniaonmosquitolarvae.Fitoterapia71,553–555. Rahuman,A.A.,Gopalakrishnan,G.,Venkatesan,P.,Geetha,K.,2008.Isolationand
identificationofmosquitolarvicidalcompoundfromAbutilonindicum(Linn.) Sweet.Parasitol.Res.102,981–988.
Schulz,K.H.,Hausen,B.M.,Wallhöfer,L.,Schmidt-Löffler,P.,1975. Chrysanthemen-allergie.2.ExperimentelleUntersuchungenzurIdentifizierungderAllergene. Arch.Dermatol.Forsch.251,235–244.
Sertoli,A.,Campolmi,P.,Fabbri,P.,Gelsomini,N.,Panconesi,E.,1985.Eczemacaused bycontactwithChrysanthemummorifoliumRamat.G.Ital.Dermatol.Venereol. 120,365–370.
Simas,N.K.,Lima,E.D.C.,Conceic¸ão,S.D.R.,Kuster,R.M.,deOliveiraFilho,A.M.,Lage, C.L.S.,2004.Naturalproductsfordenguetransmissioncontrol–larvicidal activ-ityofMyroxylonbalsamum(redoil)andofterpenoidsandphenylpropanoids. Quim.Nova27,46–49.
Simas,N.K.,Lima,E.D.C.,Kuster,R.M.,Lage,C.L.S.,DeOliveiraFilho,A.M.,2007. Poten-tialuseofPipernigrumethanolextractagainstpyrethroid-resistantAedesaegypti
larvae.Rev.Soc.Bras.Med.Trop.40,405–407.
Simas,N.K.,Dellamora,E.C.L.,Schripsema,J.,Lage,C.L.S.,Filho,A.M.O., Wessjo-hann,L.,Porzel,A.,Kuster,R.M.,2013.Acetylenic2-phenylethylamidesandnew isobutylamidesfromAcmellaoleracea(L.)R.K.Jansen,aBrazilianspicewith larvicidalactivityonAedesaegypti.Phytochem.Lett.6,67–72.
Spindola,K.C.W.,2015.CassiaaustraliseDendranthemagrandiflorum:plantas orna-mentaiscomo fontede substânciasantivirais einseticidase produtos de inovac¸ão.Tesededoutorado,ProgramadePós-graduac¸ãoemQuímicade Pro-dutosNaturais,UniversidadeFederaldoRiodeJaneiro,RiodeJaneiro,174pp. Takahashi,M.,Sato,T.,1979.StudiesonthecomponentsofChrysanthemum
mori-foliumRamat.var.sinenseMakinoformaesculentumMakino.II.Componentsof oneofBrands“Mottenohoka”.Yakuga.Zasshi99,90–91.
Tunón,H.,Thorsell,W.,Bohlin,L.,1994.Mosquitopepellingactivityofcompounds occurringinAchilleamillefoliumL.(Asteraceae).Econ.Bot.48,111–120. Uehara, A., Nakata, M., Kitajima,J., Iwashina,T., 2012. Internal and external
flavonoidsfromtheleavesofJapaneseChrysanthemumspecies(Asteraceae). Biochem.System.Ecol.41,142–149.