Contents lists available atScienceDirect
Toxicology in Vitro
journal homepage:www.elsevier.com/locate/toxinvit
Mebendazole, an antiparasitic drug, inhibits drug transporters expression in
preclinical model of gastric peritoneal carcinomatosis
Laine Celestino Pinto
a,1, Caroline de Fátima Aquino Moreira-Nunes
b,1, Bruno Moreira Soares
a,
Rommel Mário Rodriguez Burbano
a, José Alexandre Rodrigues de Lemos
a,c,
Raquel Carvalho Montenegro
a,b,⁎aBiological Science Institute, Federal University of Para, Belem, Pará, Brazil
bLaboratory of Pharmacogenomics, Nucleus of Research and Development of Medicines (NPDM), Federal University of Ceara, Fortaleza, Ceará, Brazil cFoundation Center for Hemotherapy and Hematology of Pará (HEMOPA), Laboratory of Genetics and Molecular Biology, Belem, Pará, Brazil
A R T I C L E I N F O
Keywords: Mebendazole Inhibition Drug transporter Gastric cancer cells
A B S T R A C T
The present study aimed to investigate whether MBZ down-regulates drug transporter expression (ABCB1,
ABCC1,SLC47A1). mRNA expression level ofABCB1,ABCC1andSLC47A1was evaluated by qPCR and protein expression levels MDR-1 was performed by western blotting in malignant ascites cells (AGP-01) treated with MBZ for 24 h. The mRNA expression level ofABCB1andABCC1significantly decreased at a 1.0μM of MBZ compared to negative control, whileSLC47A1extremely decreased at all tested concentrations of MBZ. Protein expression levels MDR-1 significantly decreased at a 1.0μM of MBZ compared to negative control. Therefore, our results showed MBZ may play an important role in inhibiting MDR gene expression in malignant ascites cells.
1. Introduction
As one of the most common cancers, gastric cancer has the third highest lethality and fourth highest morbidity of all cancers worldwide (Ferlay et al., 2013). Generally, patients with gastric cancer have locally advanced or metastatic disease at diagnosis, owing absence of specific symptoms in the early tumor stages, the frequent detection of the dis-ease at advanced stages, and the limited therapeutic options (Liu et al., 2014). Although there have been advances in the treatment strategies for gastric cancer, the prognosis of gastric cancer is still poor; the 5-year survival rate is only 20%–30% because most cases are diagnosed in an advanced stage (Kamangar et al., 2006; Tahara et al., 2010; Yoong et al., 2011).
Peritoneal carcinomatosis (PC) or malignant ascites (MA) indicates the presence of malignant cells in the peritoneal cavity and is a grave prognostic sign. It represents an advanced state of several types of cancer, especially in gastric cancer (Sangisetty and Miner, 2012). There is a lack of randomized controlled trials identifying the most favorable therapy, mainly because PC is highly resistant to the current ther-apeutics in clinics, and therefore evidence-based therapeutic guidelines have not been established yet (Sugiyama et al., 2011). Therefore, there is an urgent need to introduce new therapeutic agents in the
management of peritoneal carcinomatosis.
Multidrug resistance (MDR) is usually the main cause for failure of chemotherapy against malignant tumors, including gastric cancer. There are quite a number of different mechanisms accounting for drug resistance, and MDR protein family plays an essential role (Zhang and Fan, 2010).
More proteins associated with this process of resistance are owned family called ABC-ATP-biding cassette family. These transmembrane proteins use the energy of ATP hydrolysis to remove active drug within cells (Choi, 2005).
So far, studies in cell culture have consistently shown that the pri-mary mechanism MDR (Multiple Drug Resistance) in most cultured tumor cells involves at least three major genes: ABCB1 (Multidrug Resistance Protein 1 - MDR-1) which encoding the P-glycoprotein (P-gp), ABCC1 (multidrug resistance associated protein 1 - MRP1) and ABCG2 (Breast Cancer Resistance Protein - BCRP) (Abbott, 2003; Schinkel and Jonker, 2003; Szakács et al., 2006; Widmer et al., 2003; Zhou et al., 2001).
Additionally, a member of the subfamily of transporter called SLC47A1 or organic cations multidrug extrusion and toxin (MATE1) has been recognized as an important protein responsible for the efflux of various drugs (Moreira-Nunes et al., 2013).
http://dx.doi.org/10.1016/j.tiv.2017.06.007
Received 18 April 2017; Received in revised form 6 June 2017; Accepted 8 June 2017
⁎Corresponding author at: Laboratory of Pharmacogenomics, Nucleus of Research and Development of Medicines, Federal University of Fortaleza, Coronel Nunes de Melo st, n 1000, Rodolfo Teófilo, CEP: 60416-000 Fortaleza, Ceará, Brazil.
1These authors contributed equally to this study.
E-mail address:rc_montenegro@pq.cnpq.br(R.C. Montenegro).
Available online 09 June 2017
0887-2333/ © 2017 Elsevier Ltd. All rights reserved.
The study of efflux of drugs can have an important therapeutic role for overcoming drug resistance, and can provide a rationale for the use of a combination of drugs which target the molecular overlap, but do not necessarily share the same mechanisms transport (Apperley, 2007). Mebendazole (MBZ) is available for treating roundworm, common hookworm, American hookworm, pinworm, and whipworm in humans and animals, acting by depolymerizing tubulin and therefore disrupting the functions of microtubules. Moreover, MBZ has been proved to in-hibit the growth of different cancer cellsin vitroandin vivo, including gastric cancer (Bai et al., 2011; Doudican et al., 2008; Martarelli et al., 2008; Mukhopadhyay et al., 2002; Pinto et al., 2015).
Based upon thesefindings and the clinically proved safety of MBZ (Lacey, 1988; Sajid et al., 2006; Van der Westhuizen et al., 1984) this work analyzed the effects of MBZ on drug transporters expression in a human gastric cancer cell line model.
2. Material and methods
2.1. Gastric cancer cells (GC)
Our group established and characterized cytogenetically a gastric cancer cell line obtained from an asciticfluid (AGP-01, malignant as-cites) from a patient with primary tumor on stomach (intestinal-type adenocarcinoma) (Fig. 1(A), (B), (C)), each of which exhibited a com-posite karyotype with several clonal chromosome alterations similar to the respective primary tumor from the stomach as described previously (Leal et al., 2009). Cell line was maintained in DMEM medium sup-plemented with 10% fetal bovine serum, 2 mM glutamine, 100 U/mL penicillin, and 100 mg/mL streptomycin at 37 °C with 5% CO2.
2.2. Chemicals
MBZ (Medley®500 mg) was dissolved in DMSO to a concentration of 10 mg and stored at−20 °C as a master stock solution.
Our previous data demonstrated that MBZ strongly inhibited the growth of AGP-01 cell line after 72 h (Pinto et al., 2015).
2.3. Protein and mRNA preparation
To quantitate mRNA levels, cells (4 × 104) treated with MBZ (0.1μM, 0.5μM and 1.0μM) based in IC50 of MBZ (Pinto et al., 2015) and untreated cells were incubated in a 12 well plate for 24 h at 37 °C. RNA was extracted using TRIzol® (Life Technologies) according to manufacturer's instructions and reversely transcribed to cDNA using High Capacity cDNA Reverse Transcriptase (Life Technologies) in a GeneAmp® PCR System 9700 thermal cycler (Applied Biosystems®). The RNA concentration and quality were determined using a NanoDrop spectrophotometer (Kisker, Germany) and 1% agarose gels, respec-tively. Samples were stored at−80 °C until use.
To quantitate protein levels, cells were seeded into 6-well plates at a density of 1 × 106cells/well for 24 h at 37 °C. After 24 h of incubation,
cells were treated with MBZ (0.1μM, 0.5μM and 1.0μM) for 24 h for protein purification. Total protein was isolated from samples using the
AllPrep DNA/RNA/Protein Kit (Qiagen, Germany) according to the manufacturer's instructions. The protein pellet was dissolved in a buffer containing 7 M urea, 2 M thiourea, 4% CHAPS, 50 mM DTT, 1% Protease Inhibitor Cocktail (Sigma-Aldrich, USA), and 0.5% each Phosphatase Inhibitor Cocktail 1 and 2 (Sigma-Aldrich, USA), as pre-viously performed by (Leal et al., 2012). The protein concentrations were determined by the method of Bradford (Sigma-Aldrich, USA).
2.4. Quantitative real-time polymerase chain reaction (qPCR)
The genes selected for expression analysis were:ABCB1,ABCC1and SLC47A1 and β-actin was used as internal control. Such genes are commercially available as TaqMan® Gene expression assays (Life Technologies, EUA), PCR primers and probe sequence of each gene are listed inTable 1.
qPCR was performed usingABI 7500 Real-Time PCRsystem (Applied Biosystems®). For each sample was used concentrations following: 3μL of cDNA, 1μL of each primer/probe, 12,5μL of TaqMan® Gene Expression Master Mix (Life Technologies, EUA) and 8,5μL of Ultra-pure H2O. Each assay was performed at least three times in triplicate
according to Minimum Information for Publication of Quantitative Real-Time PCR Experiments- MIQE Guidelines (Bustin et al., 2009).
The expression level was calculated using the 2−ΔΔCT(delta delta threshold cycle) method, the expression level of the gene of interest is reported relative to the reference gene for each sample (Schmittgen and Livak, 2008).
2.5. Western blotting assay
Reduced protein (25μg) from each sample was separated by 12.5% homogeneous sodium dodecyl sulfate-polyacrylamide gel electrophor-esis (SDS-PAGE) and electroblotted onto a polyvinylidene fluoride (PVDF) membrane (Hybond-P; GE Healthcare, USA). The PVDF mem-brane was blocked with phosphate-buffered saline containing 0.1% Tween 20 and 5% low fat milk and incubated overnight at 4 °C with the corresponding P-glycoprotein Monoclonal Antibody (dilution 1:50; MA1-26529; Thermo Fisher Scientific, USA). After extensive washing, a peroxidase-conjugated secondary antibody was added for 1 h at room temperature. Immunoreactive bands were visualized using the Western blotting Luminol reagent, and the images were acquired using an
Table 1
Sequence of oligonucleotides used for qPCR.
Gene Sequence (5′–3′) NCBI reference sequence (RefSeq)
ABCB1 TGACAGCTACAGCACGGAAG NM_000927.4 TCTTCACCTCCAGGCTCAGT
ABCC1 GAGAGTTCCAAGGTGGATGC NM_004996.3 AGGGCCCAAAGGTCTTGTAT
SLC47A1 CGCAGCGCGCGAGTCACATGG NM_018242.2 GGCCTGTGAATTGTGTGTAAGCTC
ImageQuant 350 digital image system (GE Healthcare, Sweden). ACTB was used as a loading reference control.
2.6. Statistical analysis
All statistical analyses were conducted using GraphPad Prism 5.0 software. The data were expressed as mean ± standard deviation and one-way ANOVA test was performed to determine the significance of differences between control samples and treated samples. The Pearson linear correlation test was used to correlate MBZ concentrations and variation of gene expression for each tested genes. The results were considered to be statistically significant atp< 0.05.
3. Results
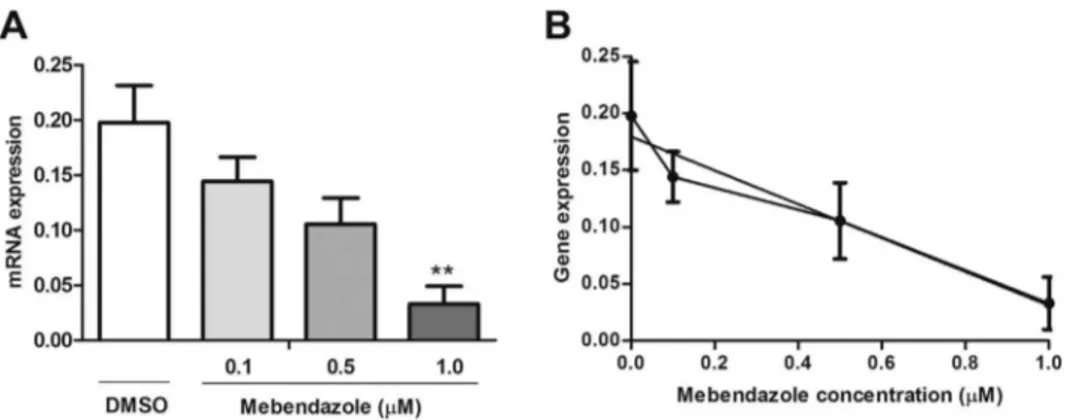
The mRNA expression level ofABCB1,ABCC1 andSLC47A1was evaluated by qPCR in malignant ascites cells (AGP-01) treated with MBZ for 24 h. The mRNA expression level of ABCB1significantly de-creased at a concentration 1.0μM of MBZ (0.0330 ± 0.0282) com-pared to negative control (0.1442 ± 0.03135,p < 0.01) and showed high correlation (p= 0,0266, R2 0.95) as shown inFig. 2(A) and (B). The mRNA expression ofABCC1was also reduced at 1.0μM of MBZ (0.1025 ± 0.0850) compared to negative control (1.224 ± 0.0469 p < 0.01), showing a decrease in a concentration-dependent manner and high correlation (p= 0.0075, R2 0.99) as seen inFig. 3(A) and (B). The mRNA expression level ofSLC47A1was extremely decreased at all tested concentrations of MBZ (0.1μM = 0.0197 ± 0.0020, 0.5μM = 0.0210 ± 0.0059, 1.0μM = 0.0093 ± 0.0052) compared to negative control (0.2028 ± 0.0078, p< 0.0001). There was no significant correlation as shown inFig. 4(A) and (B).
Western Blot analysis was performed to access the protein expres-sion levels of MDR-1 after treatment with MBZ for 24 h, and the results showed that the protein levels of MDR-1 was also decreased at a con-centration 1.0μM of MBZ compared to negative control (1.073 ± 0.04726, p< 0.0001), as shown in the mRNA expression (Fig. 5(A) and (B)).
These results indicate that MBZ may play an important role in a reduction of MDR gene expression in gastric cancer cells.
4. Discussion
MBZ has been extensively used to treat gastrointestinal parasitic infections in humans and animals, and in this capacity, it has shown remarkable efficacy and safety (Lacey, 1988; Sajid et al., 2006; Van der Westhuizen et al., 1984). Furthermore, clinical data from long-term MBZ therapy in the treatment of alveolar echinococcosis suggest that MBZ should also exhibit minimal toxicity even at high doses (Müller et al., 1982; Reuter et al., 2000). Therefore, MBZ was identified as a potentially effective agent in gastric cancerin vitro(Pinto et al., 2015). The 5-year survival rate for advanced or metastatic gastric cancer is nearly 5%–20%, with a median overall survival being < 1 year (Kamangar et al., 2006; Wagner et al., 2006). With the development of new anticancer drugs, such as taxanes, CPT-11, oxaliplatin, gefitinib and S-1, significant improvements in the efficacy of chemotherapy against gastric cancer have been achieved (Macdonald, 2006). How-ever, some patients still fail onfirst-line chemotherapy and will relapse and eventually develop resistance to currently available treatment op-tions due to the acquisition of multidrug resistance (MDR) (Zhang and Fan, 2007). Therefore, it is necessary tofind markers which could ac-curately predict the risk of gastric cancer, and give the evidence for early prediction of the clinical outcome so as to improve the clinical management of gastric cancer patients.
Benzimidazole have been claimed to interact specifically with P-gp and have been shown to be substrates for P-glycoprotein mediated drug efflux (Nare et al., 1994). This is in full agreement with the fact that benzimidazole pharmacokinetics is influenced by MDR transporterin vivo(Jonker et al., 2000). Indeed, triclabendazole and triclabendazole sulfoxide inhibit the transport activity of P-gp and these benzimidazoles were less toxic in cells overexpressing P-gp, which suggests that they are driven out by P-gp. However, triclabendazole sulfone, albendazole, mebendazole, oxfendazole and thiabendazole failed to have any effect on P-gp activity (Dupuy et al., 2010). On the contrary, in our study,
Fig. 2.mRNA expression level ofABCB1in AGP-01 cells after 24 h of treatment with MBZ 0.1μM, 0.5μM and 1.0μM. The bars represent the mean ± standard error of mean of three independent experiment in triplicate. **p< 0.01 compared with negative con-trol by ANOVA followed by Tukey test (A). Correlation betweenABCB1gene expression and MBZ concentra-tions (B).
MBZ inhibited mRNA and protein expression levels ofABCB1in ma-lignant ascites cells (AGP-01) and the mechanism involved in this process is not known.
In the human intestine, Pgp is strongly expressed on the apical surface of the superficial columnar epithelial cells of the ileum and colon, and its expression level decreases gradually proximally into the jejunum, duodenum and stomach (Ho et al., 2003). The levels of both DNA methylation and histone deacetylation regulate MDR1 gene ex-pression (El-Osta et al., 2002; Nakayama et al., 1998; Kusaba et al., 1999). An important consequence of epigenetic mechanisms is the local silencing of gene expression.
Du et al. (2005) showed that RP L6 could regulate MDR in gastric cancer cells by suppressing drug-induced apoptosis.Robey et al. (2008) reported an initial phase I studies of CBT-1, an orally-administered, bisbenzylisoquinoline plant alkyloid as P-gp inhibitor. CBT-1 at 1μM completely reversed P-gp-mediated resistance to vinblastine, paclitaxel and depsipeptide. In our study, MBZ performed an important function as ABCB1/ABCC1/SLC47A1 inhibitor showing that it could regulate expression of MDR in malignant ascites and probably in gastric cancer cells.
MATE1 is recognized as an important protein responsible for efflux of several drugs in some cell types, such as liver, heart and hemato-poietic stem cells, although their main expression is evident in the kidneys (Moreira-Nunes et al., 2013).
Among the drug transport mediated by MATE1 protein, as are some chemotherapeutic oxaliplatin and imatinib mesylate (Chen et al., 2009; Moreira-Nunes et al., 2013; Yokoo et al., 2007), antibiotics asfl uor-oquinolones (Ohta et al., 2009) cimetidine (Matsushima et al., 2009; Tanihara et al., 2007), among others.
The expression ofSLC47A1was extremely decreased in cells treated with MBZ. Although the mechanism involved in this process is un-known. Tissue-specific expression, regulations associated with various diseases and inter-individual variations in MATE1 depend it on several transcription factors and also DNA methylation. Factors other than Sp1 and AP-1 would also play a role in the transcriptional regulation of MATE1 (Yonezawa and Inui, 2011). In conclusion, MBZ overcome ABCB1,ABCC1andSLC47A1gene expression in malignant ascites cells (AGP-01). However, additional investigation of toxicology, pharmaco-kinetics and antitumor effect of MBZ might support the use of MBZ as an alternative drug in gastric cancer patients.
Conflict of interest
The authors declare that there are no conflicts of interest.
Transparency document
The http://dx.doi.org/10.1016/j.tiv.2017.06.007 associated with this article can be found, in the online version.
Acknowledgments
The authors are grateful to the Brazilian Agencies CNPq (3112240/ 2016-0; 235135/2014-3), CAPES, FAPESPA and Federal University of Para for fellowships andfinancial support.
Fig. 4.mRNA expression level ofSLC47A1in AGP-01 cells after 24 h of treatment with MBZ 0.1μM, 0.5μM and 1.0μM. The bars represent the mean ± standard error of mean of three independent experiment in triplicate. **p< 0.0001 compared with negative control by ANOVA followed by Tukey test (A). Correlation betweenSLC47A1 gene expression and MBZ concentrations (B).
Neuro-Oncology 13, 974–982.
Bustin, S.A., Benes, V., Garson, J.A., Hellemans, J., Huggett, J., et al., 2009. The MIQE Guidelines: minimum information for publication of quantitative real-time PCR ex-periments. Clin. Chem. 55, 611–622.
Chen, Y., Teranishi, K., Li, S., Yee, S.W., Hesselson, S., et al., 2009. Genetic variants in multidrug and toxic compound extrusion-1, hMATE1, alter transport function. Pharmacogenomics 9, 127–136.
Choi, C.H., 2005. ABC transporters as multidrug resistance mechanisms and the devel-opment of chemosensitizers for their reversal. Cancer Cell Int. 5, 30.
Doudican, N., Rodriguez, A., Osman, I., Orlow, S.J., 2008. Mebendazole induces apoptosis via Bcl-2 inactivation in chemoresistant melanoma cells. Mol. Cancer Res. 6, 1308–1315.
Du, J., Shi, Y., Pan, Y., Jin, X., Lin, C., et al., 2005. Regulation of multidrug resistance by ribosomal protein L6 in gastric cancer cells. Cancer Biol. Ther. 4, 242–247. Dupuy, J., Alvinerie, M., Menez, C., Lespine, A., 2010. Interaction of anthelmintic drugs
with P-glycoprotein in recombinant LLC-PK1-mdr1a cells. Chem. Biol. Interact. 186, 280–286.
El-Osta, A., Kantharidis, P., Zalcberg, J.R., Wolffe, A.P., 2002. Precipitous release of methyl-CpG binding protein 2 and histone deacetylase 1 from the methylated human multidrug resistance gene (MDR1) on activation. Mol. Cell. Biol. 22, 1844–1857. Ferlay, J., Soerjomataram, I., Ervik, M., Dikshit, R., Eser, S., Mathers, C., et al., 2013.
GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. International Agency for Research on Cancer, Lyon, France (Internet). Han, Z., Hong, L., Han, Y., Wu, K., Han, S., et al., 2007. Phospho Akt mediates multidrug
resistance of gastric cancer cells through regulation of P-gp, Bcl-2 and Bax. J. Exp. Clin. Cancer Res. 26, 261–268.
Ho, G.T., Moodie, F.M., Satsangi, J., 2003. Multidrug resistance 1 gene (Pglycoprotein 170): an important determinant in gastrointestinal disease? Gut 52, 759–766. Hu, W.-Q., Peng, C.-W., Li, Y., 2009. The expression and significance of P-glycoprotein,
lung resistance protein and multidrug resistance-associated protein in gastric cancer. J. Exp. Clin. Cancer Res. 28, 144.
Jonker, J.W., Smit, J.W., Brinkuis, R.F., Maliepaard, M., Beijnen, et al., 2000. Role of breast cancer resistance protein in the bioavailability and fetal penetration of topo-tecan. J. Natl. Cancer Inst. 92, 1651–1656.
Kamangar, F., Dores, G.M., Anderson, W.F., 2006. Patterns of cancer incidence, mortality, and prevalence acrossfive continents: defining priorities to reduce cancer disparities in different geographic regions of the world. J. Clin. Oncol. 24, 2137–2150. Kusaba, H., Nakayama, M., Harada, T., Nomoto, M., Kohno, K., et al., 1999. Association of
5′CpG demethylation and altered chromatin structure in the promoter region with transcriptional activation of the multidrug resistance 1 gene in human cancer cells. Eur. J. Biochem. 262, 924–932.
Lacey, E., 1988. The role of the cytoskeletal protein, tubulin, in the mode of action and mechanism of drug resistance to benzimidazoles. Int. J. Parasitol. 18, 885–936. Leal, M.F., Nascimento, J.L.M., da Silva, C.E.A., Lamarão, M.F.V., Calcagno, D.Q., et al.,
2009. Establishment and conventional cytogenetic characterization of three gastric cancer cell lines. Cancer Genet. Cytogenet. 195, 85–91.
Leal, M.F., Calcagno, D.Q., Demachki, S., Assumpcao, P.P., Chammas, R., Burbano, R.R., et al., 2012. Clinical implication of 14-3-3 epsilon expression in gastric cancer. World J. Gastroenterol. 18, 1531–1537.
Liu, Z., Zhang, J., Gao, Y., Pei, L., Zhou, J., Gu, L., et al., 2014. Large-scale character-ization of DNA methylation changes in human gastric carcinomas with and without metastasis. Clin. Cancer Res. 20, 4598–4612.
Macdonald, J.S., 2006. Gastric cancer—new therapeutic options. N. Engl. J. Med. 355, 76–77.
Martarelli, D., Pompei, P., Baldi, C., Mazzoni, G., 2008. Mebendazole inhibits growth of human adrenocortical carcinoma cell lines implanted in nude mice. Cancer Chemother. Pharmacol. 61, 809–817.
Matsushima, S., Maeda, K., Inoue, K., Ohta, K.Y., Yuasa, H., et al., 2009. The inhibition of human multidrug and toxin extrusion 1 is involved in the drug-drug interaction caused by cimetidine. Drug Metab. Dispos. 37, 555–559.
Moreira-Nunes, C.F.A., Azevedo, T.C.B., Beltrão, A.C.S., Francês, L.T.V.M., Sousa, R.G.M.A., et al., 2013. Differentially expressed genes responsible for insensitivity of CD34 + cells to kinase inhibitors in patients with chronic myeloid leukemia. BMC Proc. 7, O1.
Mukhopadhyay, T., Sasaki, J., Ramesh, R., Roth, J., 2002. Mebendazole elicits a potent antitumor effect in human cancer cell lines both in vitro and in vivo. Clin. Cancer Res. 8, 2963–2969.
Müller, E., Akovbiantz, A., Ammann, R.W., Bircher, J., Eckert, J., et al., 1982. Treatment
of human echinococcosis with mebendazole. Preliminary observations in 28 patients. Hepato-Gastroenterology 29, 236–239.
Nakayama, M., Wada, M., Harada, T., Nagayama, J., Kusaba, H., et al., 1998. Hypomethylation status of CpG sites at the promoter region and overexpression of the humanMDR1gene in acute myeloid leukemias. Blood 92, 4296–4307.
Nare, B., Liu, Z., Prichard, R.K., Georges, E., 1994. Benzimidazoles, potent anti-mitotic drugs: substrates for the P-glyprotein transporter in multidrug-resistant cells. Biochem. Pharmacol. 48, 2215–2222.
Ohta, K.Y., Imamura, Y., Okudaira, N., Atsumi, R., Inoue, K., Yuasa, H., 2009. Functional characterization of multidrug and toxin extrusion protein 1 as a facilitative trans-porter forfluoroquinolones. J. Pharmacol. Exp. Ther. 328, 628–634.
Pinto, L.C., Soares, B.M., Pinheiro, J.J., Riggins, G.J., Assumpção, P.P., Burbano, R.M., Montenegro, R.C., 2015. The anthelmintic drug mebendazole inhibits growth, mi-gration and invasion in gastric cancer cell model. Toxicol. in Vitro 29, 2038–2044. Reuter, S., Jensen, B., Buttenschoen, K., Kratzer, W., Kern, P., 2000. Benzimidazoles in the
treatment of alveolar echinococcosis: a comparative study and review of the litera-ture. J. Antimicrob. Chemother. 46, 451–456.
Robey, R.W., Shukla, S., Finley, E., Oldham, R.K., Barnett, D., et al., 2008. Inhibiton of P glycoprotein (ABCB1)- and multidrug resistance-associated protein 1 (ABCC1)-mediated transport by the orally administered inhibitor, CBT-1((R)). Biochem. Pharmacol. 75, 1302–1312.
Sajid, M.S., Iqbal, Z., Muhammad, G., Iqbal, M.U., 2006. Immunomodulatory effect of various anti-parasitics: a review. Parasitology 132, 301–313.
Sangisetty, S.L., Miner, T.J., 2012. Malignant ascites: a review of prognostic factors, pa-thophysiology and therapeutic measures. World J. Gastrointest. Surg. 4, 87–95. Schinkel, A.H., Jonker, J.W., 2003. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv. Drug Deliv. Rev. 55, 3–29. Schmittgen, T.D., Livak, K., 2008. Analyzing real-time PCR data by the comparative Ct
method. Nat. Protoc. 3, 1101–1107.
Sugiyama, M., Kakeji, Y., Tsujitani, S., Harada, Y., Onimaru, M., 2011. Antagonism of VEGF by genetically engineered dendritic cells is essential to induce antitumor im-munity against malignant ascites. Mol. Cancer Ther. 10, 540–549.
Szakács, G., Paterson, J.K., Ludwig, J.A., Booth-Genthe, C., Gottesman, M.M., 2006. Targeting multidrug resistance in cancer. Nat. Rev. Drug Discov. 5, 219–234. Tahara, T., Shibata, T., Nakamura, M., Yamashita, H., Yoshioka, D., Okubo, M.,
Yonemura, J., Maeda, Y., Maruyama, N., Kamano, T., et al., 2010. Association be-tween IL-17A, -17F and MIF polymorphisms predispose to CpG island hyper-me-thylation in gastric cancer. Int. J. Mol. Med. 25, 471–477.
Tanihara, Y., Masuda, S., Sato, T., Katsura, T., Ogawa, O., Inui, K., 2007. Substrate spe-cificity of MATE1 and MATE2-K, human multidrug and toxin extrusions/H(+)-or-ganic cation antiporters. Biochem. Pharmacol. 74, 359–371.
Van der Westhuizen, B., Newcomb, K., Guerrero, J., 1984. Anthelmintic efficacy of me-bendazole suspension against induced helminth infections in South African sheep and cattle. Am. J. Vet. Res. 45, 779–782.
Wagner, A.D., Grothe, W., Haerting, J., Kleber, G., Grothey, A., Fleig, W.E., 2006. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J. Clin. Oncol. 24, 2903–2909.
Widmer, N., Colombo, S., Buclin, T., Decosterd, L.A., 2003. Functional consequence of
MDR1expression on imatinib intracellular concentrations. Blood 102, 1142. Yokoo, S., Yonezawa, A., Masuda, S., Fukatsu, A., Katsura, T., Inui, K., 2007. Differential
contribution of organic cation transporters, OCT2 and MATE1, in platinum agent-induced nephrotoxicity. Biochem. Pharmacol. 74, 477–487.
Yonezawa, A., Inui, K.-I., 2011. Importance of the multidrug and toxin extrusion MATE/ SLC47A family to pharmacokinetics, pharmacodynamics/toxicodynamics and phar-macogenomics. Br. J. Pharmacol. 164, 1817–1825.
Yoong, J., Michael, M., Leong, T., 2011. Targeted therapies for gastric cancer: current status. Drugs 71, 1367–1384.
Zhang, D., Fan, D., 2007. Multidrug resistance in gastric cancer: recent research advances and ongoing therapeutic challenges. Expert. Rev. Anticancer. Ther. 7, 1369–1378. Zhang, D., Fan, D., 2010. New insights into the mechanisms of gastric cancer multidrug
resistance and future perspectives. Future Oncol. 6, 527–537.
Zhang, Y., Qu, X., Hu, X., Yang, X., Hou, K., et al., 2009. Reversal of P-glycoprotein-meidiated multi-drug resistance by the E3 ubiquitin ligase Cbl-b in human gastric adenocarcinoma cells. J. Pathol. 218, 248–255.