ww w . e l s e v i e r . c o m / l o c a t e / b j i d
The
Brazilian
Journal
of
INFECTIOUS
DISEASES
Review
article
Natural
killer
cells
in
hepatitis
B
virus
infection
Shao-fei
Wu
a,1,
Wen-jing
Wang
b,1,
Yue-qiu
Gao
a,∗aDepartmentofHepatopathy,ShuguangHospitalAffiliatedtoShanghaiUniversityofTraditionalChineseMedicine,Shanghai,China bDepartmentofGynecology,ShuguangHospitalAffiliatedtoShanghaiUniversityofTraditionalChineseMedicine,Shanghai,China
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received2February2015
Accepted5May2015
Availableonline25June2015
Keywords:
NKcells
HBV Review
a
b
s
t
r
a
c
t
Naturalkillercellsareauniquetypeoflymphocyteswithcytotoxiccapacity,andplay
impor-tantrolesagainsttumorsandinfections.Recently,naturalkillercellshavebeenincreasingly
valuedintheireffectsinhepatitisBvirusinfection.SincehepatitisBvirusisnotcytopathic,
thesubsequentantiviralimmuneresponsesofthehostareresponsibleforsustainingthe
liverinjury,whichmayresultincirrhosisandevenhepatocellularcarcinoma.Many
stud-ieshaveconfirmedthatnaturalkillercellsparticipateinanti-hepatitisBvirusresponses
bothintheearlyphaseafterinfectionandinthechronicphaseviacytolysis,degranulation,
andcytokinesecretion.However,naturalkillercellsplaydichotomicroles:theyexert
antivi-ralandimmunoregulatoryfunctionswhilstcontributetothepathogenesisofliverinjury.
Here,wereviewtherolesofnaturalkillercellsinhepatitisBvirusinfection,introducing
noveltherapeuticstrategiesforcontrollinghepatitisBvirusinfectionviathemodulationof
naturalkillercells.
©2015ElsevierEditoraLtda.Allrightsreserved.
Introduction
HepatitisBvirus(HBV)infectionisoneofthemostcommon
chronicviralinfectionsinhumans.HBVisnotcytopathic,i.e.
whencellularimmuneresponsesaredeficientor
pharmaco-logicallysuppressed,HBVcanreplicateathighlevelsinvivo
withoutdetectablepathologicalconsequences.1,2Ithasbeen
shownthatliverinjuryisinfactduetotheantiviralimmune
response ofthe host. In chronic HBV infection, prolonged
progressmaycausepersistentliverinflammation,eventually
resultingincirrhosisandhepatocellularcarcinoma(HCC).3
Itiswellknownthatvirus-specificCD8+ cytotoxicT
lym-phocytesandCD4+T-helpercellsplayeffectiveandregulatory
∗ Correspondingauthor.
E-mailaddress:gaoyueqiu@hotmail.com(YQ.Gao).
1 Theseauthorscontributedequallytothisstudyandsharethefirstauthorship.
roles inanti-HBV immunity.4 However, the roles ofinnate
immunityhavetriggeredextensivedebates.Innateimmunity
isconsideredtocomposethefirst-linehostdefenseagainst
pathogens,includingsensingdangersignals,controllingviral
replication and disseminationvery early afterinfection,as
well as for timely orchestration of virus-specific adaptive
responses.5However,ithasbeenreportedthatHBVdoesnot
modulatehostcellulargenetranscription,andwould
there-foreinduceneitherinnateantiviralresponseinhepatocytes
nor intrahepatic innate immune responses.6 Furthermore,
a portion of chronically infected patients undergo a
long-lasting immunetolerance phasethat is characterizedbya
lack ofclinical symptoms and high HBV load.7 Hence, the
innateimmunityisdeemedtoaccountforthe“absence”of
http://dx.doi.org/10.1016/j.bjid.2015.05.006
anti-HBVresponse.Nevertheless,somefindingsare
inconsis-tentwiththeseconclusions,suggestinganactiveroleofinnate
immunityinHBVclearance.Natural killer(NK)cellsare an
essentialpartoftheinnateimmunesystem,andparticipate
intheantiviralresponsesdespitethedichotomic
character-isticstheydisplay.8 Here,wewillreviewtherecentstudies
regardingalteredNKcellsphenotypesandfunctionsinHBV
infection.
General
features
of
NK
cells
HumanNKcellsaregenerallydefinedasagroupof
lympho-cytesthatexpressCD56butlacktheTcellreceptor(TCR)–CD3
complex.They representabout 15% ofall peripheralblood
lymphocytesandthisproportioncanrisetomorethan30%
in the liver.9 NK cells exert their effects mainly through
therecognitionandkillingoftargetcells andthe secretion
ofcytokinessuch asinterferon-␥(IFN-␥)and tumor
necro-sisfactor-␣ (TNF-␣), whichcanmodulate antiviral immune
responses.Byproducingantiviralcytokinesandchemokines,
NKcellsalsoplayanimportantroleinbridgingthe innate
andadaptive immuneresponses.10 NKcellscan befurther
divided into two subsets, the CD56bright and the CD56dim
subsets, according to the cell membrane density of CD56.
CD56dim NKcells,expressinghighlevels ofthe low-affinity
Fc␥-receptorCD16andthekillerimmunoglobulin-like
recep-tor(KIR),representsabout 90%ofallbloodNKcells,whilst
CD56bright NK cells constitute less than 10% of all blood
NK cells and do not express CD16and KIR.11 Referring to
functions,CD56dimNKcellsefficientlykilltargetcellsby
degra-nulationbutsecretelowlevelsofcytokines;ontheotherhand,
CD56brightNKcellsproducealargeamountofcytokinesupon
stimulationbut areless cytotoxic.However,CD56bright cells
exhibitsimilarorenhancedcytotoxicityagainsttargetcells
afterprolongedactivationcomparedwithCD56dimcells.12In
addition,CD56brightNKcellsconstitutivelyexpressthe
high-andintermediate-affinityinterleukin-2receptors(IL-2R)and
expandinvitroandinvivoinresponsetolowdosesofIL-2.9,13,14
Onthe contrary,restingCD56dim NKcellsonlyexpressthe
intermediate-affinityIL-2Randproliferateweaklyinresponse
tohighdosesofIL-2invitro,evenafterinductionofthe
high-affinityIL-2R.9,11,13
NKcellsexpressanarrayofreceptorsincludinginhibitory
andactivatingreceptors,inducingnegativeandpositive
sig-nalswhencombinedwiththeirligands,whichcandetermine
whether or notNK cells become activated, enabling them
todetect infectedor neoplastic cells whilesparing normal
cells.15MHCclassImolecules,encodedbyhighlyconserved
genesinheritedindependently andexpressedbymost
nor-malcells,arenaturalligandsforNKcellinhibitoryreceptors.
Thus,thestrengthoftheactivatingsignalsonencountering
normalcellsisdampenedbyinhibitorysignals,andNKcells
areleftquiescent.However,MHCclassImoleculesareoften
down-regulatedoncancerousorvirus-infectedcells,
deliver-ing insufficientinhibitory signals, thus activatingNK cells.
Thisisknown asthe “missing-self” mechanism ofNKcell
activation.Inanothercondition,NKcellsareselectively
acti-vatedby‘stressed’cells,whichexpressupregulatedactivating
ligands for NK cells and thereby overcome the inhibitory
signalingdeliveredbyMHCclassImolecules.Thisisknown
asthe“stress-inducedself”triggeringofNKcellactivation16,17
(Fig.1).Inbothconditions,NKcellactivationleadsto
degranu-lationand/orcytokinesecretion.Inaddition,NKcellscanbe
activateduponstimulationbyIL-12,IL-15,IL-18andIFN-␣/.14
NK
cells
in
the
early
immune
responses
to
HBV
infection
Innateimmunityisthefirstbarrieragainstinfectionand
neo-plasticgrowth,andisdistinguishedbytherapidparticipation
in the earlyresponse toantigenswithout presensitization.
NKcells,asanimportantelementoftheinnateimmunity,
display at least three effector functions that contribute to
infectioncontrol:theycandirectlykillinfectedcellsby
releas-ingcytolyticgranules,induceapoptosisoftargetcellsthrough
crosslinkingdeathreceptorsandligands,andproducea
vari-ety of immunomodulative cytokines (Fig. 2). Studies have
confirmedthatNKcellstakepartintheantiviralresponses
tocertainRNAviruses(e.g. HIV,HCV)andDNAviruses(e.g.
CMV).18–20However,HBViscurrentlyconsideredasa“stealth”
virus anditsinfection ischaracterizedbyalackofobvious
symptomsandliverdamagethatevenlastsforseveralyears.
Onthisaccount,patientsattheveryearlystageofinfection
are almost unrecognizable.On the other hand,only
chim-panzeeandtupaiacanbeinfectednaturallybyHBV,providing
insufficientanimalmodelsforitsstudy.Althoughwoodchuck
infectedwiththewoodchuckhepatitisvirus (WHV)offersa
favorablemodel,21whetheritisanentirelyrepresentativeof
thehumansettingisyettobediscussed.Mousemodelsof
per-sistentHBVinfectionsthatareanalogoustothoseofhuman
chronicHBV infectionshavebeen established,22,23 butwith
the sameproblemsaswiththe woodchuckmodel.Todate,
takingintoconsiderationthesefactors,ourcognitionofthe
anti-HBVinnateimmuneresponsesattheearliest
presymp-tomaticstagesisstilllimited.
SomestudiessuggestthatNKcellsandeveninnate
immu-nity donotsignificantly contributetothe initialcontrolof
HBVinfection.Studiesinchimpanzeesandwoodchuckshave
shownthatHBVdoesnotinducesignificantchangesin
intra-hepaticgeneexpressionduringentryandreproductioninthe
liver,andtheviralloaddoesnotdecreaseuntiltheonsetofthe
adaptiveimmuneresponse,severalweekslater.24,25Withthe
developmentofinfection,theclearanceofHBV-infected
hepa-tocytesbyadaptiveimmunitycorrelateswithelevatedlevels
ofIFN-␥andTNF-␣intheliver,2whichcouldbeproducedby
activatedNKcells.However,follow-upexperimentsindicate
thatratherthanNKcells,CD8+cells,whicharealsosources
ofIFN-␥andTNF-␣,arethemaineffectorcellsresponsiblefor
viralclearance.26
However, some other experiments showed
contradic-tory results. NK cells express mRNAs coding for toll-like
receptors(TLR),TLR1toTLR9,whichrecognizecertain
non-self molecules and initiate immune responses.27 In vitro,
HBV-plasmid DNA promotes NK cell activation including
cytotoxicity and IFN-␥ production in the liver through
TLR/IFN-␣-mediatedsignalingpathways.28Inastudycarried
out ina high-titer HBV replication mouse model, NK cells
wererequiredtoeliminateHBVinfection,presumablythrough
NK cell
Inhibitory receptor
MHC I molecule
Activating ligand
Healthy cell
a
b
c
Unactivated Missing self Stress-induced self Stressed cell Stressed cell Activating
receptor
–
+
+
–
+
Fig.1–RecognitionofhealthyandstressedcellsbyNKcells.(a)MHCImoleculesareexpressedbymostnormalcells,which transmitinhibitorysignals.Thestrengthofinhibitorysignalsovercomesactivatingsignals,andNKcellsareleft
unactivated.(b)MHCImoleculesareoftendown-regulatedonstressedcells,offeringinsufficientinhibitorysignals,thus activatingNKcells.Thisisthe“missing-self”mechanismofNKcellactivation.(c)Somestressedcellsup-regulateactivating ligandsratherthandown-regulateMHCImolecules,whichdeliveroverwhelmingactivatingsignalsandactivatetheNK cells.Thisisthe“stress-inducedself”mechanismofNKcellactivation.
afterinfectionwithhigh titersofWHV,NKcellswere
acti-vated and virus load was reduced significantly, suggesting
thattheinnateresponseisactivatedintheliversoonafter
exposuretoacertaindoseofWHV.29 Owingtothedelayed
appearanceofsymptomsorthetotallyasymptomaticnature
ofHBVinfection,studiesoftheearlyimmuneeventsin
HBV-infectedpeopleare rare. Astudy oftwoblood donorswho
wereidentified byaccidenttohaveseroconvertedwiththe
appearanceofhepatitisBsurfaceantigen(HBsAg)and
sub-sequentriseofHBVviremiawithoutalanineaminotransferse
(ALT)elevation,haveshownthatNKcellsareabletomount
anearlyand efficientresponsetoHBV,andthatthe innate
immunesystemisabletorecognizeHBVfromthebeginningof
theinfection,whichprobablycontributestothetimely
induc-tionofadaptiveresponses.30InacutehepatitisB,activation
receptor-expressingNKcellsarepreferentiallyenriched,while
the inhibitory receptor-expressing NK cells are reduced.31
Moreover, according to two independent studies, NK cells
displayedincreasedordecreasedcytolyticactivityandIFN-␥
production.31,32TheseresultsrevealthatNKcellsparticipate
intheearly-stageresponseafterHBVinfection.
However,mostofthesestudieswerecarriedoutinanimal
models,whichmaynotfullyrepresenttherealityinhumans.
Ontheotherhand,datafromhumanbeingsareinsufficient.
Hence,theroleofNKcellsintheearlyphaseofHBVinfection
isstilltobedemonstratedinhumans.
Contribution
of
NK
cells
to
HBV-induced
liver
injury
InHBVinfection,NKcellsalwaysplaycontradictoryroles,as
theyexertantiviralandimmunoregulatoryfunctionswhilst
contributetothepathogenesisofliverinjury.Theapoptosis
ofHBV-infectedhepatocytesisinfavorofself-protectionasit
helpstoviralclearance.Butinthecaseofsustainedinfection,
thecontinuousinjurytotheliveristhepreconditionforliver
fibrosis andHCC.Inthe immune-activatedstageofchronic
hepatitisB(CHB),NKcellsskewtowardacytolyticactivity,
whichcorrelatespositivelywiththeseverityofliverdamage.33
EnhancedcytolyticactivityofNKcellscanbepartlyattributed
toalteredlevelsofcertaincytokines33andtotherecognition
betweenNKG2Danditsligands.34
Other than cytotoxic effectors-mediated liver damage,
apoptotic signal transmission is also an important factor
Signaling receptors/ ligands
Perforin/ granzyme
Cytokine receptors
Cytokine secretiors
Cytokines
NK cell
Cytolysis Intercellular
signaling
Fig.2–NKcellsmediatetheirfunctionsthroughatleastthreemechanisms:releasingperforin/granzymeforcytolysis, deliveringintercellularsignalingthroughreceptor–ligandcrosslinking,andsecretingcytokines.
littleornoTNF-relatedapoptosis-inducingligand(TRAIL)on
theirsurface35andhepatocytesexpressminimalTRAIL
death-inducing receptors.36 However, in patients with HBV and
liverinflammation,TRAIL-expressingNK cellsare enriched
in the liver and the hepatocytes express upregulated
lev-elsoftheTRAILdeath-inducingreceptor,indicatingthatNK
cellsmaycontributetoliverinflammationbyTRAIL-mediated
death of hepatocytes.37 Besides, after being activated, NK
cells may induce massive HBV-infected hepatocyte
degen-eration through the Fas/Fas ligand interaction,38 and are
even involved in the disease progression of HBV-related
acute-on-chronicliverfailure(ACLF).39Intrahepatic
PD-1/PD-L1up-regulationiscloselyassociatedwiththeviralloadand
inflammatory responses, which decreases simultaneously
withinflammationremission,implyingthatthePD-1/PD-L1
systemparticipatesinliverinjury.40,41AlthoughNKcell
dys-functionisreportedtocorrelate withPD-1up-regulation,42
directevidencesarelackingtocertifythatPD-1altersNKcell
functioninHBVinfection.
NK
cells
in
chronic
HBV
infection
ThecontroversialroleofNKcellsintheacutephaseofHBV
infectionhasbeenunderlinedabove.Inchronicinfection,NK
cellsdisplayvaryingchangesinproportion,phenotypeand/or
function,whichdifferbetweenstudies.ThedefectsinNKcells
arereflectedinmanyaspects:(1)thepercentagesofhepatic
and peripheral NK cells are reduced in immune-activated
CHBpatients,withorwithoutchangesintheirsubsets33,43,44;
(2) altered expression of activating or inhibitory receptors
onNKcells33,43–46;(3)up-regulationofsomemoleculeswith
inhibitoryeffectssuchasTcellimmunoglobulinandmucin
domain containing molecule-3 (Tim-3);47 (4) maintained or
evenenhancedcytolyticactivity,whichiscorrelated tothe
severity of liver injury33,43,46; and (5) impaired capacity to
produce cytokines such as IFN-␥ and TNF-␣.43,44,46,47 Viral
loadreductionthroughanti-viraltherapypartlyrestoresNK
cells numbers, cytokine production and inhibitory
recep-tor expression.43,45,48,49 Although these studies are mostly
consistent, there are discordancesin somedetails suchas
thechanges intheexpressionofsomereceptors33,43–46 and
changesincytolyticactivity.Thereasonsforthesedifferences
are notclearyet,but maybeascribed toethnicvariations,
samplesize,diseaseprocessandseverity.Despiteinconsistent
conclusionsbetweenstudies,NKcellsdisplayintactoreven
increasedcytotoxicitybutimpairedanti-viralcytokines
secre-tionduringchronicHBVinfection,andthesechangesmaybe
partlyrectifiedafterviralloadreduction.
The factors contributing to NK cell dysfunction were
demonstratedbymanystudies.NKcellsexpressmRNAsforall
HBV-specific CD8+ T cell
TRAIL receptor TRAIL Perforin
NKG2D/2B4
mDC
pDC
IFN-α↓ OX40L ↑
Activ ating cytokine
↓ Inhibitor
y
cytokine
↑
NKG2D/2B4 ligand
NK cell
Dysfunction
Macrophage/
Kupffer
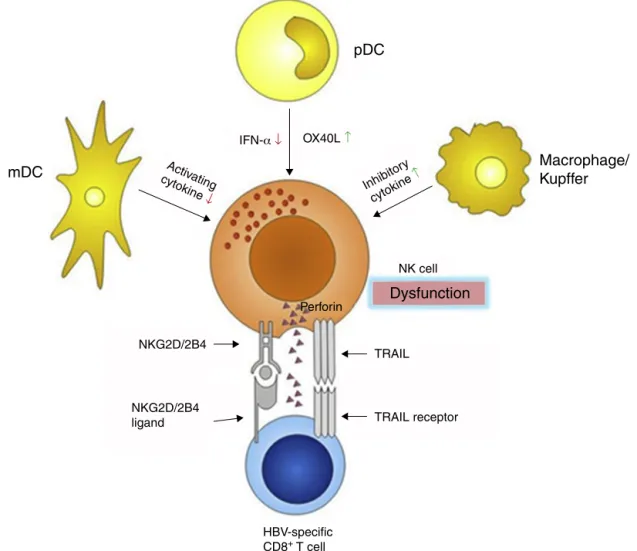
Fig.3–mDCs,pDCsandmacrophagescontributetoNKcelldysfunctionthroughalteredcytokinesecretionandsignaling receptorexpression.DisorderedNKcellssubsequentlymediateCD8+Tcellsdeath.
stimulatedtoinducetheproductionofIFN-␥.27Arecentstudy
revealedthat NKcells canbeactivated byHBV-based DNA
plasmidsinaTLR-dependentmanner,displaying enhanced
NKcelleffectorfunctionsincludingcellularcytotoxicityand
IFN-␥production.26However,inthepersistentHBVinfection
phase,NKcellssufferfunctionaldisordersthatcanberestored
withviralreplicationsuppression.Itcanbededucedthat
dur-inglong-lastingvirusreplication,NKcellsareinfluencedby
pathologicalcircumstancesinducedbytheHBVinfection.
NKcellsinteractwithsomeotherimmunecellsthrough
receptor–ligandcombinationand/orregionalcytokine
secre-tion.Dendriticcells (DCs)are the mostimportant
antigen-presentingcells,andaredividedintomyeloiddendriticcells
(mDC) and plasmacytoid dendritic cell (pDC). DCs bridge
theinnateandadaptiveimmunities,andconsequentlyplay
pivotal roles in eliminating external antigens. During HBV
infection,bothmDCsandpDCsdisplayfunctionaldefects,50–52
whichinfluencethecrosstalkwithNKcells,making
ineffi-cienttheNKcellsactivation.mDCsaresubstantiallyimpaired
intheirabilitytoactivateNKcellsviadecreasedmDC-derived
cytokine secretion, which in turn fail to secrete adequate
amountsofIFN-␥.52pDCsarepoorNKactivators,potentially
throughanOX40L/IFN-␥-dependentpathway53,54(Fig.3).
BesidesDCs,intrahepaticKupffercellsalsoparticipatein
the anti-HBVimmune response, partlythrough interfering
withthe NKcells.Kupffercellsare themainsourceofthe
immune-suppressive cytokine interleukin-10 (IL-10), which
maintainsthehumoralimmunetoleranceduringpersistent
HBV infection55 (Fig.3).IL-10actsasaninhibitorycytokine
blunting NK activation, which restrains IFN-␥ secretion.56
However,anotherstudy demonstratedthat IL-10had a
sig-nificant effect on NK cytotoxicity rather than on IFN-␥ or
TNF-␣productionorNKcellactivatoryreceptorexpression.57
BlockingtheIL-10pathwaymaycorrectdefectivecapacityof
NK cells toproduceIFN-␥.58 Furthermore,plasma levelsof
transforminggrowthfactor-1(TGF-1)isalsoelevatedduring
HBV infection,59 which mayimpair NKcell-mediated
cyto-toxiccapacityandIFN-␥productionbydown-regulatingthe
expressionoftheactivatingreceptorsNKG2Dand2B4.
More-over,theirintracellularadaptorproteins,respectivelyDAP10
andSAP,arealsodecreased.60
Since the immunesystem is areticular and syntrophic
entirety,NKcelldysfunctionalsoinfluencesthefunctionof
the other parts of the system, especially effector T cells.
CD8+ T cells are the main effector cells responsible for
infection.25However,inthechronicinfectionstage,theyare
markedlydiminishedinnumberandexhaustiveinfunctions
inpatients who failed tocontrol virus replication.61,62 The
decreaseinCD8+ effectorTcellscanbepartlyattributedto
NKcells,becauseNKcellscannotonlymediateCD8+Tcells
deaththrough up-regulationof TRAILreceptors,63 but also
limitCD8+ TcellsimmunitybyNKG2D-and2B4-dependent
perforin-mediatedlysis64,65(Fig.3).
Unfortunately,therestorationofHBV-specificCD8+Tcells
isdifficulttoachieve.Evenifafavorablevirologicalresponseis
obtainedafteranti-viraltreatment,HBV-specificCD8+Tcells
remainatlowlevelsandexpressanexhaustedphenotype.66,67
This“permanentsignature”mayresultfromchronicexposure
toHBV-relatedantigens,whichinturnchangesthe
differen-tiationprogramofCD8+ Tcells.Unlikewhatisobservedin
HBVinfection,typeIinterferoncanprotectanti-viralCD8+T
cellsfromeliminationbyNKcell-mediatedperforin
expres-sioninlymphocyticchoriomeningitisvirusinfection.68These
diverse mechanisms may partly explain whyCHB is
clini-callydifficulttomanage,withanunsatisfyingresponserate
toIFNtreatment.Furthermore,althoughnotyetobservedin
HBV infection,NK cells may preventactivatedCD4+Tcells
frombeingkilledbytheinteractionbetweenNKG2Aandits
ligand.69
NKcellsmaynotbeasimportantasDCsinbridgingthe
innateandadaptive immunities,buttheirrolesareunique,
andcorrelationalstudiesareattractingmoreandmore
atten-tioninHBVinfection.
NK
cells
as
potential
targets
for
immunotherapy
Intheclinicalsetting,currentlyavailableantiviraltreatment
forCHB infection can bedivided into two classes of
ther-apeutic agents:nucleos(t)ide analogs (NAs)and IFN-␣. NAs
includenucleoside(lamivudine,telbivudineandentecavir)or
nucleotide(adefovirandtenofovir)analogs.Themajor
advan-tagesofNAsaregoodtoleranceandpotentantiviralactivity
associatedwithhighratesofon-treatmentresponseto
ther-apy.TheadvantagesofIFNincludeafinitecourseoftreatment,
absenceofdrugresistance,andanopportunitytoachievea
durableresponsetotherapy.Obviously,theadvantagesofone
agentare the disadvantages ofthe other. Besides, the two
agentshavebeenconfirmedtocontributelittletothe
restora-tionofHBV-specificCD8+Tcells.Sincecurrenttherapiesare
farfromsufficientfromeconomicalandeffectivenesspoints
ofview,noveltherapeuticstrategiesare ingreatneed.Itis
unclearwhyindividualsresponddifferentlytoIFN,70butitis
supposedtobedue,atleastinpart,toNKcell
characteris-ticsincludingcellnumbers,receptorexpressionandfunction
alteration.67,71Similarresultswerealsoobtainedwiththeuse
ofNAs,43,45indicatingthatthemodulationofNKcellfunction
mayhelptosuppressHBVreplication.AberrantDNA
methyla-tionisanearlyandubiquitouseventduringHCCdevelopment,
andcanbedetectedeveninprecancerouslivertissuessuch
aschronichepatitis,livercirrhosis, ordysplasticnodules.72
Arecentstudyconfirmedthatthisprocesswasclosely
asso-ciatedwith NKcell activity.73 Hence,regulation ofNK cell
functionisapotentialwaytotreatCHBandtopreventthe
occurrenceofcirrhosisandHCC.
IthasbeendiscussedabovethatNKcellfunctional
alter-ations include at least three aspects: decreased cytokine
secretion, increased cytotoxicity and apoptosis-mediating
capacity(Fig.3).Therefore,weshouldturntoincreasing
anti-viralcytokineproductionandprotectothercellsfrombeing
killed,targetingontheinteractionbetweenNKcells’
recep-torstotheirligandsandcytokinesinthemicroenvironment.
Astheimmunesystemisacomplexentity,theinfluenceon
everypartofthissystemshouldbeconsideredtoformulate
newstrategiesinordertoavoidimmuneoverreaction.
StrategiesbasedontheregulationofNKcell functionto
treatCHBwereonlycarriedoutinanimalexperimentssofar.
NKcellsmayachieveenhancedanti-viralviabilityor
allevi-atedhepatocytelysisbyblockadeofsomeoftheiractivating
orinhibitoryreceptorsandligandinteractions.45,74Increased
expressionofPD-1 inthe liverresultsinimmunedisorder,
which istothedisadvantageofHBV clearance.PD-1/PD-L1
blockadecouldreverseimmunedysfunctionbyaugmenting
IFN-␥ secretionand accelerating HBV elimination invivo.75
IL-10andTGF-1havebeenshowntorestrainNKcells
func-tion,andblockadeofIL-10and/orTGF-1restoredthecapacity
ofNKcellstoproduceIFN-␥,thereby enhancingtheir
non-cytolyticantiviralcapacity.58,60ThecontributionofIFN-␥and
TNF-␣inHBVclearanceisundisputed,especiallyintheacute
phase of infection.2 However, in the immunotolerant and
immunoreactivephases,elevatedlevelsofIFN-␥andTNF-␣
arenotenoughtohelpeliminatingthevirus,butallows
sus-tainingtheliverinjury.76
Concluding
remarks
ThereasonwhyHBVinfection isdifficulttomanageinthe
clinicalsettingisbecauseofthepropertiesofthevirusitself,
butalsooftheimmuneresponsesofthehosts.Asdiscussed
above,theroleofNKcellsinHBVinfectionisstillcontroversial:
whetherNKcellsisinvolvedintheearlyphaseofHBV
infec-tion,andwhatalternations havebeen occurredtoNKcells
duringchronicinfectionarenotknownyet.Whereas,itcannot
bedeniedthatthepersistenceofHBVinfectionandongoing
liverinjurycouldbepartlyduetothedysfunctionofNKcells
andsubsequentmediationofdisordersoftheimmunesystem
anddeathofhepatocytes.Onaccountofourlimited
knowl-edge,newimmunotherapiesbasingonNKcellsare onlyin
theinfancystage.AnewsubsetofhumanNKcells,
denom-inatedasNK-22cells,wasrecentlydiscovered.77Theylocate
inmucosa-associatedlymphoidtissues,andarehard-wiredto
secreteIL-22,whichprovideprotectiontohepatocytes78,79and
promoteproliferationofliverstem/progenitorcells,80
provid-inganoveltherapeuticcandidateforchronicHBVinfection.
Insummary,onthebasisofexistingachievements,more
researchesarestillneededtodefinetheexactrolesofNKcells
inHBVinfection.Newstrategiesshouldbeaimingon
melio-rating the functionof NKcells, inhibiting viral replication,
alleviatingliverinjuryandavoidingcirrhosisandHCC.
Conflicts
of
interest
Acknowledgements
This work was supported by Science Research Project of
TwelfthFive-year-Plan“AIDSandViralHepatitisMajor
Infec-tiousDiseasesPreventionandControl”,2012ZX10005004-002
and2012ZX10005010-002-003,and NationalNatural Science
FoundationofChina,81102570and81202662.
r
e
f
e
r
e
n
c
e
s
1. GuidottiLG,ChisariFV.Immunobiologyandpathogenesisof viralhepatitis.AnnuRevPathol.2006;1:23–61.
2. GuidottiLG,RochfordR,ChungJ,ShapiroM,PurcellR,Chisari FV.Viralclearancewithoutdestructionofinfectedcells duringacuteHBVinfection.Science.1999;284:825–9.
3. FerrariC,MissaleG,BoniC,UrbaniS.Immunopathogenesis ofhepatitisB.JHepatol.2003;39Suppl1:S36–42.
4. BertolettiA,FerrariC.Innateandadaptiveimmuneresponses inchronichepatitisBvirusinfections:towardsrestorationof immunecontrolofviralinfection.Gut.2012;61:1754–64.
5. MedzhitovR,JanewayCJr.Innateimmunity.NEnglJMed. 2000;343:338–44.
6. WielandS,ThimmeR,PurcellRH,ChisariFV.Genomic analysisofthehostresponsetohepatitisBvirusinfection. ProcNatlAcadSciUSA.2004;101:6669–74.
7. FattovichG,BortolottiF,DonatoF.Naturalhistoryofchronic hepatitisB:specialemphasisondiseaseprogressionand prognosticfactors.JHepatol.2008;48:335–52.
8. HanQ,ZhangC,ZhangJ,TianZ.Theroleofinnateimmunity inHBVinfection.SeminImmunopathol.2013;35:23–38.
9. DohertyDG,NorrisS,Madrigal-EstebasL,etal.Thehuman livercontainsmultiplepopulationsofNKcells,Tcells,and CD3+CD56+naturalTcellswithdistinctcytotoxicactivities andTh1,Th2,andTh0cytokinesecretionpatterns.J Immunol.1999;163:2314–21.
10.CooperMA,FehnigerTA,TurnerSC,etal.Humannatural killercells:auniqueinnateimmunoregulatoryroleforthe CD56(bright)subset.Blood.2001;97:3146–51.
11.CooperMA,FehnigerTA,CaligiuriMA.Thebiologyofhuman naturalkiller-cellsubsets.TrendsImmunol.2001;22:633–40.
12.StrowigT,BrilotF,MunzC.NoncytotoxicfunctionsofNK cells:directpathogenrestrictionandassistancetoadaptive immunity.JImmunol.2008;180:7785–91.
13.JostS,AltfeldM.Controlofhumanviralinfectionsbynatural killercells.AnnuRevImmunol.2013;31:163–94.
14.VivierE,TomaselloE,BaratinM,WalzerT,UgoliniS. Functionsofnaturalkillercells.NatImmunol.2008;9:503–10.
15.YamagiwaS,KamimuraH,IchidaT.Naturalkillercell receptorsandtheirligandsinliverdiseases.MedMol Morphol.2009;42:1–8.
16.VivierE,UgoliniS,BlaiseD,ChabannonC,BrossayL. TargetingnaturalkillercellsandnaturalkillerTcellsin cancer.NatRevImmunol.2012;12:239–52.
17.CooperMA,ColonnaM,YokoyamaWM.Hiddentalentsof naturalkillers:NKcellsininnateandadaptiveimmunity. EMBORep.2009;10:1103–10.
18.MartinMP,GaoX,LeeJH,etal.Epistaticinteractionbetween KIR3DS1andHLA-BdelaystheprogressiontoAIDS.Nat Genet.2002;31:429–34.
19.KhakooSI,ThioCL,MartinMP,etal.HLAandNKcell inhibitoryreceptorgenesinresolvinghepatitisCvirus infection.Science.2004;305:872–4.
20.OrangeJS,FassettMS,KoopmanLA,BoysonJE,StromingerJL. Viralevasionofnaturalkillercells.NatImmunol.2002;3: 1006–12.
21.FletcherSP,ChinDJ,JiY,etal.Transcriptomicanalysisofthe woodchuckmodelofchronichepatitisB.Hepatology. 2012;56:820–30.
22.YangD,LiuL,ZhuD,etal.AmousemodelforHBV immunotoleranceandimmunotherapy.CellMolImmunol. 2014;11:71–8.
23.HuangLR,WuHL,ChenPJ,ChenDS.Animmunocompetent mousemodelforthetoleranceofhumanchronichepatitisB virusinfection.ProcNatlAcadSciUSA.2006;103:17862–7.
24.FletcherSP,ChinDJ,ChengDT,etal.Identificationofan intrahepatictranscriptionalsignatureassociatedwith self-limitinginfectioninthewoodchuckmodelofhepatitisB. Hepatology.2013;57:13–22.
25.ThimmeR,WielandS,SteigerC,etal.CD8(+)Tcellsmediate viralclearanceanddiseasepathogenesisduringacute hepatitisBvirusinfection.JVirol.2003;77:68–76.
26.ZhuR,Mancini-BourgineM,ZhangXM,BayardF,DengQ, MichelML.Plasmidvector-linkedmaturationofnaturalkiller (NK)cellsiscoupledtoantigen-dependentNKcellactivation duringDNA-basedimmunizationinmice.JVirol.
2011;85:10201–12.
27.LauzonNM,MianF,MacKenzieR,AshkarAA.Thedirect effectsofToll-likereceptorligandsonhumanNKcellcytokine productionandcytotoxicity.CellImmunol.2006;241:102–12.
28.YangPL,AlthageA,ChungJ,etal.Immuneeffectorsrequired forhepatitisBvirusclearance.ProcNatlAcadSciUSA. 2010;107:798–802.
29.GuyCS,Mulrooney-CousinsPM,ChurchillND,MichalakTI. Intrahepaticexpressionofgenesaffiliatedwithinnateand adaptiveimmuneresponsesimmediatelyafterinvasionand duringacuteinfectionwithwoodchuckhepadnavirus.JVirol. 2008;82:8579–91.
30.FisicaroP,ValdattaC,BoniC,etal.Earlykineticsofinnateand adaptiveimmuneresponsesduringhepatitisBvirus
infection.Gut.2009;58:974–82.
31.ZhaoJ,LiY,JinL,etal.Naturalkillercellsarecharacterizedby theconcomitantlyincreasedinterferon-gammaand
cytotoxicityinacuteresolvedhepatitisBpatients.PLoSONE. 2012;7:e49135.
32.LunemannS,MaloneDF,HengstJ,etal.Compromised functionofnaturalkillercellsinacuteandchronicviral hepatitis.JInfectDis.2014;209:1362–73.
33.ZhangZ,ZhangS,ZouZ,etal.Hypercytolyticactivityof hepaticnaturalkillercellscorrelateswithliverinjuryin chronichepatitisBpatients.Hepatology.2011;53:73–85.
34.ChenY,WeiH,SunR,DongZ,ZhangJ,TianZ.Increased susceptibilitytoliverinjuryinhepatitisBvirustransgenic miceinvolvesNKG2D-ligandinteractionandnaturalkiller cells.Hepatology.2007;46:706–15.
35.MirandolaP,PontiC,GobbiG,etal.ActivatedhumanNKand CD8+TcellsexpressbothTNF-relatedapoptosis-inducing ligand(TRAIL)andTRAILreceptorsbutareresistantto TRAIL-mediatedcytotoxicity.Blood.2004;104:2418–24.
36.IchikawaK,LiuW,ZhaoL,etal.Tumoricidalactivityofa novelanti-humanDR5monoclonalantibodywithout hepatocytecytotoxicity.NatMed.2001;7:954–60.
37.DunnC,BrunettoM,ReynoldsG,etal.Cytokinesinduced duringchronichepatitisBvirusinfectionpromoteapathway forNKcell-mediatedliverdamage.JExpMed.
2007;204:667–80.
38.OkazakiA,HiragaN,ImamuraM,etal.Severe necroinflammatoryreactioncausedbynaturalkiller
cell-mediatedFas/Fasligandinteractionanddendriticcellsin humanhepatocytechimericmouse.Hepatology.
2012;56:555–66.
40.XieZ,ChenY,ZhaoS,etal.IntrahepaticPD-1/PD-L1 up-regulationcloselycorrelateswithinflammationandvirus replicationinpatientswithchronicHBVinfection.Immunol Invest.2009;38:624–38.
41.GermanidisG,ArgentouN,HytiroglouP,etal.LiverFOXP3 andPD1/PDL1expressionisdown-regulatedinchronicHBV hepatitisonmaintainedremissionrelatedtothedegreeof inflammation.FrontImmunol.2013;4:207.
42.WiesmayrS,WebberSA,MacedoC,etal.DecreasedNKp46 andNKG2DandelevatedPD-1areassociatedwithaltered NK-cellfunctioninpediatrictransplantpatientswithPTLD. EurJImmunol.2012;42:541–50.
43.TjwaET,vanOordGW,HegmansJP,JanssenHL,WoltmanAM. Viralloadreductionimprovesactivationandfunctionof naturalkillercellsinpatientswithchronichepatitisB.J Hepatol.2011;54:209–18.
44.LiY,WangJJ,GaoS,etal.Decreasedperipheralnaturalkiller cellsactivityintheimmuneactivatedstageofchronic hepatitisB.PLOSONE.2014;9:e86927.
45.LiF,WeiH,WeiH,etal.Blockingthenaturalkillercell inhibitoryreceptorNKG2Aincreasesactivityofhuman naturalkillercellsandclearshepatitisBvirusinfectionin mice.Gastroenterology.2013;144:392–401.
46.OlivieroB,VarchettaS,PaudiceE,etal.Naturalkillercell functionaldichotomyinchronichepatitisBandchronic hepatitisCvirusinfections.Gastroenterology.
2009;137:1151–60,e1151–7.
47.JuY,HouN,MengJ,etal.Tcellimmunoglobulin-and mucin-domain-containingmolecule-3(Tim-3)mediates naturalkillercellsuppressioninchronichepatitisB.J Hepatol.2010;52:322–9.
48.LvJ,JinQ,SunH,etal.Antiviraltreatmentaltersthe frequencyofactivatingandinhibitoryreceptor-expressing naturalkillercellsinchronichepatitisBvirusinfected patients.MediatorsInflamm.2012;2012:804043.
49.ZhangL,WangQ,ZhaoP,HuX,JiangY.Effectsofentecaviron peripheralbloodlymphocyteprofilesinchronichepatitisB patientswithsuboptimalresponsestoadefovir.ClinExp PharmacolPhysiol.2014;41:514–23.
50.vanderMolenRG,SprengersD,BindaRS,etal.Functional impairmentofmyeloidandplasmacytoiddendriticcellsof patientswithchronichepatitisB.Hepatology.2004;40: 738–46.
51.WoltmanAM,BoonstraA,JanssenHL.Dendriticcellsin chronicviralhepatitisBandC:victimsorguardianangels? Gut.2010;59:115–25.
52.TjwaET,vanOordGW,BiestaPJ,BoonstraA,JanssenHL, WoltmanAM.RestorationofTLR3-activatedmyeloid dendriticcellactivityleadstoimprovednaturalkillercell functioninchronichepatitisBvirusinfection.JVirol. 2012;86:4102–9.
53.ShiCC,TjwaET,BiestaPJ,etal.HepatitisBvirussuppresses thefunctionalinteractionbetweennaturalkillercellsand plasmacytoiddendriticcells.JViralHepat.2012;19:e26–33.
54.MartinetJ,Dufeu-DuchesneT,BruderCostaJ,etal.Altered functionsofplasmacytoiddendriticcellsandreduced cytolyticactivityofnaturalkillercellsinpatientswithchronic HBVinfection.Gastroenterology.2012;143:1586–96,e1588.
55.XuL,YinW,SunR,WeiH,TianZ.Kupffercell-derivedIL-10 playsakeyroleinmaintaininghumoralimmunetolerancein hepatitisBvirus-persistentmice.Hepatology.2014;59:443–52.
56.TuZ,BozorgzadehA,PierceRH,KurtisJ,CrispeIN,OrloffMS. TLR-dependentcrosstalkbetweenhumanKupffercellsand NKcells.JExpMed.2008;205:233–44.
57.ParkJY,LeeSH,YoonSR,etal.IL-15-inducedIL-10increases thecytolyticactivityofhumannaturalkillercells.MolCells. 2011;32:265–72.
58.PeppaD,MiccoL,JavaidA,etal.Blockadeof
immunosuppressivecytokinesrestoresNKcellantiviral
functioninchronichepatitisBvirusinfection.PLoSPathog. 2010;6:e1001227.
59.MurawakiY,NishimuraY,IkutaY,IdobeY,KitamuraY, KawasakiH.Plasmatransforminggrowthfactor-beta1 concentrationsinpatientswithchronicviralhepatitis.J GastroenterolHepatol.1998;13:680–4.
60.SunC,FuB,GaoY,etal.TGF-beta1down-regulationof NKG2D/DAP10and2B4/SAPexpressiononhumanNKcells contributestoHBVpersistence.PLoSPathog.2012; 8:e1002594.
61.MainiMK,BoniC,LeeCK,etal.Theroleofvirus-specific CD8(+)cellsinliverdamageandviralcontrolduring persistenthepatitisBvirusinfection.JExpMed. 2000;191:1269–80.
62.BoniC,FisicaroP,ValdattaC,etal.Characterizationof hepatitisBvirus(HBV)-specificT-celldysfunctioninchronic HBVinfection.JVirol.2007;81:4215–25.
63.PeppaD,GillUS,ReynoldsG,etal.Up-regulationofadeath receptorrendersantiviralTcellssusceptibletoNK cell-mediateddeletion.JExpMed.2013;210:99–114.
64.WaggonerSN,TaniguchiRT,MathewPA,KumarV,WelshRM. Absenceofmouse2B4promotesNKcell-mediatedkillingof activatedCD8+Tcells,leadingtoprolongedviralpersistence andalteredpathogenesis.JClinInvest.2010;120:1925–38.
65.LangPA,LangKS,XuHC,etal.Naturalkillercellactivation enhancesimmunepathologyandpromoteschronicinfection bylimitingCD8+T-cellimmunity.ProcNatlAcadSciUSA. 2012;109:1210–5.
66.WherryEJ,BarberDL,KaechSM,BlattmanJN,AhmedR. Antigen-independentmemoryCD8Tcellsdonotdevelop duringchronicviralinfection.ProcNatlAcadSciUSA. 2004;101:16004–9.
67.MiccoL,PeppaD,LoggiE,etal.Differentialboostingofinnate andadaptiveantiviralresponsesduring
pegylated-interferon-alphatherapyofchronichepatitisB.J Hepatol.2013;58:225–33.
68.XuHC,GrusdatM,PandyraAA,etal.TypeIinterferon protectsantiviralCD8+TcellsfromNKcellcytotoxicity. Immunity.2014;40:949–60.
69.LuL,IkizawaK,HuD,WerneckMB,WucherpfennigKW, CantorH.RegulationofactivatedCD4+TcellsbyNKcellsvia theQa-1-NKG2Ainhibitorypathway.Immunity.
2007;26:593–604.
70.BusterEH,HansenBE,LauGK,etal.Factorsthatpredict responseofpatientswithhepatitisBeantigen-positive chronichepatitisBtopeginterferon-alfa.Gastroenterology. 2009;137:2002–9.
71.MahdaviM,AmirrasouliH,AlavianSM,etal.Impactof pegylatedinterferon-alfa-2aonperforinlevelinpatientswith chronichepatitisB;preliminarystudy.HepatitisMonthly. 2013;13:e11903.
72.GaoW,KondoY,ShenL,etal.VariableDNAmethylation patternsassociatedwithprogressionofdiseasein hepatocellularcarcinomas.Carcinogenesis.2008;29: 1901–10.
73.OkamotoY,ShinjoK,ShimizuY,etal.Hepatitisvirus infectionaffectsDNAmethylationinmicewithhumanized livers.Gastroenterology.2014;146:562–72.
74.HuangM,SunR,WeiH,TianZ.Simultaneousknockdownof multipleligandsofinnatereceptorNKG2Dpreventsnatural killercell-mediatedfulminanthepatitisinmice.Hepatology. 2013;57:277–88.
75.TzengHT,TsaiHF,LiaoHJ,etal.PD-1blockagereverses immunedysfunctionandhepatitisBviralpersistenceina mouseanimalmodel.PLoSONE.2012;7:e39179.
77.CellaM,FuchsA,VermiW,etal.Ahumannaturalkillercell subsetprovidesaninnatesourceofIL-22formucosal immunity.Nature.2009;457:722–5.
78.ZenewiczLA,YancopoulosGD,ValenzuelaDM,MurphyAJ, KarowM,FlavellRA.Interleukin-22butnotinterleukin-17 providesprotectiontohepatocytesduringacuteliver inflammation.Immunity.2007;27:647–59.
79.ZhangY,CobleighMA,LianJQ,etal.Aproinflammatoryrole forinterleukin-22intheimmuneresponsetohepatitisB virus.Gastroenterology.2011;141:1897–906.