The relationship between
Helicobacter pylori
genes
cag
E and
vir
B11
and gastric cancer
Valeska Portela Lima
a,*
, Marcos Antonio Pereira de Lima
a, Ma´rcia Vale´ria Pitombeira Ferreira
a,
Marcos Aure´lio Pessoa Barros
b, Sı´lvia Helena Barem Rabenhorst
aaDepartment of Pathology and Forensic Medicine, Federal University of Ceara´, Porangabussu Campus, Alexander Barau´na Street, 949, Fortaleza, Ceara´ 60183-630, Brazil bSaint House of Mercy, Fortaleza, Brazil
1. Introduction
Helicobacter pyloriis a Gram-negative bacterium that has been associated with diverse pathologies of varying severity, such as chronic gastritis, peptic ulcer, mucosa-associated lymphoid tissue (MALT) lymphoma, and gastric carcinoma.1 Although most
infected persons remain asymptomatic, 15–20% of H. pylori -positive individuals will develop the associated diseases. This could be dependent on environmental factors, host genetic factors or specific properties of the microorganism.1
There is some evidence that the presence of certain virulence factors is important in the organism’s ability to cause different diseases. Several bacterial virulence factors have been suggested, including the vacuolating cytotoxin (VacA) and the
cytotoxin-associated gene A antigen (CagA). However, data supporting these findings are insufficient to explain the variety and severity of the related diseases. One important virulence factor ofH. pyloriis the cag pathogenicity island (cag-PAI). This contains 31 genes, including the
cagA gene, but also codes for six proteins of a putative type IV secretion system, specialized in the transfer of a variety of protein complexes across the bacterial membrane to the extracellular space or into the host cells.2,3One of the cag-PAI genes iscagE, located in
the right half of the cag-PAI.1,4–6Sozzi et al.6and Ikenoue et al.7have
suggested that this gene is a more accurate marker of an intact cag-PAI than othercaggenes. Another gene that codifies proteins of a type IV secretion system isvirB11. This gene is located in the left half of the cag-PAI. The VirB11 protein has a ring-shaped structure composed of six monomeric units. It is important for the transportation of protein complexes and exhibits ATPase activity.8,9
Several studies have described an association betweenH. pylori cagE and gastritis, duodenal ulcer, and peptic ulcer disease.10–12Only a
few studies have described an association with gastric cancer, but the number of cases included has been small and therefore the test
A R T I C L E I N F O
Article history:
Received 17 February 2009
Received in revised form 6 September 2009 Accepted 8 September 2009
Corresponding Editor:Sunit K. Singh, Hyderabad, India
Keywords:
Gastric cancer
Helicobacter pylori
Genotype
S U M M A R Y
Background: The association betweenHelicobacter pylorigene diversity and gastric cancer has been poorly reported, although it is one of the important ways to explain the gastric pathogenesis. The aim of this study was to investigate the frequency ofcagE andvirB11 genes inH. pyloriisolated from patients with gastric cancer and to analyze the histology profiles.
Materials and methods:The presence ofH. pyloriand subtypes (cagE andvirB11) was detected by PCR from the genomic DNA of 101 patients who had been diagnosed with gastric cancer. The cases were grouped according to the presence/absence of the genes studied and were analyzed in relation to histopathological parameters.
Results: H. pyloriinfection was detected in 94 out of 101 (93.1%) gastric carcinomas. The cases were categorized into the following groups: cagE+/virB11+, cagE+/virB11 , cagE /virB11+, and cagE /
virB11 . Frequencies were: 50% (47/94)cagE+/virB11+, 3.2% (3/94)cagE+/virB11 , 10.6% (10/94)cagE /
virB11+, and 36.2% (34/94) cagE /virB11 . Tumors in the gastric antrum were predominant. An exception was thecagE /virB11 group, in which tumors had a tendency to be located in the gastric cardia; the majority of the cardia tumors (56% (14/25)) were in this group. Intestinal histology type was the most frequent, but the cagE+/virB11 group only had diffuse tumors. H. pylori cagE+/virB11+ occurred most frequently (except at stage III), and was present at all gastric cancer stages.
Conclusions: This study is the first to include a relevant number of gastric cancer cases withH. pylori
infection, reporting the frequency and relationship ofcagE andvirB11 genes and the genesis of this tumor. The presence of these cag pathogenicity island genes shows that they are important factors for the pathogenesis and malignancy of gastric cancer related toH. pylori.
ß2009 International Society for Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
* Corresponding author. Tel.: +55 85 8805 0432; fax: +55 85 3267 3840.
E-mail address:valeskacb@yahoo.com.br(V.P. Lima).
Contents lists available atScienceDirect
International Journal of Infectious Diseases
j o u r n a l h o m e p a g e : w w w . e l s e v i e r . c o m / l o c a t e / i j i d
results have often been combined with those of other diseases.13–15
In contrast to studies ofcagE, those related to the genevirB11 are more frequently in vitro, with rare reports in vivo.6,16Therefore, the
aim of this study was to investigate the frequency ofcagE andvirB11 genes inH. pyloriisolates from patients with gastric cancer and to analyze the histology profiles.
2. Materials and methods
2.1. Clinical specimens
This study was approved by the ethics committee of the Federal University of Ceara´ and all subjects signed an informed consent form before inclusion. Samples from 101 patients with gastric carcinoma who had undergone gastrectomy were collected from two hospitals in the state of Ceara´, Brazil: Walter Cantı´deo Hospital at the Federal University of Ceara´ and Saint House of Mercy in Fortaleza, both located in Fortaleza, the state capital. Histological classification was done according to the Lauren classification and the tumors were staged according to the TNM criteria (tumor, node, metastasis) by the study team of pathologists.
2.2. DNA extraction
Genomic DNA was extracted from frozen tumor tissue using cetyltrimethyl ammonium bromide (CTAB), as per the method of Foster and Twell, with some modifications.17DNA extraction was
done only in fragments that presented more than 80% of tumor cells and the quality was analyzed by 1% agarose gel electropho-resis with ethidium bromide staining.
2.3. H. pylori and cagE and virB11 gene detection
H. pyloriinfection was detected by amplification of the urease C (ureC) gene using oligonucleotide primers for PCR, as described by Lage et al.18The presence of thecagE andvirB11 genes was identified
in theH. pylori-positive samples using the primers described by Sozzi et al.6The PCR mixtures for amplification of both genes were
prepared in a volume of 25
m
l using MasterMix (Taq DNApolymerase, dNTPs and MgCl2) according to the manufacturer’s
instructions (Promega), adding 0.8% of Tween 20, 0.4
m
M of eachprimer, and 1
m
l of the DNA sample. PCR products were analyzed by1% agarose gel electrophoresis with ethidium bromide staining. The sample was consideredH. pylori-positive when aureC fragment of 294 bp was amplified, whilecagE andvirB11 genes were considered positive when fragments of 509 bp and 491 bp, respectively, were detected. We used known DNA to beH. pylori-positive as a positive control. DNAse-free water was used as a negative control. DNA preservation was also confirmed by amplification of different genes using other approaches in our laboratory. Random samples were reanalyzed for confirmation of the results.
2.4. Statistical analysis
Statistical analyses were carried out using Epi Info 6.04d (Centers for Disease Control and Prevention, Atlanta, GA, USA) and SPSS 12.0 (SPSS Inc., Chicago, IL, USA) programs. Statistically significant differences were evaluated by the Chi-square test. Results were considered statistically significant when thep-values were less than 0.05.
3. Results
Among the 101 cases analyzed, 68 (67.3%) were male and 33 (32.7%) female. The average age was 62.7 years, ranging from 23 to 90 years. The most frequent tumor site was the gastric non-cardia
(76/101 (75.2%) vs. cardia 25/101 (24.8%)). Intestinal and diffuse types presented similar frequencies: 58 (57.4%) and 43 (42.6%), respectively.
3.1. Detection of H. pylori and presence of cagE and virB11 genes
H. pylori infection was detected in 94 of the 101 (93.1%) gastric carcinomas.H. pylori-positive cases were more frequent-ly male (64/94 (68.1%)). H. pyloriinfection was detected more frequently in non-cardia tumors (73.4% (69/94)) than in cardia tumors (26.6% (25/94)). Among these H. pylori-infected cases, the intestinal histology type was slightly more frequent (58.5% (55/94)). The cagE gene was found in 53.2% (50/94) and the
virB11 gene in 60.6% (57/94) of the cases. Additionally, thecagE andvirB11 genes had a significant positive correlation (r= 0.728,
p= 0.000).
3.2. Relationship between H. pylori infection and cagE and virB11 genes with gastric cancer
To investigate the relationship between theH. pyloriinfection and the presence ofcagE andvirB11 genes in gastric cancer, we divided the cases into four groups according to the presence of these genes: cagE+/virB11+, cagE+/virB11 , cagE /virB11+, and
cagE /virB11 . The frequency of cases was 50% (47/94) incagE+/
virB11+, 3.2% (3/94) in cagE+/virB11 , 10.6% (10/94) in cagE /
virB11+, and 36.2% (34/94) incagE /virB11 .
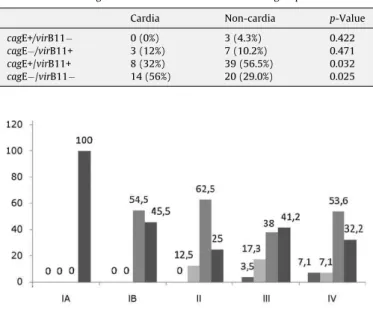
Table 1shows the distribution of the four groups according to gastric site. It is noteworthy that the cagE+/virB11+ group was predominant in non-cardia tumors (56.5%) and thecagE /virB11 group was more frequent in tumors situated in the gastric cardia (56%).
Fig. 1shows the distribution of groups according to tumor stage. ThecagE+/virB11+ group was present from stage IB, being the most frequent at stages IB, II and IV. Although thecagE /virB11 group was present from stage IA, it was more frequent at stage III. Despite the small numbers in thecagE+/virB11 andcagE /virB11+ groups, the former was restricted to the advanced stages (III and IV), while the latter was represented at almost all stages.
Fig. 1.Distribution of cases by tumor stage (IA, IB, II, III and IV) in the four defined groups:cagE+/virB11 ,cagE /virB11+,cagE+/virB11 andcagE /virB11 . Table 1
Correlation between gastric sites and the four determinate groups
Cardia Non-cardia p-Value
cagE+/virB11 0 (0%) 3 (4.3%) 0.422
cagE /virB11+ 3 (12%) 7 (10.2%) 0.471
cagE+/virB11+ 8 (32%) 39 (56.5%) 0.032
Since the gastric cancer tumor grading included lymph node involvement and metastases as well as tumor size (TNM), we analyzed the data for tumor size alone to better verify whetherH. pyloriwas present at the beginning of tumorigenesis (Fig. 2). In this distribution, it was possible to observe that the cagE+/virB11+ group was the most frequent at tumor sizes T2 and T3.
Fig. 3 shows the distribution of cases by lymph node involvement. ThecagE+/virB11+ group was the most frequent in N0, N1 and N2 cases. The majority of theH. pylori-infected gastric cancers did not show metastasis (Fig. 4).
4. Discussion
Several studies have reported the influence of particular virulence genes (mainlyvacA andcagA) on the clinical outcome ofH. pyloriinfection in different geographical regions. Neverthe-less, the clinical relevance of these putative virulence-associated genes ofH. pyloriis still a matter of controversy. The cytotoxin-associated genecagA, which codes for the outer membrane protein CagA, was initially considered an important virulence factor, due to its association with some gastrointestinal diseases.19–21However,
subsequent studies have provided more inconsistent results.22,23
Miehlke et al.22demonstrated that up to 80% of subjects without
ulcer disease in Texas were infected with H. pyloristrains that possessedcagA. In China and Japan,cagA-positive strains are nearly universally present and not associated with disease complica-tions.24,25In children, infection withcagA-positive strains has not
been consistently associated with peptic ulcer disease.26–28These
findings suggest that polymorphism of the cagA gene can be relevant,3,9but the presence of other possibleH. pylorivirulence
genes may also be. The other virulence factor ofH. pylorimost studied isvacA, which presents genotype and geographic variation, with the genotype vacA s1m1 associated with more severe gastrointestinal diseases.29,30Even so, the presence of cagA and vacA has not been sufficient to explain diseases related toH. pylori
infection.
Therefore, the pathogenicity ofH. pyloristrains could be related to the presence of othercag-PAI genes. Among these genes,cagE andvirB11 stand out: these genes are located on different sides of the cag-PAI, so they can also be used as markers of the presence of this island, as proposed by some authors;1,2,4,6 they also have pathogenic potential due to their critical functions.1,8 Thus, we
examined the frequency ofH. pyloriand the relationship between
cagE andvirB11 genes and the histological data.
The current study demonstrated the frequency of H. pylori
infection to be 93.1% in gastric cancer cases. This frequency is similar to that reported in another study from Brazil by Thomazini et al.,31which reported a frequency of 95%, and a study from
Turkey by Saribasak et al.,32which reported a frequency of 100%.
Both of these studies used PCR to detectH. pyloriinfection in gastric cancers. In addition, Nomura et al.,33found 94% prevalence ofH. pyloriinfection in gastric cancer by serology, in a study conducted in Hawaii. On the other hand, these frequencies are higher than those found in the majority of previous studies, which have reported frequencies between 52% and 69%. However, most of these have used serology and histology to detect H. pylori
infection.34–36
ThecagE gene was found in 53.2% ofH. pylori-positive gastric cancer cases. This is the first study to analyze the frequency ofcagE in gastric cancer separately.Table 2shows the frequency of the
cagE gene found in studies of gastrointestinal diseases.11–13,21,37–39
These studies show that the frequency of thecagE gene is highest in more severe diseases, such as ulcer and gastric cancer, than in gastritis, suggesting that this gene may be important in the progression of these diseases. Likewise, thevirB11 gene was found in a significant (60.6%) number of the gastric cancer cases analyzed. So far, there have been no studies linking the presence of gastric cancer tovirB11. The only two studies found in the literature at the time of writing were those of Tomasini et al.16and Sozzi et al.,6
both of which studied dyspeptic Italian patients with duodenal ulcer, non-atrophic gastritis and atrophic gastritis. These studies found thevirB11 gene to be present in 90% and 94.7% of the cases, respectively, both higher than the frequency found in the present study.
The frequency of thecagE gene found in the present study was lower than in others (Table 2), but this could be due to the small number of gastric cancer cases analyzed in those studies. Also, the
Fig. 3.Distribution of cases by lymph node involvement (N0, N1, N2, N3, Nx) in the four defined groups:cagE+/virB11 ,cagE /virB11+,cagE+/virB11 andcagE /
virB11 .
Fig. 4.Distribution of cases by presence/absence of metastasis (M0, M1, Mx) in the four defined groups:cagE+/virB11 ,cagE /virB11+,cagE+/virB11 andcagE /
virB11 .
frequency of thevirB11 gene was lower than that found in studies of other gastric diseases. Besides the significance of the number of cases studied, these differences may be related to regional variations in circulatingH. pyloristrains, since the bacterium is known to have great genomic variability.1
Together, these studies point to the importance of these genes in the progression and severity of gastric diseases. In fact, the VirB11 protein is strategically located in the type IV secretion system, associated with ATPase activity, andcagE is considered as contributing to the construction of the cag type IV secretion system and inducing the secretion of cytokines, such as interleukin-8, from infected host epithelial cells.
To verify the influence ofcagE andvirB11 genes ofH. pylorion gastric cancer, we divided the cases into four groups based on the presence or absence of these genes. Although the gastric antrum was the most prevalent site of gastric cancer withH. pyloriinfection, the
cagE /virB11 group showed a slight increase in incidence of tumors located in the gastric cardia. Since this region is associated with the development of tumors withoutH. pyloriinfection,40,41the
strains found in the cardia region may be less virulent.
Reports of the association ofH. pyloriwith histological types of gastric cancer are controversial and there are no reports associating histological subtypes and H. pylori genotypes. The intestinal type was prevalent in thecagE /virB11+,cagE+/virB11+, andcagE /virB11 groups. However, in spite of the small number of cases, thecagE+/virB11 group was only found in the diffuse type. Some studies have shown an association betweenH. pylori
and the intestinal type,33,42while others have observed a similar
distribution between the two histological types.42,43 Palestro et
al.,44reported thatH. pyloriinfection may also be involved in the
pathogenesis of diffuse gastric cancer. These variations could be due to the presence of strain factors that were not analyzed here, associated with host genetic predisposition, such as mutation in the E-cadherin gene.45,46
There are only a few studies reporting the relationship between gastric cancer andH. pyloriinfection and tumor stage, such as that of Luo et al.47They found a higher frequency ofH. pyloriinfection
at stage II. In the present study, we found the presence ofH. pylori
beginning at the early stages with cagE+/virB11+ and cagE /
virB11 . The presence ofH. pylori cagE+/virB11+ from the earlier stages and the high frequency at all stages suggest that this strain can be more pathogenic than others and also that this strain can be important in gastric tumorigenesis. This aspect was confirmed by the presence of theH. pylori cagE+/virB11+ in tumors of a small size. Studies reporting high frequencies of these genes in more aggressive gastric diseases support this idea.6,11–13,16On the other
hand, the presence of thecagE /virB11 group could be due to other virulence factors ofH. pylorior even associated with other risk factors, such as particular foods, alcohol and smoking, which were not studied.
Finally, this study is the first to include a relevant number of gastric cancer cases with H. pylori infection, reporting the frequency and relationship ofcagE andvirB11 genes and also to analyze the presence of these genes focusing on histological aspects. The results presented here indicate that these genes, which are part of the cag-PAI, are important virulence factors ofH. pyloriin bacterial pathogenesis.
Conflict of interest
No conflict of interest to declare.
References
1. Covacci A, Telford JL, Gludice GD, Parsonnet J, Rappouli R.Helicobacter pylori
virulence and genetic geography.Science1999;284:1328–33.
2. Akopyants NS, Clifton SW, Kersulyte D, Crabtree JE, Youree BE, Reece CA, et al. Analyses of the cag pathogenicity island ofHelicobacter pylori.Mol Microbiol
1998;28:37–53.
3. Covacci A, Rappuoli R. Tyrosine-phosphorylated bacterial proteins: Trojan horses for the host cell.J Exp Med2000;191:587–92.
4. Censini S, Lange C, Xiang Z, Crabtree JE, Ghiara P, Borodovsky M, et al. cag, a pathogenicity island ofHelicobacter pylori, encodes type I-specific and disease-associated virulence factors.Proc Natl Acad Sci U S A1996;93:14648–53. 5. Segal ED, Lange C, Covacci A, Tompkins LS, Falkow S. Induction of host signal
transduction pathways by Helicobacter pylori. Proc Natl Acad Sci U S A
1997;94:7595–9.
6. Sozzi M, Tomasini ML, Vindigni C, Zanussi S, Tedeschi R, Basaglia G, et al. Heterogeneity of cag genotypes and clinical outcome ofHelicobacter pylori
infection.J Lab Clin Med2005;146:262–70.
7. Ikenoue T, Maeda S, Ogura K, Akanuma M, Mitsuno Y, Imai Y, et al. Determina-tion ofHelicobacter pylorivirulence by simple gene analysis of the cag patho-genicity island.Clin Diagn Lab Immunol2001;8:181–6.
8. Krause S, Pansegrau W, Lurz R, de la Cruz F, Lanka E. Enzymology of type IV macromolecule secretion systems: the conjugative transfer regions of plasmids RP4 and R388 and the cag pathogenicity island ofHelicobacter pyloriencode structurally and functionally related nucleoside triphosphate hydrolases. J Bacteriol2000;182:2761–70.
9. Backert S, Moese S, Selbach M, Brinkmann V, Meyer TF. Phosphorylation of tyrosine 972 of theHelicobacter pyloriCagA protein is essential for induction of a scattering phenotype in gastric epithelial cells.Mol Microbiol2001;42: 631–44.
10. Tummuru MK, Sharma SA, Blaser MJ.Helicobacter pyloripicB, a homologue of theBordetella pertussistoxin secretion protein, is required for induction of IL-8 in gastric epithelial cells.Mol Microbiol1995;18:867–76.
11. Day AS, Jones NL, Lynett JT, Jennings HA, Fallone CA, Beech R, et al. cagE is a virulence factor associated withHelicobacter pylori-induced duodenal ulcera-tion in children.J Infect Dis2000;181:1370–5.
12. Chomvarin C, Namwat W, Chaicumpar K, Mairiang P, Sangchan A, Sripa B, et al. Prevalence ofHelicobacter pylori vacA,cagA,cagE,iceA andbabA2 genotypes in Thai dyspeptic patients.Int J Infect Dis2008;12:30–6.
13. Ali M, Khan AA, Tiwari SK, Ahmed N, Rao LV, Habibullah CM. Association between cag-pathogenicity island inHelicobacter pyloriisolates from peptic ulcer, gastric carcinoma, and non-ulcer dyspepsia subjects with histological changes.World J Gastroenterol2005;11:6815–22.
14. Yamazaki S, Yamakawa A, Okuda T, Ohtani M, Suto H, Ito Y, et al. Distinct diversity ofvacA,cagA, andcagE genes ofHelicobacter pyloriassociated with peptic ulcer in Japan.J Clin Microbiol2005;43:3906–16.
15. Tan HJ, Rizal AM, Rosmadi MY, Goh KL. Distribution ofHelicobacter pylori cagA,
cagE andvacA in different ethnic groups in Kuala Lumpur, Malaysia.J Gastro-enterol Hepatol2005;20:589–94.
Table 2
Frequencies ofcagE found in the literature
Author, year [Ref.] Frequency (n/N) Population Disease
Lima et al., 2008 [present study] 53.2% (50/94) Brazilian Gastric cancer
Ali et al., 2005[13] 100% (14/14) India Gastric cancer
Erzin et al., 2006[37] 81.8% (27/33) Turkey Gastric cancer
Chomvarin et al., 2008[12] 93.8% (15/16) Thailand Gastric cancer
Day et al., 2000[11] 92.3% (12/13) Canada Duodenal ulcer
Erzin et al., 2006[37] 67.9% (19/28) Turkey Duodenal ulcer
Chomvarin et al., 2008[12] 90% (18/20) Thailand Gastric ulcer
Chomvarin et al., 2008[12] 85.7% (12/14) Thailand Duodenal ulcer
Audibert et al., 2001[38] 64.7% (99/153) France Ulcer/gastritis
Lehours et al., 2004[39] 56.4% (22/39) France Gastritis
Erzin et al., 2006[37] 26.7% (8/30) Turkey Dyspepsia
Chomvarin et al., 2008[12] 87.1% (54/62) Thailand Non-ulcer gastritis
16. Tomasini ML, Zanussi S, Sozzi M, Tedeschi R, Basaglia G, De Paoli P. Heteroge-neity of cag genotypes inHelicobacter pylori isolates from human biopsy specimens.J Clin Microbiol2003;41:976–80.
17. Foster GD, Twell DJ. Plant gene isolation: principles and practice. Chichester, UK: Wiley; 1996. p. 426.
18. Lage AP, Godfroid E, Fauconnier A, Burette A, Butzler JP, Bollen A, et al. Diagnosis ofHelicobacter pyloriinfection by PCR: comparison with other invasive tech-niques and detection ofcagA gene in gastric biopsy specimens.J Clin Microbiol
1995;33:2752–6.
19. Crabtree JE, Taylor JD, Wyatt JI, Heatley RV, Shallcross TM, Tompkins DS, et al. Mucosal IgA recognition ofHelicobacter pylori120 kDa protein, peptic ulcera-tion, and gastric pathology.Lancet1991;338:332–5.
20. Ching CK, Wong BC, Kwok E, Ong L, Covacci A, Lam SK. Prevalence of CagA-bearingHelicobacter pyloristrains detected by the anti-CagA assay in patients with peptic ulcer disease and in controls.Am J Gastroenterol1996;91:949–53. 21. Mo´dena JL, Acrani GO, Micas AF, Castro M, Silveira WD, Mo´dena JL, et al. Correlation between Helicobacter pylori infection, gastric diseases and life habits among patients treated at a university hospital in Southeast Brazil.Braz J Infect Dis2007;11:89–95.
22. Miehlke S, Kibler K, Kim JG, Figura N, Small SM, Graham DY, et al. Allelic variation in thecagA gene ofHelicobacter pyloriobtained from Korea compared to the United States.Am J Gastroenterol1996;91:1322–5.
23. Graham DY, Genta RM, Graham DP, Crabtree JE. Serum CagA antibodies in asymptomatic subjects and patients with peptic ulcer: lack of correlation of IgG antibody in patients with peptic ulcer or asymptomaticHelicobacter pylori
gastritis.J Clin Pathol1996;49:829–32.
24. Pan ZJ, Berg DE, van der Hulst RW, Su WW, Raudonikiene A, Xiao SD, et al. Prevalence of vacuolating cytotoxin production and distribution of distinct
vacAalleles inHelicobacter pylorifrom China.J Infect Dis1998;178:220–6. 25. Maeda S, Ogura K, Yoshida H, Kanai F, Ikenoue T, Kato N, et al. Major virulence
factors, VacA and CagA, are commonly positive in Helicobacter pylori isolates in Japan.Gut1998;42:338–43.
26. Queiroz DM, Soares TF, Rocha GA, Carvalho AS, Lage AP, Mendes EN, et al. Difference in cytotoxin production byHelicobacter pyloristrains isolated from adults and children with peptic ulcer.Proceedings from Helicobacter pylori: basic mechanisms to clinical cure [abstract 49]. 1996.p. 49.
27. Loeb M, Jayaratne P, Jones N, Sihoe A, Sherman P. Lack of correlation between vacuolating cytotoxin activity,cagA gene inHelicobacter pylori,and peptic ulcer disease in children.Eur J Clin Microbiol Infect Dis1998;17:653–6.
28. Mitchell HM, Hazell SL, Bohane TD, Hu P, Chen M, Li YY. The prevalence of antibody to CagA in children is not a marker for specific disease.J Pediatr Gastroenterol Nutr1999;28:71–5.
29. Atherton JC, Cao P, Peek Jr RM, Tummuru MK, Blaser MJ, Cover TL. Mosaicism in vacuolating cytotoxin alleles ofHelicobacter pylori. Association of specific vacA types with cytotoxin production and peptic ulceration.J Biol Chem1995;270: 17771–7.
30. Cover TL. The vacuolating cytotoxin of Helicobacter pylori. Mol Microbiol
1996;20:241–6.
31. Thomazini CM, Pinheiro NA, Pardini MI, Naresse LE, Rodrigues MA.Helicobacter pyloriand gastric cancer: distribution ofcagA andvacA genotypes in patients with gastric carcinoma.J Bras Patol Med Lab2006;42:25–30.
32. Saribasak H, Salih BA, Yamaoka Y, Sander E. Analysis ofHelicobacter pylori
genotypes and correlation with clinical outcome in Turkey.J Clin Microbiol
2004;42:1648–51.
33. Nomura A, Stemmermann GN, Chyou PH, Kato I, Perez-Perez GI, Blaser MJ.
Helicobacter pyloriinfection and gastric carcinoma among Japanese Americans in Hawaii.N Engl J Med1991;325:1132–6.
34. Pereira LP, Waisberg J, Andre´ EA, Zanoto A, Mendes Ju´nior JP, Soares HP. Detection ofHelicobacter pyloriin gastric cancer.Arq Gastroenterol2001;38: 240–6.
35. Araujo-Filho I, Brandao-Neto J, Pinheiro LA, Azevedo IM, Freire FH, Medeiros AC. Prevalence ofHelicobacter pyloriinfection in advanced gastric carcinoma.Arq Gastroenterol2006;43:288–92.
36. Mangia A, Chiriatti A, Ranieri G, Abbate I, Coviello M, Simone G, et al.H. pylori
status and angiogenesis factors in human gastric carcinoma.World J Gastro-enterol2006;12:5465–72.
37. Erzin Y, Koksal V, Altun S, Dobrucali A, Aslan M, Erdamar S, et al. Prevalence of
Helicobacter pylori vacA,cagA,cagE,iceA,babA2 genotypes and correlation with clinical outcome in Turkish patients with dyspepsia.Helicobacter2006;11:574– 80.
38. Audibert C, Burucoa C, Janvier B, Fauche`re JL. Implication of the structure of the
Helicobacter pyloricag pathogenicity island in induction of interleukin-8 secre-tion.Infect Immun2001;69:1625–9.
39. Lehours P, Me´nard A, Dupouy S, Bergey B, Richy F, Zerbib F, et al. Evaluation of the association of nineHelicobacter pylorivirulence factors with strains in-volved in low-grade gastric mucosa-associated lymphoid tissue lymphoma.
Infect Immun2004;72:880–8.
40. Hansen S, Vollset SE, Derakhshan MH, Fyfe V, Melby KK, Aase S, et al. Two distinct aetiologies of cardia cancer; evidence from premorbid serological markers of gastric atrophy andHelicobacter pyloristatus.Gut2007;56:918– 25.
41. Buruk F, Berberoglu U, Pak I, Aksaz E, Celen O. Gastric cancer andHelicobacter pyloriinfection.Br J Surg1993;80:378–9.
42. Smith MG, Hold GL, Tahara E, El-Omar EM. Cellular and molecular aspects of gastric cancer.World J Gastroenterol2006;12:2979–90.
43. Hansson LE, Engstrand L, Nyre´n O, Evans Jr DJ, Lindgren A, Bergstro¨m R, et al.
Helicobacter pyloriinfection: independent risk indicator of gastric adenocarci-noma.Gastroenterology1993;105:1098–103.
44. Palestro G, Pellicano R, Fronda GR, Valente G, De Giuli M, Soldati T, et al. Prevalence ofHelicobacter pyloriinfection and intestinal metaplasia in subjects who had undergone surgery for gastric adenocarcinoma in Northwest Italy.
World J Gastroenterol2005;11:7131–5.
45. Rogers WM, Dobo E, Norton JA, Van Dam J, Jeffrey RB, Huntsman DG, et al. Risk-reducing total gastrectomy for germline mutations in E-cadherin (CDH1): pathologic findings with clinical implications. Am J Surg Pathol2008;32: 799–809.
46. Schwarz A. Preventive gastrectomy in patients with gastric cancer risk due to genetic alterations of the E-cadherin gene defect. Langenbecks Arch Surg
2003;388:27–32.
47. Luo B, Wang Y, Wang XF, Gao Y, Huang BH, Zhao P. Correlation of Epstein–Barr virus and its encoded proteins withHelicobacter pyloriand expression ofc-met