Dessulfurização de dibenzothiofeno por Gordonia alkanivorans estirpe 1B
Texto
(2) On the Cover - Photo of colonies of Gordonia allkanivorans..
(3) UNIVERSIDADE DE LISBOA FACULDADE DE CIÊNCIAS DEPARTAMENTO DE BIOLOGIA VEGETAL. Dessulfurização de dibenzotiofeno por Gordonia alkanivorans estirpe 1B. Luís Manuel Gonçalves Alves. ORIENTADORES:. Professor Doutor Rogério Paulo de Andrade Tenreiro Professor Auxiliar da Faculdade de Ciências da Universidade de Lisboa. Doutor José António dos Santos Pereira de Matos Investigador Auxiliar do Instituto Nacional de Engenharia, Tecnologia e Inovação. DOUTORAMENTO EM BIOLOGIA (MICROBIOLOGIA). 2007.
(4) Na presente dissertação incluem-se resultados que foram alvo de publicação com outros autores. Para efeitos do disposto no nº 2 do Art. 8º do Decreto-Lei 388/70, O autor da dissertação declara que interveio na concepção e execução do trabalho experimental, na interpretação dos resultados e na redacção dos manuscritos publicados ou enviados para publicação. Lisboa, 03 de Agosto, 2007. -----------------------------------------------(Luís Manuel Gonçalves Alves).
(5) Para os meus pais, a minha esposa e o meu filho com carinho.. "A ciência nunca está concluída, está cada vez mais próxima da compreensão total e rigorosa da natureza, mas nunca chega a alcançá-la.” Carl Sagan.
(6)
(7) Abbreviations 2-HBP – 2-Hydroxybiphenyl (2-hidroxibifenilo) 4,6-dmDBT – 4,6-dimethylDBT (4,6-dimetilDBT) 4-mDBT – 4-methylDBT (4-metilDBT) Ala – Alanine aa – Amino acid BDS – Biodesulfurization (biodessulfurização) bp – Base pair BT – Benzothiophene rDNA – Ribosomal DNA DNS – Dinitrosalysilic acid DBT – Dibenzothiophene (dibenzotiofeno) DBTS – Dibenzothiophene sulfone DCW – Dry cell weight DO – Densidade óptica DSMZ – Deutsche Sammlung von Mikroorganismen und Zellkulturen GmbH dsz – Desulfurization DszA – DBT-monooxygenase DszB – DBTS-monooxygenase DszC – 2'-Hydroxybiphenyl2-sulfinate desulfinase DszD – Flavin-reductase DMF – Dimethylformamide DNA – Deoxyribonucleic acid Fig – Figure FMN – Flavin mononucleotide FP – Filter paper Gly – Glycine GC – Gas chromatography HDS – Hydrodesulfurization (hidrodessulfurização). HPBS – 2-(2′-hydroxyphenyl) benzene sulfinate HPLC – High performance liquid chromatography Ile – Isoleucine IPTG – Isopropyl β-D-1thiogalactopyranoside Lys – Lysine LRP – Lamas da reciclagem de papel LB – Luria-Bertani broth µ – Specific growth rate MS – Mass spectroscopy Asn – Asparagine NADH – Nicotinamide adenine dinucleotide OD– Optical density ppm – Parts per million (partes por milhão) PCR – Polymerase chain reaction rbs – Ribossome biding site rpm – Revolutions per minute RPS – Recycled paper sludge Ser – Serine SD – Standard deviation SDS-PAGE – sodium dodecyl sulfate polyacrylamide gel electrophoresis SFM – sulfur-free mineral Thr – Threonine tds – Thermal desulfurization U – International units Val – Valine Trp – Tryptophan v/v – Volume/volume w/v – Weight/volume w/w – Weight/ weight.
(8)
(9) Resumo A crescente utilização de combustíveis fósseis levou a um aumento da emissão de óxidos de enxofre para a atmosfera, os quais são um dos principais causadores das chuvas ácidas. A legislação já aprovada prevê que em 2009 o nível máximo de enxofre nos combustíveis seja apenas 10 ppm, enquanto actualmente esse valor se situa nos 150 ppm. O processo de hidrodessulfurização (HDS) utilizado nas refinarias é baseado em técnicas físico-químicas muito dispendiosas, além de apresentar limitações na remoção do enxofre orgânico. Quanto mais estrita for a legislação sobre os níveis máximos de enxofre nos combustíveis fósseis, mais compostos recalcitrantes à HDS necessitam de ser removidos. Isto implica um aumento da intensidade do tratamento físico-químico e inerentemente dos seus custos. Como resultado, os compostos recalcitrantes à HDS representam uma barreira significativa para a obtenção de níveis de enxofre muito baixos nalgumas fracções petrolíferas. Diversas entidades governamentais e companhias petrolíferas já reconheceram a dificuldade de cumprir as regulamentações ambientais de uma maneira eficiente e económica usando a tecnologia convencional de HDS uma vez que as unidades de HDS para a dessulfurização profunda são extremamente dispendiosas de construir e operar. Por isso, é muito importante o estudo de novos processos de dessulfurização que possam de alguma maneira substituir ou complementar a HDS. A dessulfurização biológica poderá ser uma dessas tecnologias a implementar nos próximos anos pela indústria petrolífera. A alternativa ao tratamento físico-químico passa pelo recurso a processos biológicos (biodessulfurização) mais eficazes para a dessulfurização dos combustíveis fósseis, nomeadamente ao nível da remoção do enxofre ligado covalentemente a matrizes orgânicas. A biodessulfurização (BDS) ocorre em condições de funcionamento mais amenas sob condições de pressão atmosférica e temperatura ambiente, apresentando maior especificidade de reacção devido à natureza dos biocatalisadores, não requerendo. 7.
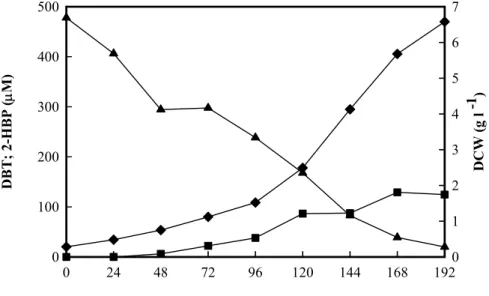
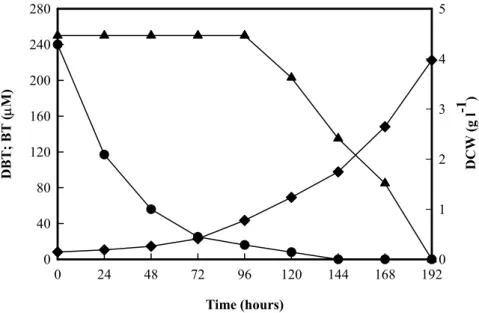
(10) hidrogénio molecular e permitindo a manutenção no processo da emissão de CO2 a um nível baixo. Por estes motivos, a remoção de enxofre por processos biocatalíticos é hoje em dia considerada como alternativa ou como complemento do processo de HDS convencional usado na indústria petrolífera. Deste modo, na última década e meia tem-se verificado um aumento dos estudos envolvendo a utilização de microrganismos com a capacidade de remover especificamente o enxofre deste tipo de compostos. A grande maioria dos trabalhos de BDS foi efectuada com estirpes de Rhodococcus erythropolis, especialmente a estirpe IGTS8 a qual se tornou a estirpe referência neste tipo de estudos. Assim, foi obtido um excelente conhecimento da BDS de dibenzotiofeno (DBT) e compostos análogos em termos de fisiologia bacteriana, enzimologia e biologia molecular. No entanto este conhecimento resumiu-se inicialmente ao género Rhodococcus e só mais recentemente se começou a estudar bactérias pertencentes a outros géneros. Neste trabalho foi isolada e seleccionada uma bactéria a partir de solos contaminados por hidrocarbonetos, que demonstrou uma boa capacidade de dessulfurização de DBT. Este composto é utilizado como modelo na maioria dos estudos de BDS. A bactéria seleccionada foi identificada como Gordonia alkanivorans estirpe 1B após estudos de caracterização de microbiologia clássica, bioquímica e biologia molecular. Embora esta espécie tenha sido descrita pela primeira vez em 1998, nenhum trabalho de BDS associado a esta tinha sido publicado. O crescimento desta bactéria, num meio de cultura com glucose como fonte de carbono e DBT como única fonte de enxofre, permite a dessulfurização deste composto formando-se 2-hidroxibifenilo (2-HBP) e libertando-se o enxofre na forma de sulfito. A taxa de dessulfurização específica obtida foi de 1,03 µmol g-1(biomassa) h-1 para uma taxa específica de crescimento de 0,019 h-1. Esta bactéria tem ainda a capacidade de utilizar outros compostos tiofénicos, tais como o benzotiofeno, tiofeno, 4-metil e 4,6-dimetildibenzotiofeno.. 8.
(11) Foi identificado e sequenciado o operão de G. alkanivorans estirpe 1B, responsável pela capacidade de dessulfurização de dibenzotiofeno, sendo constituído por três genes. As sequências nucleotídicas obtidas apresentam uma semelhança na ordem dos 85 a 90% em relação às sequências dos genes homólogos de Rhodococcus erythropolis IGTS8, permitindo concluir que G. alkanivorans estirpe 1B utiliza a via metabólica 4S. A principal vantagem desta via metabólica é não ocorrer diminuição do potencial energético do composto dessulfurizado, facto importante para a dessulfurização de combustíveis fósseis. Tendo em vista a utilização de fontes de carbono alternativas obtidas a partir de resíduos agro-industriais na formulação de meios de cultivo, foi estudado o efeito da presença ou ausência de alguns iões metálicos que compõem o meio de cultivo utilizado no crescimento laboratorial desta bactéria. Para os iões metálicos estudados, apenas a ausência de cobre e de zinco no meio de cultura diminuiu a quantidade de 2-HBP produzida. No entanto, a ausência de zinco reduziu a biomassa produzida, indicando que este ião pode ter uma importância relevante para o metabolismo de G. alkanivorans estirpe 1B. Crescimentos da estirpe 1B, em meios de cultura contendo sulfato ou DBT como fonte de enxofre, permitiram verificar que o ião zinco apenas estimula o metabolismo bacteriano na presença de DBT. Isto sugere que o ião zinco tem um papel importante no sistema enzimático envolvido na dessulfurização. Estes resultados foram confirmados após estudos envolvendo células de G. alkanivorans estirpe 1B pré-crescidas na presença ou ausência de zinco. De facto, no ensaio com células pré-crescidas com zinco, foi possível obter uma produtividade específica de 2-HBP de 2,29 µmol g-1(biomassa) h-1, valor este 7,6 vezes superior ao obtido no ensaio com células pré-crescidas na ausência de zinco. A produtividade obtida na ausência de zinco sofreu um incremento de 70% quando as células pré-crescidas foram incubadas com 1 mg l-1 de zinco. Estes resultados permitiram verificar a necessidade de aumentar a quantidade de zinco inicialmente utilizado no meio de cultura (0,5 mg l-1) para 10 mg l-1 de modo a maximizar a taxa de dessulfurização de DBT.. 9.
(12) A aplicação industrial da BDS depende do custo inerente à produção de biocatalisadores, o qual ainda é muito elevado para permitir que este processo seja viável em termos económicos. Assim, o estudo de fontes de carbono alternativas resultantes da hidrólise de materiais agro-industriais pode ser uma das estratégias para reduzir esses custos. Neste trabalho foram utilizadas lamas da reciclagem de papel (LRP) uma vez que é um material rico em celulose (35%). Os polissacáridos das LRP foram hidrolisados com uma mistura enzimática, a qual foi previamente dialisada para remoção de compostos contendo enxofre. O hidrolisado obtido continha, respectivamente, 36,3 e 53,9 g l-1 de glucose e açúcares totais, com um rendimento de hidrólise de 72%. O hidrolisado de LRP foi inicialmente utilizado como fonte de carbono (10 g l-1 glucose), resultando um elevado crescimento bacteriano (DO600 nm = 10 após 4 dias de cultura) e no caso do hidrolisado obtido com a mistura enzimática dialisada obteve-se uma produtividade específica de 2-HBP de 1,1 µmol g-1(biomassa) h-1. Nestas condições G. alkanivorans estirpe 1B apresentou uma taxa de dessulfurização um pouco superior à obtida após crescimento em glucose comercial. Este hidrolisado foi ainda testado como fonte de outros nutrientes para além de fonte de carbono. Obteve-se um bom crescimento bacteriano (DO600 nm = 9 após 5 dias de cultura) e dessulfurização de DBT quando o hidrolisado de LRP foi suplementado apenas com fosfatos e amónia. No entanto, a melhor suplementação foi a que incluiu adicionalmente magnésio e 10 mg l-1 de zinco, onde a taxa específica de crescimento foi de 0,03 h-1. Apesar de neste trabalho não ter sido estudada a dessulfurização de nenhum combustível fóssil, procedeu-se no entanto a um estudo de dessulfurização de um combustível modelo. Este modelo era constituído por DBT, 4-metilDBT e 4,6-dimetilDBT dissolvidos em n-heptano. G. alkanivorans estirpe 1B, utilizando o hidrolisado de LRP como fonte de nutrientes, diminuiu o enxofre total presente neste combustível de 6 mM para 2,23 mM após 7 dias de cultivo, para uma taxa específica de crescimento de 0,062 h-1. Esta estirpe dessulfuriza preferencialmente DBT, seguido de 4-metilDBT e por fim 4,6-dimetilDBT,. 10.
(13) embora a dessulfurização dos 3 compostos ocorra simultaneamente. A taxa específica máxima de dessulfurização obtida foi de 22,2 µmol g-1(biomassa) h-1, valor semelhante aos descritos para algumas estirpes selvagens de R. erythropolis e Gordonia sp. Estes resultados permitiram mostrar que o hidrolisado de LRP pode ser utilizado como fonte de nutrientes para a dessulfurização de DBT e seus derivados por G. alkanivorans estirpe 1B, apresentando uma eficiência semelhante à obtida no meio de cultura convencional com glucose comercial. A utilização de LPR apresenta um duplo benefício: as LRP podem ser usadas como fonte de nutrientes num processo biotecnológico contribuíndo para a resolução do problema ambiental deste resíduo. Em conclusão, os resultados obtidos na melhoria da actividade de dessulfurização por G. alkanivorans estirpe 1B e a utilização de um meio de cultura menos dispendioso revelaram-se promisores para uma futura aplicação biotecnológica desta bactéria.. Palavras-Chave: Biodesulfurização, Combustíveis fósseis, Dibenzotiofeno, Gordonia alkanivorans, Fontes de carbono alternativas.. 11.
(14)
(15) Abstract The decreased availability of low sulfur crude oils has resulted in a need to refine heavier, higher sulfur crude. The combustion of this crude produces sulfur oxides that contribute to air pollution and, consequently, many countries have changed their legislation to obtain a progressive reduction in sulfur content of fossil fuels. The microbiological process, biodesulfurization, offers the potential for an alternative/complementary method for lowering the sulfur content of petroleum products. A mesophilic bacterium identified as Gordonia alkanivorans strain 1B was isolated from soil samples contaminated with hydrocarbons. This strain has the ability to desulfurize dibenzothiophene (DBT) and also other sulfur compounds, namely, benzothiophene, DBT sulfone, 4-methylDBT and 4,6-dimethylDBT. The identification, sequencing and characterization of desulfurization genes of strain 1B allowed to confirm that the desulfurization of DBT occurs through the specific sulfur pathway 4S. In order to use cheaper alternative nutrient sources to decrease the costs associated to the production of biocatalysts, the culture medium requirements were studied, mainly the metal ion composition. It was found that zinc ion may have an important role for one or more desulfurization enzymes. The increase of zinc concentration from 0.5 to 10 mg l-1 allowed an improvement of DBT desulfurization of about 26%. Polysaccharides of recycled paper sludge were hydrolyzed with an enzyme formulation and the hydrolyzate obtained was used for nutrient source supplementation of culture medium to grow Gordonia alkanivorans strain 1B. Under these conditions the bacterium was able to desulfurize a model oil containing DBT, 4-methylDBT and 4,6-dimethylDBT, with a reduction on total sulfur from 6 to 2.23 mM. The maximum specific desulfurization rate of strain 1B in model oil was 22.2 µmol g-1(DCW) h-1 very similar to some reported wild type Rhodococcus erythropolis and Gordonia sp. strains. 13.
(16) The improvement of desulfurization activity in addition to the use of a less expensive culture medium is an important achievement for a future application of this bacterium. Keywords: Biodesulfurization, Fossil fuels, Dibenzothiophene, Gordonia alkanivorans, Alternative carbon sources.. 14.
(17) Contents.
(18)
(19) CONTENTS. Chapter 1 General introduction …………………………………………………………. 21 [Partially in press – Enzimas em Biotecnologia – Produção, Aplicações e Indústria (Chapter 18)] Fossil fuels desulfurization…………………………………………………………..21 Sulfur in fossil fuels……………………………………..……………………..21 The sulfur problematics……….…..……………………………………………22 Regulations………………….………………..………………………………...24 Hydrodesulfurization…….……...………………………………………………24 Biodesulfurization………………………………………………………………27 Desulfurizing microorganisms……..………..………………………………….28 Model compounds biodesulfurization……………………………………………….29 Benzothiophene biodesulfurization…………………………………………….30 Dibenzothiophene biodesulfurization…………………………………………..31 Dibenzothiophene metabolic pathways……....……………………………32 4S pathway enzymatics…………………………………………………….38 4S pathway genetics………………………………………………………..41 Biodesulfurization application……………………………………………………….45 Oil biodesulfurization…………………………………………………………...47 Coal biodesulfurization………………………………………………………….49 Bottlenecks for biodesulfurization application…………………………………...50 Scope of the thesis…………………………………………………………………...51. Chapter 2 Desulfurization of dibenzothiophene, benzothiophene and ……..…..……………71 other thiophene analogues by a newly isolated bacterium Gordonia alkanivorans strain 1B [Paper published – Appl. Biochem. Biotechnol. 2005, 120: 199-208] 17.
(20) CONTENTS. Chapter 3 Sequencing, cloning and expression of the dsz genes required……………….…… 89 for dibenzothiophene sulfone desulfurization from Gordonia alkanivorans strain 1B [Paper published – Enzyme Microb. Technol. 2007, 40: 1598-1603]. Chapter 4 Effect of zinc and other metal ions on the performance ……….…………………110 of dibenzothiophene desulfurization by Gordonia alkanivorans strain 1B [Paper submitted –J. Ind. Microbiol. Biotechnol.]. Chapter 5 Dibenzothiophene desulfurization by Gordonia alkanivorans ………...…...……126 strain 1B using recycled paper sludge hydrolyzate [Paper submitted – Chemosphere]. Chapter. 6. Global analysis and conclusions ………...….…………………….………...…149. 18.
(21) Chapter. 1.
(22)
(23) GENERAL INTRODUCTION. FOSSIL FUELS DESULFURIZATION SULFUR IN FOSSIL FUELS After food, fossil fuel is humanity’s most important source of energy and many of the benefits we enjoy from our way of life are due to fossil fuel use. Indeed, about 85% of our energy comes from fossil fuels, another 8% comes from nuclear power and 7% from all other sources, mostly hydroelectric power and wood. There are three major fuels - coal, oil, and natural gas. Oil leads with a share of about 40% of the total world consumption, followed by coal (24%) and natural gas (22%). Almost all fossil fuels are used by burning, which causes pollution producing waste products due to impurities in the fuel, especially particulates and various gases such as sulfur dioxide, nitrogen oxides and volatile organic compounds (Gupta et al., 2005). Organic compounds containing sulfur (S) constitute an important fraction of fossil fuels and due to their low biodegradability are considered recalcitrant compounds. In crude oil the contaminant S is present in inorganic form, e.g. hydrogen sulfide and elemental sulfur (Kropp & Fedorak, 1998), although the organic forms are predominant with over 200 sulfur containing organic compounds (Lu et al., 1999). These compounds can be divided in three groups: (I) aliphatic and aromatic thiols, and its oxidation products (bisulfides); (II) aliphatic and aromatic thioethers; (III) heterocycles based in thiophenic ring: thiophene, benzothiophene (BT), dibenzothiophene (DBT) and its alkyl derivatives (Kropp & Fedorak, 1998). Petroleum recovered from different reservoirs varies widely in compositional and physical properties (Van Hamme et al., 2003) containing between 0.04 and 5% (w/w) sulfur, and in general, crude oils of higher density contain a higher percent of sulfur (Kropp et al., 1997). The organosulfur compounds in petroleum include thiols, thioethers, and thiophenes, but the. 21.
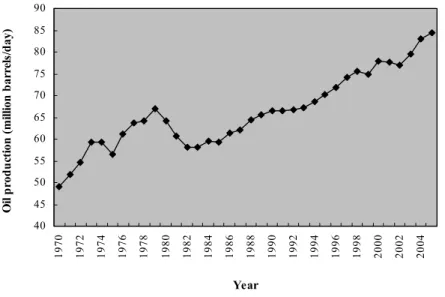
(24) GENERAL INTRODUCTION. sulfur compounds that predominate in the so-called heavy fractions, where sulfur content is the highest, are primarily the condensed thiophenes (Lu et al., 1999). In some cases the sulfur content is very high, e.g., the crude oil in California and Utah at the USA and in Germany contain 5.5, 13.9 and 9.6% of sulfur, respectively (Kropp & Fedorak, 1998). Sulfur in gasoline is mainly found in thiophenic and non-thiophenic compounds, and in diesel oil is found in benzo and dibenzothiophenes (Oldfield et al., 1998). Total sulfur content varies among ranks of coal from 0.3% to 6%; on average, organic and inorganic sulfur comprise equal amounts (Wang & Krawiec, 1994), although there are some exceptions (Fairbairn & Bushell, 1992). The inorganic sulfur in coal is, predominantly, in the form of pyrite while organic sulfur is present in several forms; the principal moieties are thiols, sulfides, disulfides, and thiophenes (Wang & Krawiek, 1994).. THE SULFUR PROBLEMATICS The growth of industrial civilization, in particular the use of fossil fuels for energy, has led to the environmental pollution with a range of compounds of non-biological origin. This concern will become crucial at least partially owing to the decreasing availability of lowsulfur fuels (Konishi et al., 1997). In 1990 about 65 million barrels of oil were produced each day, with an average sulfur content of about 1.1% (Monticello, 1998). Since then the oil production has risen (Fig. 1) and in 2005 about 85 million barrels were produced each day (Energy Information Administration’s primary report of recent international petroleum statistics, USA). There is no indication that this trend will slow down any time in the near future. Recent estimates of the worldwide reserves of fossil fuels indicate that the proven reserves of natural gas, crude oil and coal are sufficient to continue at this rate for at least the next 70 years (Kerr, 2000).. 22.
(25) GENERAL INTRODUCTION. Oil production (million barrels/day). 90 85 80 75 70 65 60 55 50 45 2004. 2002. 2000. 1998. 1996. 1994. 1992. 1990. 1988. 1986. 1984. 1982. 1980. 1978. 1976. 1974. 1972. 1970. 40. Year. Fig. 1. World oil production during the last 25 years. Data were obtained from the Energy Information Administration’s primary report of recent international petroleum statistics (USA).. Most of the hydrocarbons mined from the Earth are burned for energy and since most liquid and solid (i.e., oil and coal) reserves are contaminated with sulfur, direct combustion of this fuel will lead to the release of vast amounts of sulfur oxides into the atmosphere (Monticello, 2000). These oxides (together with acidic nitrogen oxides) are responsible for poor air-quality, acid rain (Oldfield et al., 1998) and for ozone layer depletion (Malik et al., 2001b). In addition, SO2 is responsible for various health hazards, such as respiratory tract cancer and cardio-respiratory diseases (Malik et al., 2001b). Concentrations of SO2 higher than 100 ppm in the atmosphere are harmful to the respiratory system of humans and a short-period exposure to 400-500 ppm is lethal (Schmidt et al., 1973). The combination of SO2 with dust in the atmosphere or with fog increases the noxious effect. The plant kingdom is also very sensitive to the SO2 concentration; exposure to 1-2 ppm SO2 provokes damages in few hours (Schmidt et al., 1973).. 23.
(26) GENERAL INTRODUCTION. In oil spill accidents, some sulfur heterocyclic compounds are introduced in the environment. Some of these compounds (e.g. benzothiophene and its derivatives) present mutagenic and carcinogenic activities and acute toxicity to the organisms living in that ecosystem. Condensed thiophenes are bioacumulated in organism tissues, which associated with their mutagenic, carcinogenic and toxic potential, significantly contribute to the negative impact in the oil spills (Kropp & Fedorak, 1998).. REGULATIONS During the last decade a drastic reduction in the allowed sulfur concentration in transportation fuels was observed. In Portugal this sulfur reduction has also been observed. Considering the values for diesel, in 1995 the maximum concentration of sulfur allowed was 2000 ppm, decreasing to 500 ppm in 1996 (Portaria nº 949/94 25th October). In 2001 this value was set at 350 ppm (Decreto-Lei nº 104/2000 3rd June) and nowadays is 50 ppm (Decreto-Lei nº 235/2004 16th December). Considering the values for gasoline, in 1995 the maximum concentration of sulfur allowed was 1000 ppm for leaded gasoline and 500 ppm for unleaded gasoline (Portaria nº 1489/95 29th December). In 2002 this value was set at 150 ppm (Decreto-Lei nº 104/2000 3rd June) and nowadays is 50 ppm (Decreto-Lei nº 235/2004 16th December). In the near future, several countries along the world only allow the use of transportation fuels with the maximum concentration of sulfur of 15 ppm and with a target value of 10 ppm in 2010 (Swaty, 2005; Rashtchi et al., 2006). In Portugal this value was regulated to be 10 ppm in January 2009 (Decreto-Lei nº 235/2004 16th December).. HYDRODESULFURIZATION The environmental restrictions in the industrialized countries require the use of fossil fuels with low sulfur content. However, the supply of low sulfur crude oils is being exhausted and 24.
(27) GENERAL INTRODUCTION. consequently they rule a higher price on the market than higher sulfur crudes (Gray et al., 1996). Thus, one of the strategies to reduce the emission levels to the atmosphere is to remove the sulfur from fossil fuels before their combustion. Refineries remove organic sulfur from crude oil-derived fuels by hydrodesulfurization (HDS). HDS is a catalytic process that converts organic sulfur to hydrogen sulfide gas by reacting crude oil fractions with hydrogen at pressures between 150 and 3,000 psi and temperatures between 290 and 455 °C in the presence of metal catalysts (Grossman et al., 1999). The metal catalysts mainly used are CoMo/Al2O3 and sometimes NiMo/Al2O3. At these conditions, sulfur concentration can be reduced from 1-5% to 0.1% (Izumi et al., 1994). Although HDS can easily remove the inorganic sulfur or simple organic sulfur compounds, it is not effective for removing complex polycyclic sulfur compounds (Ohshiro & Izumi, 1999; del Olmo et al., 2005). Molecules such as 4,6-dimethyldibenzothiophene (4,6dmDBT), with alkyl groups adjacent to the sulfur atom, are often used as model molecules in deep HDS studies because they are very difficult to desulfurize and create problems in deep HDS (Niquille-Röthlisberger & Prins, 2006). Indeed, it was shown that DBT and its alkylated derivatives still remain in HDS treated gas oil (Onaka et al., 2001a). The imposition of increasingly stringent restrictions on the levels of sulfur allowed in transportation fuels implies that more recalcitrant compounds to the HDS process must be removed. To achieve this goal it is necessary to increase the intensity of physical-chemical treatment and inherently its costs (Grossman et al., 2001). Deep hydrodesulfurization technology must be implemented to attain this low level of sulfur (Prins et al., 2006). This technology involves much higher pressures, temperatures and residence times (Reichmuth et al., 2000), requiring the use of sophisticated and expensive catalysts (Klein et al., 1999). In these conditions, the process to remove the organic sulfur is too expensive (McFarland, 1999). In addition, increasing the severity of HDS also elicits undesirable effects on fuel quality, as other chemical components are reduced at the higher temperatures and pressures. 25.
(28) GENERAL INTRODUCTION. needed to achieve low sulfur levels (Folson et al., 1999), thereby reducing the octane number of the fuel (Reichmuth et al., 2000). In general, the distribution of sulfur in crude oil is such that the proportion of sulfur increases along with the boiling point of the distillate fraction. As a result, the higher the boiling range of the fuel the higher the sulfur content will tend to be. For example, a middledistillate-range fraction, e.g. diesel fuel, will typically have higher sulfur content than the lower-boiling-range gasoline fraction (Grossman et al., 1999). Organic sulfur compounds in the lower-boiling fractions of petroleum, e.g. the gasoline range, are mainly thiols, sulfides, and thiophenes, which are readily removed by HDS. However, middle-distillate fractions, e.g. the diesel and fuel oil range, contain significant amounts of benzothiophenes and dibenzothiophenes, which are considerably more difficult to remove by HDS (Grossman et al., 2001). Moreover, 4- and 6-substituents in DBT extremely retard the desulfurization reaction because of its steric hindrance. Probably, these substituents located near sulfur in DBT will inhibit the adsorption of this atom to the catalyst surface (Kabe et al., 1992). Due to their resistance to HDS, these compounds represent a significant barrier to reaching very low sulfur levels in middle- and heavydistillate-range fuels (Grossman et al., 2001). The governments and the petroleum refining companies have recognized that it is difficult to meet the environmental regulations in a cost-effective way by the conventional hydrodesulfurization technology because hydrodesulfurization units for the high extent of desulfurization are extremely expensive to build and operate (Ohshiro & Izumi, 1999). In fact, attempts are underway worldover to bring down the severity of refining operations through the development of milder physical and chemical processes (Bhatia & Sharma, 2006). For these reasons, it is of great importance to study new desulfurization processes that can substitute or complement HDS. Biological desulfurization might be one of those technologies to be implemented by oil industry.. 26.
(29) GENERAL INTRODUCTION. BIODESULFURIZATION Biodesulfurization (BDS) constitutes one possibility to be implemented instead of HDS process, due to the specificity of microorganisms to eliminate sulfur from HDS recalcitrant compounds (del Olmo et al., 2005). Biocatalytic processes are noted for their mild operating conditions, greater reaction specificity afforded by the nature of biocatalysis (Kaufman et al., 1998) and for not requiring molecular hydrogen (Reichmuth et al., 2000), allowing the maintenance of CO2 emissions at a low level (Ishii et al., 2000b). For these reasons, the sulfur removal by biocatalytic processes is considered an alternative or a complementary step for the conventional HDS process used in the refining industry of fossil fuels. The first biodesulfurization studies were reported in the 50s and 60s, but without significant results. Only in the last 15 years BDS studies presented a development that allows considering a future application of microorganisms to desulfurize fossil fuels. Biological desulfurization of petroleum may occur either oxidatively or reductively. In the oxidative approach, organic sulfur is converted to sulfate, and this may be removed in process water. This route is attractive because it does not require further processing of the sulfur. In the reductive desulfurization scheme, organic sulfur is converted into hydrogen sulfide, which may then be catalytically converted into elemental sulfur; this is also an approach of utility at the refinery (Kaufman et al., 1998). Oil and coal are complex substrates and the fact that a microorganism can metabolize DBT in laboratorial conditions does not necessarily imply that it can remove the organic sulfur from the fossil fuels. It is necessary to take into account the problem of microbial sulfur compounds accessibility in addition to the existence of steric hindrance associated to the structure of these compounds that difficult the activity of microbial enzymatic systems. A fundamental aspect to the BDS application is the possibility of keeping intact the carbon structure of the fossil fuel. Thus, the selected microorganisms must have the capability to use DBT as sulfur source but not as carbon source.. 27.
(30) GENERAL INTRODUCTION. DESULFURIZING MICROORGANISMS Several procaryotes presenting the ability to utilize sulfur from poliaromatic hydrocarbon compounds have been described: Acinetobacter sp. (Malik, 1978), Agrobacterium sp. (Constanti et al., 1996), Arthrobacter sp. (Dahlberg et al., 1993; Lee et al., 1995), Bacillus sp. (Kirimura et al., 2001), Beijerinckia sp. (Laborde & Gibson, 1977), Brevibacterium sp. (Van Afferden et al., 1990), Corynebacterium sp. (Omori et al., 1992), Desulfomicrobium sp. (Onadera-Yamada et al., 2001), Desulfovibrio sp. (Kim et al., 1990; Yeong et al., 1990; Sohrabi et al., 2006), Gordonia sp. (Rhee et al., 1998; Santos et al., 2006; Jia et al. 2006), Klebsiella sp. (Dudley & Frost, 1994), Mycobacterium sp. (Furuya et al., 2001; Takada et al., 2005; Li et al., 2007), Nocardia sp. (Wang & Krawiec, 1994), Paenibacillus sp. (Konishi et al., 1997), Pseudomonas sp. (Setti et al., 1992; De Fatima et al., 1996; Luo et al., 2003), Rhodococcus sp. (Denome et al., 1993a; Izumi et al., 1994; Ohshiro et al., 1994: Lee et al., 1995; Matsui et al., 2002), Rhizobium sp. (Malik, 1978; Frassinetti et al., 1998), Sinorhizobium sp. (Tanaka et al., 2001), Sphingomonas sp. (Darzins & Mrachko, 2000; Gai et al., 2007), Sulfolobus sp. (Kargi, 1987; Ju & Kankipati, 1998), Xanthomonas sp. (Constanti et al., 1996). The majority of the BDS studies involve aerobic microorganisms despite some anaerobic bacteria have also been described. However, the low rate and extent of petroleum desulfurization by currently available anaerobic cultures and the lack of knowledge on the biochemistry and genetics of such microorganisms makes the development of a commercial anaerobic process unlikely (Kilbane, 2006). The genus Gordonia The first report describing this genus in biodesulfurization studies was the work involving Gordona sp. strain 213E (Gilbert et al., 1998). The genus Gordona was proposed by Tsukamura (1971), but the three original species of the genus were subsequently reclassified in the genus Rhodococcus (Goodfellow & Alderson, 1977). However, Rhodococcus species could be divided into these two aggregate groups by serological and chemotaxonomic. 28.
(31) GENERAL INTRODUCTION. properties, such as mycolic acid composition and menaquinone profiles. Stackebrandt et al. (1988) found that the two aggregate groups are phylogenetically distinct on the basis of 16S rRNA sequences and revived the genus Gordona. Nowadays, the name Gordonia, instead of Gordona, is being recognized because it is etymologically correct (Stackebrandt et al., 1997). The members of the genus Gordonia are widely distributed in nature (Takeuchi & Hatano, 1988). Other Gordonia species have also been isolated from activated sludge in aeration tanks of biological sewage-treatment plants (Lemmer & Kroppenstedt, 1984) and from the packing material of a biofilter used for biological odor abatement (Bendinger et al., 1995; Klatte et al., 1996). Nevertheless, little is known about Gordonia species or strains that degrade toxic aromatic compounds, unlike the genus Rhodococcus, a phylogenetic neighbor which is a very important taxon from the point of view of bioremediation (Finnerty, 1992).. MODEL COMPOUNDS BIODESULFURIZATION Crude oil can contain 50% of organic sulfur in the form of condensed thiophenes (Frassinetti et al., 1998) that after refining at high temperature lead to DBT concentrations higher than 70% of total sulfur (Kropp & fedorak, 1998). The straight-run middle-distillate feed stock contains 95% of the organosulfur compounds as thiophenic compounds, that include thiophene, BT and DBT. Each of these basic aromatic structures can have a variety of alkyl substituents, thereby increasing the number of unique compounds (Folsom et al., 1999). Furthermore, these compounds are recalcitrant and persisting in biosphere, which are released in the environment through industrial processes, including gasification and liquefaction of coal and refining of crude oil, and through oil spilling accidents (Berthou & Vignier, 1986). Alkyl-substituted DBTs seem to be the most difficult to remove among all organosulfur compounds, with certain isomers surviving even to deep HDS treatment (Amorelli et al., 29.
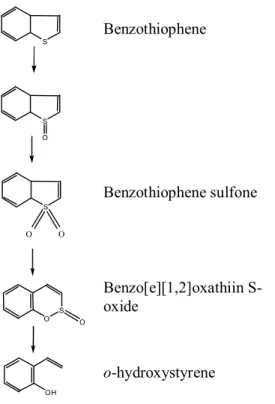
(32) GENERAL INTRODUCTION. 1992). DBT can be regarded as a model for the main aromatic organosulfur nucleus of the coal matrix (Oldfield et al., 1998). In this context, for more than 4 decades, dibenzothiophene has been the model compound for the biodegradation or biodesulfurization studies of sulfur heterocycles (Bressler & Fedorak, 2001). In the majority of the reported works on biodesulfurization, DBT and other analogous compounds with alkyl groups are utilized as carbon and energy source or as sulfur source (Monticello et al., 1985). Another advantage to use this compound as model is the fact that it does not present any mutagenic potential (Kropp & Fedorak, 1998). Although DBT is the preferential model compound, some reports refer benzothiophene in BDS studies (Matsui et al., 2001). The importance of BT as model compound is relevant due to the fact that it presents a different metabolic pathway.. BENZOTHIOPHENE BIODESULFURIZATION Studies on the carbon-sulfur bond-targeted type of BT desulfurization have only been performed in some bacteria: Desulfovibrio desulfuricans (Setti et al., 1993), Gordonia sp. 213E (Gilbert et al. 1998), Paenibacillus sp. A11-2 (Konishi et al. 2000a), Rhodococcus sp. T09 (Matsui et al. 2000) and Sinorhizobium sp. KT55 (Tanaka et al. 2001). The pathway for BT desulfurization by Paenibacillus sp. A11-2 (Konishi et al., 2000a) and Sinorhizobium sp. KT55 (Tanaka et al., 2001) is BT → BT sulfoxide → BT sulfone → benzo[e][1,2]oxathiin S-oxide → o-hydroxystyrene (Fig. 2). BT suffers a double oxidation of the sulfur atom forming the corresponding sulfoxide and sulfone. Conversion of BT S,Sdioxide into benzo[e][1,2]oxathiin S-oxide can be explained by oxidative cleavage of one of the two C-S bonds in BT S,S-dioxide and circularization of the cleavage product under acidic conditions. In the final step, benzo[e][1,2]oxathiin S-oxide seems to lose its sulfur by the desulfination reaction (Konishi et al., 2000a).. 30.
(33) GENERAL INTRODUCTION. Benzothiophene S. S O. Benzothiophene sulfone S. O. O. Benzo[e][1,2]oxathiin Soxide (F. S O. O. o-hydroxystyrene OH. Fig. 2. The postulated pathway of benzothiophene desulfurization by Paenibacillus sp. strain A11-2 (adapted from Konishi et al., 2000a).. DIBENZOTHIOPHENE BIODESULFURIZATION Despite the obvious chemical similarity between DBT and BT, the two desulfurization pathways are mutually exclusive. Thus BT cannot be desulfurized via the DBT-specific pathway and DBT cannot be desulfurized through the BT-specific pathway (Gilbert et al. 1998). Indeed, cells of R. erythropolis IGTS8 pre-grown in BT cannot desulfurize DBT. This suggests that the enzymatic system of BT desulfurization could be different from DBT desulfurization system. However, it was reported that the first step of BT desulfurization (sulfur oxidation) could be catalyzed by the same monooxygenase involved in DBT desulfurization (Kobayashi et al., 2000). Therefore, the organisms having these metabolic pathways are complementary in terms of their potential roles in development of a microbial fuel desulfurization technology (Oldfield et al., 1998).. 31.
(34) GENERAL INTRODUCTION. It has been reported that DBT is aerobically or anaerobically metabolized by microorganisms. Desulfovibrio desulfuricans M6, a sulfate-reducing bacterium, degraded DBT anaerobically, and biphenyl was isolated as the major degradation product (Kim et al., 1990). An anaerobic process for sulfur removal will be attractive because it does not liberate sulfate as a by-product that must be disposed by some appropriate treatment. However, anaerobic microorganisms effective enough for the practical petroleum desulfurization have not been found yet (Ohshiro & Izumi, 1999). There are many reports concerning aerobic DBT metabolism. DBT desulfurization occurs inside the cell with its entrance in the cytoplasm possibly from the organic phase, after transient adsorption (Monticello, 1998). However, Gallardo et al. (1997) reported that DBT is present in the aqueous phase before its entrance into the cell. The solubility of DBT in water is very low, about 0.005 mM, although it can be increased with the surfactants produced by the cells. Increased rates of DBT desulfurization in higher hydrocarbon fractions were reported, and this might suggest transfer of DBT through the interface between the aqueous and hydrocarbon phase or adsorption of cells at the interface (Maghsoudi et al., 2001). On the other hand, the presence of a significant amount of bacterial cells in the organic phase and in the water/organic interphase in 1-10 µl droplets during desulfurization of DBT in high hexadecane concentrations was reported (Kaufman et al., 1998), presumably due to biosurfactant production, which will have impact on separations in a commercial process (McFarland, 1999).. Dibenzothiophene metabolic pathways To current knowledge, DBT metabolism by aerobic microorganisms can be divided in 3 different pathways: Kodama pathway (Kodama et al., 1973), Van Afferden pathway (Van Afferden et al., 1990) and 4S pathway (Denome et al., 1993a).. 32.
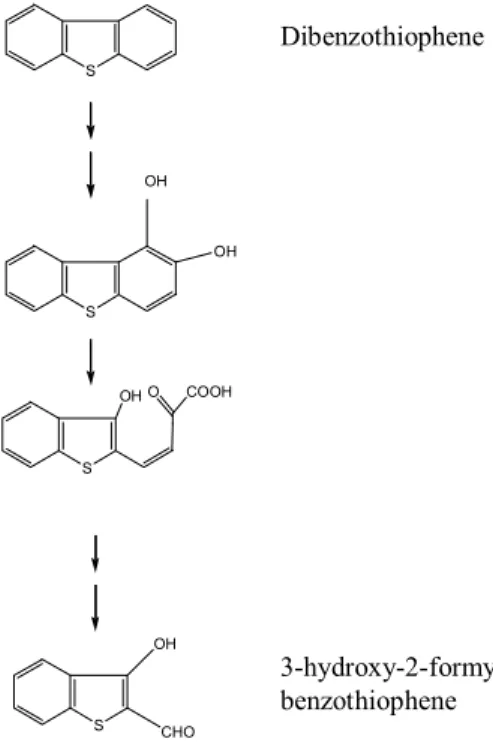
(35) GENERAL INTRODUCTION. Kodama pathway In 1973, Kodama and co-workers reported that DBT was partially degraded by strains of Pseudomonas sp. through some oxidations by a mechanism similar to the naphtalene degradation (Fig. 3). The dihydroxylation of a DBT aromatic ring causes the destruction of that ring, obtaining 3-hydroxy-2-formyl-benzothiophene as final product, in which the thiophenic nucleus containing the sulfur atom persists (Mormile & Atlas, 1988). This final product presents biological toxicity levels similar to the initial substrate (Gallagher et al., 1993).. Dibenzothiophene S. OH. OH. S. OH O. COOH. S. OH. S. 3-hydroxy-2-formylbenzothiophene. CHO. Fig. 3. Kodama pathway – Dibenzothiophene is partially degraded by attack of a specific aromatic dioxygenase. The product, 3-hydroxy 2-formyl benzothiophene, which retains the sulfur moiety, is not further degraded (adapted from Kodama et al., 1973).. 33.
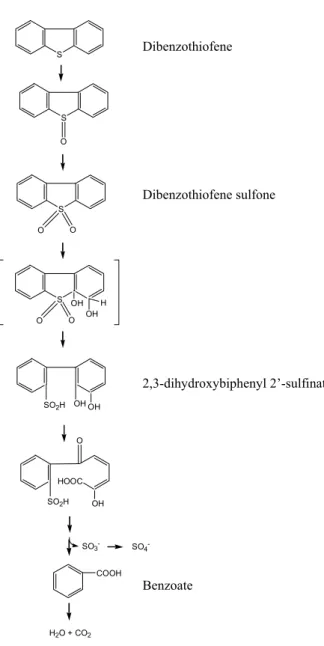
(36) GENERAL INTRODUCTION. More. recently,. 3-hydroxy-2-formyl-benzothiophene. was. totally. biodegraded. and. mineralized by a mixed bacterial culture (Bressler & Fedorak, 2001). This is the most widely used pathway by bacteria that can metabolize DBT (Kilbane & Jackowsky, 1992). Since the DBT analogous compounds present in fossil fuels contain alkyl and aryl groups in those positions, this metabolic pathway cannot degrade them. Thus, this is a metabolic pathway without practical interest to biodesulfurization. Van Afferden pathway In 1990, Van Afferden et al. described a different metabolic pathway by Brevibacterium sp., in which DBT is converted, in stoichiometric quantities, to benzoate and sulfite (Fig. 4). DBT is initially oxidized to DBT sulfone (DBTS), followed by the opening of the thiophenic ring initiated by aromatic dioxygenase action, to yield 2,3-dihydroxybiphenyl 2’sulfinate. A second round of dioxygenase attack opens the 2,3-dihydroxybenzene nucleus. Benzoate is mineralized to CO2 and H2O, remaining in the final only 9% of the carbon from DBT as dissolved organic carbon in the medium (Van Afferden et al., 1990). DBT is used as nutrient either as carbon and sulfur sources. This DBT degradation pathway has only a partial interest in terms of BDS of fossil fuels, due to the complete mineralization of the carbon structure of the hydrocarbon. This necessarily provokes a decrease of the potential chemical energy of the fossil fuels. Unfortunately, this pathway has not been studied in more detail and there is no further information concerning its enzymology or genetics. Although its less importance in terms of BDS, the bacteria using this metabolic pathway are potentially useful in the formulation of mixed microbial inocula for poliaromatic hydrocarbon bioremediation processes.. 34.
(37) GENERAL INTRODUCTION. Dibenzothiofene. S. S O. Dibenzothiofene sulfone S O. O. S OH. H OH. O. O. 2,3-dihydroxybiphenyl 2’-sulfinate SO2H. OH OH. O. HOOC SO2H. OH. SO3COOH. SO4-. Benzoate. H2O + CO2. Fig. 4. Van Afferden Pathway – Following oxidation of dibenzothiophene to dibenzothiophene sulfone, thiophene ring-opening is triggered by the action of an angular dioxygenase. The product of this reaction is 2,3-dihydroxybiphenyl 2’sulfinate. In a second round of dioxygenase attack, the catechol ring is opened and the product degraded to benzoate. Ultimately, benzoate is mineralized to CO2 and water (adapted from Van Afferden et al., 1993).. 35.
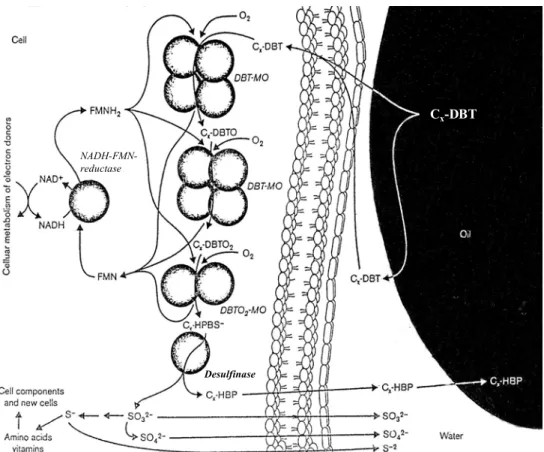
(38) GENERAL INTRODUCTION. 4S pathway The third reported DBT desulfurization pathway is the usually denominated 4S pathway, due to the formation of 4 sulfur compounds during the metabolic pathway (sulfoxidesulfone-sulfonate-sulfate) (Kilbane, 1989; Kilbane & Jackowski, 1992; Omori et al., 1992). This pathway is specific for the removal of the sulfur atom present in DBT, in which the thiophenic group is progressively oxidized without degradation of the carbon structure. The enzymatic system involved in the desulfurization of DBT was reported for the first time by Ohshiro et al. (1994). 4S pathway is a multienzymatic system with four different activities (Gray et al., 1996) as depicted in Fig. 5. DBT desulfurization is an energetically expensive process, since it has been estimated to require 4 mol NADH per mol of DBT desulfurized (Oldfield et al., 1997). The molecular oxygen is also an important factor to the activity of the first two enzymes of this pathway. Ohshiro et al. (1995) reported that DBT degradation was repressed in a reaction mixture with high concentration of cells, in which there is oxygen limitations. The final product (2-hydroxybiphenyl; 2-HBP) concentration is the main limiting factor to the DBT biodesulfurization (Omori et al., 1992). 2-HBP is the active ingredient in the disinfectant Lysol and, perhaps not surprisingly, was found to be toxic to Arthrobacter sp. strain ECRD-1 at a concentration of 50 mg l-1 when applied to freshly inoculated cultures (Lee et al., 1995). 2-HBP presents a strong inhibitory effect on the cells in the process of DBT desulfurization, due to the retroactive inhibition of the desulfurization enzymes (Nekodzuka et al., 1997). This inhibition was reported in a study using a Gordonia sp. strain CYKS1 in which the presence of 0.2 mM 2-HBP in the culture medium increased by 50% the cell duplication time (Rhee et al., 1998). Even a small concentration of 2-HBP decreased about 20% the desulfurization and cell growth rates (Kim et al., 2004).. 36.
(39) GENERAL INTRODUCTION. Cx-DBT NADH-FMNreductase. Desulfinase. Fig. 5. A conceptual diagram of some of the steps in the desulfurization of oil by bacterial cells. DBT is converted to 2-HBP due to the activity of four enzymes. DBTO – dibenzothiophene sulfoxide; DBTO2 – dibenzothiophene sulfone; HPBS – 2-(2′hydroxyphenyl) benzene sulfinate; DBT-MO – dibenzothiophene monooxygenase; DBTO2MO – DBT sulfone monooxygenase (adapted from Monticello, 2000).. However, in bibliography is extensively reported that these effects are reduced in biphasic media because 2-HBP shows a really high affinity for organic solvents (Caro et al., 2007; Kobayashi et al., 2001; Yang et al., 2005). Without reduction of DBT carbon content, the microorganisms that utilize 4S pathway are very promising for application in BDS processes (Rhee et al., 1998), namely bacteria belonging to the genera Arthrobacter, Brevibacterium, Gordonia, Paenibacillus and. 37.
(40) GENERAL INTRODUCTION. Rhodococcus (Duarte et al., 2001; Mohebali et al., 2007), as well as Agrobacterium (Oldfield et al., 1998). The enzymatic activities involved in DBT desulfurization are associated to the soluble fraction and none of them are associated to the membrane fraction (Gray et al., 1996), as opposed to the enzymes involved in the metabolism of other very hydrophobic molecules, such as alkanes (Monticello, 2000). The microorganisms that use this pathway to metabolize DBT can release a potentially noxious atom from the thiophenic compound producing a biodegradable compound (sulfate), only with a slight reduction of the hydrocarbon calorific value (Wang & Krawiec, 1996). In an in-vitro desulfurization assay, it was shown that the only intermediate accumulated to any extent was 2-(2-hydroxyphenyl) benzenesulfinate, being produced at a rate about five times faster than its consumption. Therefore, the last step in the pathway (catalyzed by the desulfinase) was rate limiting (Gray et al., 1996). The original Rhodococcus IGTS8 desulfurizing enzymes have little activity towards thiophenes or benzothiophenes, and thus gasoline desulfurization will require new biocatalysts with improved efficiency toward thiophenic sulfur (McFarland, 1999). This enzyme system will act not only on DBT but also on thioxanthen-9-one, as well as on DBT derivatives, 4-methyl DBT, 2-ethyl DBT, 3-ethyl DBT, 3,4,6-trimethyl DBT, 3,4,6,7tetramethyl DBT (Kobayashi et al., 2000), 4,6-dimethyl DBT, 2,8-dimethyl DBT and 3,4benzo DBT (Ohshiro et al., 1997).. 4S pathway enzymatics The first enzyme of 4S pathway is a DBT monooxygenase (DszC) which catalyses the DBT oxidation to DBTS in two steps. The second is also a monooxygenase (DszA) which converts DBTS to 2-(2’-hydroxyphenyl) benzenesulfinate, and in the final step a liase 38.
(41) GENERAL INTRODUCTION. (DszB) converts it to 2-hydroxybiphenyl and sulfite. In this metabolic pathway, a FMNreductase (DszD) has an important role in the activity of the monooxygenases, since it is responsible for the maintenance of reduced flavin levels (Fig. 5). DBT monooxygenase This enzyme is classified as unspecific monooxygenase (EC 1.14.14.1) by the Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NCIUBMB). There is not yet a specific classification for this particular enzyme. This enzyme catalyzes the sequential conversion of the DBT to a DBT sulfone using FMNH2, as a cosubstrate (Xi et al., 1997): DBT → DBT-sulfoxide → DBT-sulfone The first oxidation step (constant rate 0.06 min−1) occurs with one-tenth of the rate of the second step (constant rate 0.5 min−1). DBT monooxygenase from R. erythropolis D-1 is a homohexamer with a subunit molecular weight of 45 kDa. Its activity is maximal at a temperature of 40 °C and a pH of 8.0. DBT monooxygenase was also purified from the thermophilic bacterium Paenibacillus sp. strain A11-2. The molecular mass of the purified enzyme and its subunit were determined to be 200 kDa and 43 kDa by gel filtration and sodium dodecyl sulfate polyacrylamide gel electrophoresis, respectively, indicating a tetrameric structure. The optimal temperature and pH for this enzyme are 65 ºC and pH 9 (Konishi et al., 2002). DBT sulfone monooxygenase The enzyme classification of DBT sulfone monooxygenase is the same than the DBT monooxygenase (EC 1.14.14.1). DBT sulfone monooxygenase is widely studied and has been purified from some bacterial strains (R. erythropolis IGTS8, R. erythropolis D-1, Paenibacillus sp. strain A11-2 and Bacillus subtilis WU-S2B). This monooxygenase,. 39.
(42) GENERAL INTRODUCTION. present in strain D-1, has a molecular weight of 97 kDa consisting of two subunits with identical masses of 50 kDa (Ohshiro et al., 1999) and oxidizes DBT sulfone to 2-(2′hydroxyphenyl) benzene sulfinate (HPBS) using FMNH2 as a co-substrate (Xi et al., 1997) with a reaction rate 5-10-fold higher than DszC (Gray et al., 1996). Being thermophilic, the enzymes isolated from Paenibacillus sp. strain A11-2 (Konishi et al. 2000b) and Bacillus subtilis WU-S2B (Ohshiro et al., 2005) exhibit different characteristics from the enzyme isolated from the mesophiles. This thermophilic enzyme has an optimum temperature of 45 °C and is stable till 60 °C. It works at an optimum pH of 5.5. 2'-hydroxybiphenyl-2-sulfinate desulfinase 2'-hydroxybiphenyl-2-sulfinate desulfinase (EC 3.13.1.3) is the rate-limiting enzyme of 4S pathway and catalyzes the conversion of HPBS to 2-HBP. After cleavage, the sulfur product is incorporated into the biomass of the microorganism (Watkins et al., 2003). It is the least studied enzyme as it is produced in very small amounts (Gupta et al., 2005). This enzyme was purified from R. erythropolis IGTS8 and it consists of a monomer with a subunit molecular weight of 40 kDa, showing enzyme activity over a wide temperature range (25– 50 °C), with the optimum at 35 °C (Watkins et al., 2003). The working pH range for this enzyme is 6.0–7.5, with the optimum at pH 7.0. A cysteine residue is shown to be present in the sequence of this enzyme and it is found to be critical for enzyme activity (Nakayama et al., 2002). This enzyme was also purified and characterized from thermophilic bacteria, such as Paenibacillus sp. strain A11-2 (Konishi & Maruhashi, 2003). The molecular mass of the purified enzyme is 39 kDa, with a monomeric structure. The optimal temperature and pH for the reaction involving this enzyme are 55 ºC and 8, respectively.. 40.
(43) GENERAL INTRODUCTION. Flavin reductase Flavin reductase (EC 1.5.1.30), also named NADH-FMN oxidoreductase, is associated to the supply of free FMNH2 needed to 4S monooxygenases activity. All the monooxygenases of 4S pathway showed a requirement for equimolar quantities of flavin reductase for their respective oxygenation reactions. Xi et al. (1997) reported an enhanced desulfurization activity of DBT monooxygenase and DBT sulfone monooxygenase, under in vitro conditions, with increased concentrations of flavin reductase. The purified flavin reductase of R. erythropolis D1 contains no chromogenic cofactors and it was found to have a molecular mass of 86 kDa and four identical 22-kDa subunits. The optimal temperature and pH for enzyme activity were 35 °C and 6.0, respectively, and the enzyme retained 30% of its activity after heat treatment at 80 °C for 30 min (Matsubara et al., 2001). The flavin reductase purified from the thermophilic strain Paenibacillus sp. strain A11-2 (Konishi et al. 2000b) was also characterized. It is a homodimer with a subunit molecular weight of 25 kDa. This enzyme was completely FMN-dependent, and FAD could not act as a cofactor. This enzyme has the optimal temperature and pH at 55 °C and 5.5, respectively.. 4S pathway genetics Although the sox (sulfur oxidation) designation was first used, the dsz (desulfurization) designation for the 4S pathway genes has generally been adopted because several other unrelated genes were already labeled sox. To avoid misunderstanding with those other genes, the dsz designation (Dsz for gene product) has generally been accepted (Gupta et al., 2005). Rhodococcus erythopolis IGTS8 and Rhodococcus sp. strain X309 were among the first strains to be characterized at the molecular level (McFarland, 1999). There are indications. 41.
(44) GENERAL INTRODUCTION. of the conservative nature of the dsz genotype and of the establishment of differences and similarities among desulfurization strains isolated from different geographic locations (Denis-Larose et al., 1997). Complete sequence identity of the dsz operon was observed between Arthrobacter sp. DS7 and Rhodococcus sp. IGTS8 (Serbolisca et al., 1999). However, evidence for the presence of bacteria with divergent dszA gene sequences in an oil-polluted soil was reported (Duarte et al., 2001). The work performed by Denome et al. (1993b) indicates that a single genetic pathway controls the metabolism of dibenzothiophene, naphthalene and phenanthrene in Pseudomonas sp. strain C18. The majority of genetic studies involving desulfurization genes were performed with Rhodococcus strains and, therefore, it is important to increase the knowledge of regulatory mechanisms for other bacterial genus. In this context, some works were more recently reported using the bacteria Bacillus subtilis (Kirimura et al., 2004), Burkholderia sp. (Di Gregorio et al., 2004), Gordonia nitida (Park et al., 2003), Mycobacterium sp. G3 (Nomura et al., 2005; Takada et al., 2005), Mycobacterium phlei (Kirimura et al., 2004) and Paenibacillus sp. A11-2 (Ishii et al., 2000a). dsz operon The dsz genes of Rhodococcus sp. are arranged in an operon-regulated system located on a circular plasmid of approximately 150 kb (Oldfield et al., 1998), 120 kb (Santos et al., 2007) or on a 100-kb plasmid in other strains (Gupta et al., 2005). In this plasmid-encoded pathway, three genes (dszABC) arranged in an operonic manner and spanning a 4-kb region are responsible for the metabolism of DBT to 2-HBP and sulfate (Denis-Larose et al., 1997), though some differences occur at gene expression level (Li et al., 1996). It is transcribed in the same direction, coding for three proteins DszA, DszB, DszC, respectively (Piddington et al., 1995). The termination codon for dszA and the initiation codon for dszB overlap 2-bp and there is no such overlap between dszB and dszC (Oldfield et al., 1998),. 42.
(45) GENERAL INTRODUCTION. existing a 13-bp gap (Piddington et al., 1995). Although expressed as an operon, DszB is present at concentrations several-fold less in the cytoplasm, as compared with DszA and DszC (Li et al. 1996). These genes, when cloned on a Dsz negative phenotype, confer the ability to desulfurize DBT to 2-HBP. The evidences that support the hypothesis that the cluster is expressed as an operon are: first, disabling or removing the promoter region prevented expression of all measurable enzymatic activities; second, replacing the promoter region with alternative promoters relieved the sulfur repression normally observed at each step of desulfurization; and third, replacing the native promoter region with the E. coli tac promoter allowed expression of DBT desulfurization in E. coli (Piddington et al., 1995). Analogous to the dsz operon in mesophiles, the tds (thermal desulfurization) operon is located on a 8.7-kb DNA fragment in the thermophile Paenibacillus sp. A11-2 (Ishii et al. 2000a; Konishi et al. 2000a). The tdsA, tdsB, and tdsC nucleotide sequences and the deduced amino acid sequences showed significant homology to the dszA, dszB and dszC genes of R. erythropolis IGTS8. Several gram-positive and gram-negative organisms are known to have desulfurization genes; and they show 70% homology (McFarland 1999). dsz operon regulation Promoter and regulatory regions of the dsz operon were also studied and it was found that synthesis of enzymes is strongly repressed in the presence of readily bioavailable sulfur (Li et al. 1996), i.e., sulfate, sulfide, methionine and cysteine. The promoter for the dsz gene cluster in strain IGTS8 is located in a 385-bp region immediately upstream of dszA. This 385 bp of 5’ untranslated dsz DNA contains a number of interesting elements; it appears to contain a promoter and at least three regions that affect Dsz activity. The first, at -263 to 244, reduced repression, but deletions did not affect repression or gene expression. The second region, between -144 and -121, was able to bind a protein that could be an activator; deletion of this region reduced gene expression but not repression. The third region,. 43.
(46) GENERAL INTRODUCTION. between -57 and -98, may be a repressor binding site that overlaps the promoter -10 and -35 regions (Li et al., 1996). To date, no repressor or activator proteins that act upon the regulatory sequence of dsz have been isolated (Reichmuth et al., 2000). The three genes are transcribed in the same direction beginning in the position -46 bp. The enzyme production is induced by DBT and its analogs, DBT sulfone or thioxanthen 9one, and it is strongly repressed by sulfate or the compounds with sulfur, such as methionine and cysteine, even in the presence of DBT (Kayser et al., 1993; Ohshiro et al., 1996). Sitedirected mutagenesis will be a better way to construct mutants with full promoter activity and no repression (Ohshiro & Izumi, 1999). Gene expression Many environmental pollutants are readily degraded by naturally occurring microbes. Very often, however, the rate of degradation may not be optimal for practical large-scale bioremediation. Genetic engineering of biodegradative pathways offers the potential of expanding the existing capabilities found in nature. Expression of biocatalytic pathways in foreign microorganisms can significantly enhance the efficiency of the biodegradation process (Chen et al., 1999). This is the case of the dsz operon expression in Pseudomonas aeruginosa EGSOX that, in addition to the ability to produce a biosurfactant that increase the aqueous concentrations of hydrophobic compounds, allows DBT desulfurization 4-fold faster than the wild strain of R. erythropolis (Gallardo et al., 1997). It has been shown (Rambosek et al. 1999) that the reconstruction of several promoters containing the R. erythropolis IGTS8 dsz genes, with the dszD gene, helps to increase the rate of DBT desulfurization. The same conclusion was reported in the work involving R. erythropolis KA2-5-1 (Konishi et al., 2005a). In addition, when flavin reductase, flavin mononucleotide reductase or various oxidoreductases were over-expressed in recombinant constructs, the desulfurization rate increased up to 100-fold (Gray et al., 1996).. 44.
(47) GENERAL INTRODUCTION. BIODESULFURIZATION APPLICATION An industrial-scale process for petroleum biodesulfurization using aerobic microorganisms has not yet been demonstrated. However, through an improved understanding of the biochemistry and genetics of the desulfurization pathway, it is anticipated that improved biocatalysts with activities suitable for an industrial process will be developed (Kilbane, 2006). Until the present date, studies on sulfur oxidative pathways have mainly been focused in model compounds, which limit the ability to demonstrate the commercial potential of BDS (Grossman et al., 2001). However, some reported works involved several fractions of crude oil refining, including gasoline and diesel (Rhee et al., 1998; Folsom et al., 1999; Grossman et al., 1999; Grossman et al., 2001; Furuya et al., 2003; Guobin et al., 2005; Li et al., 2005). Efforts to increase the rate of sulfur removal from aromatic sulfur heterocycles have been possible due to the use of genetic engineering techniques or the use of immobilization matrices (Gupta et al., 2005). The selection of the petroleum feedstock in biodesulfurization will play a large role in the overall economic viability of the process. BDS may be utilized as a pretreatment to crude oil before entering pipelines. It may also be applied as an alternative to hydrotreating the crude at the refinery or it may be applied in the polishing of refinery products such as diesel or gasoline. As pretreatment, the BDS unit may be used to treat marginally sour crudes (0.60.7% S), converting them to sweet crudes (<0.5% S). For this application, the extent of desired desulfurization is quite low, and this may serve as an attractive initial niche for BDS (Kaufman et al., 1998). Inherent to all of the current bioprocessing of fossil feedstocks schemes is the need to contact a biocatalyst-containing aqueous phase with an immiscible or partially miscible organic substrate (Van Hamme et al., 2003). Factors such as liquid-liquid and gas-liquid mass transport, amenability for continuous operation and high throughput, capital and 45.
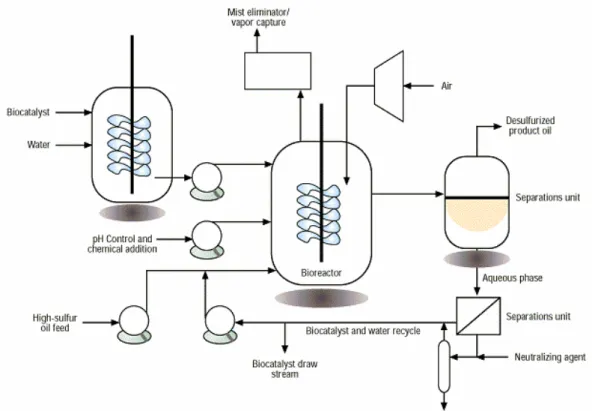
(48) GENERAL INTRODUCTION. operating costs, as well as ability for biocatalyst recovery and emulsion breaking, are significant issues in the selection of a reactor for aqueous-organic contacting (Kaufman et al., 1998). Biodesulfurization studies of fossil fuels usually involved intact cells as biocatalyst, which avoids the Dsz enzymes purification and facilitate the BDS industrialization. The immobilization of cells can be used to desulfurize DBT efficiently (Shan et al., 2005) being the life-time of immobilized cell biocatalysts more than 600 h (Hou et al., 2005). Traditionally, impeller-based stirred reactors are used for such mixing, because of their ease of operation and wide acceptance in the chemical and biological processing industries (Fig. 6).. Fig. 6. A conceptual process flow diagram for the biodesulfurization process (adapted from Monticello, 1998). 46.
(49) GENERAL INTRODUCTION. This kind of reactors promotes the contact between the aqueous and organic phases by imparting energy to the entire bulk solution, achieving water or oil droplet sizes of 100-300 µm in diameter when surfactants are not present. To obtain droplets of about 5 µm, the energy consumed by the reactor will be 5-fold higher (Kaufman et al., 1997). In BDS processes, oil is mixed with an aqueous medium that contains biocatalytically active bacterial cells. Recovery of oil from the oil–water–bacteria mixture follows the biodesulfurization step as a separated batch process (Konishi et al., 2005b). To date, there are some microbial desulfurization studies at laboratory scale involving petroleum fractions and coal. Energy BioSystems Corporation (ECB) was the only commercial venture dedicated to the development of biodesulfurization technology (Kilbane, 2006). EBC’s concept for a biodesulfurization process was not only to treat diesel, but also to produce a value-added surfactant byproduct to achieve a more economical process (Monticello, 2000). There was a plan to construct a demonstration-scale biodesulfurization process at the Petro Star refinery in Valdez, Alaska. The date for the construction of a demonstration plant was progressively postponed (Kilbane, 2006).. OIL BIODESULFURIZATION Early biodesulfurization research used model compounds like DBT, sometimes in aqueous systems bearing little resemblance to the conditions the biocatalyst would encounter in commercial applications (Ohshiro & Izumi, 1999). In fact, the desulfurization rates of diesel oil were much smaller than those obtained for pure DBT (Rhee et al., 1998). Biodesulfurization of petroleum results in total sulfur removals between 30 and 70% for mid-distillates (Grossman et al., 1999), 24 to 78% for hydrotreated diesel (Rhee et al., 1998; Maghsoudi et al., 2001; Ma et al., 2006), 20-60% for light gas oils (Chang et al., 1998; Ishii et al., 2005) and 25-60% for crude oils (McFarland, 1999).. 47.
(50) GENERAL INTRODUCTION. Taking into account that BDS can be a complementary method to HDS, the study of fractions of pre-desulfurized oil is important. Grossman et al. (2001) reported a treatment by Rhodococcus sp. strain ECRD-1 of middle distillate oil whose sulfur content was virtually all substituted DBTs containing 669 ppm of total sulfur. Analysis of the sulfur content of the treated oil revealed that 92% of the sulfur had been removed, reducing the sulfur content from 669 ppm to 56 ppm. In addition, studies of desulfurization with Rhodococcus erythropolis I-19, involving hydrodesulfurized middle distillate oil, showed that after 0, 1, 3 and 6 h, the sulfur concentrations were 1850, 1620, 1314 and 949 ppm, respectively. The first 230-ppm drop in total sulfur, observed after 1 h, corresponded primarily to a biotransformation of DBT and midboiling- range sulfur compounds. Between 1 and 3 h, another 300-ppm sulfur reduction occurred, with some evidence for more highly alkylated DBTs being affected. At 3 h, most of the DBT and much of the C1-DBTs were consumed. Between 3 and 6 h, desulfurization shifted to the higher-boiling-range sulfur compounds, resulting in an additional 365-ppm drop in total sulfur. Analysis of this middle distillate oil biodesulfurized from 1850 to 615 ppm sulfur showed the majority of the remaining sulfur to be thiophenes (75%), with 11% sulfides, 2% sulfoxides and 12% sulfones (Folsom et al., 1999). More recently, Zhang et al. (2007) reported a total reduction of 97% (to 6 µg ml–1) of the sulfur content of previous hidrodesulfurized diesel oil. There are also some studies on desulfurization of oil fractions involving thermophilic bacteria such as Paenibacillus sp. (Konishi et al., 1997). When Paenibacillus sp. strains A11-1 and A11-2 were cultured in the presence of light gas oil containing 800 ppm of total sulfur at a high temperature, the bacteria grew well. Light gas oil is known to contain small amounts of sulfur and limited species of heterocyclic organosulfur compounds composed mainly of alkylated DBT derivatives. In conformity with the stimulated bacterial growth, the content of sulfur in the oil phase was significantly decreased, indicating that both Paenibacillus strains can desulfurize at high temperatures from the processed light gas oil. However, these strains presented a very low desulfurization rate (Onaka et al., 2001b),. 48.
(51) GENERAL INTRODUCTION. lower than the desulfurization rate obtained with R. rhodochrous (Konishi et al., 1997). The use of thermophilic bacteria has some advantages since it is not necessary to cool-down the oil fractions after the HDS, which makes this process less expensive (Konishi et al., 2000b). Another advantage is the fact of reducing the possibility of contamination by undesirable bacteria that can negatively affect the BDS process (Kirimura et al., 2001). Although the obtained removals are significant, this level of desulfurization is insufficient to meet the required sulfur levels for all oil derivatives (Grossman et al., 1999).. COAL BIODESULFURIZATION The isolation of mesophilic bacterial strains belonging to the genera Chryseomonas, Moraxella, Pseudomonas, Xanthomonas (Gómez et al., 1999), Leptospirillum and Thiobacillus, and thermophilic prokaryotic strains, as Acidianus brierleyi, Metallosphaera sedula, Sulfolobus acidocaldarius and Thiobacillus caldus (Kargi & Weissman, 1987; Schippers et al., 1999), allowed, in general, a high efficiency of metabolization of sulfur present in coal, mainly pyritic sulfur. Thiobacillus ferrooxidans is the most widely used organism for biodesulfurization of coal (Olson & Kelly, 1986). It is an aerobic chemoautotrophic bacterium which derives its metabolic energy from oxidation of reduced iron and sulfur components of the pyrite (Lees et al., 1996). The effect of particle size, pulp density, cell density and various other process parameters on biodesulfurization process of coal has been extensively studied (Malik et al., 2001b). Bioleaching is a complex phenomenon governed by a chain of reactions representing direct (bacterial) and indirect attack on sulfide (Schippers et al., 1999). During this process, the insoluble pyrite is solubilized to aqueous ferrous sulfate and other intermediate products that are subsequently oxidized to ferric iron and soluble sulfate (Malik et al., 2001a). Therefore, the iron solubilization and sulfur removal are linked to each other. Usualy, the optimal coal. 49.
Imagem
Documentos relacionados
Os gerentes atuando como facilitadores da comunicacão interpessoal para incrementar a criacão do conhecimento organizacional: este capítulo.. inicia com uma abordagem
Thus, it could be concluded that sugarcane cells cultivated in suspension responded to chitin hydrolyzate producing lignin in the first 6 h of elicitation, but
Table 1 - Antibacterial activity of the crude (0.48 mg protein) and dialyzed (0.30 mg protein) purple fluid of Aplysia dactylomela.. Data are reported as the mean ± SD for 3
Protein peak was tested for hemolytic activity and the fractions under peak showing activity were pooled together, lyophilized and dialyzed against 10 mM sodium
This study enabled us to unveil that the family interview is an important stage, since it deals with the possibility of donating organs and tissues to save and/ or improve
By examining the return characteristics of 10 different, long minus short, accounting-based anomaly portfolios, we find that a portfolio that rebalances daily, based
Portanto, pelo texto, o segurado que preencher os requisitos para se aposentar por tempo de contribuição poderá abrir mão do fator previdenciário e optar pela
The biomass accumulation of shoot and roots and macro- and micronutrient contents, as well as the number of mycorrhizal spores in the soil, were negatively affected by the increase