www.jped.com.br
ORIGINAL
ARTICLE
Circulating
endothelial
progenitor
cells
in
obese
children
and
adolescents
夽
,
夽夽
António
Pires
a,∗,
Paula
Martins
a,
Artur
Paiva
b,
Ana
Margarida
Pereira
c,
Margarida
Marques
d,
Eduardo
Castela
a,
Cristina
Sena
c,
Raquel
Seic
¸a
caServiceofPediatricCardiology,HospitalPediátricodeCoimbra,CentroHospitalareUniversitáriodeCoimbra(CHUC),Coimbra,
Portugal
bInstitutoPortuguêsdoSangueeTransplantac¸ão,Coimbra,Portugal
cLaboratóriodeFisiologia,InstitutodeImagemBiomédicaeCiênciasdaVida,FaculdadedeMedicina,UniversidadedeCoimbra,
Coimbra,Portugal
dLaboratóriodeEstatística,FaculdadedeMedicina,UniversidadedeCoimbra,InstitutodeImagemBiomédicaeCiênciasdaVida,
Coimbra,Portugal
Received1October2014;accepted13January2015 Availableonline29August2015
KEYWORDS
Pediatricobesity; C-reactiveprotein; Monocyte
chemoattractant protein-1; E-selectin; Asymmetric dimethylarginine; Endothelial progenitorcells
Abstract
Objective: Thisstudy aimed toinvestigate therelationship between circulating endothelial
progenitorcellcountandendothelialactivationinapediatricpopulationwithobesity.
Methods: Observational and transversal study, including120 children andadolescents with
primary obesityofbothsexes, aged6---17 years,whowere recruitedatthis Cardiovascular
RiskClinic.Thecontrolgroupwasmadeupof41childrenandadolescentswithnormalbody
massindex.Thevariablesanalyzedwere:age,gender,bodymassindex,systolicanddiastolic
bloodpressure,high-sensitivityC-reactiveprotein,lipidprofile,leptin,adiponectin,
homeo-stasismodelassessment-insulinresistance,monocytechemoattractantprotein-1,E-selectin,
asymmetricdimethylarginineandcirculatingprogenitorendothelialcellcount.
夽 Pleasecitethisarticleas:PiresA,MartinsP,PaivaA,PereiraAM,MarquesM,CastelaE,etal.Circulatingendothelialprogenitorcellsin obesechildrenandadolescents.JPediatr(RioJ).2015;91:560---6.
夽夽StudylinkedtotheHospitalPediátrico,CentroHospitalareUniversitáriodeCoimbra(CHUC);LaboratóriodeFisiologiaandLaboratório deEstatística,InstitutodeImagemBiomédicaeCiênciasdaVida,FaculdadedeMedicina,UniversidadedeCoimbra;andtotheInstituto PortuguêsdoSangueeTransplantac¸ão,Coimbra,Portugal.
∗Correspondingauthor.
E-mail:pires1961@gmail.com(A.Pires). http://dx.doi.org/10.1016/j.jped.2015.01.011
Results: Insulinresistancewascorrelatedtoasymmetricdimethylarginine(=0.340;p=0.003),
which was directly,but weakly correlated toE-selectin (=0.252;p=0.046).High
sensitiv-ity C-reactiveprotein wasnot foundto becorrelated tomarkersofendothelial activation.
Systolicbloodpressurewasdirectlycorrelated tobody massindex(=0.471;p<0.001)and
thehomeostasismodelassessment-insulinresistance(=0.230;p=0.012),andinversely
corre-latedtoadiponectin(=−0.331;p<0.001)andhigh-densitylipoproteincholesterol(=−0.319;
p<0.001).Circulatingendothelialprogenitorcellcountwasdirectly,butweaklycorrelated,to
bodymassindex(r=0.211;p=0.016),leptin(=0.245;p=0.006),triglyceridelevels(r=0.241;
p=0.031),andE-selectin(=0.297;p=0.004).
Conclusion: Circulatingendothelialprogenitorcellcountiselevatedinobesechildrenand
ado-lescentswithevidenceofendothelialactivation,suggestingthat,duringinfancy,endothelial
repairingmechanismsarepresentinthecontextofendothelialactivation.
©2015SociedadeBrasileiradePediatria.PublishedbyElsevierEditoraLtda.Allrightsreserved.
PALAVRAS-CHAVE
Obesidadeinfantil; ProteínaCreativa; Proteínaquimiotática demonócitos-1; E-seleticna; Dimetilarginina assimétrica; Célulasprogenitoras endoteliais
Célulasprogenitorasendoteliaiscirculantesemcrianc¸aseadolescentesobesos
Resumo
Objetivo: Oobjetivodesteestudofoi investigararelac¸ãoentreosnúmerosdecélulas
pro-genitoras endoteliaiscirculantese aativac¸ãoendotelial em uma populac¸ãopediátrica com
obesidade.
Métodos: Estudoobservacionaletransversal,incluindo120crianc¸aseadolescentescom
obesi-dadeprimáriadeambosdesexos,comidadesentre6e17anos,recrutadosdenossaClínica
deRiscosCardiovasculares.Ogrupodecontrolecontoucom41crianc¸aseadolescentescom
índicedemassacorporalnormal.Asvariáveisanalisadasforam:idade,sexo,índicedemassa
corporal,pressãoarterialsistólicaediastólica,proteínaCreativadealtasensibilidade,perfil
lipídico,leptina,adiponectina,resistênciaàinsulinaparaavaliac¸ãodomodelodehomeostase,
proteínaquimiotáticademonócitos-1,E-seleticna,dimetilargininaassimétricaenúmerosde
célulasendoteliaisprogenitorascirculantes.
Resultados: Aresistênciaàinsulinafoicorrelacionadaadimetilargininaassimétrica(p=0,340;
p=0,003),que foidiretamentecorrelacionada, porém deformamuitaamena àE-seleticna
(=0,252; p=0,046). Nãoconstatamos quea proteína C reativa de alta sensibilidade está
correlacionada amarcadores de ativac¸ãoendotelial. A pressãoarterial sistólicafoi
direta-mentecorrelacionadaaoíndicedemassacorporal=0,471;p<0,001)eàresistênciaàinsulina
paraavaliac¸ãodomodelodehomeostase(=0,230;p=0,012)einversamentecorrelacionada
a adiponectina(=-0,331; p<0,001)e lipoproteína de alta densidade-colesterol =-0,319;
p<0,001).Osnúmerosdecélulasprogenitorasendoteliaiscirculantesforamdiretamente
cor-relacionados,porémdeformamuitoamenaaoíndicedemassacorporal(r=0,211;p=0,016),
àleptina(=0,245;p=0,006),aosníveisdetriglicerídeos(r=0,241;p=0,031)eàE-seleticna
=0,297;p=0,004).
Conclusão: Osnúmerosdecélulasprogenitorasendoteliaiscirculantessãoelevadosemcrianc¸as
eadolescentesobesoscomcomprovac¸ãodeativac¸ãoendotelial,sugerindoque,nainfância,os
mecanismosdereparac¸ãoendotelialestãopresentesnocontextodaativac¸ãoendotelial.
©2015SociedadeBrasileiradePediatria.PublicadoporElsevierEditoraLtda.Todososdireitos
reservados.
Introduction
Inobesity,variousinflammatoryagents,suchasC-reactive protein1---3andleptin,4disturbtheproductionofnitricoxide
via the inhibition of its rate-limitingenzyme, endothelial nitric oxide synthase (eNOS).5,6 Other endogenous
sub-stances, such as asymmetric dimethylarginine (ADMA), a competitive antagonist of eNOS, also play a role in fur-thercompromisingnitricoxidebioavailability.Itsproduction is stimulated by inflammatory agents, such as C-reactive
protein.7 Unlike nitricoxide,it canbe easilyassayed and
usedasasurrogatefornitricoxidebioavailability.
Inpatientswithcardiovascularriskfactors,suchas arte-rial hypertension and insulin resistance, the count and function of EPCs is reduced. In these, the risk of cardio-vasculareventsisincreased.9,10
Inflammatorymarkersalsoappearstoreducethenumber ofEPCs,implyingapossibleroleinobesity.11,12
The aims of the present study were two fold. Firstly, to demonstrate that endothelial activation is present in childhoodobesity;andsecondly,thatrepairingmechanisms, throughEPCactivation,arenot,atthisearlystage, compro-mised.
Methods
Subjects
The authors conducted an observational and transversal analysis, in a cohort of obese children and adolescents, recruitedrandomly,andfollowed-upataCardiovascularRisk Clinic.Allparentsgavetheirinformedconsentforthe chil-drentoparticipateinthestudy,whichhadbeenapproved bythelocalEthicsCommittee.
Theinclusioncriteriaforthestudy groupwereprimary obesity(bodymassindex[BMI]abovethe95thpercentilefor sexandage)inchildrenandadolescentsaged6---17years, withoutrecent or chronic illnesses.The exclusion criteria included secondary causes of obesity,acute infectious or inflammatorydisorders(within amonth of sampling),and chronicdisorders.
Thecontrolgroupincludedhealthychildrenand adoles-cents,unrelated tothe studygroup, withinthe sameage rangewitha normal BMI(percentil 5---85), recruited from theCardiologyClinicwheretheyweresentforevaluationof murmurs,butprovedtohavenosubjacentcardiac anoma-lies. All had undergone a 12-h fast prior to the clinical evaluationandbloodsampling.
The study group comprised 120 and the control group 41children andadolescents,of both sexes. Basedonthis samplesize,asignificancelevel(p-value)of0.05,apower of 0.80,and an effect size of 0.52were obtained. These valueswerecalculatedusingtheG*Power3.1.5program.
The variables analyzed were: age, gender, body mass index,systolicbloodpressure,diastolicbloodpressure,total cholesterol, high-density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), triglycerides, high-sensitivity C-reactive protein (hsCRP), monocyte chemoattractant protein-1 (MCP-1), lipid profile, leptin, adiponectin, homeostasis model assessment-insulin resis-tance(HOMA-IR),E-selectin,asymmetricdimethylarginine, andcirculatingEPCcount.
Clinicalandanthropometricevaluation
Weight(inkilogramstothenearest100g)wasdetermined usingaSECA220® digitalweightscale(MedicalScalesand Measuring Systems, Hamburg, Germany) and for standing height(incentimeterstothenearest0.1cm)astadiometer includedinthesameequipmentwasused,withthechildren wearingonly undergarments. Body mass indexwas calcu-lated basedon the formula:BMI=(weight/height2).13 The
WorldHealthOrganization(WHO)BMIpercentilechartswere
usetodefineobesity(ifBMI>percentil95;normalweight: BMIbetweenpercentil5---85).
The criteria for metabolic syndrome in children and adolescentsweredefinedaccordingtotheInternational Dia-betesFederationconcensus.14
Bloodcollectionandbiochemicalanalysis
Fastingvenousbloodsamples(15mL)wereobtainedto esti-matethehematologicalparameters.Bloodspecimenswere collected in vacutainertubes withor without ethylenedi-aminetetraaceticacid(EDTA)asneeded.Serumandplasma were preparedand then frozen (−80◦C)for storage until
analysis. High-sensitivity C-reactive protein was deter-mined by immunonephelometry from serum samples and processedintheBNProSpec® System(SiemensHealthcare DiagnosticsInc,Munich,Germany)analyzer(undetectedif <0.02mg/dL).
Monocyte chemoattractant protein-1, asymmetric dimethylarginineandE-selectinweredeterminedbyELISA using commercially available enzyme-linked immunosor-bent assaykits OptEIATM (BD BiosciencesPharmingen, CA, USA).
Leptin and adiponectin levels were determined using commerciallyavailableenzyme-linkedimmunosorbentassay kits(eBioscience--- SanDiego,CA,UnitedStatesand BioVen-dor--- Brno,CzechRepublic,respectively).
Insulin levels were determined by chemiluminescence fromserumsamplesandprocessedintheIMMULITE2000® (SiemensHealthcareDiagnosticsInc,Munich,Germany) ana-lyzer and glucose levels were determined from plasma samplesanalyzedintheVITROS5.1FS®(OrthoClinical Diag-nostics,Johnson&Johnson,NY,USA)systembymicroslide technology.
The HOMA-IR was calculated based on the formula; HOMA-IR=insulin(mU/L)×glucose(mmol/L)/22.5, consid-ering 3 as the cut-off value for the diagnosis of insulin resistance.15
Total cholesterol, low-density lipoprotein cholesterol (LDL-C),high-density lipoprotein cholesterol (HDL-C),and triglyceride (TG) concentrations were measured using an automatedbiochemicalanalyzerVITROS5.1FS®(Ortho
Clin-icalDiagnostics,Johnson&Johnson,NY,USA).
Fordeterminationof circulating EPC count,peripheral blood (PB) was collected in EDTA tubes, stored on ice, and processed within2h. Identification and characteriza-tionofcirculatingEPCwasperformedusingananti-CD146 conjugated withflouresceinisothiocyanate(clone:P1H12, BectonDickinson(BD),CA,USA),anti-KDRconjugatedwith phycoeritrin (clone: 89106, R&D System, Headquarters, Minneapolis, USA), anti-CD34 peridinin chlorophyll pro-tein cyanine 5.5 (clone: 8G12, BD Bioscience, CA, USA), anti-CD133allophycocyanin(clone:293C3,MiltenyiBiotec, Bergisch Gladbach, Germany), and CD45 krome orange (clone: J.33, Immunotech --- Beckman Coulter, Marseille, France)combinationofmonoclonalantibodies(mAb).
Forsamplestaining,adirectimmunofluorescence tech-nique was used. Data acquisition was performed in a FACSCantoII® flowcytometer(BDBiosciencesPharmingen,
CA, USA) using the FACSDiva® software (BD Biosciences
International Society for Hematotherapy and Graft Engi-neering (ISHAGE) sequential gating strategy proposed by Schmidt---Luckewasfollowed.
CirculatingEPCSwereidentifiedaccordingtoaminimal antigenicprofilethatincludesatleastonemarkerof stem-ness/immaturity(CD34andCD133),plusatleastonemarker ofendothelialcommitment(KDRandCD146).CD45staining wasusedtoexcludeleukocytes.
Fordataanalysis,theInfinicytTMsoftware,V.1.5
(Cytog-nosSL,Salamanca,Spain)wasused.
Statisticalanalysis
ThedatawasanalyzedusingtheIBMSPSS20software(IBM Corp.Released 2011.IBM SPSSStatisticsfor Windows,NY, USA).The descriptiveanalysisof theparametric variables wasdone bycalculating themean±standard error of the mean.Student’st-testandMann---WhitneyUtestwereused tocalculate thedifferences in the demographic,clinical, andhematologicalparametersbetweentheobeseand con-trolgroups,dependingonthenormalityofdistribution.For categoricalresponsevariables,differencesbetweenthetwo groups were assessed using the chi-squared test. Logistic regression was used when clinical variables were con-trolledbetweenthetwogroups.Toestablishthecorrelation betweentheparametersintheobesegroup,Spearman’sand Pearsons’scorrelationswereused.Theresultswere consid-eredstatisticallysignificantatp<0.05.
Results
Comparativeanalysisbetweentheobeseand controlgroups
Onehundredandtwentyobesechildrenandadolescents,61 boysand59girls,withagesbetween6and17years(mean age11.65years±2.96)wereincludedinthestudy.The con-trolgroup wasmade upof41 healthy,non-obesechildren andadolescents,29boysand12girls,withinthesameage group(meanage12.73years±2.77).
We firstly compared the two group’s anthropometric, clinical and analytical parameters. As demonstrated in
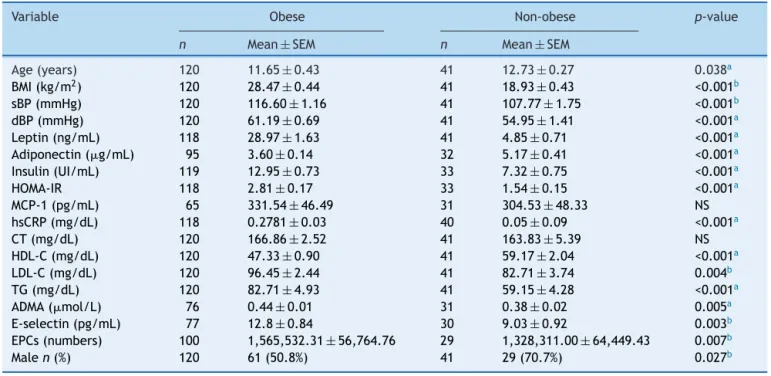
Table1,allthemeansoftheanalyzedparameterswere sig-nificantlyhigher in the obese group,including E-selectin, ADMAandcirculatingEPCS,exceptforadiponectinand HDL-C(Fig.1).Totalcholesterolandmonocytechemoattractant protein-1showednosignificantstatisticaldifferences.
As age (p=0.038) and gender (p=0.027) were signif-icantly different between the two groups, the analysis wasrepeatedusinglogisticregression,andtheresultsdid not differ significantly, namely, MCP-1 (p=0.334), ADMA (p=0.005),E-selectin(p=0.008),andEPCs(p=0.043).Total cholesterolandMCP-1continuedtoshownosignificant dif-ferences.
Circulating EPCcount were directly,but weakly corre-lated,toBMI(r=0.21;p=0.016),leptin(=0.25;p=0.006),
triglyceride levels (r=0.24; p=0.031), and E-selectin (=0.30;p=0.004).
Table1 Anthropometric,clinical,andanalyticalparametersoftheobeseandnon-obesegroups.
Variable Obese Non-obese p-value
n Mean±SEM n Mean±SEM
Age(years) 120 11.65±0.43 41 12.73±0.27 0.038a
BMI(kg/m2) 120 28.47±0.44 41 18.93±0.43 <0.001b
sBP(mmHg) 120 116.60±1.16 41 107.77±1.75 <0.001b
dBP(mmHg) 120 61.19±0.69 41 54.95±1.41 <0.001a
Leptin(ng/mL) 118 28.97±1.63 41 4.85±0.71 <0.001a
Adiponectin(g/mL) 95 3.60±0.14 32 5.17±0.41 <0.001a
Insulin(UI/mL) 119 12.95±0.73 33 7.32±0.75 <0.001a
HOMA-IR 118 2.81±0.17 33 1.54±0.15 <0.001a
MCP-1(pg/mL) 65 331.54±46.49 31 304.53±48.33 NS
hsCRP(mg/dL) 118 0.2781±0.03 40 0.05±0.09 <0.001a
CT(mg/dL) 120 166.86±2.52 41 163.83±5.39 NS
HDL-C(mg/dL) 120 47.33±0.90 41 59.17±2.04 <0.001a
LDL-C(mg/dL) 120 96.45±2.44 41 82.71±3.74 0.004b
TG(mg/dL) 120 82.71±4.93 41 59.15±4.28 <0.001a
ADMA(mol/L) 76 0.44±0.01 31 0.38±0.02 0.005a
E-selectin(pg/mL) 77 12.8±0.84 30 9.03±0.92 0.003b
EPCs(numbers) 100 1,565,532.31±56,764.76 29 1,328,311.00±64,449.43 0.007b
Malen(%) 120 61(50.8%) 41 29(70.7%) 0.027b
BMI, bodymassindex; sBP, systolicbloodpressure; dBP,diastolic blood pressure;HOMA-IR, homeostasismodel assessment-insulin resistance;MCP-1,monocytechemoattractantprotein-1;hsCRP,high-sensitivityC-reactiveprotein;CT,totalcholesterol;HDL-C,high densitylipoproteincholesterol;LDL-C,lowdensitylipoproteincholesterol;TG,triglycerides;ADMA,asymmetricdimethylarginine;EPCs, circulatingendothelialprogenitorcellcount;NS,non-significantp-value;n,samplenumber;SEM,standarderrorofthemean.
Obese 0
500,000 1,000,000
Mean endothelial progenitor cell numbers
1,500,000 2,000,000
*
Non-obese
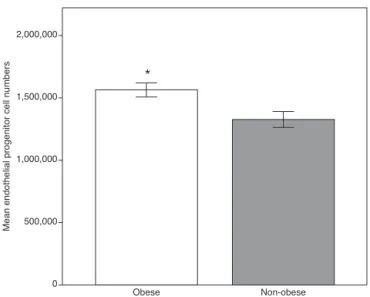
Figure1 Endothelialprogenitorcellcountintheobeseand
thenon-obesegroups.Thehigherlevelsintheobesegroupmay
reflecttheresponsebyrepairingmechanismssecondarytoearly
endothelial activation. Data expressed as means±standard
error of the mean. Obese group, n=100; non-obese group,
n=29.*Student’st-test,p<0.01vs.nonobesegroup.
Analysisoftheobesegroup
Bodymassindexwasdirectlyandmoderatelycorrelatedto MCP-1(=0.51;p<0.001),systolicbloodpressure(=0.47;
p<0.001),leptin(=0.40;p<0.001),andHOMA-IR(=0.35;
p<0.001);weaklycorrelatedtohsCRP(=0.22;p=0.018); and inversely but weakly correlated to adiponectin (=−0.28;p=0.007)andHDL-C(=−0.26;p=0.004).
Monocyte chemoattractant protein-1 was directly cor-related tosystolic blood pressure (=0.34; p=0.005), as shown in Fig. 2. High sensitivity C-reactive protein was
90.00 .000 500,000 1,000,000 1,500,000 2,000,000
R2 linear=0.099
100.00 110.00 120.00
sBP
MCP-1
130.00 140.00 150.00
Figure 2 Correlation between monocyte chemoattractant
protein-1andsystolicbloodpressure.Inobesity,cardiovascular
riskfactorsclusterandcontributetotheinflammatoryprocess,
asobservedinthecorrelationbetweensystolicbloodpressure
andMCP-1inthe presentstudy.sBP,systolic bloodpressure;
MCP-1,monocytechemoattractantprotein-1.
weaklycorrelatedtoleptin (=0.32;p<0.001) andLDL-C (=0.23;p=0.011).
Systolic blood pressure was inversely correlated to adiponectin (=−0.33; p=0.001) and directly correlated toHOMA-IR (=0.23;p=0.012) and,asexpected, moder-atelycorrelatedtoage(=0.50;p<0.001).Diastolicblood pressurehadlesspronouncedcorrelations.
TheHOMA-IRwascorrelatedtoADMA(=0.34;p=0.003) providing the most direct link to a marker of endothe-lial activation. This was evident particularly at HOMA-IR values above 3 (p=0.003), the cut-off value we used to define insulin resistance. It was also directly cor-related to leptin (=0.39; p<0.001), systolic blood pressure (=0.23;p<0.012), anddiastolic blood pressure (=0.20;p<0.033),andinverselycorrelatedtoadiponectin (r=−0.21;p=0.040).
Metabolic syndrome was observed in 11.7% (n=14) of the obese patients. As expected, being part of the definition,systolic(p=0.005),butnotdiastolicblood pres-sure (p=0.728), waselevated, aswere triglyceridelevels (p<0.001),HDL-Cwaslower(p=0.007),andnosignificant differenceswereobservedinglucoselevels.Regardingthe markers of endothelial activation andrepair mechanisms, ADMA was directly, but weakly correlated to E-selectin (=0.25; p=0.046),and thelatter wasweaklycorrelated tocirculatingEPCcount(=0.22;p=0.047).
Discussion
The present study clearly demonstrates that, unlike their lean counterparts, obese children and adolescents with evidence of ongoing low-grade inflammation and endothelialactivation haveraised EPCcount.Correlations between adiposity, inflammatory markers, and obesity-related comorbidities such as arterial hypertension and insulinresistancewerealsoobserved.
Monocytechemoattractantprotein-1regulatesmigration and infiltration of monocytes andmacrophages16 into the
vascularwallandplaysanimportantrolein inflammation-mediateddiseases,suchasobesity.17 Asscanttranslational
researchhasbeendoneinthisfield,theauthorswere inter-ested in evaluating the relationship of MCP-1 to obesity relatedcomorbidities.UnlikethefindingsbyBreslinetal.,18
nodifferenceswereobservedinMCP-1levelsbetweenthe obeseandcontrolgroupsnorwhatanassociationwith dys-lipidemia retrieved (data not shown), possibly due to a different sample size(39 obesechildren andadolescents) and ethnicity (American-Mexican). However, in the obese group, MCP-1,unlike hsCRP, hada directcorrelation with systolic blood pressure. This trend was not found in the controlgroup.Itis,thustemptingtopostulatethatin child-hood obesity,MCP-1 contributes to arterial hypertension, andimplicitlyisimplicatedinthepathophysiological mech-anismsleadingtocardiovasculardiseaselaterinlife.Based onthepresentevidence,MCP-1,comparedtohsCRP,would appeartobeamorerobustcardiovascularriskmarker. How-ever,duetothesamplesize,furtherresearchneedstobe done in thisfield.To thebestof theauthors’ knowledge, theseassociationshavenotbeenpreviouslydescribed.
highersystolicanddiastolicbloodpressurevalues(p<0.001) than thecontrol group.The prevalence of arterial hyper-tension in the obese group was9.2%, and almost a third had pre-hypertension (26.7%). Apart from MCP-1, and in agreementwiththefindingsbyMoseretal.,20systolicblood
pressurewasdirectlycorrelatedtoBMIandinverselyrelated to adiponectin and HDL-C. This cluster of mediators cor-relatesvisceraladiposity,inflammation,insulinresistance, andhypertension,someofthecomponentsofthemetabolic syndrome, found in 11.7% of the obese group. A particu-larinterestingfindingwastheinversecorrelationbetween adiponectin and arterial hypertension, thus corroborating the findings of the study by Brambilla et al.,21 in which
adiponectinwasproposedasoneofthemechanismsrelated tohypertensioninchildhoodobesity.This impliesthatthe lossofitsantiatherogenicandanti-inflammatoryproperties contributetotheunderlyingmechanismsofobesity-related hypertension.
Furthermore,thelossofadiponectin’sinsulinsensitizing properties that occurs in obesity has also been impli-cated in insulin resistance.22 Unlike the findings by Lee
etal.,23 whereadiponectinwasfoundtobeastrong
inde-pendent predictor of insulin sensitivity in obese children and adolescents of different racial backgrounds, in the present study adiponectinwas shown to becorrelated to hyperinsulinaemia(r=−0.23; p=0.033),butnot toinsulin resistance (n=38%; p=0.277), despite strikingdifferences inadiponectinlevelsbetweentheobeseandcontrolgroups (p<0.001).
The present study included only whitesubjectsfrom a differentgeographicalarea,which,togetherwiththe sam-ple size (n=45 with HOMA-IR >3), might account for the observeddifferences,although adiponectingene polymor-phisms might also play a role. This hypothesis is merely speculative,asitwasnottestedinthepresentstudy. How-ever, this rationale could not beapplied toleptin, which had a stronger correlation to hyperinsulinaemia (r=0.35;
p<0.001) and insulin resistance (p<0.001), findings in accordancewithpreviouslypublishedworks.24Aconnection
between MCP-1 (p=0.168), hsCRP (p=0.375), and insulin resistancewasnotobserved.
The authors further attempted to relate ADMA and E-selectin,bothmarkersofendothelialactivation,toinsulin resistance.Inadults,raisedlevelsofasymmetric dimethy-larginineareconsideredacardiovascularriskmarker,andas asurrogateofnitricoxidebioavailability,reflects endothe-lial integrity. A direct correlation between HOMA-IR and ADMA (r=0.35; p=0.003) was observed, particularly evi-dent intheinsulin-resistant group(p=0.003),findings not previously reported. Some reports have suggested that ADMAcontributestoinsulinresistance asdecreasednitric oxide bioavailability reduces blood flow to insulin sensi-tive tissues compromising glucose uptake in response to insulin.25Conversely,insulinresistanceitselfhasbeenfound
tocontributetowardendothelial dysfunction.Underthese circumstances, itis temptingtospeculate onADMA’s per-missive role on the interplay between insulin resistance and endothelial dysfunction, through its actions onnitric oxide bioavailability. A direct correlation between leptin andADMAwasalsoobserved,butonly ingirls. Thisis not surprising,asleptinlevelsweresignificantlyhigherinobese girls (p<0.001). Nevertheless, this finding, not previously
reported,potentially links leptin, and henceadiposity, to endothelialactivation.Comparatively,linearregressionby logarithmic transformation showed that insulin resistance hadagreaterinfluenceonADMA.
E-selectin is a specific endothelial adhesion molecule whoselevelsareraisedinendothelialdysfunction, promot-ingthemigrationofinflammatorycellstotheintima.Inthis analysis,obese children andadolescents had significantly higherlevelsthanhealthycontrols, implyingthepresence ofendothelialactivation,whichindicatesanearlystageof atherosclerosis.Consideringtheentirecohort,adirect,but weakcorrelation tohsCRP (=0.21;p=0.033) wasfound,
reinforcing the role of inflammation in endothelial dys-functionandinagreementwithpublishedliterature,26 but
asimilar pattern wasnot demonstrated withinthe obese group,possiblydue toitshomogeneity.Wealsoobserved, withintheobesegroup,adirectcorrelationbetweenADMA and circulating EPC count. We found these correlations particularly interesting as they highlight various patho-physiological pathways related to endothelial activation, namely,inflammatorycelladhesiontriggeredbyE-selectin, increasedexpression of ADMA, whocompetitively inhibits eNOS,reducingnitricoxidebioavailabilityand,ultimately, repairofinjuredtissueby EPCS.The authorsbelievethat therelationshipbetweenE-selectinandADMA,inchildhood obesity,hasnotbeenpreviouslypublished.
Theterm EPCsshouldbereservedforaprogenitorcell restrictedspecifically totheendothelial lineage.In 1997, Asahara et al.27 reported for the first time on the
exist-enceof EPCs.To date,nospecific markerhasbeen found toclearlyidentifythesecells,whichmakestheirisolation controversial,astheiridentificationisbasedoncellsurface markersshared byother hematological celllines.As such variousmethodshavebeen usedtoidentifyEPCs.Inorder tominimizefalsepositivesvariouscellsurfacemarkershave beenintegratedtoidentifythesecells,namelythoselinked toimmaturecelllines(e.g.CD34)withendothelial commit-ment(e.g.kinaseinsertdomainreceptor).
Asreportedby variousauthors,thecountandfunction ofcirculatingEPCSnotonlycorrelateinverselywith cardio-vascularriskfactors,butalsomaypredicttheoccurrenceof cardiovascularevents,particularlyintheadultpopulation.28
Data regarding EPCs and childhood obesity is scarce. A reportbyJungetal.29observedadirectcorrelationto
E-selectin.Contrarytothepresentstudy,thiscorrelationonly appliedtoobeseadolescents.Inthepresentstudy,EPCsand E-selectin were directly correlated in the pre-adolescent obesegroup,afindingnotpreviouslyreportedandthat rein-forcesthefactthatendothelialdysfunctionispresentinvery youngindividuals.
Inthisanalysis,theobesegrouphadasignificantlyhigher numberofEPCs,implyingthatelevatedEPCsinobese chil-dren and adolescents represent an attempt at repairing underlyingvascular damage. Thus,at this stage,it would bepossibletoreversetheunderlyingendothelialdamageif stepsaretakentocontrolweight.
damage. The inference is that therapeutic interventions aimedatweightlossoughttobeaggressivelyinstitutedin order to reverse endothelial damage and obesity-related comorbidities, such as arterial hypertension and insulin resistance.
Thisstudyhasseverallimitations.Firstlyitssamplesize, particularlyitscontrolgroup.Italsoonlyincludeda white-onlypopulation,limitedtoaparticulargeographicalarea, theformerduetolocalracedistribution.Thestudyincluded bothgenders,aswellas,differentagegroups.Intheresults, these differences were adjusted for, and therefore their interferencewasnullified.
Conflict
of
interest
Theauthorsdeclarenoconflictsofinterest.
References
1.Kapiotis S, Holzer G, Schaller G, Haumer M, Widhalm H, WeghuberD,et al.A proinflammatorystateis detectablein obese children and is accompanied by functional and mor-phological vascular changes. Arterioscler Thromb Vasc Biol. 2006;26:2541---6.
2.PasceriV,WillersonJT,YehET.Directproinflammatoryeffect ofC-reactiveproteinonhumanendothelialcells.Circulation. 2000;102:2165---8.
3.Soriano-Guilén L, Hernández-Garcia B, Pita J, Dominguez-Garrido N, Del Rio-Camacho G, Rovira A. High-sensitivity C-reactive protein is a good marker of cardiovascular risk in obese children and adolescents. Eur J Endocrinol. 2008;159:R1---4.
4.Braunersreuther V, Mach F, Steffens S. The specific role of chemokines in atherosclerosis. Thromb Haemost. 2007;97:714---21.
5.Montero D, Walther G, Perez-Martin A, Roche E, Vinet A. Endothelialdysfunction,inflammation,andoxidativestressin obesechildrenandadolescents:markersandeffectoflifestyle intervention.ObesRev.2012;13:441---55.
6.AvogaroA,deKreutzenbergSV.Mechanismsofendothelial dys-functioninobesity.ClinChimActa.2005;360:9---26.
7.vanderZwanLP,SchefferPG,DekkerJM,StehouwerCD,Heine RJ,TeerlinkT.Systemicinflammationislinkedtolowarginine andhighADMAplasmalevelsresultinginanunfavourableNOS substrate-to-inhibitor ratio:theHoornStudy. ClinSci(Lond). 2011;121:71---8.
8.YoderMC.Humanendothelialprogenitorcells.ColdSpringHarb PerspectMed.2012;2:a006692.
9.Müller-EhmsenJ,BraunD,SchneiderT,PfisterR,WormN, Wiel-ckensK,etal.Decreasednumberofcirculatingprogenitorcells inobesity:beneficialeffectsofweightreduction.EurHeartJ. 2008;29:1560---8.
10.Hill JM, Zalos G, Halcox JP, Schenke WH, Waclawiw MA, Quyyumi AA, et al. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N Engl J Med. 2003;348:593---600.
11.TsaiTH,ChaiHT,SunCK,YenCH,LeuS,ChenYL,etal.Obesity suppressescirculatinglevelandfunctionofendothelial progen-itorcellsandheartfunction.JTranslMed.2012;10:137.
12.VermaS,KuliszewskiMA,LiSH,SzmitkoPE,ZuccoL,WangCH, etal.C-reactiveproteinattenuatesendothelialprogenitorcell survival,differentiation, and function:furtherevidenceof a mechanisticlinkbetweenC-reactiveproteinandcardiovascular disease.Circulation.2004;109:2058---67.
13.ButteNF,GarzaC,deOnisM.Evaluationofthefeasibilityof internationalgrowth standards for school-aged children and adolescents.JNutr.2007;137:153---7.
14.ZimmetP,AlbertiG,KaufmanF,TajimaN,SilinkM,Arslanian S,etal.Themetabolicsyndromeinchildrenandadolescents. Lancet.2007;369:2059---61.
15.Romualdo MC, de Nóbrega FJ, Escrivão MA. Insulin resis-tance in obese children and adolescents. J Pediatr (Rio J). 2014;90:600---7.
16.Deshmane SL, Kremlev S, Amini S, Sawaya BE. Monocyte chemoattractantprotein-1(MCP-1):anoverview.JInterferon CytokineRes.2009;29:313---26.
17.RullA,CampsJ,Alonso-VillaverdeC,JovenJ.Insulinresistance, inflammation,andobesity:roleofmonocytechemoattractant protein-1(orCCL2)intheregulationofmetabolism.Mediators Inflamm.2010;2010,pii:326580.
18.BreslinWL,JohnstonCA,StrohackerK,CarpenterKC,Davidson TR,MorenoJP,et al.ObeseMexican Americanchildrenhave elevatedMCP-1,TNF-␣,monocyteconcentration,and dyslipid-emia.Pediatrics.2012;129:e1180---6.
19.Kotchen TA. Obesity-related hypertension: epidemiology, pathophysiology, and clinicalmanagement. Am JHypertens. 2010;23:1170---8.
20.MoserDC,GiulianoIdeC,TitskiAC,GayaAR,Coelho-e-SilvaMJ, LeiteN.Anthropometricmeasuresandbloodpressureinschool children.JPediatr(RioJ).2013;89:243---9.
21.Brambilla P, Antolini L, Street ME, Giussani M, Galbiati S, Valsecchi MG, et al. Adiponectin and hypertension in normal-weightandobesechildren.AmJHypertens.2013;26: 257---64.
22.ChiarelliF,MarcovecchioML.Insulinresistanceandobesityin childhood.EurJEndocrinol.2008;159:S67---74.
23.LeeS,BachaF,GungorN,ArslanianSA.Racial differencesin adiponectinin youth: relationship to visceralfat and insulin sensitivity.DiabetesCare.2006;29:51---6.
24.SteinbergerJ,SteffenL,JacobsDRJr,MoranA,HongCP,Sinaiko AR.Relationofleptintoinsulinresistancesyndromeinchildren. ObesRes.2003;11:1124---30.
25.SydowK,MondonCE,CookeJP.Insulinresistance:potentialrole oftheendogenousnitricoxidesynthaseinhibitorADMA.Vasc Med.2005;10:S35---43.
26.BruyndonckxL,HoymansVY,VanCraenenbroeckAH,VissersDK, VrintsCJ,RametJ,etal.Assessmentofendothelial dysfunc-tioninchildhoodobesityandclinicaluse.OxidMedCellLongev. 2013;2013:174782.
27.AsaharaT,MuroharaT,SullivanA,SilverM,vanderZeeR,Li T,etal.Isolation ofputativeprogenitorendothelialcellsfor angiogenesis.Science.1997;275:964---7.
28.WernerN,KosiolS,SchieglT,AhlersP,WalentaK,LinkA,etal. Circulatingendothelialprogenitorcellsandcardiovascular out-comes.NEnglJMed.2005;353:999---1007.