O R I G I N A L A R T I C L E EXPERIMENTAL/SPECIAL TOPICS
Effects of Kaurenoic Acid and Arginine on Random Skin Flap
Oxidative Stress, Inflammation, and Cytokines in Rats
Joaquim Jose´ de Lima Silva1•De´bora Gramosa Pompeu2• Naiara Coelho Ximenes3•Antoniella Souza Gomes Duarte4• Nilce Viana Gramosa5•Krishnamurti de Morais Carvalho6• Gerly Anne de Castro Brito4• Sergio Botelho Guimara˜es1
Received: 9 May 2015 / Accepted: 18 August 2015 / Published online: 25 September 2015
ÓSpringer Science+Business Media New York and International Society of Aesthetic Plastic Surgery 2015
Abstract
Background Kaurenoic acid (KA), a diterpene extracted from copaı´ba oil-resin, is known to have potent antioxidant and anti-inflammatory properties. L-Arginine (LA) is an amino acid and a nitrogenous precursor for the synthesis of nitric oxide (NO). NO paper in wound healing has already been well documented. The aim of this study was to investigate the protective effects of LA and KA against ischemia reperfusion injury in a randomized skin flap model in rats.
Methods A modified McFarlane flap model measuring 2.5 wide98 cm long was established in 36 anesthetized rats and evaluated within 3 groups: group control, group L-arginine, and group kaurenoic acid. Each group was
subdivided into two subgroups (T1 and T2, n =6 each). Samples were collected 24 h (T1)/48 h (T2) postopera-tively for oxidative stress (glutathione), as non-protein thiols, malondialdehyde (MDA), NO2, inflammation
[myeloperoxidase (MPO)], and cytokines TNF-aand IL-1b
assays.
Results KA promoted a significant decrease of TNF-a
and IL-1 expression and MPO activity atT1/T2 time points. NSGH levels increased significantly in KA-treated rats, while MDA levels decreased significantly in the same rats. Arginine promoted a significant decrease in MDA levels at theT1 time point and a significant increase in non-protein thiols concentrations at T1/T2 time points. NO2
concen-tration also decreased at theT1 time point.
& Sergio Botelho Guimara˜es sergiobotelho@terra.com.br Joaquim Jose´ de Lima Silva
cirurgiaoplastico@joaquimjose.med.br De´bora Gramosa Pompeu
deboragpompeu@gmail.com Naiara Coelho Ximenes naiaracx@gmail.com
Antoniella Souza Gomes Duarte asouzagomes@yahoo.com.br Nilce Viana Gramosa
nilcegramosapompeu@yahoo.com.br Krishnamurti de Morais Carvalho carvalhokris@gmail.com Gerly Anne de Castro Brito gerlybrito@hotmail.com
1 Department of Surgery, Federal University of Ceara´ (UFC), Rua Professor Costa Mendes, 1608/38andar, Bloco Dida´tico, Fortaleza, CE 60430-140, Brazil
2 Course of Chemical Engineering (UFC), Av. da Universidade, 2853 - Benfica, Fortaleza, CE 60020-181, Brazil
3 Faculty of Pharmacy, Dentistry and Nursing (UFC), Rua Alexandre Barau´na, 949 - Rodolfo Teo´filo, Fortaleza, CE 60430-160, Brazil
4 Department of Morphology (UFC), Rua Delmiro de Farias s/n - Rodolfo Teo´filo, Fortaleza, CE 60416-030, Brazil 5 Department of Organic and Inorganic Chemistry (UFC),
Campus do Pici, bloco 940 Bairro Pici, Fortaleza, CE 60451-970, Brazil
6 Clinical Pharmacology and Molecular Laboratory, School of Medicine, Ceara State University (UECE), Av. Dr. Silas Muguba, 1700 - Campus do Itaperi, Fortaleza, CE 60740-000, Brazil
Conclusions KA may attenuate the oxidative stress and the inflammation, thereby reducing tissue damage induced by ischemia/reperfusion in rats subjected to dorsal skin flaps.
No Level Assigned This journal requires that authors assign a level of evidence to each submission to which Evidence-Based Medicine rankings are applicable. This excludes Review Articles, Book Reviews, and manuscripts that concern Basic Science, Animal Studies, Cadaver Studies, and Experimental Studies. For a full description of these Evidence-Based Medicine ratings, please refer to the Table of Contents or the online Instructions to Authors
http://www.springer.com/00266.
Keywords Cutaneous flapsKaurenoic acidL-Arginine
Inflammatory responseOxidative stressRatsWistar
Introduction
Necrosis of the distal part of skin flaps is an unsolved problem, even today, in reconstructive plastic surgery and aesthetics [1]. Many attempts were made to improve the survival rate of skin flaps after ischemia–reperfusion injury using substances that decrease or inhibit the formation of free radicals or neutrophil-mediated damage [1,2].
L-arginine, 2-amino-5-guanidinovaleric acid (LA), a semi-essential amino acid, is the nitrogenous precursor for the synthesis of nitric oxide (NO) by a NADPH-dependent NO synthase (NOS) and regulates vital metabolic pathways [3]. NO paper in wound healing has already been well documented [4,5]. LA is known to promote wound healing and has several immunomodulatory effects [6].
Copaifera langsdorffii Desf. (Leguminosae) is a large tree that grows abundantly in northern Brazil. Pharmaco-logic studies of the copaı´ba oil-resin demonstrated anti-inflammatory activity and healing of skin wounds of its largest representative diterpene, named kaurenoic acid (KA) [7, 8]. Phytochemical studies showed a discrete presence (8 %) of essential oils and an important occur-rence (70 %) of two diterpenes [kaurenoic (30 %) and politic (40 %) acids] in the copaı´ba oil-resin [7,8].
Several studies have demonstrated the broad spectrum of biological activities of KA, including antibacterial activity [9]; anticonvulsant properties [10]; and anti-in-flammatory, antioxidant, and anti-peroxidative activities [11]. Lima-Silva et al. [12] investigated the effects of the copaı´ba oil-resin in an experimental model of randomized skin flaps. The oil-resin was obtained from theCopaifera langsdorffii genus (Leguminosae) and administered by gavage to rats. The researchers concluded that the copaı´ba oil-resin presented a discrete antilipoperoxidative activity and an intense antioxidant activity as well as an
anti-inflammatory activity in the experimental model studied. Bearing in mind that LA is known to promote wound healing [6] and that the oral administration of KA to rats has been reported to confer protection against ischemia– reperfusion injury, this study aimed to investigate the effect of KA and LA on oxidative stress and inflammation using a modified McFarlane skin flap model [13].
Materials and Methods
Ethical Considerations, Animal Preparation, and Experimental Groups
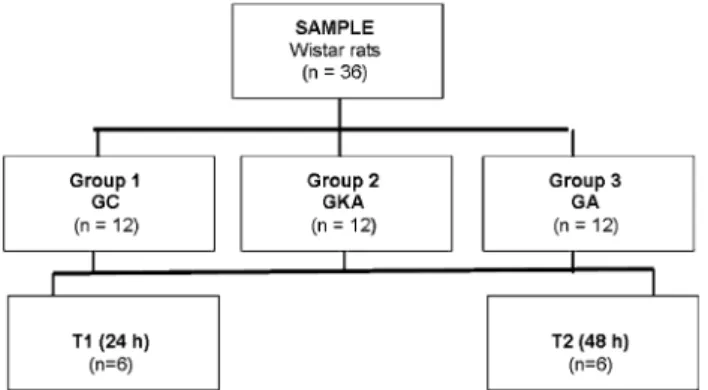
All applicable institutional and/or national guidelines for the care and use of animals were followed. Approval by the local ethics committee (protocol #64/2008) was obtained. Thirty-six male Wistar rats weighing 170–285 g were used in this study. Animals were housed in separate cages at 25°C and were fed with rat chow and tap water ad libitum.
Rats were randomly allocated into three groups: group control (GC), groupL-arginine (GA), and group kaurenoic acid (GKA). Each group was subdivided into two sub-groups (T1;T2).
Experimental Protocol
Animals were anesthetized with an intraperitoneal injection of a combination of ketamine (100 mg/ml) and xylazine (20 mg/ml) at a total dose of 0.2 ml/100 g of body weight. Dorsal hair was removed with an electric clipper, and all surgical procedures were performed under sterile condi-tions. A McFarlane-type caudally based skin flap, mea-suring 2.5 wide98 cm long as proposed by Sarifakioglu et al. [13], was designed on the dorsum of the rat using the iliac crest as a constant anatomic landmark to ensure proper positioning. The flap was elevated beneath the panniculus carnosus, and each flap was then sutured back to its donor site immediately with continuous 5/0 nylon sutures.
KA was administered by gavage (100 mg/kg in DMSO 3 %) and LA (0.5 g/kg in distilled water) was administered intraperitoneally (i.p.) to GKA and GA rats, respectively, 2 h before surgery (subgroups T1 and T2) and again 24 h postoperatively (subgroupT2). Control group rats received saline 2.0 ml i.p. 2 h before surgery (subgroupsT1 andT2) and again 24 h postoperatively (subgroup T2). Tissue samples measuring 10 cm2were collected from the mid 1/3 portion of the skin flap at postoperative day 1 (subgroup
T1) and postoperative day 2 (subgroupT2). Tissue samples were snap-frozen in liquid nitrogen and stored in glass tubes at-70°until subsequent preparation and analysis of
Chemicals
LA, (S)-2-amino-5-guanidinopentanoic acid, was pur-chased from Sigma-Aldrich, USA. KA was provided by Prof. Nilce Viana Pompeu de Sousa, Brazil (Department of Organic and Inorganic Chemistry, Federal University of Ceara´, Brazil) who extracted the KA from the copaiba oil (Copaifera langsdorffii Sp.). IL-1b and TNF-aELISA Kit DuoSet kit was purchased from R&D Systems, Min-neapolis, MN, USA.
Biochemical Assays
Skin homogenates were assayed for levels of IL-1b and TNF-a cytokines; GSH, as non-protein thiols (NPSH), MDA, myeloperoxidase activity (MPO) activities; and NO2. The levels of IL-1band TNF-ain the samples were
determined with a commercial ELISA DuoSet kit (R&D Systems, Minneapolis, MN, USA) according to the man-ufacturer’s recommendations. Non-protein SH (NPSH, namely GSH) levels were measured by the Sedlak and Lindsay procedure [14]. The MDA levels were assayed for the products of lipid peroxidation by monitoring thiobar-bituric acid reactive substance formation as previously described [15]. MPO activity in skin samples was mea-sured according to the technique described by Bradley, Christensen, and Rothstein [16], using one hydrogen per-oxide 1 % as a substrate for the MPO. NO was indirectly determined by measuring nitrite content in the skin homogenate using Griess reagent [17].
Statistical Analysis
Statistical analysis was performed using Graphpad Prism 5.0 (GraphPad Software, San Diego California USA,www. graphpad.com). All data were tested for distribution using the Kolmogorov–Smirnov test with Dallal–Wilkinson– Lillie forpvalue. One-way analysis of variance followed by a Dunnett test was used for comparing groups.p\0.05
was considered to be significant (Fig.1).
Results
Effects of Kaurenoic Acid andL-Arginine
on the Expression of TNF-aand IL-1 in the Skin of Rats
TNF-a expression decreased significantly at the T1 time point in GKA compared with GC (115.1±33.94 vs. 193.9±40.53, p\0.01) (Fig.2) and at 48 h
postopera-tively (T2) (105.4±25.93 vs. 208.7±58.13, p\0.01)
(Fig.3). IL-1 expression was significantly decreased at the
T1 time point in GKA compared with GC (73.5±30.53 vs. 358.0±105.71,p\0.001) (Fig.4) and at 48 h
post-operatively (T2) (169.1±55.94 vs. 332.6±88.58,
p\0.001) (Fig.5).
Effects of Kaurenoic Acid andL-Arginine on MDA
Concentrations in the Skin of Rats
Both KA and LA promoted a significant decrease of MDA concentrations at the T1 time point (0.011±0.003 vs. 0.056±0.011,p\0.001 and 0.017±0.008 vs. 0.056±
0.011,p\0.001, respectively) (Fig. 6). KA also promoted a
significant decrease of MDA at 48 h postoperatively (T2) (0.015±0.004 vs. 0.031±0.008,p\0.01) (Fig.7).
Effects of Kaurenoic Acid andL-Arginine on NPSH
Concentrations in the Skin of Rats
Both KA and LA promoted a significant increase in NPSH concentrations at the T1 time point (21.220 ±4.59 vs. Fig. 1 Animal distribution, treatments, and sample colleting time points. GCgroup control,GKA group kaurenoic acid,GAgroupL -arginine
7.185±3.66, p\0.001 and 14.570±4.98 vs. 7.185±
3.66, p\0.05, respectively) (Fig.8) and at 48 h
postop-eratively (T2) (19.920±3.75 vs. 5.830±2.99, p\
0.0001 and 12.710±5.83 vs. 5.830±2.99, p\0.05)
(Fig.9).
Effects of Kaurenoic Acid andL-Arginine on NO2 Levels in the Skin of Rats
There was a significant reduction of the NO2level in GKA
(6.67±3.00 vs. 13.6±4.87, p\0.01) and GA Fig. 3 Expression of TNF-a in rat skin at T2 time point. Bars
represent mean±SD values for each group (GCgroup control,GKA group kaurenoic acid,GAgroupL-arginine). TNF-aexpressed as pg/ mL ANOVA/Dunnett tests. **p\0.01, compared with control
Fig. 4 Expression of IL-1 in rat skin atT1 time point.Barsrepresent mean±SD values for each group (GCgroup control, GKA group kaurenoic acid, GA group L-arginine). IL-1 expressed as pg/mL ANOVA/Dunnett tests. **p\0.001, compared with control
Fig. 5 Expression of IL-1 in rat skin atT2 time point.Barsrepresent mean±SD values for each group (GCgroup control, GKA group kaurenoic acid, GA group L-arginine). IL-1 expressed as pg/mL ANOVA/Dunnett tests. **p\0.001, compared with control
Fig. 6 Levels of MDA in rat skin at T1 time point.Barsrepresent mean±SD values for each group (GCgroup control, GKA group kaurenoic acid,GAgroupL-arginine). MDA expressed as microMol malondialdehyde/g/skin. ANOVA/Dunnett tests. **p\0.001,
com-pared with control; ***p\0.0001, compared with control
Fig. 7 Levels of MDA in rat skin at T2 time point.Barsrepresent mean±SD values for each group (GCgroup control, GKA group kaurenoic acid,GAgroupL-arginine). MDA expressed as microMol malondialdehyde/g/skin ANOVA/Dunnett tests. **p\0.001,
(3.83±2.38 vs. 13.6 ±4.87, p\0.0001) at the T1 time
point (Fig.10). No significant changes occurred at the T2 time point (p[0.05).
Effects of Kaurenoic Acid andL-Arginine on MPO
Activity in the Skin of Rats
KA promoted a significant decrease of MPO activity, at the
T1 time point (0.867±0.39 vs. 4.312±2.51, p\0.05)
and at theT2 time point (0.734 ±0.55 vs. 3.849±1.93,
p\0.05) (Figs.11,12).
Fig. 8 Levels of non-protein thiols (NPSH) in rat skin atT1 time point.Barsrepresent mean±SD values for each group (GCgroup control, GKA group kaurenoic acid, GA group L-arginine). NPSH expressed as mg NPSH/g/skin. ANOVA/Dunnett tests. *p\0.05,
compared with control; ***p\0.0001, compared with control
Fig. 9 Levels of non-protein thiols (NPSH) in rat skin atT2 time point.Barsrepresent mean±SD values for each group (GCgroup control, GKA group kaurenoic acid, GA group L-arginine). NPSH expressed as mg NPSH/g/skin. ANOVA/Dunnett tests. *p\0.05,
compared with control; ***p\0.0001, compared with control
Fig. 10 NO2 levels in rat skin at T1 time point. Bars represent mean±SD values for each group (GC group control,GKA group kaurenoic acid,GAgroupL-arginine). Values expressed as micromol NO2/24 h. ANOVA/Dunnett tests. **p\0.001, compared with control
Fig. 11 Myeloperoxidase (MPO) levels in rat skin atT1 time point.Bars represent mean±SD values for each group (GCgroup control,GKA group kaurenoic acid, GAgroupL-arginine). Values expressed as U MPO/g skin. ANOVA/Dunnett tests. *p\0.05, compared with control
Fig. 12 Myeloperoxidase (MPO) levels in rat skin atT2 time point. Barsrepresent mean±SD values for each group (GCgroup control, GKAgroup kaurenoic acid,GAgroupL-arginine). Values expressed as U MPO/g skin. ANOVA/Dunnett tests. *p\0.001, compared with
Discussion
The inflammatory process involves the release of pro-in-flammatory and anti-inpro-in-flammatory cytokines. Cytokines are released in a cascade. Initial cytokines released include pro-inflammatory tumor necrosis factor-a (TNF-a) and interleukin-1 IL-1ß. TNF-a is a member of a group of cytokines that stimulate the acute phase reaction in sys-temic inflammation and is produced mainly by activated macrophages, although it can be produced by other cell types, such as CD4?lymphocytes and NK cells [18]. Studies have demonstrated that after endotoxin infusion, the levels of TNF-apeak in 60–90 min [9,10]. Interleukin-1 (IL-Interleukin-1) consists of two different proteins, IL-Interleukin-1aand IL-1b. IL-1b is produced, processed, and secreted from activated immune cells and plays a major role in the initiation of local and systemic inflammatory processes [19,20].
We tested the hypothesis that KA, an active fraction of the copaiba oil-resin, may attenuate skin flap oxidative stress and inflammation. We verified that the use of KA promoted a significant decrease of TNF-a(Figs.2,3) and IL-1 (Figs.4,5) expressions, atT1 andT2 time points. The use of LA did not alter TNF-a or IL-1 expression.
GSH is the dominant NPSH in mammalian cells; it is the primary endogenous free radical scavenger in the human body and is the brain’s major antioxidant system. Reduc-tion of GSH levels in the presence of increased concen-trations of free radicals ensures an increased potential for cellular oxidative stress [21]. In our study, the use of KA promoted intense antioxidative and anti-peroxidative effects considering that NSGH levels increased signifi-cantly in KA-treated rats, while MDA levels decreased significantly in the same rats. The production of NO, synthesized from LA, is usually evaluated by measuring nitrates and nitrites as the final stable oxidized products of its metabolism [18,20].
MPO, a leukocyte-derived heme peroxidase, is primarily hosted in human polymorphonuclear neutrophils, mono-cytes, and tissue macrophages. MPO is stored in the azurophilic granules of PMN and monocytes. During cel-lular activation and degranulation, MPO is released into phagocytic vacuoles as well as into the extracellular space. MPO activity not only enhances host defenses by mediat-ing efficient microbial killmediat-ing but also can contribute to progressive tissue damage in chronic inflammatory states [22]. Preventing or decreasing neutrophil invasion in ischemic tissue by blocking any step of neutrophil activa-tion reduces tissue MPO enzyme activity [23].
We hypothesize that the significant decrease in MPO activity seen in GKA at theT1 andT2 time points could be related to a decrease in the generation of free radicals (ROS) and reactive nitrogen species (RNS) induced by KA.
It is known that early after tissue trauma, a significant burst of ROS and RNS generation takes place in the early period of reperfusion after ischemic insult resulting in an activated inflammatory reaction including neutrophil accumulation [24].
The production of NO, synthesized from LA by NOS catalytic action, is usually evaluated by measuring nitrates and nitrites as the final stable oxidized products of its metabolism [25]. The sum of nitrites and nitrates (named NOx) present in a biological fluid has been considered a good indicator of NO formation [26,27].
NO2levels decreased at the 24 h time point in the skin
of rats treated with KA suggesting an antioxidative activity of the diterpene.
Conclusion
In light of our study, we conclude that both KA and LA present moderate antilipoperoxidative action along with intense antioxidant activity during ischemia and reperfu-sion of randomized skin flaps in rats. Anti-inflammatory activity was also found in rats treated with KA.
Compliance with Ethical Standards
Conflict of interest The authors declare that they have no conflict of interest.
References
1. Hart K, Baur D, Hodam J, Lesoon-Wood L, Parham M, Keith K, Vasquez R, Ager E, Pizzarro J (2006) Short- and long-term effects of sildenafil on skin flap survival in rats. Laryngoscope 116:522–528
2. Huemer GM, Wechselberger G, Otto-Schoeller A, Gurunluogh R, Piza-Katzer H, Schoeller T (2003) Improved dorsal random-pattern skin flap survival in rats with a topically applied combi-nation of Nonivamide and Nicoboxil. Plast Reconstr Surg 111(3):1207–1211
3. Wood KC, Hsu LL, Gladwin MT (2008) Sickle cell disease vasculopathy: a state of nitric oxide resistance. Free Radic Biol Med 44(8):1506–1528
4. Lee RH, Eferon D, Tantry U, Barbul A (2001) Nitric oxide in the healing wound: a time-course study. J Surg Res 101:104–108 5. Dal Secco D, Paron JA, de Oliveira SHP, Ferreira SH, Silva JS,
de Queiroz-Cunha F (2004) Neutrophil migration in inflamma-tion: nitric oxide inhibits rolling, adhesion and induces apoptosis. Nitric Oxide 9:153–164
6. Zandifar A, Seifabadi S, Zandifar E, Beheshti SS, Aslani A, Javanmard SH (2015) Comparison of the effect of topical versus systemicL-arginine on wound healing in acute incisional diabetic rat model. J Res Med Sci 20(3):233–238
8. Paiva LA, de Alencar Cunha KM, Santos FA, Gramosa NV, Silveira ER, Rao VS (2002) Investigation on the wound healing activity of oleo-resin from Copaifera langsdorffi in rats. Phytother Res 16(8):737–739
9. Velikova A, Bankova V, Tsvetkovab I, Kujumgievb A, Marcucci MC (2000) Antibacterial ent-kaurene from Brazilian propolis of native stingless bees. Fitoterapia 71:693–696
10. Okoye TC, Akah PA, Omeje EO, Okoye FBC, Nworu CS (2013) Anticonvulsant effect of kaurenoic acid isolated from the root bark ofAnnona senegalensis. Pharmacol Biochem Behav 109:38–43 11. Paiva LA, Gurgel LA, De Sousa ET, Silveira ER, Silva RM,
Santos FA, Rao VS (2004) Protective effect of Copaifera langsdorffiioleo-resin against acetic acid-induced colitis in rats. J Ethnopharmacol 93(1):51–56
12. de Lima Silva JJ, Guimara˜es SB, da Silveira ER, de Vasconcelos PR, Lima GG, Torres SM, de Vasconcelos RC (2009) Effects of Copaifera langsdorffii Desf. on ischemia-reperfusion of ran-domized skin flaps in rats. Aesthet Plast Surg 33:104–109 13. Sarifakioglu N, Gokrem S, Ates L, Akbuga UB, Aslan G (2004)
The influence of sildenafil on random skin flap survival in rats: an experimental study. Br J Plast Surg 57:769–772
14. Sedlak J, Lindsay RH (1968) Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman’s reagent. Anal Biochem 25(1):192–205
15. Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86(1):271–278
16. Bradley PP, Christensen RD, Rothstein G (1982) Cellular and extracellular myeloperoxidase in pyogenic inflammation. Blood 60:618–622
17. Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR (1982) Analysis of nitrate, nitrite, and [15 N] nitrate in biological fluids. Anal Biochem 126(1):131–138 18. Perry RT, Collins JS, Wiener H, Acton R, Go RCP (2001) The
role of TNF and its receptors in Alzheimer’s disease. Neurobiol Aging 22(6):873–883
19. Dinarello CA (2009) Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol 27:519–550 20. Frey BN, Andreazza AC, Kunz M, Gomes FA, Quevedo J,
Sal-vador M, Gonc¸alves CA, Kapczinski F (2007) Increased oxidative stress and DNA damage in bipolar disorder: a twin-case report. Prog Neuropsychopharmacol Biol Psychiatry 31(1):283–285 21. Dringen R (2000) Metabolism and functions of glutathione in
brain. Prog Neurobiol 62:649–671
22. Hansson M, Olsson I, Nauseef WM (2006) Biosynthesis, pro-cessing, and sorting of human myeloperoxidase. Arch Biochem Biophys 445:214–224
23. Cetin C, Ko¨se AA, Aral E, Colak O, Erc¸el C, Karabag˘li Y, Alatas¸ O, Eker A (2001) Protective effect of fucoidin (a neutrophil rolling inhibitor) on ischemia reperfusion injury: experimental study in rat epigastric island flaps. Ann Plast Surg 47(5):540–546 24. Park JW, Qi WN, Liu JQ, Urbaniak JR, Folz RJ, Chen LE (2005) Inhibition of iNOS attenuates skeletal muscle reperfusion injury in extracellular superoxide dismutase knockout mice. Micro-surgery 25:606–613
25. Moshages H, Kok B, Huizenga JR, Jansen PL (1995) Nitrite and nitrate determinations in plasma: a critical evaluation. Clin Chem 41:892–896
26. Zunic G, Pavlovic R, Malicevic Z, Savic V, Cernak I (2000) Pulmonary blast injury increases nitric oxide production, disturbs arginine metabolism, and alters the plasma free amino acid pool in rabbits during the early posttraumatic period. Nitric Oxide 4(2):123–128