Revisão
*e-mail: npelopes@fcfrp.usp.br
#This paper is dedicated to Prof. Hans Viertler
LYCHNOPHORINAE (ASTERACEAE): A SURVEY OF ITS CHEMICAL CONSTITUENTS AND BIOLOGICAL ACTIVITIES#
Larissa Costa Keles, Nathalya Isabel de Melo, Gabriela de Paula Aguiar, Kamila Akemi Lima Wakabayashi, Carlos Eduardo de Carvalho, Wilson Roberto Cunha e Antônio Eduardo Miller Crotti
Núcleo de Pesquisas em Ciências Exatas e Tecnológicas, Universidade de Franca, 14404-600 Franca - SP, Brasil João Luis Callegari Lopes e Norberto Peporine Lopes*
Departamento de Física e Química, Faculdade de Ciências Farmacêuticas de Ribeirão Preto, Universidade de São Paulo, Av. do Café, s/n, 14040-903 Ribeirão Preto – SP, Brasil
Recebido em 1/7/10; aceito em 21/9/10; publicado na web em 27/10/10
LYCHNOPHORINAE (ASTERACEAE): A SURVEY OF ITS CHEMICAL CONSTITUENTS AND BIOLOGICAL ACTIVITIES. This work reviews the current literature about the chemical constituents and the biological activities of the subtribe Lychnophorinae (Vernonieae, Asteraceae). The notable secondary metabolites are sesquiterpene lactones of furanoheliangolide (goyazensolide and eremantholide types) and lavonoids. Some of its most investigated activities include its anti-inlammatory, analgesic, antimicrobial and cytotoxic activities, specially for the Lychnophora and Eremanthus species. The data presented on this paper not only displayed the role played by the Lychnophorinae species as a source of bioactive compounds, but also reinforced the need of further studies involving the species of such subtribe.
Keywords: Lychnophorinae; Lychnophora; sesquiterpene lactones.
INTRODUCTION
The subtribe Lychnophorinae belongs to the tribe Vernonieae of Asteraceae (Compositae). Most of its genera is found on the Brazilian’s rupestrian vegetation (Table 1), which is present on the top of mountain chains on the Central and Southeastern regions of Brazil, especially in Minas Gerais, Bahia and Goiás, all presenting many species1 with a high degree of endemism.
Due to the taxonomic complexity of the Lychnophorinae, the ge-nera of this subtribe have received considerable attention and several previous classiication had to be changed.2-4 In the most recent literatu-re literatu-review of Lychnophorinae, Robinson3 considered the following ten genera: Anteremanthus H. Robinson, Chronopappus DC, Eremanthus Less. (syn. Sphaerophora Schultz-Bip = Paralychnophora MacLeish), Vanillosmopsis Schultz-Bip, Lychnophora Mart. (syn. Haplostephium Mart.), Lychnophoriopsis Schultz-Bip. (syn Episcothamnus H. Ro-binson), Minasia H. Robinson, Piptolepis Schultz-Bip, Pithecoseris Mart., and Proteopsis Mart & Zucc. ex Schultz-Bip.
The terpenoid chemistry of this subtribe is mainly formed by furanoheliangolide sesquiterpene lactones (STL).4 The biological activities of these compounds were extensively investigated in the literature. Some species of the subtribe Lychnophorinae have also been employed in Brazilian traditional medicine. For example, the Lychnophora species, popularly known as “arnica da serra” or “falsa arnica” are used as analgesic and anti-inlammatory agents.5 Some species also have an economic importance, such as Eremanthus erythropappa, popularly known as “candeia” in Portuguese, whose essential oil is rich in α-bisabolol and it is used in several cosmetic preparations.6
Based on the economical and social importance of the subtribe Lychnophorinae, the goal of this work is to present a current litera-ture review on the secondary metabolites and biological activities of the species of such subtribe in order to provide a basis for several
different research areas such as botanic, pharmaceutical, medical and chemical ields.
SECONDARY METABOLITES OF LYCHNOPHORINAE
The chemical composition of Eremanthus and Lychnophora, the two most numerous genera of Lychnophorinae have been extensively investigated, as clearly seen by the number of papers dealing with this matter. On the other hand, some genera of this subtribe (i.e., Anteremanthus, Chronopappus and Pithecoseris) have not been submitted to phytochemical studies yet. Previous studies have been mostly focused on the investigation of extracts from the aerial parts or leaves of this subtribe, although a number of studies on roots have also been reported.7-16 The secondary metabolites mentioned earlier were usually identiied and/or isolated from extracts obtained by ma-ceration with non-polar (n-hexane or petroleum ether) or moderately polar solvents (i.e., dichloromethane, chloroform, and ethyl acetate). Nevertheless, more recent studies have focused on the chemical com-position of alcoholic and hydroalcoholic extracts.11,12,17,18 Alternative extraction methods, such as glandular trichomes microsampling19 and sonication-assisted extraction20 have also been investigated.
Figure 1 shows the main groups of secondary metabolites that have been described for the Lychnophorinae species, and theirs struc-tures are shown in Figures 5-21. Terpenoids (70.2%) and lavonoids (16.9%) are the notable secondary metabolites of Lychnophorinae, although acetylene derivatives (2.6%), quinic acid derivatives (5.0%), benzoic acid derivatives (0.4%), phenylpropanoids (1.4%), and lignans (1.2%) have also been reported. It must be mentioned that the lack of data on the chemical composition of polar fractions and hydroalcoholic and aqueous extracts have direct inluence on the number of occurrences of such metabolite groups in literature.
Table 1. Chemical constituents isolated and/or identiied from species of the subtribe Lychnophorinae*
Species Chemical constituents Ref.
Eremanthus arboreus (Gardner) MacLeish [= Vanillosmopsis arborea (Gardner) Baker]
19w-EO, 25w-EO, 27w-EO/w-Hex, 28w-Hex, 37w-EO, 39w-EO, 40w-EO, 75w-EO, 77w-EO, 80w-EO, 126ap-EtOAc,
127ap-EtOAc, 137ap-EtOAc, 235w-EO, 236w-EtOH, 237w-EtOH, 266ap-EtOAc
46
E. argenteus McLeish & H. Schumacher 94ils-CHCl
³, 96ils-CHCl³, 135ils-CHCl³, 136ils-CHCl³, 276ils-CHCl³, 291ils-CHCl³,296ils-CHCl³ 47 E. bicolor (DC.) Baker 83 ap-PE, 88ap-PE, 102ap-PE, 104ap-PE, 116ap-PE, 119ap-PE, 121ap-PE, 139ap-PE, 141ap-PE, 146ap-PE, 147r-PE,
179r-PE, 184r-PE, 185r-PE, 186r-PE, 202ap-PE, 203r-PE/ap-PE, 225r-PE, 229r-PE, 230r-PE , 231r-PE
8
E. brasiliensis (Gardner) MacLeish[ =Vanil-losmopsis brasiliensis (Gardner) Sch. Bip.]
34ap-PE, 69ap-PE, 84ap-PE, 113ap-PE, 179ap-PE, 201ap-PE, 202ap-PE, 203ap-PE, 230ap-PE, 231ap-PE, 289ap-PE 16
E. cinctus Baker 190i-CHCl
³, 203is-CHCl³, 216is-CHCl³, 219is-CHCl³, 221is-CHCl³, 224is-CHCl³,226es-CHCl³, 234l-MeOH, 257i-MeOH,
272es-MeOH, 319l-MeOH/i-MeOH
48
E. crotonoides (DC.) Sch. Bip. 34ap-PE, 45ap-PE, 52ap-PE, 69ap-PE, 83ap-PE, 86ap-PE, 88ap-PE, 91ap-PE, 104ap-PE, 105ap-PE, 116ap-PE, 119ap-PE,
121ap-PE, 135ap-PE, 200ap-PE, 202ap-PE, 203ap-PE, 215ap-PE, 216ap-PE
49
E. elaeagnus (Mart. ex. DC.) Sch. Bip. 27l-MeOH, 83l-MeOH, 84l-MeOH, 101l-MeOH, 102l-MeOH, 106l-MeOH, 107l-MeOH, 116l-MeOH/s-MeOH:H ²
O,
119l-MeOH/s-MeOH:H
²O, 125l-MeOH/s-MeOH:H²O, 141l-MeOH, 149l-EtOAc/l-MeOH, 150l-EtOAc, 151l-EtOAc, 152l-EtOAc,
179w-Hex/ap-DCM, 199s-PE, 202s-PE, 203s-PE, 215l-MeOH, 220l-MeOH, 223l-MeOH, 224l-MeOH, 226s-PE, 227s-PE,
256l-MeOH, 262l-EtOAc, 268s-MeOH:H ²
O, 269s-MeOH:H ²
O, 274l-MeOH, 282 l-MeOH, 283s-MeOH:H ²
O, 291l-EtOAc
24, 50-52
E. erythropappus (DC.) MacLeish
[Vanillosmopsis erythropappa (DC.) Sch. Bip.]
1l-EO, 2l-EO, 3l-EO, 5l-EO, 7 l-EO, 8l-EO 9l-EO, 13l-EO, 14l-EO 15l-EO, 19l-EO, 20l-EO, 27w-EtOAc, 34l-EO, 35l-EO,
36l-EO, 37l-EO, 38l-EO, 42l-EO, 45l-EO, 52l-EO, 58l-EO, 69l-EO, 71l-EO, 73l-EO, 76l-EO, 79l-EO, 83ap-Hex/w-EtOAc,
84w-EtOAc, 91w-EtOAc, 95w-EtOH, 136w-EtOH, 139w-EtOH, 143w-EtOH, 147w-EtOAc, 173w-EtOH, 174w-EtOH,
175w-EtOH, 176w-EtOH, 177w-EtOH, 178w-EtOH, 179w-EtOH, 180w-EtOH, 182w-EtOH, 184w-EtOH, 191w-EtOH,
193w-EtOH, 195w-EtOH, 196w-EtOH
53, 54
E. eriopus Sch. Bip. ex Baker 92ap-CHCl ³, 161
ap-CHCl ³, 272
ap-CHCl ³, 295
ap-CHCl
³ 55
E. glomerulatus Less. 34ap-PE, 40ap-PE, 52ap-PE, 69ap-PE,70ap-PE, 89ap-PE, 94ap-PE, 95ap-PE, 98ap-PE, 111ap-PE, 116r-PE/t-Hex:EtOAc,
117t-Hex:EtOAc, 122ap-PE, 123ap-PE, 124ap-PE, 128t-Hex:EtOAc, 129t-Hex:EtOAc, 131t-Hex:EtOAc, 132t-Hex:EtOAc,
134t-Hex:EtOAc, 135ap-PE, 147r-PE, 155ap-PE, 161ap-PE/t-Hex:EtOAc, 163ap-PE/t-Hex:EtOAc, 164ap-PE, 165t-Hex:EtOAc,
191ap-PE, 200r-PE, 202ap-PE, 203 ap-PE/r-PE, 204ap-PE, 209r-PE, 212r-PE, 231ap-PE, 293 ap-PE
8, 49, 50, 56, 57
E. goyazensis (Gardner) Sch. Bip. 84ap-EtOAc/ap-CHCl
³, 106ap-EtOAc, 107ap-EtOAc, 116ap-EtOAc,117ap-EtOAc, 139ap-EtOAc, 142ap-EtOAc, 182w-EtOH,
183w-EtOH, 282ap-EtOAc
53, 58
E. incanus (Less.) Less. 27ap-PE, 119 ap-PE, 147 ap-PE, 179ap-PE, 189 ap-PE, 202ap-PE, 203ap-PE, 242 ap-PE 8, 41, 59
E. mattogrossensis Kuntze 84ap-CHCl
³, 113ap-CHCl³, 302ap-CHCl³ 55
E. mollis Sch. Bip. 34ap-PE, 52ap-PE, 69ap-PE, 74ap-PE, 84ap-PE, 92ap-PE, 202ap-PE, 203ap-PE/r-PE, 239ap-PE, 243ap-PE, 244ap-PE,
289ap-PE, 293ap-PE
56
E. polhii (Baker) MacLeish [Vanillosmopsis pohlii Baker]
3l-EO, 5l-EO, 13l-EO, 15l-EO, 19w-EO,27w-EO, 34ap-PE, 35l-EO, 36l-EO, 45w-EO/l-EO, 52l-EO, 69ap-PE, 73l-EO,
84ap-PE,113ap-PE, 202ap-PE, 203ap-PE, 204ap-PE, 212ap-PE, 214ap-PE, 215ap-PE, 216ap-PE, 230ap-PE, 231 ap-PE
16, 60
E. seidelii MacLeish & H. Schmacher 102ap-EtOAc, 105ap-EtOAc, 106ap-EtOAc, 107ap-EtOAc/l-EtOH, 139ap-EtOAc, 141 ap-EtOAc, 142 ap-EtOAc, 282ap-EtOAc 53, 61
E. unilorus MacLeish & H. Schmacher 84t-CHCl
³, 200t-CHl³,202t-CHCl³, 203t-CHCl³, 205t-CHCl³ 215t-CHCl³, 216t-CHCl³, 218t-CHCl³, 219t-CHCl³, 220t-CHCl³,
221t-CHCl
³, 224t-CHCl³, 233t-CHCl³, 234t-CHCl³, 240t-CHCl³, 262t-MeOH, 264t-CHCl³, 272t-CHCl³, 276t-CHCl³, 289t-CHCl³,
292t-CHCl ³, 297t-CHCl³
48
E. veadeiroensis H. Rob. 56ap-CHCl
³, 179s-Hex:EtOAc, 192s-Hex:EtOAc, 200s-Hex:EtOAc/s-EtOH, 202s-EtOH, 205s-Hex:EtOAc, 206s-Hex:EtOAc,
215s-EtOH, 218s-EtOH, 220s-EtOH,223s-EtOH, 226s-EtOH, 227s-Hex:EtOAc/s-EtOH, 257ap-EtOH, 272ap-CHCl
³, 274ap-CHCl³,
276ap-CHCl
³/ap-EtOH, 319ap-CHCl³/ap-EtOH
48, 62
Lychnophora afinis Gardner 46ap-EtOH, 47ap-EtOH, 90ap-EtOH, 92ap-EtOH, 116ap-EtOH, 121ap-EtOH, 129ap-EtOH, 130ap-EtOH, 132ap-EtOH,
133ap-EtOH, 217ap-EtOH, 271ap-EtOH, 273ap-EtOH, 274ap-EtOH, 275ap-EtOH, 276ap-EtOH, 277ap-EtOH, 294ap-EtOH,
297ap-EtOH, 298ap-EtOH
9, 42, 63
L. antillana Urb. 153sl-DCM-MeOH, 154sl-DCM-MeOH, 205sl-DCM-MeOH, 222sl-DCM-MeOH 10
L. bahiensis Mattf. 83r-PE/ap-PE, 91ap-PE/r-PE, 92ap-PE, 128ap-PE, 131ap-PE, 134ap-PE,179r-PE, 184r-PE, 200ap-PE, 202ap-PE-r-PE,
203r-PE/ap-PE, 204r-PE/ap-PE, 230r-PE/ap-PE
21
L. blanchetti Sch. Bip. 41r-PE, 71r-PE, 83ap-PE, 91r-PE, 147r-PE, 148r-PE, 157ap-PE, 158ap-PE, 159ap-PE, 160ap-PE, 162ap-PE, 165ap-PE,
179r-PE/ap-PE, 188r-PE, 190r-PE,193r-PE, 202ap-PE, 203r-PE, 230r-PE/ap-PE, 242r-PE
21, 64
L. brunioides Mart. 179ap-EtOAc, 279ap-EtOA, 280ap-EtOA, 301ap-EtOAc, 305ap-EtOAc, 311ap-EtOAc, 312ap-EtOAc, 313ap-EtOAc 65
L. columnaris Mattf. 52 ap-PE, 53 ap-PE, 54 ap-PE, 55 ap-PE, 56ap-PE, 62ap-PE, 63ap-PE, 64ap-PE, 65ap-PE, 66ap-PE, 67ap-PE, 68ap-PE, 91ap-PE,
179ap-PE, 181r-PE , 183
r-PE,200ap-PE, 202ap-PE/r-PE, 203ap-PE/r-PE
230r-PE, 231r-PE
66
L. crispa Mattf. 88 ap-PE, 91ap-PE, 108ap-PE, 110ap-PE, 128ap-PE, 131ap-PE,145ap-PE, 179r-PE, 202r-PE/ap-PE, 203r-PE/ap-PE,
230r-PE/ap-PE
21
Species Chemical constituents Ref.
L. ericoides Mart. 4l-EO, 5l-EO, 6l-EO, 7 l-EO, 8l-EO, 10l-EO, 11l-EO, 12l-EO, 13l-EO, 16l-EO, 17l-EO, 18l-EO, 19l-EO, 21l-EO, 22l-EO,
23l-EO, 26l-EO, 27l-EO, 30l-EO, 37l-EO, 45l-EO, 72l-EO, 81l-EO, 82l-EO, 83l-MeOH, 84l-DCM, 91l-DCM/l-MeOH,
92l-DCM/ap-DCM/l-MeOH, 95l-DCM 102l-DCM/l-MeOH, 103l-DCM/l-MeOH, 108l-MeOH, 109l-DCM, 110l-DCM/l-MeOH,
115l-MeOH,116l-DCM, 119ap-Hex/l-DCM, 128l-DCM/l-MeOH, 131l-MeOH, 138l-DCM/l-MeOH, 139l-DCM/l-MeOH,
140ap-EtOAc, 141ap-Hex,143l-DCM, 144l-DCM, 145l-DCM/l-MeOH, 146l-MeOH, 198ap-Hex, 200 ap-Hex,
202l-DCM/ap-Hex/l-MeOH, 220l-DCM, 223l-DCM, 225l-DCM/ap-Hex,227ap-Hex, 245r-DCM, 246r-DCM, 247r-DCM, 248 r-DCM, 249r-DCM, 250r-DCM, 251r-DCM, 252r-DCM, 253r-DCM, 254r-DCM,256l-MeOH, 257l-MeOH, 258l-MeOH,
259l-MeOH 262ap-EtOAc/l-MeOH, 263 l-MeOH, 266 l-MeOH, 281ap-EtOAc, 291l-MeOH,301l-DCM/l-MeOH, 305l-DCM/l-MeOH,
311l-DCM/ap-DCM/l-MeOH,314l-MeOH,315l-MeOH, 316l-MeOH, 317l-MeOH, 318l-MeOH, 319l-MeOH, 320l-MeOH,
322l-MeOH, 323l-MeOH, 324l-MeOH, 325l-MeOH, 326l-MeOH, 327l-MeOH, 328l-MeOH, 330l-MeOH,331l-MeOH,
332r-MeOH/l-MeOH, 333r-MeOH/l-MeOH, 334r-MeOH/l-MeOH, 336l-MeOH, 337l-MeOH, 338l-MeOH
11, 12, 18, 19, 27,
68-70
L. granmogolensis (Duarte) D. J. N. Hind 84li-EtOAc, 90li-EtOAc, 92 li-EtOAc, 264 li-EtOAc, 270 li-EtOAc, 286 li-EtOAc, 303li-EtOAc, 304li-EtOAc, 307li-EtOAc, 313 li-EtOAc 31 L. hakeaefolia Mart. 91ap/PE, 147r-PE, 179r-PE, 202r-PE/ap-PE, 203r-PE/ap-PE, 229r-PE,230r-PE 13 L. markgravii Gardner 83r-DCM, 88 r-DCM, 179r-DCM, 199 r-DCM, 200 r-DCM, 202r-DCM, 215r-DCM, 218r-DCM, 220r-DCM, 223r-DCM,
226r-DCM, 267ap-DCM, 288ap-DCM, 301ap-DCM, 305ap-DCM, 319ap-EtOH
71
L. martiana Gardner 49ap-Hex:EtOAc, 51ap-Hex:EtOAc 72
L. passerina (Mart. ex DC.) Gardner 52 ap-PE, 83l-EtOH, 84ap-EtOH/l-EtOH/ap-PE, 147r-PE, 179r-PE/ ap-PE, 181r-PE, 182r-PE/ ap-PE, 183r-PE/ ap-PE, 184r-PE/ ap-PE,
187r-PE, 200r-PE/ ap-PE, 202r-PE/ ap-PE, 203r-PE/ ap-PE,215r-PE, 212r-PE, 216r-PE/ ap-PE, 217ap-PE, 223 r-PE/ ap-PE,
224r-PE/ ap-PE, 257l-EtOH, 262l-EtOH, 280l-EtOH, 281l-EtOH, 319l-EtOH, 321l-MeOH
14, 17, 37
L. phylicaefolia DC. 45ap-PE, 179r-PE/ap-PE, 202r-PE/ap-PE, 203 r-PE/ap-PE, 210 ap-PE, 229 r-PE, 230r-PE, 308ap-PE, 13 L. pinaster Mart. 46ap-Hex, 57ap-Hex, 83 ap-DCM, 202ap-Hex, 220ap-Hex,223ap-Hex, 227ap-Hex, 238 ap-EtOH:H
²O, 241ap-EtOH:H²O,
261ap-EtOH:H
²O, 281ap-DCM, 311ap-EtOH:H²O, 328ap-EtOH:H²O, 335li-H²O:MeOH
37, 73
L. pohlii Sch. Bip. 83li-DCM, 84li-DCM, 86li-DCM, 91li-DCM, 92li-DCM/l-MeOH:H
²O, 241li-MeOH, 258li-MeOH/l-MeOH:H²O, 260l-MeOH:H²O,
262li-MeOH, 267li-MeOH, 269li-H
²O:MeOH, 274li-DCM, 279li-DCM, 282li-H²O:MeOH/l-MeOH:H²O, 288li-DCM, 300l-MeOH:H²O,
301li-H²O:MeOH/l-MeOH:H²O, 311li-DCM,314li-DCM, 320li-H²O:MeOH/l-MeOH:H²O, 322l-MeOH:H²O, 323l-MeOH:H²O,
324l-MeOH:H
²O, 325l-MeOH:H²O, 332l-MeOH:H²O, 333l-MeOH:H²O, 335li-H²O:MeOH
28, 70, 74
L. pseudovillosissima Semir & Leitão 83 fl-EtOAc/ap-EtOAc, 87ap-EtOAc, 91ap-EtOAc/l-MeOH:H
²O, 166fl-EtOAc, 167 fl-EtOAc, 168 fl-EtOAc, 169 fl-EtOAc,
170 fl-EtOAc, 171fl-EtOAc, 172fl-EtOAc, 200ap-EtOAc, 202ap-EtOAc, 207ap-EtOAc, 208ap-EtOAc, 258 l-MeOH:H ²O,
260l-MeOH:H
²O, 282l-MeOH:H²O, 300l-MeOH:H²O, 319l-MeOH:H²O, 320l-MeOH:H²O, 322l-MeOH:H²O, 323l-MeOH:H²O,
324l-MeOH:H
²O, 325l-MeOH:H²O, 332l-MeOH:H²O, 333l-MeOH:H²O
28, 68, 70
L. reticulata Gardner 83l-MeOH, 202l-MeOH, 203l-MeOH, 204l-MeOH, 215l-MeOH, 216l-MeOH, 219l-MeOH, 221l-MeOH, 223l-MeOH,
224l-MeOH, 226l-MeOH, 227l-MeOH, 302l-MeOH, 305l-MeOH
75
L. rupestris Semir & Leitão 91li-AcOET, 116 li-EtOAc, 117li-EtOAc, 118li-EtOAc, 128li-EtOAc, 131li-EtOAc, 179s-EtOAc, 180s-EtOAc, 202li-HEX,
207li-HEX, 208li-HEX, 223li-HEX, 225li-HEX, 265li-EtOH, 300li-EtOH, 332s-MeOH
76, 77
L. salicifolia Mart. 43ap-PE, 44ap-PE, 45ap-PE, 46ap-PE, 48ap-PE , 49s-EtOH/li-EtOAc/ap-PE, 51li-EtOAc/ls-EtOH, 59ap-PE, 60ap-PE, 61ap-PE, 147ap-PE,
179r-PE/ap-PE, 202ls-EtOH/ap-PE/li-EtOAc, 203r-PE/ap-PE, 212r-PE, 216r-PE, 223ap-PE, 224ap-PE/r-PE, 230r-PE/ap-PE,
231ap-PE/r-PE, 276li-EtOAc, 287li-EtOAc, 297li-EtOAc
13, 14, 32, 78, 79
L. sellowii Sch. Bip. 31ap-PE, 32ap-PE, 33 ap-PE, 91ap-PE, 92ap-PE, 114ap-PE, 115ap-PE,179ap-PE, 197ap-PE, 202ap-PE, 203ap-PE, 230ap-PE 21 L. staavioides Mart. 255l-MeOH, 258l-MeOH, 264l-MeOH, 267l-MeOH, 288l-MeOH, 291l-MeOH, 301l-MeOH, 305l-MeOH, 311l-MeOH,
314l-MeOH, 332s-MeOH
39, 77
L. trichocarpha (Spreng.) Spreng. 91ap-EtOH, 116ap-EtOH, 199ap-EtOH, 200ap-EtOH, 202ap-EtOH, 220ap-EtOH, 223ap-EtOH, 227ap-EtOH 80, 81 L. unilora Sch. Bip. 59ap-PE, 91ap-PE/r-PE, 116ap-PE, 128ap-PE, 179 r-PE, 200ap-PE, 202ap-PE/r-PE, 203ap-PE/r-PE, 204ap-PE/r-PE, 208ap-PE,
210ap-PE, 212ap-PE/r-PE, 216ap-PE, 223ap-PE, 224ap-PE
14
L. villosissima Mart. 83ap-EtOH, 255ap-EtOH,258ap-EtOH/l-MeOH:H
²O, 260l-MeOH:H²O, 262ap-EtOH, 265l-DCM, 279l-DCM, 299 l-MeOH:H²O,
320 l-MeOH:H
²O, 322ap-EtOH/l-MeOH:H²O, 323l-MeOH:H²O/ap-EtOH, 324ap-EtOH/l-MeOH:H²O, 325l-MeOH:H²O, 328l-MeOH:H²O,
332ap-EtOH, 333ap-EtOH/l-MeOH:H ²O
28, 70, 82
Lychnophoriopsis candelabrum (Sch. Bip.) H. Rob.
92l-MeOH:H
²O, 209l-DCM, 220l-DCM, 223l-DCM, 232l-DCM, 258l-MeOH:H²O, 260l-MeOH:H²O, 265l-DCM, 279l-DCM,
282l-MeOH:H
²O, 284l-DCM, 285l-DCM,299 l-MeOH:H²O, 301l-MeOH:H²O, 320 l-MeOH:H²O, 322 l-MeOH:H²O, 323l-MeOH:H²O,
324l-MeOH:H
²O, 325l-MeOH:H²O, 327l-MeOH:H²O, 328l-MeOH:H²O, 333l-MeOH:H²O
15, 28
Minasia alpestris (Gardner) H. Robinson 83l-DCM/r-DMC, 84l-DCM, 86l-DCM, 87l-DCM, 88l-DCM, 98l-DCM, 99l-DCM, 100 l-DCM, 116l-DCM, 202l-DCM,
215l-DCM, 218l-DCM, 220l-DCM, 221l-DCM
7, 83
Piptolepsis ericoides (Less.) Sch. Bip. 110 r-PE, 164 r-PE, 202r-PE, 203 r-PE, 204ap-PE/r-PE, 229ap-PE/r-PE , 230 r-PE 16 P. leptospermoides (Mart. ex DC.) Sch. Bip 24ap-PE, 34ap-PE, 52ap-PE, 91ap-PE, 97ap-PE, 116ap-PE, 117ap-PE, 128ap-PE, 156ap-PE, 179r-PE, 201ap-PE,
203ap-PE/r-PE, 204ap-PE/r-PE, 216r-PE, 217r-PE, 229ap-pe
84
P. argentea Mart. & Zucc. ex Sch. Bip. 91ap-PE/r-PE, 94ap-PE/r-PE, 128ap-PE/r-PE, 135ap-PE/r-PE, 202ap-PE/r-PE, 203ap-PE/r-PE, 204ap-PE, 212ap-PE/r-PE, 214r-PE,
229r-PE, 230ap-PE/r-PE, 231ap-PE/r-PE
85
P. furnensis Semir & Leitão Filho 91ap-EtOAc, 82ap-EtOAc 86
* The superscripts following the number of the compound indicates the part of the plant and the extract from which each compound was isolated. w: wood; ap:
aerial parts; r: roots; i: inlorescences; is: internal part of stem; es: external part of stem; l: leaves; t: total plant; li: leaves and inlorescences; EO: essential oil;
Hex: hexane; EtOAc: ethyl acetate; PE: petroleum ether; DCM: dichloromethane; EtOH: ethanol; MeOH: methanol.
selowii.21 Regarding the sesquiterpenes, sesquiterpene lactones (STL) are reported to be isolated and/or identiied in 90% of the species in-vestigated. This class of secondary metabolites is divided into groups (i.e., germacranolides, eudesmanolides, guaianolides, furanohelian-golides, among others) according to their structural diversity, which results of enzyme-catalyzed and eventually selective oxidations, cyclizations, condensations and rearrangements of their common biosynthetic precursor (farnesylpyrophosphate, FPP).22 Because of their number of occurrence and structural diversity, STLs have been used as chemotaxonomic markers of various taxa of Asteraceae,23 including the subtribe Lychnophorinae.4,22
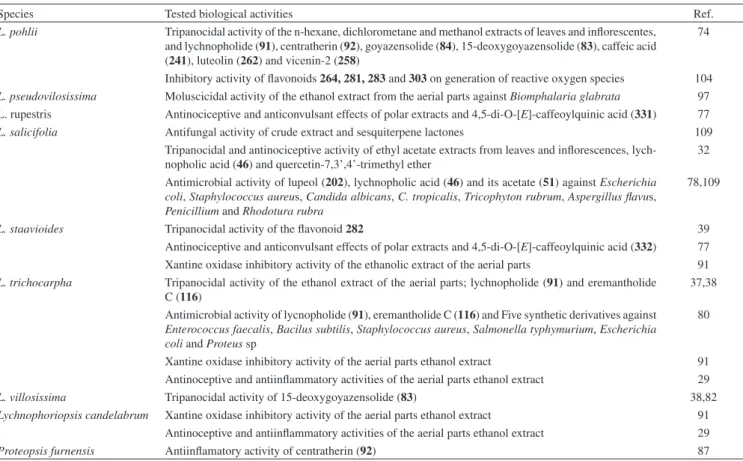
Furanoheliangolides from the goyazensolide and eremantholide types are the most common STLs in Lychnophorinae, as shown in Figure 3. They have been reported to be the major STL of all the investigated species, except for Lychnophoriopsis candelabrum, Lychnophorapseudovillosissima and L. reticulata, in which the predominance of guaianolides and eudesmanolides were observed. Furanoheliangolides of the eremantholide type has been proposed to be biosynthesized from a goyazensolide type of STL by means of a Michael-type addition to the exocyclic double bond conjugated with the lactone carbonyl (Figure 3).22,24 The conversion of goyazensolide-type into eremantholide in laboratory was recently achieved by making use of Striker’s reagent.25 Most of the furanoheliangolides from goyazensolide type occurring in Lychnophorinae have the same structural core, but they differ on the degree of oxidation and
substituents at C-8. The majority mostly exhibit exocyclic double bond between C-11 and C-13 (94.8%) and an oxygenated group (a hydroxyl or an acyloxy group) bounded at C-8 (100%), followed by common methacryloxy (52.2%), angeloxy (36.5%), tigloxy (11.3%) or their derivatives. In contrast, oxygenated groups at C-15 (26.9%) and double bonds between C-4 and C-5 (74.8%) are less common. The oxygenated groups at C-8 from mostly of the STL of Lychno-phorinae are usually α-orientated in relation to the mean plane of the ring, as reported for other subtribes of Vernonieae.22 Differently, the furanoheliangolides of the eremantholide type mostly shows double bond between C-4 and C-5 (72.7%) and hydroxyl at C-1’ (93.5%), whereas oxygenated groups at C-15 are rarely found (20.8%).
Flavonoids also have a widespread occurrence in the subtribe Lychnophorinae. Flavonols (37.4%) and lavones (38.7%) have higher percentages of occurrence than dihydrolavones (12.9%), dihydrola-vonols (9.7%) and chalcones (1.3%). Methoxyl (53.3%) and glycosyl (25.0%) are the most common substituent groups in lavones. The glycosyl groups are often bounded at C-6 and C-8 or at C-8 solely. In the case of lavonols, only 37.9% exhibit free hydroxyl group at C-3 (B ring), once this group is the precursor of methoxyl (32.8%), glycosyl (8.6%) and heteroside groups (20.7%). Chalcones have been identiied only in Lychnophora ericoides.26
Opposed to terpenoids and lavonoids, whose occurrence is wide-spread in Lychnophorinae, some classes of secondary metabolites have been restricted to some species. Lignans were isolated only from roots of Lychnophora ericoides,11 and quinic acid derivatives have been found only in some Lychnophora species (L. ericoides,12,27 L. pinaster,28 L. pohli28and L. vilosissima28) and Lychnophoriopsis candelabrum.28
BIOLOGICAL ACTIVITIES OF LYCHNOPHORINAE
Although ifty species of Lychnophorinae have been submitted to phytochemical studies, up to now only twenty-eight species were investigated regarding their biological activities, as shown in Table 2. Several biological activities from the species of the Lychnophorinae subtribe have been evaluated, and their mostly common reported activities are antimicrobial, anti-inlammatory, tripanocidal, toxicity, analgesic and antinociceptive (Figure 4).
Extracts from the Lychnophora species have been used in the Brazilian folk medicine as analgesic and anti-inlammatory. For this reason, a number of studies have focused on the evaluation of crude extracts and pure compounds for their analgesic and anti-inlammatory potential. Guzzo and co-workers29 investigated the antinociceptive activity of the ethanol aerial parts extracts of ive Lychnophora species using hot-plate and writhing tests. They reported that the Lychnophora pinaster (0.75 g/kg) and Lychnophora ericoides (1.50 g/kg) extracts signiicantly increased the time for paw licking in mice. By using the dose of 0.75g/kg for Lychnophora passerina, Lychnophoriopsis candelabrum and Lychnophora pinaster, and doses of 0.75 and 1.50 g/kg for both Lychnophora ericoides and Lychnopho-ra trichocarpha it was observed a signiicant reduction on the number Figure 1. Percentage of secondary metabolites occurrence in Lychnophorinae
(percentage was calculated in relation to the total hit number)
Figure 2. Occurrence of sesquiterpene lactones (SLTs) in Lychnophorinae (percentage was calculated in relation to the total hit number)
O
O O
R O
O O
H
-..
H+
O O
O
O O R
OH
A B
15 8
1'
11 13 4
5
15 8
1'
11 13 4
5
Table 2. Biological activities of species of the subtribe Lychnophorinae
Species Tested biological activities Ref.
Eremanthus arboreus Antiinlammatory activity of 15-hydroxyeremantholide B (126) and 15-acetoxyeremantholide B (127) 87
Gastroprotective effect of the bark essential oil 88
Anticholinesterase activity of dichloromethane:methanol and dichloromethane extracts 89
E. elaeagnus Antitumoral activity of the ethanolic extract from stems and eremantholide A (119) 24,52
Antiinlammatory activity of 15-deoxygoyazensolide (83) 87
Citotoxicity of lychnopholide (91) 90
Clastogenicity of eremanthin (179) and lychnopholide (91) 90,91
Genotoxicity of eremanthin (179) 91
Immunomodulatory activity of eremanthin (179) 92
Schistossomicidal activity of eremanthin (179), costunolide (147) and α-bisabolol (27) against
Schis-tosoma mansoni
92,93
E. erythropappus Antimicrobial activity of the essential oil of leaves and β-bisabolene (25) 35,45,94
Toxicity of extracts and essential oil of leaves 44,45
Antifungal activity of essential oil, extracts and α-bisabolol (27) 95,96
Moluscidal activity of the aerial parts ethanol extract against Biomphalaria glabrata 97
Antiedematogenic activity of the essential oil of leaves 98
Antinociceptive and antiinlammatory activities of the essential oil of leaves 36,95,99
Antiulcerogenic activity of the essential oil of leaves and α-bisabolol (27) 100
E. glomerulatus Moluscidal activity of the aerial parts ethanol extract against Biomphalaria glabrata 57,97
E. goyazensis Citotoxicity and schistossomicidal activity of goyazensolide (84) against Schistosoma mansoni 101
E. incanus Cytotoxic activity of eregoyazin (182) 41
E. mattogrossensis Antiinlamamatory activity of goyazensolide (84) and isogoyazensolide (112) 87
E. pohlii Inseticidal activity of the essential oil against Bemisia argentifolii 60
E. sphaerocephalus Moluscicidal activity of the ethanol extract from the aerial parts against Biomphalaria glabrata 97
Lychnophora afinis Antineoplastic activity and citotoxicity of lavonoids (255-321), lychnophorolide A (92) and lychno-pholic acid (46)
9,40,42
L. antillana Antineoplastic activity of crude ethanolic extract, lychnostatin 1 (153) and 2 (154) 10
L. brunioides Moluscidal and tripanocidal activity of lavonoids isolated from the ethyl acetate extract of leaves 65,102
L. diamantinana Antinociceptive and anticonvulsant effects of stems methanol extracts 77
L. ericoides Analgesic activity of the crude lyophilized aqueous extracts from stem and leaves 5 Analgesic activity of di-caffeoyquinic acids (325, 332 and 333) isolated from roots methanolic extracts 12 Analgesic and antioxidant activities of di-C-glucosyllavones isolated from hydromethanolic extract of leaves
18
Anti-inlammatory, antipyretic and antiedematogenic activities of lignans (245-254) isolated from the dichloromethane extract of roots
11
In vitro anti-inlammatory activity of centratherin (92) and goyazensolide (84) 33
Antinociceptive and anti-inlammatory activity of ethanol extract from the aerial parts 29
Antiproliferative activity of goyazensolide (84) isolated from cell culture 103
Genotoxicity of 15-deoxygoyazensolide (83) 43
Inhibitory activity of lavonoids 264, 281, 283 and 303 on generation of reactive oxygen species 104
Xantine oxidase inhibitory activity of the ethanolic extract of the aerial parts 105
L. gardneri Tripanocidal activity of crude extracts, lavonoids (255-321) and lychnopolic acid (46) 37
L. granmongolense Tripanocidal and analgesic activities of the crude hexane, ethyl acetate and and ethanol extracts from the aerial parts and centratherin (92); goyazensolide (84), lychnophorolide B (90)and the lavonoid 303
31
L. markgravii Antimicrobial activity of crude extracts from the aerial parts and lavonoids (255-321) against Strep-tococcus sp
106
Leishmanicidal activity of lavonoids 267 and 305, against Leishmania amazonensis 106
L. passerina Tripanocidal activity of the hydroalcoholic extract of aerial parts and goyazensolide (84) 37,107
Antinoceptive and antiinlammatory activities of the ethanolic extract from the aerial parts 29
Inhibitory activity of lavonoids 264, 281, 283 and 303 on generation of reactive oxygen species 104 Antioxidant activity of methanolic extracts from leaves, and quercetin (281), kaempferol (280) and
tiliroside (319)
17
Xantine oxidase inhibitory activity of the ethanolic extract of the aerial parts 105
L. pinaster Antinoceptive and antiinlammatory activities of the ethanolic extract from the aerial parts 29
Moluscicidal activity of the ethanolic extract of the aerial parts against Biomphalaria glabrata 97
Tripanocidal activity of the aqueous and hexane extracts of the aerial parts and lichnopholic acid (46) 37,108
Species Tested biological activities Ref.
L. pohlii Tripanocidal activity of the n-hexane, dichlorometane and methanol extracts of leaves and inlorescentes, and lychnopholide (91), centratherin (92), goyazensolide (84), 15-deoxygoyazensolide (83), caffeic acid (241), luteolin (262) and vicenin-2 (258)
74
Inhibitory activity of lavonoids 264, 281, 283 and 303 on generation of reactive oxygen species 104
L. pseudovilosissima Moluscicidal activity of the ethanol extract from the aerial parts against Biomphalaria glabrata 97
L. rupestris Antinociceptive and anticonvulsant effects of polar extracts and 4,5-di-O-[E]-caffeoylquinic acid (331) 77
L. salicifolia Antifungal activity of crude extract and sesquiterpene lactones 109 Tripanocidal and antinociceptive activity of ethyl acetate extracts from leaves and inlorescences,
lych-nopholic acid (46) and quercetin-7,3’,4’-trimethyl ether
32
Antimicrobial activity of lupeol (202), lychnopholic acid (46) and its acetate (51) against Escherichia
coli, Staphylococcus aureus, Candida albicans, C. tropicalis, Tricophyton rubrum, Aspergillus lavus,
Penicillium and Rhodotura rubra
78,109
L. staavioides Tripanocidal activity of the lavonoid 282 39
Antinociceptive and anticonvulsant effects of polar extracts and 4,5-di-O-[E]-caffeoylquinic acid (332) 77
Xantine oxidase inhibitory activity of the ethanolic extract of the aerial parts 91
L. trichocarpha Tripanocidal activity of the ethanol extract of the aerial parts; lychnopholide (91) and eremantholide C (116)
37,38
Antimicrobial activity of lycnopholide (91), eremantholide C (116) and Five synthetic derivatives against
Enterococcus faecalis, Bacilus subtilis, Staphylococcus aureus, Salmonella typhymurium, Escherichia
coli and Proteus sp
80
Xantine oxidase inhibitory activity of the aerial parts ethanol extract 91
Antinoceptive and antiinlammatory activities of the aerial parts ethanol extract 29
L. villosissima Tripanocidal activity of 15-deoxygoyazensolide (83) 38,82
Lychnophoriopsis candelabrum Xantine oxidase inhibitory activity of the aerial parts ethanol extract 91
Antinoceptive and antiinlammatory activities of the aerial parts ethanol extract 29
Proteopsis furnensis Antiinlamatory activity of centratherin (92) 87
Table 2. continuation
Figure 4. Biological activities of species of the subtribe Lychnopnhorinae reported in the literature. Percentage was calculated in relation to the total number of hits
of writhes induced by acetic acid. This activity was initially proposed due to the presence of sesquiterpene lactones.30 However, Grael and co-workers31 reported that extracts of the aerial parts of Lychnophora granmongolense showed no analgesic activity in the writhing model of pain, even though the STLs goyazensolide (84) and centratherin (92) were isolated from those extracts. These results, in combination with those obtained for the L. salicifolia extracts,32 suggested that not all the Lychnophora species could exhibit analgesic activity correlated to the accumulation of sesquiterpene lactones. Differently, lignans11 and di-caffeoylquinic acid derivatives,12 isolated from roots of Lychnophora ericoides exhibited interesting analgesic activity. More recently, di-caffeoylquinic acid derivatives were also identiied in the leaves of L. ericoides,26 thus indicating that these compounds
can be responsible for the analgesic activity of the hydroalcoholic extracts used by the Brazilian population.
OH
OH OH
3 4 5
7 8
14
15 16 17
1 2
9
13 6
10 HO
H
11 H
12 H
Figure 5. Monoterpenes isolated and/or identiied in species of the subtribe Lychnophorinae
lactones. In addition, Guzzo and co-workers29 investigated the etha-nolic extracts of the aerial parts of ive Lychnophora species for their anti-inlammatory and antinociceptive activities. They reported that administration of Lychnophora pinaster and Lychnophora trichocar-pha ointments, in both evaluated concentrations (5 and 10%, w/w), and Lychnophora passerina and Lychnophoriopsis candelabrum, in the concentration of 10%, signiicantly reduced the paw edema measured 3 h after carrageenan administration, suggesting, for the irst time, an anti-inlammatory activity upon topical administration of these species. However, the authors did not report the chemical composition of those extracts.
Despite the fact that sesquiterpene lactones have a widespread occurrence in the Eremanthus species, most of the biological activities of this genus are due to their essential oils from the leaves and stems. Nascimento and co-workers35 investigated the activity of Eremanthus erythropappus oil and some of its compounds and their potential synergistic interaction with ampicillin against different strains of Staphylococcus aureus. They identiied β-bisabolene as the main active constituent and reported its potential to restore the effectiveness of ampicilin against the resistant S. aureus.
Recently, the antimicrobial activity of this oil against Candida albicans and Salmonella ssp was also reported, but the major consti-tuents were β-pinene and β-caryophyllene instead of β-bisabolene.6 More recently, the essential oil of E. erythropappus was also inves-tigated for its antinociceptive and anti-inlammatory activities.36 The authors found that doses of 200 and 400 mg/kg inhibited 10.69% and 27.06% of acetic-acid-induced writhing in mice, respectively. In the formalin-induced nociception test in mice, the essential oil inhibited the irst phase of paw licking by 29.13% (400 mg/kg) and the second phase by 32.74% (200 mg/kg) and 37.55% (400 mg/kg). In the hot-plate test in mice, doses of 200 and 400 mg/kg signiicantly increased the reaction time after 30, 60 and 90 min of treatment. Doses of 200 and 400 mg/kg inhibited carrageenan-induced paw edema in rats by 15.18 and 36.61%, respectively. Doses of 200 and 400 mg/kg administered 4 h before intra-pleural injection of carrageenan signiicantly reduced exudation volume (by 20.20 and 48.70%, respectively) and leucocyte mobilization (by 5.88 and 17.29%, respectively).
The trypanocidal activity of the Lychnophora species has been extensively investigated in the literature. In this case, however, these studies are part of an intensive search for active compounds against Trypanosoma cruzi, the etiology agent of Chaga’s disease, therefore, they are not correlated with the use of species from the subtribe Lych-nophorinae in traditional medicine. The bioguided fractionation of the crude extracts of L. passerina, L. pinaster and L. trichocarpha resulted in the isolation of the bioactive sesquiterpene lactones goyazensolide (84), eremantholide C (116), lychnopholide (91), and lychnophoic acid (57).37 Goyazensolide and eremantholide C were 100% active at concentrations of 240 and 3600 µg/mL, whereas lychnopholide inhibited 50% of the grown of trypomastigote forms at concentration of 150 µg/mL.38 Besides goyazensolide, centratherin (92) and the lavonoid eridictyol (303), isolated from L. granmongolense were also found to be active against T. cruzi.31 Quercetin 3-methyl ether (291), isolated from L. staavioides showed also signiicant trypano-cidal activity.39
Flavonoids and sesquiterpene lactones have also been investi-gated for their citotoxicity40-42 and genotoxicity.43 Recent studies performed by Vasconcellos and co-workers43 demonstrated that 15-deoxygoyazensolide (83) is mutagenic in Saccharomyces ce-revisae due to the possible intercalation effect, in addition to the pro-oxidant status that exacerbates oxidative DNA damage. Studies on the toxicity of the essential oil of Eremanthus erythropappus have also been reported.44,45
CONCLUDING REMARKS
-R O
H
H H
73
H H
O OH
59
62: R=CH3 63: R=CH2OH 64: R=CHO
H
H OH
76 69*
75
H
R3 H
R1 R2
81: R1=OH, R2=H, R3=CH 3 82: R1=CH3, R2=OH, R3=H O
H
78 HO
H
77
H
H
80 H
74 H
H
72
70* 71*
O OH
65
79 H H
O
58 CO2H
H H
61 H
H
60 OH
O O
H H
OH O O
CO2R
66: R=H 67: R=CH3
O HO
CO2H 68
57
H
H H
Figure 7. Sesquiterpenes isolated and/or identiied in species of the subtribe Lychnophorinae. *Conigurations of the stereocenters were not shown in the original papers
H HO
OH
27: R=H 28: R=OH 29: R=OAc
18
21: R1=α-CH 3, R2 =H 22: R1=β-CH3, R2 =OH
O
23 19
R1 R2
H HO
30
H
25
H
24
R H
OH H H
R1
H
35 34
37
36
H
H R
2
45: R1=CH3, R2=H 46: R1=CO
2H, R2=H 47: R1=CO2H, R2=β-OH 48: R1=CO
2CH3, R2=H 49: R1=CO2H, R2=α-OH 50: R1=CO2CH3, R2=α-OH 51: R1=CO
2H, R2=α-OAc CO2R
43: R=H 44: R=CH3
H
H 52: R=CH3
53: R=CH2OH 54: R=CH2OAc 55: R=CHO 56: R=CO2H H
H
CO2H H
41
O
R2 H R1
31: R1=H, R2= CH 2 32: R1=H, R2= O 33: R1=OH, R2= O R
40
OH
39 H 20
42 H
OH
38 H
26
O O O O R1 O
R1 R2
83 84 85 86 87 H OH OAc H H 91 92 93 94 95 H OH OAc H H 96 H 88 H 90 OH OH 97 H O O O O R2 R1 R3
R1 R2 98 101 100 H OH H R3 =CH2 β-OH
=CH2 O O O
O α-CH2OCH3 99 H α-CH3 α-OH
89 H OH
83-97 98-101 O R2 A A A D D Y Y Y B C E Z W A = O
C = D =
O G = OH OH O Y = B = E = W = Z = O O O O R4 R1 O R2 102-115 R3
R2 R3 R4 R1 =CH2 =CH2 =CH2 =CH2 =CH2 102 H 103 H 108 H 107 H 106 H 110 104 111 109 H =CH2 =CH2 β-OH
α-CH3
H =CH2
β-OH β-CH3 α-CH3
α-CH3
α-CH3
α-CH3
α-CH3
α-CH3
α-CH3
α-CH3
=CH2
=CH2 105 α-CH3 β-OH
112 113 114 115 α-OH α-OH β-OH β-OH =CH2 =CH2 =CH2 =CH2 =CH2 =CH2 =CH2 Y Y A A W Y D D Z C B A A A
Figure 8. Furanoeliangolides of the goyazensolide type isolated and/or identiied from species of the subtribe Lychnophorinae
Figure 9. Furanoheliangolides of eremantholide type isolated and/or identiied from species of subtribe Lychnophorinae
136 H H Z
119 125 116 124 H 126 127 118 OAc 121 117 OH O O O O R1 O R3 O R2
R1 R2 R3
H H H H H H H H H H OAc OH H H H 134 129 H 132 OH 135 131 OH CH3 H CH3 OAc H H H 137 138-146 HO
CO2H
O O O HO OH O O O R1 R4 O O O
144α-CH3
138β-CH3
R1 R3 R4
OCH3 H
R3
128 H H
140β-CH3
141α-CH3
H
H 139α-CH3
142α-CH3
H H 143 H R2 R2 H H β-OH H H H
β-CH3 H
145α-CH3 H H
146α-CH3 H H
116-136
120 H H
123 OAc H
122 H H
130 H C2H5
133 OH C2H5 A = O C = D = O G = OH OH O Y = B = E =
W = Z =
A A A A A B B B C C D D D D Y Y Y Y Y Y Y E E E Y Y Y G W
R1 R2 R3
O O
R1
O O
R2 H
R3
166
167
168
169
170
171
R1 R2 R3
OH
OH CH3
CH3
CH3
CH3
CH3
CH3 OH
OH
OH
OH O
O
O
O
H O
O
166-172
O
O H
175 O
O H
O
O
O
O H OH
OH
R2 R1 173: R=H
174: R=β-OH R
177: R1=CH3, R2=OH 178: R1=OH, R2=CH
3 176
172 CH3 OH Ac
Figure 11. Eudesmanolides isolated and/or identiied from species of subtribe Lychnophorinae
198
R
HO
H H H
199 200
R = H
197 198-200
Figure 13. Steroids isolated and/or identiied in species of the subtribe Lychnophorinae
147 O
O
OH
O
O OR
O O
O
OHCl O
OHOAc
149:R=
149-152
O O
R2 R6R5
R4
O R1
153-160
153
R1 R2 R3 R4 R5
H β-CH3 R3
R6
OH α-OMeAcr OAc CH3
154 H β-CH3 H α-OMeAcr OAc CH3
155 β-OH β-CH3 H β-OAng CH3 OAc
156 α-OH α-CH3 H α-OAng OAc CH3
157 β-OH α-CH3 H α-OAng OAc CH3
150:R=
151: R=
152: R=
158 β-OCH3 α-CH3 H α-OMeAcr OAc CH3
159 H α-CH3 H α-OAng OAc CH3
160 H α-CH3 H α-OAng OAc CH3
161
R1 R2 R3 R4
β-CH3 Ang OAc CH3
O O
R1 R4
R3 O
O
161-162
R2
162 α-CH3MeAcrCH3 OAc
O
O O
R4 R3 R1
O
Ang
163-165
R2
163 β-CH3 OAc CH3
164 α-CH3β-OH CH3 OAc H
165 α-CH3 H CH3 OAc R1 R2 R3 R4
148
O O O
O
OHOH
Figure 12. Guaianolides isolated and/or identiied in species of the subtribe Lychnophorinae
O
O H
OH
O
188 189 190
R
R
191
O R2
O R3
H
H R1
R4
R1 R2 R3
179
180
181
182
183
=O α-CH3
α-CH3 β-CH3 =CH2 =CH2
=CH2 H
=O
=CH2
H
H α-CH3
α-CH3 =CH2
R4
H OAc
H
H
H
O R2
O R3
H
H R1
R1 R2 R3
187 186
=O =O
α-CH3
α-CH3
α-CH3
=CH2
O
O H
H
O OH
O
O
193 O
O H
H
O
O
O
184 H =CH2 =CH2
185 =O =CH2 =CH2
O
O H
O
192
O
O H
H
OH
OH
194 H
O
O H
H
O
O O O
O
O H
H OH
195 196
179-183
184-187
Figure 14. Triterpenes isolated and/or identiied in species of the subtribe Lychnophorinae R1
H R3
R2
R1 R2 R3
202
203
209
208 207
211
213 204
205
206
OH
OAc
OH
OH OH
OAc
OAc =O
OH
OAc CH3
CH3 CH3
CH3
CH3
CH3
CH3 CH3
CO2H
CO2H
∆
----12(13)
9(11)
12 13 11
9
12(13)
3
R
H
H
H R1
H
H
H
R2
R
223: R=OH
224: R=OAc
220: R1=OH, R2=CH
3
221: R1=OAc, R2=CH3
222: R1=OH, R2=CO
2H
225: R = α−OH
226: R = β-OH
227: R= =O H
H H
214 OAc CH3 9(11)
210 OH CH3 12(13)
212 OAc CH3 12(13)
202-214 201
OH
CHO
R
H
H
H
215: R=OH, ∆20,30 216: R=OAc, ∆20,30 217: R= =O, ∆20,30 218: R=OH, ∆20,21 219: R=OAc, ∆20,21
H 20
21 30
O OH
HO HO
O
O OH
O
OH O
OH H
O O
H H
Glu
228
Figure 15. Triterpene saponin isolated and/or identiied in species of the subtribe Lychnophorinae
230 231
232
CH3
HO
CH3
4 3
R
R
229 n
Figure 16. Poliisoprene and acetylene derivatives isolated and/or identiied in species of the subtribe Lychnophorinae
H H
235
H
OCH3 236
OCH3OCH3 237
R1 R2
OH
CO2H OH
OH
H OH
H OCH3 CO2H
239
CHO OH
OCH3 240
OCH3 242
R1 R2 R3
241
243 H3CO R1
R2
R3
R2 R1
H OH
244
R4
H
H H H H H R4
H H CO2H
238 H
HEDP = 235-237
238-244
O O
OCH3 OH OCH3
O O
i-Val =
HEDP CH2Oi-Val
HEDP CHO
OH OCH3
233
COOH
OCH3 OH
234
Figure 17. Benzoic acid derivatives (233 and 234) and Phenylpropanoids (235 to 244) isolated and/or identiied in species of the subtribe Lychnophorinae
Tf = Tf
OH
Tf 254 O
R2
R3 R1
O
O OCH
3 OCH3
OCH3
OH
Tf = Pi =
Tf Tf
Pi Tf
Pi Pi
Pi Pi
Tf
OH
Tf 254 O
R2
R3 R1
245 246
252 251 247 248 249 250
253
R2 R3 R1
α-OH
β-OH
=O α-OCH3
β-OCH3
α-OCH3
β-OCH3
=O
=O Tf
Tf
Pi Pi
Pi Pi
Pi Pi
Pi
Pi O
O OCH
3 OCH3
OCH3
OH
245-253
Figure 19. Flavones (254 to 276) and lavonols (277 to 300) identiied in species of the subtribe Lychnophorinae
255 256 257 258 259 260 261 262 263 264 265 266 267 268 269 270 271 272 273 274 275 276 277
R1 R2 R3 R4 R5 R6 R7 R8
H H H H H H H H H H H H H H H H H H H H H H H OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OCH3 OCH3 OCH3 OCH3 OCH3 OCH3 OCH3 OCH3 OCH3 OCH3 OCH3 H H OH OH OH OH OH OH OH OH OCH3 OCH3 H OH OH OH OH OCH3 OCH3 OCH3 OCH3 OCH3 OCH3 H H H H H H H OH OH OCH3 H OCH3 H H OH OCH3 OCH3 H H OCH3 OCH3 H H H H OH H H H H H H H H H H H H H OCH3 H H H H H Glu H Glu Glu Glu H H Glu H H H H H H H H H H H H H H H Glu H Glu Glu H Glu Glu H H H H H H H H OCH3 H OCH3 H H H OCH3 OH OH O R3
R2 O R1 R5 R8 R7 R6 R4 255 257 258 259 260 261 262 263 264 265 266 267 269 270 271 272 273 274 277
R1 R2 R3 R4 R5 R6 R7 R8
H H H H H H H H H H H H H H H H H H H H H H H OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH H H OH OH OH OH OH OH OH OH H OH OH OH OH H H H H H H H OH OH H H H OH H H H H H H OH H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H H OH OH O R3
R2 O R1 R5 R8 R7 R6 R4 278 279 280 281 282 283 284 285 OH OH OH OH OH OH OH OH H OH OH OH OH OH OH OH OH OH OH OH OH OH OCH3 OCH3 OH H OH OH OH OCH3 H H H OH OCH3 OH H H H H H H H H H H H H H H H H H H H H H H H H H H H OH 278 280 281 284 OH OH OH OH OH OH OH OH H OH OH OH OH OH OH OH OH OH OH OH OH OH OH H OH OH OH H H H OH OH H H H H H H H H H H H H H H H H H H H H H H H H H H H OH 255-300 286 287 288 289 290 OH OH OH OH OH OH OH OCH3 OCH3 OH OH OH OCH3 OCH3 H H H H H H H OH H H H H H H H H H OH OH OCH3 H OH OH 286 287 288 290 OH OH OH OH OH OH OH OH OH OH H H H H H H H OH H H H H H H H H H OH OH H OH OH OCH3 OCH3 OCH3 291 292 293 294 295 297 298 299 300 OGlu OGlu OH OH OH OH OH OH OH OH OH OH OH OCH3 OCH3 OCH3 OCH3 OCH3 OH OH OH OH OCH3 OCH3 OCH3 OH OH OH H H OCH3 H OCH3 OH OH OCH3 H H H H H H OCH3 H H H H H H H H H H H H H H H H H H H H OCH3 OH
296 OH OCH3 OCH3 OH H H H
291 292 293 294 295 297 298 299 300 OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH OH H H H OH OH H H H H H H H H H H H H H H H H H H H H H H H H H H OH
296 OH OH H H H
OCH3 OCH3 OCH3 OCH3 OCH3 OCH3 OCH3 OCH3 301 302 304 305 307 308 309 310 311 312 313 314 315 316 R2
R1 R3 R4 R5
H H H H H H H OH OH OH OH OAc OAc
OCOC2H5 OH OH OH OH OH OH H H OH OH OH OH OH OH OH OH OH OCH3 OCH3 OCH3 OH OH OH OCH3 OH OH OCH3 OCH3 H OH OH H OH OCH3 OCH3 OH H H OH H H H H H H OCH3 OH H H H H OCH3 H H H
306 H OH OCH3 OH H
303 H OH OH OH OH
O
R2 O
R5 R4 R1 R3 301 302 304 305 307 308 309 310 311 312 313 314 315 316 R2
R1 R3 R4 R5
H H H H H H H OH OH OH OH OAc OAc
OCOC2H5 OH OH OH OH OH OH H H OH OH OH OH OH OH OH OH OH OH OH OH OH OH H OH OH H OH OH H H OH H H H H H OCH3 H OH H H H H H H H
306 H OH OH H
303 H OH OH OH OH
O
R2 O
R5 R4 R1 R3 301-316 O HO OH OH O O O OH OH HO O O OH O HO OH O O OH O 321 R
319: R = H 320: R = OCH3
O O OH OH HO OH OH O OH O R
317: R = H 318: R = CH3
338 336
O
OH
O
OH OH O
OH OH O O
R1
R2
OH R2
R1
H2 C
H caf
337
O
OH OH 335
CO2H HO
O
O O R1
R2
R3
caf =
fer = O
OH OH
O
OH OCH3
322
323
324
327
328
329
331
332
333
334
R1 R2 R3
caf H H
H caf H
H H caf
H
fer H
326
H fer H
H H fer
fer fer
H
caf fer
H
caf caf
H
caf H caf
caf caf caf
OH HO2C
O
OH OH
caf
322-334 325 caf caf H
330 fer fer H
Glu
Figure 21. Quinic acid derivatives (322 to 335) and other heterosides (336 to 338) isolated and/or identiied in species of the subtribe Lychnophorinae
REFERENCES
1. Mansanares, M. E.; Forni-Martins, E. R.; Semir, J.; Caryologia 2002,
55, 367.
2. Robinson, H.; P. Biol. Soc. Wash. 1992, 105, 640. 3. Robinson, H.; Smithson. Contrib. Bot. 1999, I-IV, 1.
4. Herz, W. In Compositae: Systematics. Proceedings of the International Compositae Conference, Kew; Hind, D. J. N.; Beentje, H. J., eds.; Kew, 1996, chap. 17.
5. Bastos, M.; Cerqueira, S.; Souza, J. T.; Júnior, R. A.; Peixoto, A. B. F.;
Cienc. Cult. 1987, 39, 551.
6. Sousa, O. V.; Dutra, R. C.; Yamamoto, C. H.; Pimenta, D. S.; Rev. Bras.
Farm. 2008, 89, 113.
7. Keles, L. C.; Gianasi, F. M.; Souza, R. C.; Brito, B. L.; Schaab, E. H.; Souza, M. G. M.; Carvalho, T. C.; Martins, C. H. G.; Veneziani, R. C. S.; Cunha, W. R.; Crotti, A. E. M.; Nat. Prod. Res. 2011, 25, doi: 10.1080/14786410903533612.
8. Bohlmann, F.; Zdero, C.; King, R. M.; Robinson, H.; Phytochemistry
1980, 19, 2663.
9. Lequesne, P. W.; Menachery, M. D.; Pastore, M. P.; Kelley, C. J.; Bren-nan, T. F.; OBren-nan, K. D.; Raffauf, R. F.; Weeks, C. M.; J. Org. Chem. 1982, 47, 1519.
10. Pettit, G. R.; Herald, D. L.; Cragg, G. M.; Rideout, J. A.; Brown, P.; J.
Nat. Prod. 1990, 53, 382.
11. Borsato, M. L. C.; Grael, C. F. F.; Souza, G. E. P.; Lopes, N. P.;
Phytochemistry 2000, 55, 809.
12. Dos Santos, M. D.; Gobbo-Neto, L.; Albarella, L.; de Souza, G. E. P.; Lopes, N. P.; J. Ethnopharmacol. 2005, 96, 545.
13. Bohlmann, F.; Zdero, C.; Robinson, H.; King, R. M.; Phytochemistry
1980, 19, 2381.
14. Bohlmann, F.; Muller, L.; King, R. M.; Robinson, H.; Phytochemistry
1981, 20, 1149.
15. Dos Santos, P. A.; Lopes, J. L. C.; Lopes, N. P.; Biochem. Syst. Ecol.
2004, 32, 509.
16. Bohlmann, F.; Zdero, C.; Robinson, H.; king, R. M.; Phytochemistry
1981, 20, 731.
17. Chicaro, P.; Pinto, E.; Colepicolo, P.; Lopes, J. L. C.; Lopes, N. P.;
Biochem. Syst. Ecol. 2004, 32, 239.
18. Gobbo-Neto, L.; Santos, M. D.; Kanashiro, A.; Almeida, M. C.; Lucisano-Valim, Y. M.; Lopes, J. L. C.; Souza, G. E. P.; Lopes, N. P.;
Planta Med. 2005, 71, 3.
19. Sakamoto, H. T.; Flausino, D.; Castellano, E. E.; Stark, C. B. W.; Gates, P. J.; Lopes, N. P.; J. Nat. Prod. 2003, 66, 693.
20. Sargenti, S. R.; Vichnewski, W.; Phytochem. Anal. 2000, 11, 69. 21. Bohlmann, F.; Zdero, C.; Robinson, H.; King, R. M.; Phytochemistry
1982, 21, 1087.
22. Bohlmann, F.; Jakupovic, J.; Plant Syst. Evol. 1990, 4 (suppl.), 3. 23. Emerenciano, V. P.; Ferreira, Z. S.; Kaplan, M. A. C.; Gottlieb, O. R.;
Phytochemistry 1987, 26, 3103; Da Costa, F. B.; Terloth, L.; Gasteiger, J.; Phytochemistry 2005, 66, 345.
24. Lequesne, P. W.; Levery, S. B.; Menachery, M. D.; Brennan, T. F.; Raf-fauf, R. F.; J. Chem. Soc. Perkin Trans. 1 1978, 1572.
25. Sass, D. C.; Heleno, V. C. G.; Lopes, J. L. C.; Constantino, M. G.;
Tetrahedron Lett. 2008, 49, 3877.
26. Gobbo-Neto, L.; Lopes, N. P.; J. Agr. Food Chem. 2008, 56, 1193. 27. Gobbo-Neto, L.; dos Santos, M. D.; Albarella, L.; Zollo, F.; Pizza, C.;
Lopes, N. P.; Biochem. Syst. Ecol. 2008, 36, 473.
28. Moraes, S. L.; Gregorio, L. E.; Tomaz, J. C.; Lopes, N. P.;
Chromatographia 2009, 69 (suppl. 2), S157.
29. Guzzo, L. S.; Saude-Guimaraes, D. A.; Silva, A. C. A.; Lombardi, J. A.; Guimaraes, H. N.; Grabe-Guimaraes, A.; J. Ethnopharmacol. 2008, 116, 120.
30. Schmidt, T. J.; Bioorgan. Med. Chem. 1997, 5, 645.
31. Grael, C. F. F.; Vichnewski, W.; de Souza, G. E. P.; Lopes, J. L. C.; Albuquerque, S.; Cunha, W. R.; Phytother. Res. 2000, 14, 203. 32. Jordao, C. O.; Vichnewski, W.; De Souza, G. E. P.; Albuquerque, S.;
Lopes, J. L. C.; Phytother. Res. 2004, 18, 332.
33. Rungeler, P.; Castro, V.; Mora, G.; Goren, N.; Vichewski, W.; Pahl, H. L.; Merfort, I.; Schmidt, T. J.; Bioorgan. Med. Chem. 1999, 7, 2343. 34. Dos Santos, M. D.; Chen, G.; Almeida, M. C.; Soares, D. M.; Souza, G.
E. P.; Lopes, N. P.; Lantz, R. C.; Nat. Prod. Comm. 2010, 5, 733. 35. Nascimento, A. M. A.; Brandao, M. G. L.; Oliveira, G. B.; Fortes, I. C.
P.; Chartone-Souza, E.; Anton. Leeuw. Int. J. G. 2007, 92, 95. 36. Sousa, O. V.; Silverio, M. S.; Del-Vechio-Vieira, G.; Matheus, F. C.;
Yamamoto, C. H.; Alves, M. S.; J. Pharm. Pharmacol. 2008, 60, 771. 37. Oliveira, A. B.; Saude, D. A.; Perry, K. S. P.; Duarte, D. S.; Raslan, D.
S.; Boaventura, M. A. D.; Chiari, E.; Phytother. Res. 1996, 10, 292. 38. Saúde-Guimarães, D. A.; Faria, A. R.; Rev. Bras. Farmacogn. 2007, 17,
455.
39. Takeara, R.; Albuquerque, S.; Lopes, N. P.; Lopes, J. L. C.; Phytomedi-cine 2003, 10, 490.
40. Edwirds, J. M.; Raffauf, R. F.; Le Quesne, P. W.; J. Nat. Prod. 1979, 42, 85.
41. Herz, W.; Kumar, N.; Vichnewski, W.; Blount, J. F.; J. Org. Chem. 1980,