Effect
of
diterpenoid
kaurenoic
acid
on
genotoxicity
and
cell
cycle
progression
in
gastric
cancer
cell
lines
Plínio
Cerqueira
dos
Santos
Cardoso
a,1,
Carlos
Alberto
Machado
da
Rocha
b,*
,1,
Mariana
Ferreira
Leal
c,
Marcelo
de
Oliveira
Bahia
a,
Diego
Di
Felipe
Ávila
Alcântara
a,
Raquel
Alves
dos
Santos
d,
Natália
dos
Santos
Gonçalves
d,
Sérgio
Ricardo
Ambrósio
e,
Bruno
Coêlho
Cavalcanti
f,
Caroline
Aquino
Moreira-Nunes
a,g,
Claudia
do
Ó
Pessoa
f,
Rommel
Mário
Rodríguez
Burbano
a,h,**
aHumanCytogeneticLaboratory,BiologicalScienceInstitute,FederalUniversityofPará(UFPA),Belém,Pará,Brazil
bFederalInstituteofEducation,ScienceandTechnologyofPará(IFPA),Av.AlmiranteBarroso,1155(Marco),CEP66093-020,Belém,Pará,Brazil cDepartmentofMorphologyandGenetics,FederalUniversityofSãoPaulo(UNIFESP),SãoPaulo,SãoPaulo,Brazil
dLaboratoryofGeneticsandMolecularBiology,UniversityofFranca(UNIFRAN),Franca,SãoPaulo,Brazil eLaboratoryofBiotransformation,UniversityofFranca(UNIFRAN),Franca,SãoPaulo,Brazil
fDepartmentofPhysiologyandPharmacology,FederalUniversityofCeará(UFC),Fortaleza,Ceará,Brazil
gLaboratoryofGeneticsofHemoglobinopathiesandHematologicDiseases,FederalUniversityofCeará(UFC),Fortaleza,Ceará,Brazil hHospitalOphirLoyola(HOL),Belém,Pará,Brazil
ARTICLE INFO
Articlehistory:
Received24November2016
Receivedinrevisedform22February2017 Accepted22February2017
Keywords:
Cellcycleprogression Cometassay
Gastriccancercelllines Genetranscriptions Kaurenoicacid Micronuclei
ABSTRACT
Thegoalofourstudywastoevaluatetheeffectofkaurenoicacid,obtainedfromcopaibaoilresin,in gastriccancer(GC)andanormalmucosaofstomach(MNP01)celllines.Thecompoundwastestedat concentrationsof2.5,5,10,30and60mg/mL.Cometandmicronucleusassayswereusedtoaccessits
potential genotoxicity invitro. Moreover, we evaluated the effect of kaurenoic acid in cell cycle progressionandinthetranscriptionofgenesinvolvedinthecontrolofthecellcycle:MYC,CCND1,BCL2, CASP3,ATM,CHK2andTP53.KaurenoicacidinducedanincreaseoncellDNAdamageormicronucleus frequenciesonGCcelllinesinadose-dependentmanner.TheGCandMNP01celllinesenteringDNA synthesisand mitosisdecreasedsignificantlywithkaurenoicacid treatment,andhad anincreased growthphasecomparedwithnon-treatedcells.Thetreatmentinducedapoptosis(ornecrosis)evenata concentrationof2.5mg/mLinrelationtonon-treatedcells.GCcelllinespresentedreducedMYC,CCND1,
BCL2andCASP3transcriptionwhileATM,CHK2andTP53increasedintranscriptioninrelationto non-treatedcells,especiallyataconcentrationabove10mg/mL.ThegenetranscriptionintheMNP01
(non-treatednon-cancercellline)wasdesignatedasacalibratorforalltheGCcelllines.Inconclusion,our resultsshowedthatkaurenoicacidobtainedfromCopaiferainducesDNAdamageandincreasesthe micronucleifrequencyinadose-dependentmannerinGCcells,withasignificantgenotoxicityobserved abovetheconcentrationof5mg/mL.Moreover,thiscompoundseemstobeabletoinducecellcyclearrest
andapoptosisinGCcells.
©2017ElsevierMassonSAS.Allrightsreserved.
1.Introduction
Gastriccancer(GC)remainsthethirdleadingcauseof
cancer-related mortality worldwide, and invasion and metastasis of
gastriccancerrepresentsthemajorreasonforitspoorprognosis
[1].Multimodal therapies haveproven tobenefitpatients with
cancer.However,withoutcurativesurgery,variationsand
combi-nationsofchemotherapyand/orradiationcannotbringclinically
meaningfulsuccessnowadays[2].
*Correspondingauthor.
**Correspondingauthorat:HospitalOphirLoyola(HOL),Av.MagalhãesBarata, 992,CEP66060-281,Belém,Pará,Brazil.
E-mailaddresses:carlos.rocha@ifpa.edu.br(C.A.M.d.Rocha),rommel@ufpa.br (R.M.R.Burbano).
1Theseauthorscontributedequallytothiswork.
http://dx.doi.org/10.1016/j.biopha.2017.02.085
0753-3322/©2017ElsevierMassonSAS.Allrightsreserved.
–
Available
online
at
One of the main problems in cancer treatment is gradual
resistanceofcancercellsagainsttreatment[3].Therefore,theaim
ofimmunopharmacologicalstudieshasbeentoimprovecancer
treatmentresults[4].Thealternatesolutionfortheharmfuleffects
of synthetic agents is the use of phytotherapy [5]. Bioactive
compoundsfromplantshavealotofbiologicalactivitiesandmay
presentgreatpotentialinthedevelopmentofnewdrugsforthe
treatmentofhumandiseases[6].
Reports about medicinal plants in the Brazilian Amazonian
region have been documented in the literature, especially
concerning the oil resin extracted from the trunk of Copaifera
langsdorffiiDesfon(Leguminosae)[7,8].Thisoilresinisareputed
folkremedy used bythe nativesof Brazil for thetreatmentof
several diseases such as sore throat, urinary and pulmonary
infections,andtohastenulcerandwoundhealing[9].Usually,this
oilresinisadministeredorallyinnatura,orappliedinointment
form [10–12]. This substance has great social and economic
representationintheAmazonregion,sinceitrepresentsabout95%
oftheentireoilresinproductioncountrywide[13].
SeveralstudieshavedemonstratedthattheCopaiferaoilresin
hasmanifoldtherapeuticproperties,includinganti-inflammatory,
antitumoral,antimelanoma,antiulcerogenic,antilipoperoxidation
and antioxidant [10,12,14–16]. Furthermore, the diterpenes,
kaurenoic and polyaltic acids present in the pure oil seem to
presenthealingandanti-inflammatoryproperties[7,8].
Kaurenoicacid(ent-kaur-16-en-19-oicacid)(Fig.1)isclassified
asaditerpeneandconsideredanintermediateofthegibberellin
plantgrowthhormonebiogenesis[17].Somestudiesshowedthat
kaurenoicacidpresentsinvitroanti-parasiticandanti-microbial
activities[18–23].Moreover,thisditerpenehasanti-proliferative
actioninCEMleukemiccells,MCF-7breastandHCT-8coloncancer
cells[24].Additionally,kaurenoicacidisalsoabletoreducehuman
spermmotilitywhendirectlytestedinspermsamples,butwas
onlyweaklyspermicidal[25].
While Copaifera oil resin has its herbal properties widely
reported, there are few studies in the scientific literature that
evaluatedtheeffectsofthisoilresincomponents,especiallyinGC
celllines.Thus,thepresentstudyaimstoassessthegenotoxicityof
kaurenoicacidandtheeffectofthiscompoundinthecellcycleand
inthetranscriptionofgenesinvolved,amongotherfunctions,in
GCcelllines.
2.Materialsandmethods
2.1.Generalexperimentalprocedures
TheACP02,ACP03andAGP01celllines,previouslyestablished
andcharacterizedbyourresearchgroup,wereusedinthepresent
study[26,27].TheACP02celllinewasestablishedfroma
diffuse-typeGC,andACP03andAGP01werefromanintestinal-typeGC.
ACP03isabletostartatumorigenesisprocessinCebuspaella[28].
WealsousedtheGCcelllinePG100obtainedfromRiodeJaneiro
CellBank,Brazil,thatwaspreviouslycytogeneticlycharacterized
byourgroup[29].
Cells lines were cultivated under standard conditions in
Modified Eagle’s medium salts, supplemented with 10% fetal
bovine serum, 2mM L-glutamine and antibiotics (100IU/mL
penicillin and 100
m
g/mL streptomycin [30]). Cells weremain-tained in 25cm2 tissue culture
flasks (TPP, Trasadingen,
Switzerland)at37C inahumidi
fiedatmospherecontaining5%
CO2andwereharvestedbytreatmentwith0.15%trypsinand0.08%
EDTA in phosphate-buffered saline (PBS). Cells (3105) were
seededin5mLofcompletemediumandgrownfor2dayspriorto
treatmentwiththetestsubstance.
Cellsweretreatedwithdifferentconcentrationsofkaurenoic
acid(2.5,5,10,30and60
m
g/mL)for3h.Afterthis,thecellswerewashed withice-cold PBS and trypsinizedwith 100
m
L trypsin(0.15%) and were resuspended in complete medium. The final
concentrationofDMSOintheculturemediumwaskeptconstant,
below 0.1% (v/v). The negative control cell cultures were not
exposed tokaurenoic acid, while themethyl methanesulfonate
(Sigma Aldrich Co, St. Louis, MO, USA) was used as a positive
control (410 5M). All cell treatments were carried out with
threereplicates.
2.2.Chemicalisolation
Inthisstudy,kaurenoicacid(CAS6730-83-2)(SigmaChemical
Co., St.Louis,MO, USA) was dilutedundersterileconditions in
dimethyl sulfoxide (DMSO)and theconcentrationsused inthis
studywerebasedonpreliminarycytotoxicityassaysperformedby
our research group (unpublished data). Kaurenoic acid was
obtainedfromtheoilresinofC.langsdorffiithatgrowsabundantly
intheAmazonregionofNorthernBrazil[31].
2.3.Genotoxicityassay
TheCometassay(alkalineversion)wasperformedasdescribed
by Singhet al. [32] withsomemodifications[33,34].Aftercell
treatment,thecellswerewashedwithice-coldPBSandtrypsinized
with100
m
L trypsin(0.15%)and wereresuspendedin completemedium. An aliquot (450
m
L)of thecell suspension fromeachexperimental groupwas takenandcentrifugedat1.000rpmfor
5mininamicrocentrifuge(Eppendorf).Theresultingpelletwas
homogenizedwith300
m
Lofalowmeltingpointagarose(0.8%),spreadontomicroscopeslidespre-coatedwithanormalmelting
pointagarose(1.5%)andcoveredwithacoverslip.After5minat
4C,thecoverslipwasremovedandtheslideswereimmersedin
cold lysissolution (2.5MNaCl;100mM EDTA; 10mM Tris,10%
DMSOand1%Triton-X,pH:10)foroneweek.Afterlysis,theslides
were placed in an electrophoresis chamber and covered with
freshlymadeelectrophoresisbuffer(300mMNaOH;1mMEDTA,
pH>13).
The electrophoresis was run for 25min (34V and 300mA).
Afterward,theslideswereneutralizedbysubmersionindistilled
water(4C)for5minandfixedin100%ethanolfor3min.Staining
oftheslideswasperformedimmediatelybeforetheanalysesusing
ethidium bromide dye (20
m
g/mL). Slides were prepared induplicate,and100cellswereanalyzedpersample(50cellsfrom
eachslide)usingafluorescentmicroscope(OlympusBX41)at40
magnification.
Thedamageindex(DI)isbasedonthelengthofmigrationand
theamountofDNAinthetail.Itisconsideredasensitivemeasure
ofDNA.Fivecategories(0–4)wereusedhere:class0(nodamage),
class1(slightdamagewithataillengthshorterthanthediameter
ofthenucleus),class2(mediumdamagewithataillengthoneor
twotimesthediameterofthenucleus),class3(significantdamage
with a tail length greater than two times the diameter of the
Fig.1.Molecularstructureofkaurenoicacid(ent-kaur-16-en-19-oicacid).
nucleus)andclass4(significantdamagewithtaillengthgreater
thanthreetimesthediameterofthenucleus).Damagedindexthus
ranged from 0 (no damage: 100 cells0) to 4 (completely
damaged:100cells4)[35,36].
2.4.Mutagenicassay
The micronucleusassaywas performedaccording toFenech
[37].Threehoursaftertreatment,thecultureswerewashedtwice
withthe medium and Cytochalasin B(Sigma Chemical Co., St.
Louis,MO, USA)wasadded atafinalconcentrationof2
m
g/mL.Cultureswereharvested 21hafterCytochalasinBaddition.The
cells wereseparated fromthe bottle bytrypsinization and the
cellularsuspensionwascentrifugedat1000rpmfor5min.Then,
cellswereresuspendedin aKCl0.075Msolutionmaintainedat
4C for 3min (mild hypotonic treatment). Cell cultures were
harvested72haftertheincubation,centrifuged(5minat800rpm)
andsubmittedtohypotonicsolution(KCL0.075M).Afterward,GC
celllineswerewashedoncewith5mLofacoldmethanol:acetic
acid(5:1) (v/v) fixative and washed againwith 5mL of a cold
methanol:aceticacid(3:1)(v/v)fixative.Thecellsuspensionwas
droppedontoslidesandstainedinasolutionof5%Giemsadye
(SigmaChemicalCo.,St.Louis,MO,USA)inaphosphatebuffer(pH
6.8)for5min.Micronucleiwerescoredin2000binucleatedcells
using the criteria according to Fenech [37]. The analysis was
performedusinganopticalmicroscope(BIOVALL2000A)at100
magnification.
2.5.Cellcycleanalysis
Cell cycle distribution followed the procedure described by
Nicolettietal.[38].GClineswereincubatedat37Cfor3hinthe
darkinalysissolutioncontaining0.1%,citrate,0.1%tritonX-100
and50
m
g/mLpropidiumiodide.CellfluorescencewasdeterminedbyflowcytometryinaGuava1EasyCyteTMMiniSystemcytometer
(GuavaTechnologiesInc.,Hayward,CA,USA),usingCytoSoft4.1
software.
2.6.Genestranscriptionanalysis
Weanalyzedthetranscriptionofgenesinvolvedinthecellcycle
regulationtoevaluatetheeffectof kaurenoicacidtreatmentin
ACP02,ACP03,AGP01andPG100celllines(Table1).TotalmRNA
was isolated from all cell line samples using Trizol Reagent
(Invitrogen,Carlsbad,CA,EUA).TheextractedRNAwaspurified
usingRNeasyMiniKit(Qiagen,Valencia,CA,EUA),accordingtothe
recommended protocol. The concentration and quality were
determined using a NanoDrop spectrophotometer (Kisker,
Germany)and1%agarosegels,respectively.Sampleswerestored
at 80Cuntiluse.
RNAwas reverse-transcribed using the High-Capacity cDNA
Archive kit according to the manufacturer’s protocol (Life
Technologies,Pittsburgh,PA,USA).ComplementaryDNAwasthen
amplifiedbyreal-timereversetranscriptionquantitativePCR
(RT-qPCR) using TaqMan probes purchased as Assays-on-demand
Productsfor GeneExpression (LifeTechnologies,Pittsburgh,PA,
USA; Table 1) and a 7500 FastReal-Time PCR instrument (Life
Technologies,Pittsburgh,PA,USA).TheGAPDHgenewasselected
as an internal control for RNA input and reverse-transcription
efficiency.AllRT-qPCRswereperformedintriplicateforboththe
targetgenesandtheinternalcontrol.Therelativequantificationof
genetranscriptionwascalculatedaccordingtothemanufacturer’s
software (Life Technologies, Pittsburgh, PA, USA). The gene
Table1
SummaryofSevenTargetGenesandtheReferenceGene.
Gene symbol
Name genefunction assay*
MYC v-mycavianmyelocytomatosisviral oncogenehomolog
progressionofcellcycleprogression,apoptosisandcellulartransformation/oncogene hs00153408_m1
CCND1 cyclinD1 positiveregulationofmitoticcellcycle/oncogene hs00765553_m1
BCL2 b-cellcll/lymphoma2 anti-apoptotic/oncogene hs00608023_m1
CASP3 caspase3,apoptosis-relatedcysteine peptidase
pro-apoptotic hs00234387_m1
ATM atmserine/threoninekinase cellcyclearrestinresponsetoDNAdamageandforgenomestability/tumorsupressor hs01112355_g1 CHK2 checkpointkinase2 cellcyclecheckpointregulatorinresponsetoDNAdamageandtoreplicationblocks/
putativetumorsuppressor
hs00200485_m1
TP53 tumorproteinp53 inductionofcellcyclearrest,apoptosis,senescence,DNArepair,orchangesin metabolism/tumorsupressor
hs01034249_m1
*TaqManprobeswerepurchasedasassays-on-demandproductsforgeneexpression(LifeTechnologies,USA).
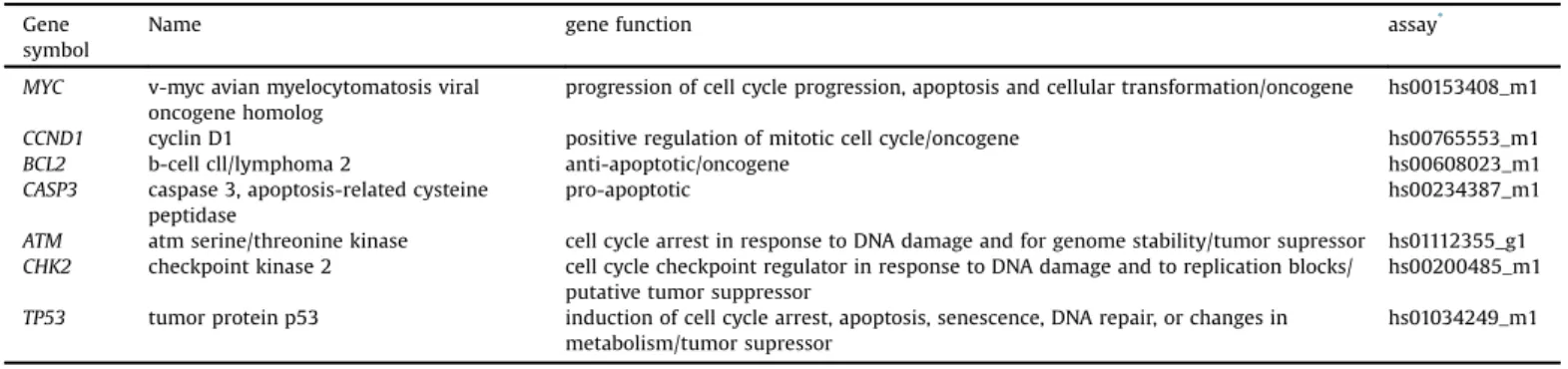
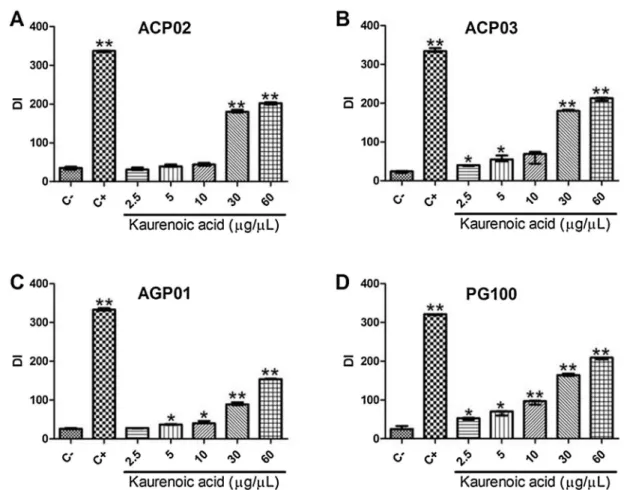
Fig.2. Genotoxicityofkaurenoicacidingastriccancercellline.A)Damageindexbycometassay;B)micronucleifrequency.DI:damageindex;MN:micronucleifrequency.C-: control,whichwerenon-treatedcells;C+:cellstreatedwithmethylmethanesulfonateon*Significantlydifferentfromthecontrol(p<0.05;byKruskal-Wallistestfollowedby Games-Howellpost-hocanalysis).**Significantlydifferentfromthecontrol(p0.001;byKruskal-WallistestfollowedbyGames-Howellpost-hocanalysis).
transcriptionintheMNP01(non-treatednon-cancercellline)was
designatedasacalibratorforalltheGCcelllines.
2.7.Statisticalanalysis
Thedataareshownastheasthemeanstandard deviation
(SD). The Shapiro-Wilk test was used to evaluate the data
distribution and to determine the appropriate subsequent test
for statisticalcomparisons. The Kruskal-Wallistest followedby
Games-Howellpost-hoctestformultiplecomparisonswasusedto
comparethestudiedgroups.Spearmancorrelationtestwasusedto
evaluate the possible correlation between kaurenoic acid
concentration and genotoxicty or gene transcription. A p-value
lessthan0.05wasconsideredsignificant[39].
3.Resultsanddiscussion
GCcelllinestreatedwithmethylmethanesulphonatepresented
asignificantincreaseintheDNAdamageindexwhencompared
withcontrolnon-treatedcells[333.5(13.5)versus25.5(7.5),
p<0.001;Fig.2A].Similarphenomenonwasrepeatedfornormal
cellsMNP01(356versus22.3,p<0.001).Morethan70%ofGCcells
treatedwiththisdrugpresentedcometclass4,whichwasdetected
inlessthan2%ofnon-treatedcells(Fig.3).
Fig.3.EffectofkaurenoicacidonthescoreofDNAdamagebycometassayingastriccancercelllines.TheDNAdamagewasclassifiedbasedonthelengthofmigrationandon theamountofDNAinthecomettail.Class0:nodamage;Class1:littledamagewithataillengthshorterthandiameterofthenucleus;Class2:mediumdamagewithatail lengthoneortwotimesthediameterofthenucleus;Class3:significantdamagewithataillengthgreaterthantwotimesthediameterofthenucleus;Class4:significant damagewithtaillengthgreaterthanthreetimesthediameterofthenucleus;C-:control,whichwerenon-treatedcells;C+:cellstreatedwithmethylmethanesulfonateon* Significantlydifferentfromthenegativecontrol(p<0.05;byKruskal-WallistestfollowedbyGames-Howellpost-hocanalysis).**Significantlydifferentfromthecontrol (p0.001;byKruskal-WallistestfollowedbyGames-Howellpost-hocanalysis).
Fig.4.Genotoxicityofkaurenoicacidbycometassayineachstudiedgastriccancercellline.A)ACP02;B)ACP03;C)AGP01;D)PG100.DI:damageindexbycometassay.C-: control,whichwerenon-treatedcells;C+:cellstreatedwithmethylmethanesulfonateon*Significantlydifferentfromthecontrol(p<0.05;byKruskal-Wallistestfollowedby Games-Howellpost-hocanalysis).**Significantlydifferentfromthecontrol(p0.001;byKruskal-WallistestfollowedbyGames-Howellpost-hocanalysis).
Kaurenoic acid induced DNA damage in a dose-dependent
manner(Fig.2).Atconcentrationsabove5
m
g/mL,kaurenoicacidinduceda significantincrease in the DNAdamage index when
comparedwithcontrolvalues:47(25.75;p=0.001),47(42.75;
p=0.006),173(69;p<0.001),205(43;p<0.001)for5, 10,30or
60
m
g/mL, respectively (Fig. 2A). Kaurenoic acid induced DNAdamageclassifiedascometclass4(Fig.3);however,lessthan30%
ofcellspresentcometclass4.
Moreover, the methyl methanesulphonate treatment also
inducedasignificantincreaseofmicronucleicomparedwiththe
control[96.5(12)versus20.5(8),p<0.001].Additionally,GC
cell lines presented a significant increase of the frequency of
micronuclei when treated with kaurenoic acid concentrations
above 5
m
g/mL: 30.5 (7.25; p=0.004), 37 (8; p<0.001), 53(19; p<0.001) and 74.5 (15.75; p<0.001), respectively
(Fig.2B).
The kaurenoic acid concentration treatment was strongly
correlatedwiththeDNAdamageindex(rS=0.912,p<0.001)and
themicronucleifrequency(rS=0.925,p<0.001).Asimilarpattern
wasobservedinresponsetothekaurenoicacidtreatmentineach
cellline(Figs.4and5).
Genotoxiceventsareconsideredcrucialstepsinthe
progres-sion of cancer. In this study, we performed comet assay and
micronucleustests in four GC cell lines treated withdifferent
concentrationsofkaurenoicacid.Toourknowledge,noprevious
studyevaluatedtheeffectofthiscompoundinGCcelllines.Here,
we observed that kaurenoic acid has a genotoxic potential
especially at higher concentrations, evidenced by increased
frequenciesofbothmicronucleiandDNAdamage.Kaurenoicacid
inducedDNAdamageinadose-dependentmanner.Ourcurrent
findings in GCcelllines areinpartinagreementwithfindings
previouslyobservedinChinesehamsterlungfibroblastcells(V79
cells)treatedwithkaurenoicacid.Therefore,thiscompoundmay
actasaDNAintercalatingagent[31],whichinduceschromosomic
alterationsandDNAbreaksinmammaliancells[40].
Few studies have evaluated the effect of kaurenoic acid.
However, the genotoxic effect of diterpens was previously
reported. Usingthecometassay,Katoet al.[41]showed thata
pimarane-type diterpene, pimaradienoic acid, induced DNA
damage at concentrations of 2.5 and 5.0
m
g/mL in V79 cells.Moreover,DNAdamagewasalsoreportedinhepatocytesofSwiss
micetreatedwith80mg/kgofthisditerpene.
Paclitaxel(Taxol1)isaditerpenoidclinicallyeffective
antineo-plasticdrugthathasbeenusedforthetreatmentofseveralhuman
cancers,includingGC[42].Thisditerpensuppressthemicrotubule
spindle dynamics, which results in mitosis inhibition and
apoptosis induction [43,44]. Branham et al. [45] previously
described that paclitaxel is able to induce DNA damage in
lymphocytes treatedwith concentrationsof 10
m
gorhigher. Ingastrointestinal cell lines (HT29 and MKN45), 0.01
m
g/mL ofpaclitaxelseemstobecytotoxicbyMTTassay
([2-(2-methoxy-4-
nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazo-lium,Monosodiumsalt](WST-8)colorimetricassay)[46].
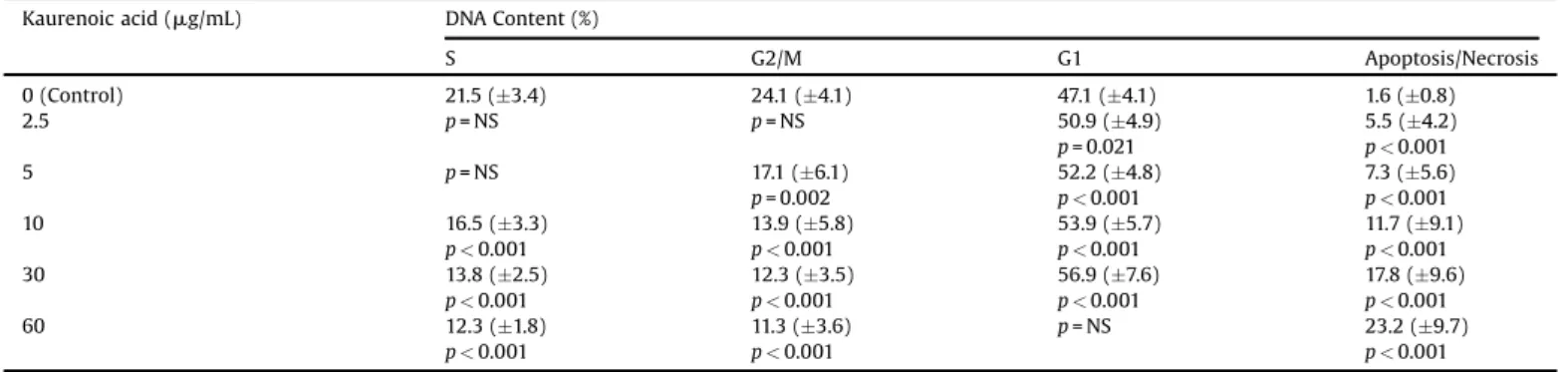
Themethylmethanesulphonatetreatmentinducedapoptosis
ornecrosis[sub-G1;40.2(4.7)versus1.6(0.8);p<0.001]and
reducedtheGCcellsentering mitosis[G2/M;12.1(1.7)versus
24.1(4.11);p<0.001]inrelationtocontrols.TheGCcellsentering
DNAsynthesisandmitosisdecreasedsignificantlywithkaurenoic
acidtreatment,alongwithanincreaseinthegrowthphase(G1)
comparedwithcontrols.Inaddition,kaurenoicacidtreatmentwas
able to significantly induce apoptosis (or necrosis) even at
concentrations of 2.5
m
g/mL in relation to non-treated cellsFig.5. Effectofkaurenoicacidonmicronucleifrequencyineachgastriccancercellline.A)ACP02;B)ACP03;C)AGP01;D)PG100.MN:micronucleifrequency.C-:control, whichwerenon-treatedcells;C+:cellstreatedwithmethylmethanesulfonateon*Significantlydifferentfromthecontrol(p<0.05;byKruskal-Wallistestfollowedby Games-Howellpost-hocanalysis).**Significantlydifferentfromthecontrol(p0.001;byKruskal-WallistestfollowedbyGames-Howellpost-hocanalysis).
(Table2andFig.6).Asimilarpatternwasobservedinresponseto
thekaurenoicacidtreatmentineachcellline.
TofurtherunderstandtheeffectofkaurenoicacidinGCcells,
weevaluatedthecellcycleprogressionandthetranscriptionof
genes involved, among other functions, in the control of this
process.KaurenoicacidwasabletoinducecellcyclearrestatG1
andinduceapoptosisinadose-dependentmanner,reducingthe
DNA synthesis and mitosis processes. The gene transcription
analysesareinagreementwiththesefindings.
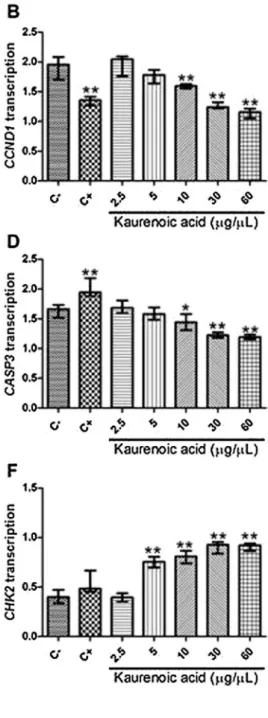
TheGCcelllinespresentedanincreasedtranscriptionofMYC
[fold-change=2.66 (0.42); Fig. 7A], CCND1 [fold-change=1.96
(0.19);Fig.7B],BCL2[fold-change=2.08 (0.21);Fig.7C],and
CASP3[fold-change=1.66(0.10);Fig.7D]inrelationtothe
non-neoplasticMNP01cells that wereusedas a calibratorfor gene
transcription analysis. On the other hand, the GC cell lines
presented a reduced transcription of ATM [fold-change=0.36
(0.14);Fig. 7E], CHK2 [fold-change=0.40 (0.11);Fig. 7F] and
TP53 [fold-change=0.27 (0.11); Fig. 7G] in relationto MNP01
cells.
TheGCcellstreatedwithmethylmethanesulphonatepresented
asignificantlyreducedtranscriptionofCCND1[1.34(0.11)versus
1.95 (0.19), p<0.001; Fig.7B],BCL2 [1.85 (0.18) versus 2.08
(0.21), p=0.048; Fig. 7C] in relation to non-treated GC cells.
Conversely, CASP3 transcription was increased in the GC cells
treated withmethyl methanesulphonatein relation tocontrols
[1.94(0.15)versus1.66(0.10),p<0.001;Fig.7D].Moreover,we
observed a significantly reduced MYC transcription in ACP02,
ACP03, AGP01 and PG100 cell lines treated with methyl
methanesulphonate in relation to non-treated cells (p<0.05),
wheneachcelllinewasevaluatedseparately.Additionally,methyl
methanesulphonate induced a significant reduction of ATM
transcriptioninACP02andPG100cells(p<0.05),andanincrease
ofCHK2transcriptioninACP03andPG100cells(p<0.05).
Table 3 shows us how the kaurenoic acid affected the
transcription of genes involvedin the controlof the cellcycle.
MYCtranscriptionwassignificantlyreducedinGCcelllinestreated
with 30 and 60
m
g/mL of kaurenoic acid compared withnon-treatedcells(Fig.7A).Lowconcentrationsofkaurenoicacidwere
alsoabletoreduceMYCtranscriptioninsomecelllines.Moreover,
GCcelllinestreatedwith10,30and60
m
g/mLofkaurenoicacidalsopresentedreducedCCND1(Fig.7B),BCL2(Fig.7C),andCASP3
(Fig. 7D) transcription in relation to non-treated cell lines.
Conversely, GC cell lines treated with10, 30 and 60
m
g/mL ofkaurenoicacidpresentedincreasedATM(Fig.7E),CHK2(Fig.7F),
andTP53(Fig.7G)transcriptioninrelationtonon-treatedcelllines.
Inaddition,5
m
g/mLofkaurenoicacidalsoinducedincreasedCHK2transcriptioninGCcelllinesinrelationtocontrols(Fig.7F).
Moreover, we observed an inverse correlation between
kaurenoic acid concentration treatment and MYC (rS= 0.770,
p<0.001), CCND1 (rS= 0.880, p<0.001), BCL2 (rS= 0.826,
p<0.001) and CASP3 (rS= 0.828, p<0.001) transcription. On
the other hand, kaurenoic acid concentration treatment was
directly correlated with ATM (rS=0.863, p<0.001), CHK2 (rS=
0.871,p<0.001)andTP53(rS=0.721,p<0.001)transcription.
Weobservedthat MYC,ATM,CHK2,CCND1,BCL2,CASP3, and
TP53transcriptionslightlyvariedinresponsetothekaurenoicacid
treatmentineachcellline.Inthisanalysis,wefirstnormalizedthe
studiedgenestranscriptioninGCcelllinesbytheirtranscriptionin
non-neoplastic cells. As expected, the GC cell lines presented
increasedtranscriptionoftheoncogenesMYC,CCND1andBCL2and
reduced transcription of the ATM, CHK2 and TP53 tumor
suppressors.
TheresponsetokaurenoicacidslightlyvariedamongGCcell
lines,whichwouldbeexpectedconsideringthedifferencesinthe
geneticbackground[26–29].Ingeneral,higherconcentrationsof
thiscompoundinducedlowerMYC,CCND1andBCL2transcription
Table2
Effectofkaurenoicacidoncellcycleprogressionandinductionofapoptosisornecrosis.
Kaurenoicacid(mg/mL) DNAContent(%)
S G2/M G1 Apoptosis/Necrosis
0(Control) 21.5(3.4) 24.1(4.1) 47.1(4.1) 1.6(0.8)
2.5 p=NS p=NS 50.9(4.9)
p=0.021
5.5(4.2)
p<0.001
5 p=NS 17.1(6.1)
p=0.002
52.2(4.8)
p<0.001
7.3(5.6)
p<0.001
10 16.5(3.3)
p<0.001
13.9(5.8)
p<0.001
53.9(5.7)
p<0.001
11.7(9.1)
p<0.001
30 13.8(2.5)
p<0.001
12.3(3.5)
p<0.001
56.9(7.6)
p<0.001
17.8(9.6)
p<0.001
60 12.3(1.8)
p<0.001
11.3(3.6)
p<0.001
p=NS 23.2(9.7)
p<0.001
NS=notstatisticallysignificant.
Fig.6.Kaurenoicacideffectoncellcycleprogressionofgastriccancercelllines.C-:control,whichwerenon-treatedcells;C+:cellstreatedwithmethylmethanesulfonateon* Significantlydifferentfromthecontrol(p<0.05;byKruskal-WallistestfollowedbyGames-Howellpost-hocanalysis).**Significantlydifferentfromthecontrol(p0.001;by
Kruskal-WallistestfollowedbyGames-Howellpost-hocanalysis).
Fig.7.Kaurenoicacideffectongenetranscriptioningastriccancercelllines.A)MYC;B)CCDN1;C)BCL2;D)CASP3;E)ATM;F)CHK2;G)TP53.Thedrugtreatmentsandgene transcription analyses were performed in ACP02, ACP03, AGP01 and PG100 cell lines. C-: control, which were non-treated cells; C+: cells treated with methylmethanesulfonateon*Significantlydifferentfromthecontrol(p<0.05;byKruskal-WallistestfollowedbyGames-Howellpost-hocanalysis).**Significantly differentfromthecontrol(p0.001;byKruskal-WallistestfollowedbyGames-Howellpost-hocanalysis).
inGCcelllines,reinforcingthatkaurenoicacidalsoactsinmitosis inhibition.
AlthoughreductionofCASP3wasdetectedinGCcellinrelation
tonon-cancerous cells and withthe kaurenoic acidtreatment,
higherconcentrationofthiscompoundinducedhigherATM,CHK2
and TP53in GC cell lines.These genes present several rolesin
humancells,includingcellcyclearrestandinductionofapoptosis
in response toDNAdamage as observed bycell cycleanalysis.
Considering that the DNA damage also increased with the
kaurenoicacidconcentrationtreatment,ourresultssuggestthat
kaurenoicacidmayalsoactasanapoptosisinducerinresponseto
DNAdamage.Furtherinvestigationsarestillnecessary,however,
kaurenoicacidmaypresentantineoplasticactivityasthediterpene
paclitaxel.
4.Conclusion
OurresultsshowedthatkaurenoicacidobtainedfromCopaifera
inducesDNAdamageandincreasesthemicronucleifrequencyina
dose-dependentmannerinGCcells,withasignificantgenotoxicity
observed above the concentration of 5
m
g/mL. Moreover, thiscompound seems to be able to induce cell cycle arrest and
apoptosisinGCcells.
Conflictofinterest
Theauthorsdeclarethatthereisnoconflictofinterest.
Acknowledgements
We gratefully acknowledge the financial support of the
Conselho NacionaldeDesenvolvimentoCientíficoeTecnológico
(CNPq)andFundaçãodeAmparoàPesquisadoEstadodeSãoPaulo
(FAPESP),forgrantsandfellowshipaward.
References
[1]L.Chen,L.Min,X.Wang,J.Zhao,H.Chen,J.Qin,etal.,LossofRACK1promotes metastasisofgastriccancerbyinducingamiRNA-302c/IL-8signalingloop, CancerRes.15(2015)3832–3841.
[2]Y.Y.Choi,S.H.Noh,J.H.Cheong,Evolutionofgastriccancertreatment:fromthe goldenageofsurgerytoaneraofprecisionmedicine,Med.J.56(2015)1177– 1185.
[3]Z.Wang,N.Wang,J.Chen,J.Shen,Emergingglycolysistargetinganddrug discoveryfromchinesemedicineincancertherapy,Evid.BasedComplement Alternat.Med.2012(2012)173–175.
[4]A.Azadmehr,R.Hajiaghaee,A.Afshari,Z.Amirghofran,M.Refieian-Kopaei,H. Darani,etal.,Evaluationofinvivoimmuneresponseactivityandinvitro anti-cancereffectbyScrophulariamegalantha,Med.PlantsRes.5(2011)2365–2368. [5]M.A.Harun-ur-Rashid,G.Sadik,A.A.Rahman,Biologicalactivitiesofanew
derivativefromIpomoeaturpithum,Pak.J.Biol.Sci.5(2002)968–969. [6]A.P. Balbani, D.H. Silva,J.C. Montovani, Patentsof drugsextracted from
Brazilianmedicinalplants,Exp.Opin.Ther.Pat.19(2009)461–473.
[7]M.R.R.Tappin,J.F.G.Pereira,L.A.Lima,A.C.Siani,J.L.Mazzei,M.F.S.Ramos, Quantitativechemicalanalysisforthestandardizationofcopaibaoilbyhigh resolutiongaschromatograpy,Quim.Nova27(2004)236–240.
[8]E.ComelliJúnior,J.Skinovski,M.F.Sigwalt,A.B.Branco,S.R.Luz,C.P.Baulé, Rupturepointanalysisofintestinalanastomotichealinginratsunderthe actionofpureCopaíba(CopaiferaIangsdorfii)oil,ActaBras.Cir.25(2010)362– 367.
[9]L.C.DiStasi,C.A.H.Lima,PlantasmedicinaisnaAmazôniaenaMataAtlântica, FundaçãoEditora,SãoPaulo,2003,pp.604.
[10]L.A.F.Paiva,L.A.Gurgel,R.M.Silva,A.R.Tomé,N.V.Gramosa,E.R.Silveira,etal., Antiinflammatoryeffectofkaurenoicacid,aditerpenefromCopaifera langsdorffiionaceticacid-inducedcolitisinrats,Vascul.Pharmacol.39(2002) 303–307.
[11]M.W.Biavatti,D.Dossin,F.C.Deschamps,M.P.Lima,Copaibaoil-resinanalysis: contributiontoqualitycontrol,Rev.Bras.Farmacol.16(2006)230–235. [12]J.J.deLimaSilva,S.B.Guimaraes,E.R.daSilveira,P.R.L.Vasconcelos,G.G.Lima,
S.M.Torres,etal.,EffectsofCopaiferalangsdorffiiDesf:on ischemia-reperfusionofrandomizedskinflapsinrats,Aesthet.Plast.Surg.33(2009) 104–109.
[13]R.D.Medeiros,G.Vieira,Sustainabilityofextractionandproductionofcopaiba (CopaiferamultijugaHayne)oleoresininManaus,AM,Brazil,For.Ecol.Manage. 256(2008)282–288.
[14]A.Ohsaki,L.T.Yan,S.Ito,H.Edatsugi,Y.IwataD.Komoda,Theisolationandin vivopotentantitumoractivityofclerodanediterpenoidfromtheoleoresinof theBrazilianmedicinalplant,CopaiferalangsdorfiDesfon,Bioorg.Med.Chem. Lett.4(1994)2889–2892.
[15]N.M. Gomes, C.M. Rezende, S.P. Fontes, M.E. Matheus, P.D. Fernandes, Antinociceptiveactivityofamazoniancopaibaoils,J.Ethnopharmacol.109 (2007)486–492.
[16]A.O.dosSantos,M.A.Costa,T.Ueda-Nakamura,B.P.Dias-Filho,V.F.da Veiga-Júnior,M.M.deSouzaLima,etal.,Leishmaniaamazonensis:effectsoforal treatmentwithcopaibaoilinmice,Exp.Parasitol.129(2011)145–151. [17]D. Barton, K. Nakanshi, O. Meth-Cohn, Comprehensive Natural Products
Chemistry,Elsevier,Oxford,1999,pp.234.
[18]K.Boakye-Yiadom,N.I.Fiagbe,J.S.Aymin,Antimicrobialpropertiesofsome WestAfricanmedicinalplantsiv:antimicrobialactivityofxylopicacidand otherconstituenteofthefruitsofXylopiaaethiopica(Annonaceae),Llyodia40 (1977)543–545.
[19]S.C.Davino,A.M.Giesbrecht,N.F.Roque,Antimicrobialactivityofkaurenoic acidderivatesubstitutedoncarbon-15,Braz.J.Med.Biol.Res.22(1989)1127– 1129.
[20]R.Batista,E.Chiari,A.B.deOliveira,Trypanosomicidalkaurenediterpenes fromWideliapaludosa,PlantaMed.65(1999)283–284.
[21]A.C.de Melo,B.B.Cota,A.B. deOliveira,F.C.Braga,HPLCquantitationof kaurenediterpenesinXylopiaspecies,Fitoterapia72(2001)40–45. [22]M. Wilkens, C. Alarcon, A. Urzua, L. Mendoza, Characterization of the
bactericidalactivityofthenaturalditerpenekaurenoicacid,PlantaMed.68 (2002)452–454.
[23]M.Cotoras,C.Folch,L.Mendoza,Characterizationoftheantifungalactivityon Botrytiscinereaofthenaturalditerpenoidskaurenoicacidand 3beta-hydroxy-kaurenoicacid,J.Agric.FoodChem.52(2004)2821–2826.
[24]L.V. Costa-Lotufo,G.M.A.Cunha, P.A.M.Farias,G.S. Viana,K.M. Cunha, C. Pessoa,etal.,Thecytotoxicandembryotoxiceffectsofkaurenoicacid,a diterpeneisolatedfromCopaiferalangsdorffiioleo-resin,Toxicon40(2002) 1231–1234.
[25]A.Valencia,A.Wens,H.Ponce-Monter,N.Pedron,A.J.Gallegos,A.F.Guijane, etal.,ZoapatleXII.InvitroeffectofkaurenoicacidisolatedfromMontana frutescensandtwoderivativesuponhumanspermatozoa,J.Ethnopharmacol. 18(1986)89–94.
[26]M.F.Leal,J.L.MartinsdoNascimento,C.E.daSilva,M.F.VitaLamarão,D.Q. Calcagno,A.S.Khayat,Establishmentandconventionalcytogenetic characterizationofthreegastriccancercelllines,CancerGenet.Cytogenet.195 (2009)85–91.
[27]M.F.Leal,D.Q.Calcagno,J.F.BorgesdaCosta,T.C.R.Silva,A.S.Khayat,E.S.Chen, etal.,MYC,TP53,andchromosome17copy-numberalterationsinmultiple Table3
Effectofkaurenoicacidonthetranscriptionofgenesinvolvedinthecontrolofcellcycleprogression.Valuesrepresenttherelationshipbetweenthetranscriptionofeachgene intheGCcelllinesandthetranscriptionofthesamegeneintheMNP01(non-treatednon-cancercellline).
Kaurenoicacid(mg/mL) MYC CCND1 BCL2 CASP3 ATM CHK2 TP53
0(Control) 2.66(0.42) 1.96(0.19) 2.08(0.21) 1.66(0.10) 0.36(0.14) 0.40(0.11) 0.27(0.11)
2.5 p=NS p=NS p=NS p=NS p=NS p=NS p=NS
5 p=NS p=NS p=NS p=NS p=NS 0.75(0.07)
p<0.001
p=NS
10 p=NS 1.59(0.04)
p<0.001
1.56(0.17)
p<0.001
1.42(0.21)
p=0.016
0.57(0.02)
p=0.005
0.81(0.07)
p<0.001
0.45(0.11)
p=0.002
30 2.07(0.08)
p<0.001
1.23(0.06)
p<0.001
1.47(0.14)
p<0.001
1.23(0.07)
p<0.001
0.89(0.08)
p<0.001
0.92(0.07)
p<0.001
0.50(0.09)
p<0.001
60 1.27(0.14)
p<0.001
1.16(0.07)
p<0.001
1.32(0.17)
p<0.001
1.19(0.07)
p<0.001
0.95(0.04)
p<0.001
0.93(0.04)
p<0.001
0.48(0.03)
p<0.001
NS=notstatisticallysignificant.
gastriccancercelllinesandintheirparentalprimarytumors,J.Biomed. Biotechnol.2011(2011)231–238.
[28]F.daCosta,M.F.Leal,T.C.Silva,E.F.AndradeJúniro,A.P.Rezende,J.A.Muniz, etal.,ExperimentalgastriccarcinogenesisinCebusapellanonhumanprimates, PLoSOne6(2011)e21988.
[29]H.F.Ribeiro,D.F.Alcântara,L.A.Matos,J.M.C.Sousa,M.F.Leal,M.A.C.Smith, etal.,Cytogeneticcharacterizationandevaluationofc-MYCgeneamplification inPG100,anewBraziliangastriccancercellline,Braz.J.Med.Biol.Res.43 (2009)717–721.
[30]G.Speit,B.Habermeier,R.Helbig,Differencesintheresponsetomutagens betweentwoV79sublines,Mutat.Res.325(1994)105–111.
[31]B.C.Cavalcanti,L.V.Costa-Lotufo,M.O.Moraes,R.R.Burbano,E.R.Silveira,K.M. Cunha,etal.,Genotoxicityevaluationofkaurenoicacid,abioactive diterpenoidpresentinCopaibaoil,FoodChem.Toxicol.44(2006)388–392. [32]N.P. Singh,M.T.McCoy, R.R.Tice, E.L.Schneider, Asimpletechnique for quantificationoflowlevelsofDNAdamageinindividualcells,Exp.CellRes. 175(1988)184–191.
[33]A.Hartmann,G.Speit,Asimpletechniqueforquantificationoflowlevelsof DNAdamageinindividualcells,Toxicol.Lett.90(1997)183–188.
[34]A.R. Collins, The comet assay for DNA damage and repair: principles, applications,andlimitations,Mol.Biotechnol.26(2004)249–261. [35]A.Collins,M.Dusinska,M.Franklin,M.Somorovská,H.Petrovská,S.Duthie,
Cometassayinhumanbiomonitoringstudies:reliability,validation,and applications,Environ.Mol.Mutagen.30(1997)139–146.
[36]J.daSilva,T.R.O.Freitas,V.Heuser,J.R.Marinho,B.Erdtmann,Genotoxicity biomonitoringincoalregionsusingwilfrodentCtenomystorquatusbyassay andmicronucleustest,Environ.Mol.Mutagen.35(2000)270–278.
[37]M.Fenech,Theinvitromicronucleustechnique,Mutat.Res.455(2000)81–95. [38]I.Nicoletti,G.Migliorati,M.C.Pagliacci,F.Grignani,C.Riccardi,J.Immunol.
Methods139(1991)271–279.
[39]M.Ayres, M.Ayres Júnior, D.L.Ayres, A.Santos, Biostat5.0: Aplicações estatísticasnasáreasdasciênciasbiológicasemédicas,SociedadeCivil Mamirauá,Brasília,2007.
[40]F.Palitti,Mechanismsoforiginofchromossomalaberrations,Mutat.Res.404 (1998)133–137.
[41]F.H.Kato,N.I.Viana,C.B.Santini,C.G.deSouza,R.C.Veneziani,S.R.Ambrósio, Assessmentoftheinvitroandinvivogenotoxicandantigenotoxiceffectsof pimaradienoicacidinmammaliancells,Mutat.Res.749(2012)87–92. [42]Y.I.Bang,S.A.Im,K.W.Lee,J.Y.Cho,E.-K.Song,K.H.Lee,etal.,Randomized,
double-blindphaseIItrialwithprospectiveclassificationbyATMproteinlevel toevaluatetheefficacyandtolerabilityofolaparibpluspaclitaxelinpatients withrecurrentormetastaticgastriccancer,J.Clin.Oncol.20(2015)3858– 3865.
[43]J.J.Manfredi,S.B.Horwitz,Taxol:anantimitoticagentwithanewmechanism ofaction,Pharmacol.Ther.25(1984)83–125.
[44]L.A.Amos,J.Löwe,HowTaxolstabilisesmicrotubulestructure,Chem.Biol.6 (1999)R65–R69.
[45]M.T.Branham,S.B.Nadin,L.M.Vargas-Roig,D.R.Ciocca,DNAdamageinduced bypaclitaxelandDNArepaircapabilityofperipheralbloodlymphocytes evaluatedbythealkalinecometassay,Mutat.Res.560(2004)11–17. [46]Y.Toiyama,Y.Inoue,J.Hiro,E.Ojima,H.Watanabe,Y.Narita,etal.,Therangeof
optimalconcentrationandmechanismsofpaclitaxelinradio-enhancementin gastrointestinalcancercelllines,CancerChemother.Pharmacol.59(2007) 733–742.