w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Short-term
carcinogenesis
evaluation
of
Casearia
sylvestris
Cleide
A.S.
Tirloni
a,
Giseli
K.
Traesel
a,
Francislaine
A.R.
Lívero
c,
Salvador
D.V.
Neto
a,
Ronaldo
de
Faria
Junior
a,
Thaís
C.
Paim
a,
Joyce
A.
Santos
b,
Silvia
A.
Oesterreich
a,
Ariany
C.
Santos
a,
Roosevelt
I.C.
Souza
a,
Euclides
L.
Cardozo
Junior
c,
Arquimedes
Gasparotto
Junior
a,∗aFaculdadedeCiênciasdaSaúde,UniversidadeFederaldaGrandeDourados,Dourados,MS,Brazil
bFaculdadedeCiênciasExataseTecnológicas,UniversidadeFederaldaGrandeDourados,Dourados,MS,Brazil cLaboratóriodeFarmacologiaeToxicologiadeProdutosNaturais,UniversidadeParanaense,Umuarama,PR,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received31January2017
Accepted17May2017
Availableonline7August2017
Keywords:
Genotoxicity Mutagenesis
Short-termcarcinogenesis
Toxicity
Tumormarkers
a
b
s
t
r
a
c
t
CaseariasylvestrisSw.,Salicaceae,isanimportantmedicinalplantwidelyusedinBrazilforthetreatment ofvariouscardiovasculardisorders.ThisspecieswasincludedasofinterestbyBrazilianUnifiedHealth System.Althoughpreclinicalstudiesdescribedcardiovascularprotectiveeffectsandapparentabsenceof toxicity,nostudieshaveevaluateditscarcinogenicpotential.Inthisstudy,weproposedashort-term car-cinogenesisevaluationofC.sylvestrisinWistarrats,aimingtocheckthesafetyofthisspeciestouseitas proposedbyBrazilianUnifiedHealthSystem.C.sylvestrisleaveswereobtainedandthecrudeextractwas preparedbymacerationfrommethanol/water.Wistarratswereorallytreatedfor12weekswith50,250 or500mgkg−1ofcrudeextractorvehicle.Bodyweight,dailymorbidityandmortalityweremonitored. Bloodandbonemarrowsampleswerecollectformicronucleustest,cometassayandtumormarkers evaluation.Vitalorganswereremovedtomacroandhistopathologicalanalyses.Thecrudeextractdid notinducemutagenicandgenotoxiceffectsandnoalterationswereobservedinimportanttumor mark-ers.Finally,nodetectablesignsofinjurythroughgrosspathologyorhistopathologicalexaminations wereobserved.Ourresultscertifytheabsenceofthecrudeextracttoxicity,indicatingitssafety,evenat prolongedexposureasproposedbyBrazilianUnifiedHealthSystem.
©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Thisisanopen accessarticleundertheCCBY-NC-NDlicense(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Itisestimatethatabout80%oftheworld’spopulationdepends ontraditional practicestoprimary health care,and 85%of this portionusesplants(WHO,2011).Consideringthedifficultaccessof thesepopulationstoconventionalformsoftreatment,theWorld HealthOrganization(WHO)suggeststheadoption oftraditional practicesasatoolformaintaininghealthandencouragesthe devel-opmentofpublicpoliciestoinsertthemintotheofficialhealth systemofits191membercountries,includingBrazil(WHO,2011). Inthiscontext,BrazilianMinistryofHealthproposedactionsfor developmentofpublicpoliciesfortheinclusionofmedicinalplants intheBrazilianUnifiedHealthSystem(SUS),allowingthe expan-sionofdiseaseprevention,maintenanceandrecoveryofhealth. Thepurposeistoexpandthetherapeuticoptionstousers, ensur-ingaccesstomedicinalplantsinviewofacompletehealthcare
∗ Correspondingauthor.
E-mail:arquimedesjunior@ufgd.edu.br(A.GasparottoJunior).
(Carvalhoet al.,2011).Oneofthestrategies toattendthis was
theelaborationofFormHerbalMedicinesoftheBrazilian Phar-macopoeia,whichsupportsthehandlingpracticesanddispensing intheSUS,contemplating47speciesofnaturalplants,including theCaseariasylvestrisSw.,Salicaceae(Anvisa,2011).
Caseariasylvestrisisdistributedintropicalregionsandoccurs in almost all Brazilian territory, where is popularly known as ‘guac¸atonga’(TorresandYamamoto,1986).Traditionally,itsleaves and barks are used for diarrhea, fever, hyperlipidemia, inflam-mation, obesity, skin diseases and tonic health (Ferreira et al., 2011).Betweenpharmacologicalpropertiesthereareanti-cancer
(Ferreira et al., 2016), antihyperlipidemic (Schoenfelder et al.,
2008), antiinflammatory and antioxidant (Albano et al., 2013), antiatherogenic(Brantetal.,2014),besidesnoacuteorprolonged toxicologicaleffects(Amenietal.,2015).
Despite theimportance of medicinalplants forhumancare, theirtoxicologicalpotentialisfrequentlyevaluatedslightly and not as priority (Ferreira et al., 2007).Most of phyto-derivative commercializedmedications,called“natural”,areusedbythe pop-ulationwithouthavingcarriedoutadetailedstudyofthechemical
http://dx.doi.org/10.1016/j.bjp.2017.05.009
0102-695X/©2017SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://
composition,efficacyandsafetyofuse.Thisresultislimited knowl-edgeaboutsideeffects,especiallyonthegeneticmaterial(Varanda,
2006).
Carcinogenicevaluationofthemedicinalplantsisessentialto establishcontrolmeasuresinwidespreaduse,beforetheplants beingconsideredtherapeuticagents.Carcinogenesistestarepart of the regulatory process of new drug development, and life-spanstudiesinrodentshavebeenconsideredthegoldstandard since early in the 20th century. However, they require many financialresources,nearly thousandanimals,and take approxi-mately2–3 years from initiation toreport (Cohen and Arnold, 2016).Currently,short-termassays,(1–3monthsofduration),have beenproposedbyregulatoryauthoritiesasinexpensiveandrapid alternative toevaluate carcinogens substances (Bajpayee et al.,
2005).
So,despitetheC.sylvestrispresentimportantpreclinical phar-macological effects no data on its carcinogenic potential have beenpublished.Then,weproposedapreclinicalshort-term car-cinogenesisevaluationofC.sylvestris,aimingtocheckthesafety of this species to use it in herbal medicines as proposed by SUS.
Materialandmethods
Plantmaterialandextractpreparation
LeavesofCaseariasylvestrisSw.,Salicaceae,wereharvestedin abotanicalgardenof VeraCruz do Oeste,Brazil(620–650mof altitude,S25103028′′–W53152037′′)duringthesummerof2013.
Thespecimenwasidentifiedbytaxonomistsanddepositedinthe HerbariumofBotanicalMuseumofCuritiba,Brazil(no.359503). Leavesweredriedinaforceddraftoven(45◦C,48h).Thecrude
extractwaspreparedbymacerationfrommethanol/water(70:30 v/v)atroomtemperaturefor15days(Brantetal.,2014).Thesolvent waseliminatedbyarotaryevaporatorandlyophilized(yielding 17.14%).Thefreeze-driedextract(MECS)wasdissolvedinfiltered waterbeforetheexperiments.
Liquidchromatography–massspectrometry(LC–MS)
MECSwasexaminedbyultra-high performanceliquid chro-matography.Samplesat2mgml−1concentrationwerepreparedin H2O–MeOH(7:3v/v)andtheanalysiswascarriedoutona reversed-phaseHSS-C18column.Thebinarysolventwascomposedof0.1% aqueousformicacidandmethanol(v/v).Thelinearsolventgradient ataflowrateof0.4mlmin−1wasdevelopedbyincreasingmethanol percentagefrom0%to38%(10min), thento60%at13minand returningtoinitialconditionat14min,re-equilibratedfor3min. Thecolumnwasheatedat60◦Candsampleskeptatroom
tempera-ture.2lwasinjectedandcompoundsweredetectedat200–400
byhigh-resolutionmassspectrometry(HR-MS).
HR-MSanalysesweredevelopedonanelectrosprayionization massspectrometryoperatinginthenegativeionizationat atmo-sphericpressure.Thesourcetemperaturewas350◦CandN
2stream wasusedfor sampledesolvation withsheath gasand auxiliary flowrateat60and20arbitraryunits,respectively.Theionization parameterswere:sprayvoltageof3.5kV,capillaryof−20Vand tubelensof−130V.Formassaccuracy,externalcalibrationwas performedandresolutionwassetat30,000FWHM(atm/z400)in LC–MSmode.Acquisitionwasobtainedintotalioncurrentmode, withmassrangeofm/z100–1000.Compoundfragmentationwas obtainedbycollision-induceddissociationusingheliumandenergy
Carcinogenesisandmutagenesisevaluation
Animals
Femaleand maleWistar rats,12weeks old,werehousedat 22±2◦C,12hlightdarkcycle,55±10%humidityconditions,with
adlibitumaccesstowater andchow. Theethicalcommittee on animaluseoftheFederalUniversityofGrandeDourados(UFGD) approvedalltheprocedures(no.11/2015).
Experimentaldesignandsamplecollection
Ratsweredistributedintoeightgroups(n=8–10)fortreatment withMECS(50,250and500mgkg−1,p.o.,thoughgavage,1mlkg−1) orvehicle(filteredwater,1mlkg−1,controlgroup)daily,during12 weeks.Thedoseswereselectedbasedonpreviousdatareporting cardiovasculareffectsofMECS(Brantetal.,2014).Aten-timelower dosefromthehighestdosewasalsotested.
Rats morbidity and mortality, aggressiveness, eyes and ear pallidness,convulsions,salivation,motoractivity,breathing, heart-beat,diarrhea,comaandinjuryweremonitored.Bodyweight(BW), consumeofchowandwaterwereweeklycontrolled.
Attheendoftheexperiment,theanimalswerefood-deprived for12handanesthetizedwithisoflurane.Bloodsampleswere col-lectedfromcaudalveinforgenotoxicitytestandafterdecapitation fortumormarkersanalyses.Liver,spleen,kidneysandlungswere removed for determining therelative weights, gross pathology andhistopathologicalanalyses.Therightfemurwascollectedfor micronucleusassay.
Grosspathologyandhistopathologicalevaluation
Sampleswereharvested,fixedin10%formalin,dehydratedwith alcoholandxylene,embeddedinparaffinwax,sectioned(4m)
andstainedwithhematoxylin/eosin.Thesliceswereanalyzedbya veterinarypathologist,withaslicescanner,at10×magnification.
Micronucleustest
The proximal epiphyses were cut off from femurs and the spinalcordwasremovedbydrainwithfetalbovineserum.After centrifugation (5min, 1000×g) the pelletwas homogenized, a dropofthiswasplacedonaslideandthesmearwasperformed. Slices were dried at room temperature and fixed in absolute methylalcohol(10min)and24hlatterwerestainedwithgiemsa (15min),washedindistilledwaterandblindanalyzed.Two thou-sandpolychromaticerythrocytes(MN/PCE)/animalwerecounted using an optical microscope (1000× magnification). The poly-chromatic/normochromaticerythrocytesratio(PCE/NCEratio)was calculatedbyanalyzing100randomerythrocytes/animal(OECD,
1997).
Cometassay
Bloodofrats (20l) treatedwithMECSduring 12weeks or
cyclophosphamide (20mgkg−1, p.o., through gavage, 1mlkg−1, 24hbeforesamplecollection)washomogenizedin120lof1.5%
low-melting-pointagarosegeland transferredtosliceswith5% agarosegel.Afterimmersioninalysisbuffer(2.5MNaCl,100mM EDTA,and10mMTris[pH 10.0]with1%Triton X-100and10% dimethylsulfoxide)for4h, sliceswereincubatedinanalkaline buffer(300mMNaOHand1mMEDTA,pH>13,4◦C)for20min.
Nucleoidswereelectrophoresed(20min,25V,300mA,inthedark at 4◦C), whereupon the alkali was neutralized with 0.4M Tris
nucleoiddiameter).Readingswereusedtocalculatedamagedcell (sumof0–3classesdamage)andscoreofdamage(thevalueofthe damagemultipliedbytheclassnumberandthenthesumofthe threeclasses)(OECD,2014).
Tumormarkers
PlasmaticlevelsoftumormarkersCA15-3(breast),CA19-9 (pancreas,biliaryanddigestivetract),CA125(ovariesandliver),CA 27-29(breast),CA72-4(stomachandovarian),alpha-fetoprotein (AFP;livercancer),squamouscellcarcinomaantigen(SCC;cervix squamouscell,head,neck,esophagusandlung);and thyrocalci-tonin(TC;thyroid)weredeterminedbyelectrochemiluminescence usinganautomatedanalyzer.ResultsareexpressedasUl−1 (CA 15-3,CA19-9,CA125,CA27-29),ngl−1(AFPandSCC)orpgml−1 (TC).
Statisticalanalyses
Differencesweredeterminedbyone-wayanalysisofvariance (ANOVA)orKruskalWallisfollowedbyBonferroni’sorDunn’spost hoc. Weighgain of rats was analyzedby two-way ANOVA fol-lowedbyBonferroni’sposthoc.Thelevelofsignificancewas95% (p<0.05).Thedataareexpressedasmean±standarderrorofthe mean(S.E.M.).Graphsweredrawnandstatisticalanalysiswas car-riedoutusingtheGraphPadPrismsoftwareversion5.0forMacOS X(GraphPad®Software,SanDiego,CA,USA).
Results
Phytochemicalcharacterization
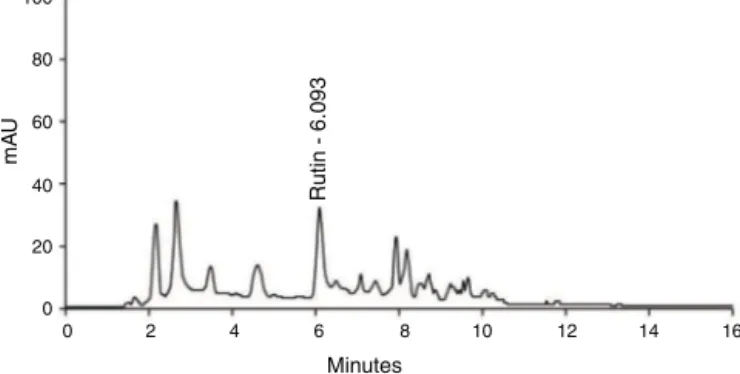
BioactiveclerodanediterpenestypicalofgenusCaseariawere identified,highlightingthecasearvestrinsA–CandcasearinsBand G. Gallic acidderivatives such as isobutyl gallate-3,5-dimethyl etherandmethylgallate-3,5-dimethyletherhavealsobeen identi-fied.Furthermore,thefingerprintobtainedfromMECSshowedfive peakswithabsorbanceat335nmcompatiblewithflavonoid glyco-sides.ThepeakRt6.093issimilartorutinanditsaveragecontent wasfoundtobe20.2mgg−1(Fig.1).
Bodyweight,relativeorgansweightandclinicalevaluation
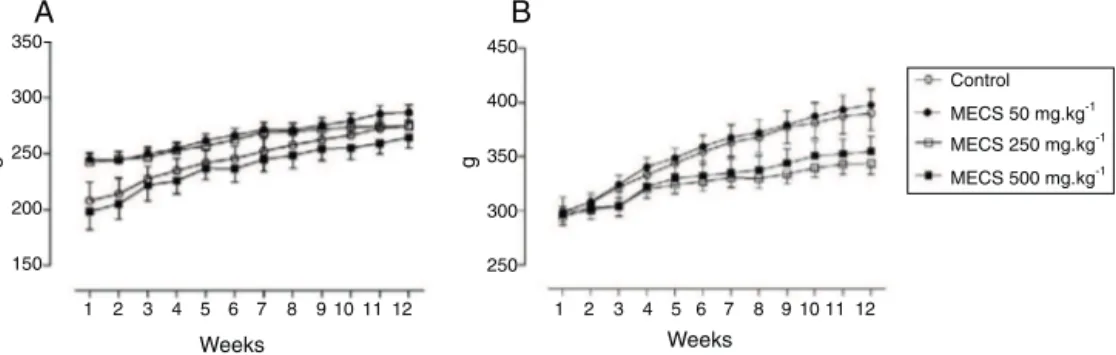
TreatmentwithMECSdidnotalter theBW (g)of femaleor malerats(Fig.2AandB,respectively).Therelativeweightofliver, spleenandkidneyswerethesamebetweenallgroupsfound (Sup-plementary Table). Moreover, no alterations in chow or water consumption,andbehavioralwerefound.
100
80
60
40
20
0 2 4 6 8 10 12 14 16
0
Minutes
mA
U
Rutin - 6.093
Fig.1.UPLCanalysisofMECSobtainedfromCaseariasylvestris.
Grosspathologyandhistopathologicalanalyses
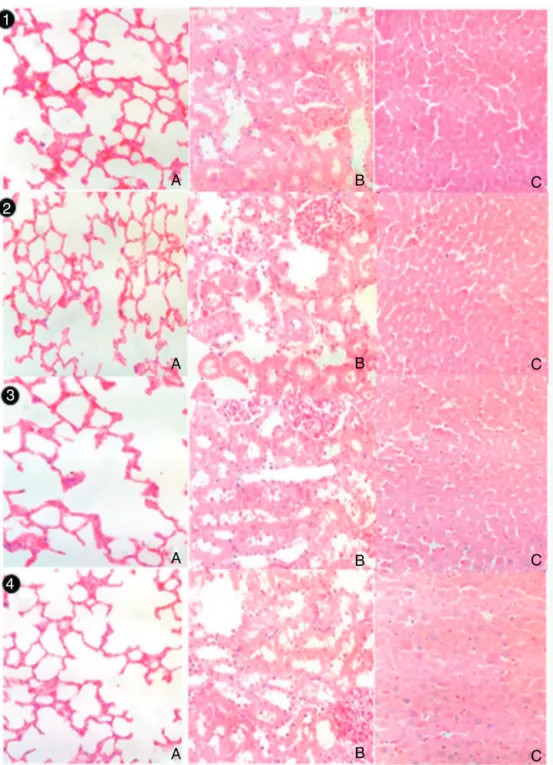
MECSdidnotinducelung,kidneyorlivermacroscopic alter-ations(datanotshown).Thehistologicalexaminationconfirmed theabsenceofdamageinlung(A),kidney(B)orliver(C)infemale or male rats treated with vehicle (Figs. 3 and 4, respectively),
MECS50mgkg−1(Figs.3and4,respectively),MECS250mgkg−1
(Figs.3and4,respectively)orMECS500mgkg−1 (Figs.3and4,
respectively).
Micronucleustest
CyclophosphamideincreasedthenumberofMN-PCEinfemale andmaleratsby283.9%(Fig.5AandB,respectively);anddecreased thePCE/NCEratioby99%infemaleandmale(Fig.5CandD, respec-tively)when compared withcontrol group.MECSdidnot alter the values of MN-PCE or PCE/NCEratio in female (Fig. 5A and C, respectively)ormale rats(Fig.5Band D,respectively)when comparedwithC-.Illustrativeimageofnormochromatic erythro-cyte,polychromaticerythrocyteandmicronucleusarepresentedin
Fig.5E(C-),F(MECS50mgkg−1),G(MECS250mgkg−1)orH(MECS 500mgkg−1).
Cometassay
Classesandnumberofdamagedcellsfoundinthecometassay offemaleandmalearepresentedinTables1and2,respectively.No alterationswereobservedinthenegativecontrolgroup. Cyclophos-phamideinducedanintensedamageinallsamples.In contrast, MECSdidnotinducedalterations.
Tumormarkers
MECSdidnotaltertheplasmaticlevelsofCA15-3,CA19-9, CA125, CA27-29,CA72-4,SCCand TC offemale ormale rats
Table1
Numberofdamagedcellsandclassesofdamageoffemaleratstreatedwithvehicle(C−,negativecontrol),cyclophosphamide(C+,positivecontrol)orfreeze-driedextract
ofCaseariasylvestris50,250and500mgkg−1.
Groups Damagecells(%) Classesofdamage Score
1 2 3 4
C− 10.2±0.64 89.8±1.22 7.1±0.70 2.7±0.63 0.4±0.16 13.7±1.84
C+ 87.0±3.75a 8.5
±2.21a 20.1
±8.01a 63.1
±7.51a 8.52
±4.52a 194.1
±8.91a
MECS50mgkg−1 8.7
±3.01 91.3±0.94 6.5±0.87 1.9±0.31 0.3±0.21 11.20±2.61
MECS250mgkg−1 7.8±0.55 92.1±1.41 5.5±0.79 1.6±0.43 0.7±0.33 11.67±2.34
MECS500mgkg−1 7.2±0.49 93.0±0.88 5.2±0.63 1.8±0.33 0.2±0.13 9.40±2.75
Valuesexpressedasmean±S.E.M.,n=8–10.Class0,nodamage;class1,tailofcometshorterthanthediameterofnucleoid;class2,tailofcometonceortwicethediameter
ofnucleoid;class3,tailofcometmorethantwicethediameterofnucleoid.C−,negativecontrol,C+,cyclophosphamide,positivecontrol.Differencesbetweengroupswere
evaluatedbyone-wayANOVAfollowedbyBonferroni’stest.
ap<0.05whencomparedwithC
A
B
350350 300
300 250
200
250 400 450
150
1 2 3 4 5 6 7 8 9 10 11 12 1 2 3 4 5 6 7 8 9 10 11 12
Weeks Weeks
g g
Control MECS 50 mg.kg-1
MECS 250 mg.kg-1
MECS 500 mg.kg-1
Fig.2.EffectofCaseariasylvestrisextract(MECS)onbodyweightgain(g)offemale(A)ormale(B)rats.Animalsreceived(orally)vehicle(controlgroup)or50,250or
500mgkg−1MECS.Treatmentswereperformedonceaday,during12weeks.Valuesareexpressedasmeans±S.E.M.(n=8–10).Statisticalcomparisonwasperformedusing
two-wayANOVAfollowedbyBonferroni’stest.
1
3
4
A
B
C
C
C
C
A
B
A
B
A
B
2
Fig.3.Lung(A),kidney(B)orliver(C)histologyoffemaleratstreatedwithvehicleorC.sylvestrisextract(MECS).Animalsreceived(1)vehicle(controlgroup),(2)50mgkg−1
MECS,(3)250mgkg−1MECSor(4)500mgkg−1MECS.Treatmentswereperformedonceaday,during12weeks.SlideswerestainedwithHematoxylinandEosin.40×
1
2
3
4
A
B
C
A
B
C
A
B
C
A
B
C
Fig.4. Lung(A),kidney(B)orliver(C)histologyofmaleratstreatedwithvehicleorC.sylvestrisextract(MECS).Animalsreceived(1)vehicle(controlgroup),(2)50mgkg−1
MECS,(3)250mgkg−1MECSor(4)500mgkg−1MECS.Treatmentswereperformedonceaday,during12weeks.SlideswerestainedwithHematoxylinandEosin.40
×
magnification.
Table2
Numberofdamagedcellsandclassesofdamageofmaleratstreatedwithvehicle(C−,negativecontrol),cyclophosphamide(C+,positivecontrol)orfreeze-driedextractof
Caseariasylvestris50,250and500mgkg−1.
Groups Damagecells(%) Classesofdamage Score
1 2 3 4
C− 11.99±0.74 87.9±0.75 9.39±0.56 1.8±0.33 0.8±0.25 15.39±3.62
C+ 91.0±2.91a 9.0±2.91a 19.6±7.82a 62.0±9.6a 9.40±3.57a 171.8±2.91a
MECS50mgkg−1 10.59±0.61 89.5±1.08 7.19±0.91 2.5±0.52 0.9±0.40 14.89±3.14
MECS250mgkg−1 10.49±0.76 89.6±1.16 8.39±1.22 1.8±0.36 0.3±0.15 12.89±3.45
MECS500mgkg−1 9.73
±0.55 91.7±0.76 6.79±0.52 2.44±0.90 0.5±0.22 13.17±3.20
Valuesexpressedasmean±S.E.M.,n=8–10.Class0,nodamage;class1,tailofcometshorterthanthediameterofnucleoid;class2,tailofcometonceortwicethediameter
ofnucleoid;class3,tailofcometmorethantwicethediameterofnucleoid.C−,negativecontrol,C+,cyclophosphamide,positivecontrol.Differencesbetweengroupswere
evaluatedbyone-wayANOVAfollowedbyBonferroni’stest.
A
B
E
F
G
H
C
D
15
10
5
0
C- C+
C+ 50 250 500
MECS(mg.kg-1)
C- 50 250 500 MECS(mg.kg-1)
C+ C- 50 250 500
MECS(mg.kg-1)
MN-PCEs
15
10
5
0
C- 50 250 500 C+ MECS(mg.kg-1)
MN-PCEs
2.5 2.0 1.5 1.0 0.5 0.0
2.0 1.5
1.0 0.5
0.0
∗ ∗
∗ PCEs/NCEs r ∗
atio
PCEs/NCEs r
atio
Fig.5.EffectofCaseariasylvestrisextract(MECS)onthecountsof(AandC)femaleand(BandD)maleMN-PCEsandPCE/NCEratio,respectively.Cytotoxicityofvehicle
and50,250or500mgkg−1MECSispresentedinpanelsE,F,GandH,respectively.Arrowsindicatenormochromaticerythrocyte;angledarrowsindicatepolychromatic
erythrocytes;andsquareindicatemicronucleusinpolychromaticerythrocyte.SlideswerestainedwithGiemsaandshownwith1000×magnification.Statisticalcomparison
wasperformedusingone-wayANOVAfollowedbyBonferroni’stest.Valuesareexpressedasmean±S.E.M.(n=8–10).*p<0.05whencomparedwithcontrolgroup.
MN-PCE,micronucleatedpolychromaticerythrocytes;PCE/NCE,polychromatictonormochromaticerythrocytesratio.C−,controlgroup;C+,cyclophosphamide,positivecontrol
group.
Table3
Tumormarkersoffemaleratstreatedwithvehicle(controlgroup)orfreeze-driedextractofCaseariasylvestris50,250and500mgkg−1during12weeks.
Tumormarker Groups
Control MECS50mgkg−1 MECS250mgkg−1 MECS500mgkg−1
CA15-3 1.08±0.07 1.07±0.05 0.96±0.05 1.01±0.06
CA19-9 10.01±0.29 9.12±0.35 9.55±0.45 9.34±0.33
CA125 13.34±0.55 13.91±0.41 14.40±0.69 14.33±0.62
CA27-29 6.20±0.49 5.23±0.37 5.09±0.28 5.22±0.43
CA72-4 1.34±0.12 1.43±0.19 1.42±0.13 1.21±0.12
AFP 1.73±0.15 1.26±0.07a 1.21±0.07a 1.18±0.07a
SCC 0.35±0.05 0.30±0.04 0.37±0.04 0.42±0.04
TC 4.36±0.37 4.00±0.25 4.35±0.39 4.05±0.26
Valuesexpressedasmean±S.E.M.,n=8–10.CA,cancerantigen;AFP,alpha-fetoprotein;SCC,squamouscellcarcinomaantigen;TC,thyrocalcitonin.Unit:CA15-3,CA19-9,
CA125,CA27.29andCA72-4:Ul−1;AFP:ngml−1;SCC:ngml−1;TC:pgml−1.Differencesbetweengroupswereevaluatedbyone-wayANOVAorKruskalWallisfollowedby
Bonferroni’sorDunn’stest.
ap<0.05whencomparedwithcontrol.
Table4
Tumormarkersofmaleratstreatedwithvehicle(controlgroup)orfreeze-driedextractofCaseariasylvestris50,250and500mgkg−1during12weeks.
Tumormarker Groups
Control MECS50mgkg−1 MECS250mgkg−1 MECS500mgkg−1
CA15-3 1.01±0.05 1.02±0.12 0.94±0.03 1.04±0.06
CA19-9 10.17±0.31 9.82±0.37 9.74±0.46 9.80±0.37
CA125 14.18±0.43 13.71±0.42 13.12±0.40 13.09±0.49
CA27-29 6.00±0.46 5.37±0.42 5.37±0.39 5.67±0.27
CA72-4 1.26±0.09 1.36±0.13 1.38±0.10 1.39±0.09
AFP 1.10±0.06 1.13±0.08 1.35±0.08 1.21±0.06
SCC 0.35±0.03 0.35±0.04 0.45±0.04 0.40±0.05
TC 4.00±0.25 4.53±0.37 4.55±0.38 4.30±0.30
Valuesexpressedasmean±S.E.M.,n=8–10.CA,cancerantigen;AFP,alpha-fetoprotein;SCC,squamouscellcarcinomaantigen;TC,thyrocalcitonin.Unit:CA15-3,CA19-9, CA125,CA27.29andCA72-4:Ul−1;AFP:ngml−1;SCC:ngml−1;TC:pgml−1.Differencesbetweengroupswereevaluatedbyone-wayANOVAorKruskalWallisfollowedby Bonferroni’sorDunn’stest.
(Tables 3and4,respectively).Theplasmatic levelsof AFPwere
significantlyreducedinfemaleratstreatedwithalldosesofMECS.
Discussion
Inthiswork,ashort-termcarcinogenesisstudywasconducted with a popular herbal preparation obtained from C. sylvestris. Throughaseriesofdetailedprotocols,weobservedthattheMECS
oftreatment.Furthermore,nosignificantincreaseintumor mark-erslevels, signsof tumorormalignant cells ingross pathology norhistopathologicalchangesinvitalorgansofWistarratswere detected.
reportedin28.4%ofthem(Sponchiadoetal.,2016).Thishigh inci-denceofpositiveresultsshouldalerttotheimportanceofassessing thegenotoxicandmutagenicpotentialofherbalmedicinesbefore applyingthemastherapeuticagents.
Importantmarkersoftheactionofgenotoxiccompoundsarethe presenceofmicronucleiorthedecompressionofpartsofcellular DNA.Themicronucleiareoriginatedfromchromosomefragments thatareacentricorthataredelayedinrelationtotheothersintheir migrationtowardthepolesofthemitoticspindle.Thesechanges maybeinducedbyagentsthatarecapableofbreakingDNAor inter-feringwithspindle formation.Similarly,thepresenceof simple breaks,alkalinelabilesitesandcrosslinksresultingfromtheaction ofgenotoxiccompounds,altersthecellstructureoftheDNA,which isnormallysupercoiledandstronglycompacted,causingrelaxation inpartsofthemolecule(Tafazolietal.,2017).Inviewofthe above-mentioneddata,theOrganizationforEconomicCo-Operationand Development(OECD)recommendsthemicronucleustestandthe cometassayasanimportanttoolcapableofidentifyingpotentially genotoxicagents(OECD,1997,2014).Therefore,theabsenceof sig-nificantchangesintheseassaysprovidedveryconsistentdataon thelowgenotoxicityoftheMECS.
Anotherimportantaspectofthisstudywastheinvestigationof thecarcinogenicpotentialofMECS.Forthis,weinitiallyoptedfor thedosagesofdifferenttumormarkers,andlater,foradetailed histopathologicalanalysisofdifferentvitalorgans.Tumormarkers (orbiologicalmarkers)areproteinsorpiecesofproteinpresentin tumors,bloodorotherbiologicalfluidstowhichchangesintheir concentrationsarerelatedtothegenesisandgrowthofneoplastic cells.Ingeneral,tumormarkerscanbeproducedbynormalcells aswellasbycancercells,althoughareproducedatveryhigh lev-elsbytumorcells(Duff,2001).Thesemarkersmaybeusefulin thediagnosisandprognosisofdifferenttypesoftumors,aswell asassistinthedevelopmentofnewtreatmentmodalities(Touitou
andBogdan,1998).
Inourstudy,noneoftheanimalsreceivingMECSshoweda sig-nificantincreaseindifferenttumormarkerslevelswhencompared toanimalstreatedonlywiththevehicle.Ontheotherhand,female ratstreatedwithMECS(atalldoses)showedasignificant reduc-tioninAFPlevels.AFPisanimportantfetalserumprotein,whichis normallysynthesizedintheliver,yolksacandfetalgutwith func-tionsofplasmatransportandmaintenanceofoncoticpressure.In general,levelsabove500ng/mlarehighlysuggestiveofliver malig-nancyandvalueswellbelowthatleveldonot,ontheirown,have considerableclinicalsignificance(Sauzayetal.,2016).Generally, tumormarkersarecomplementarytestsandshouldalwaysbeused accompaniedbyothermethodsfordiagnosisortherapeutic mod-ification.Inourcase,inacomplementarywaytotumormarkers, allthevitalorgansofanimalstreatedwiththeMECSwere exam-inedthroughdetailedhistopathologicalanalysis,and,innoneof thecases,morphoanatomicalterations suggestiveof tumorand malignancywereobserved.
Takentogether,ourstudyindicatesthatthemethanolicextract fromC.sylvestrisissafeatthetesteddoses,withnomutagenic, genotoxicorcarcinogeniceffects.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare
thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata
appearinthisarticle.
Righttoprivacyandinformedconsent.Theauthorsdeclarethat
nopatientdataappearinthisarticle.
Funding
ThisworkwassupportedbyFundac¸ãodeApoioao Desenvolvi-mentodeEnsino,Ciência,TecnologiadoEstadodoMatoGrossodo Sul(FUNDECT,59/300.046/2015),andConselhoNacionalde Desen-volvimentoCientíficoeTecnológico(CNPq,449464/2014-8).
Authors’contributions
CAST:invivoexperimentsanddataanalyses.ELCJ:LC–MS anal-yses.SDVN,RDFJandTCP:invivoexperiments.JAS,GKTandSAO: mutagenicityanalyses.ACSandRICS:histopathologicalanalyses. FARL:dataanalysis,datadiscussionandmanuscriptpreparation. AGJ:datadiscussionandmanuscriptcorrection.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,atdoi:10.1016/j.bjp.2017.05.009.
References
Albano,M.N.,daSilveira,M.R.,Danielski,L.G.,Florentino,D.,Petronilho,F.,Piovezan,
A.P.,2013.Anti-inflammatoryand antioxidantpropertiesofhydroalcoholic
crudeextractfromCaseariasylvestrisSw.(Salicaceae).J.Ethnopharmacol.147, 612–617.
Ameni,A.Z.,Latorre,O.A.,Torres,L.M.,Górniak,S.L.,2015.Toxicitystudyabouta
medicinalplantCaseariasylvestris:acontributiontotheBrazilianUnifiedHealth System(SUS).J.Ethnopharmacol.175,9–13.
Anvisa, 2011. Formulário de Fitoterápicos da Farmacopéia Brasileira, Agência
NacionaldeVigilânciaSanitária.Anvisa,Brasília,pp.1–126.
Bajpayee,M.,Pandey,A.K.,Parmar,D.,Dhawan,A.,2005.Currentstatusof
short-termtestsforevaluationofgenotoxicity,mutagenicity,andcarcinogenicityof environmentalchemicalsandNCEs.Toxicol.Mech.Methods15,155–180.
Brant,N.M.F.,Gasparotto,F.M.,Araújo,V.O.,Maraschin,J.C.,Ribeiro,R.C.L.,Lourenc¸o,
E.L.B.,GasparottoJunior,A.,2014.CardiovascularprotectiveeffectsofCasearia
sylvestrisSwartzinSwissandC57BL/6LDLr-nullmiceundergoinghighfatdiet. J.Ethnopharmacol.154,419–427.
Carvalho,A.C.B.,Perfeito,J.P.S.,Silva,L.V.C.,Ramalho,L.S.,deOliveiraMarques,R.F.,
Silveira,D.,2011.RegulationofherbalmedicinesinBrazil:advancesand
per-spectives.Bras.J.Pharm.Sci.47,467–474.
Cohen,S.M.,Arnold,L.L.J.,2016.Criticalroleoftoxicologicpathologyinashort-term
screenforcarcinogenicity.Toxicol.Pathol.29,215–227.
Duff,M.J.,2001.Clinicalusesoftumormarkers:acriticalreview.Crit.Rev.Clin.Lab.
Sci.38,225–262.
Ferreira,P.M.,Bezerra,D.P.,Silva,J.N.,daCosta,M.P.,Ferreira,J.R.,Alencar,N.M.,
Figueiredo,I.S.,Cavalheiro,A.J.,Machado,C.M.,Chammas,R.,Alves,A.P.,Moraes,
M.O.,Pessoa,C.,2016.Preclinicalanticancereffectivenessofafractionfrom
CaseariasylvestrisanditscomponentcasearinX:invivoandexvivomethods andmicroscopyexaminations.J.Ethnopharmacol.186,270–279.
Ferreira,P.M.P.,Carvalho,A.F.F.U.,Souza,D.F.,Magalhães,J.F.,Martins,A.R.,
Mar-tins,M.A.C.,Queiroz,M.G.R.,2007.WaterextractofMoringaoleiferaseeds:a
toxicologicalapproach.REPM1,45–57.
Ferreira,P.M.P.,Costa-Lotufo,L.V.,Moraes,M.O.,Barros,F.W.A.,Martins,A.M.A.,
Cavalheiro,A.J.,Bolzani,V.S.,Santos,A.G.,Pessoa,C.,2011.Folkusesand
phar-macologicalpropertiesofCaseariasylvestris:amedicinalreview.An.Acad.Bras. Cienc.83,1373–1384.
OECD,1997.Guideline474:mammalianerythrocytemicronucleustest.In:OECD
(Ed.), Guideline for Testing of Chemicals. Organization for Economic Co-OperationandDevelopment,Paris,pp.1–10.
OECD,2014.Guideline489:invivomammalianalkalinecometassay.In:OECD(Ed.),
GuidelineforTestingofChemicals.OrganizationforEconomicCo-Operationand Development,Paris,pp.1–25.
Sauzay, C.,Petit,A., Bourgeois,A.M.,Barbare, J.C.,Chauffert, B.,Galmiche,A.,
Houessinon,A.,2016.Alpha-foetoprotein(AFP):amulti-purposemarkerin
hepatocellularcarcinoma.Clin.Chim.Acta463,39–44.
Schoenfelder,T.,Pich,C.T.,Geremias,R.,Avila,S.,Daminelli,E.N.,Pedrosa,R.C.,
Bet-tiol,J.,2008.AntihyperlipidemiceffectofCaseariasylvestrismethanolicextract.
Sponchiado,G.,Adam,M.L.,Silva,C.D.,Soley,B.S.,deMello-Sampayo,C.,Cabrini,
D.A.,Correr,C.J.,Otuki,M.F.,2016.Quantitativegenotoxicityassaysforanalysis
ofmedicinalplants:asystematicreview.J.Ethnopharmacol.178,289–296.
Tafazoli,S.,Vo,T.D.,Petersen,A.,Constable,A.,Coulet,M.,Phothirath,P.,Lang,J.,
Bald-win,N.,2017.Genotoxicity,acuteandsubchronictoxicityevaluationofsavory
foodingredients.Regul.Toxicol.Pharmacol.87,71–87.
Torres,R.B.,Yamamoto,K.,1986.TaxonomiadasespéciesdeCaseariaJacq.
(Flacour-tiaceae)doestadodeSãoPaulo.Rev.Bras.Bot.9,239–258.
Touitou,Y.,Bogdan,A.,1998.Tumormarkerinnonmalignantdiseases.Eur.J.Cancer
Clin.Oncol.24,1083–1091.
Varanda,E.A.,2006.Atividademutagênicadeplantasmedicinais.Rev.Ciênc.Farm.
BásicaApl.27,1–7.
WHO,2011.TheWorldMedicinesSituation2011:TraditionalMedicines:Global