w ww . e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Metanolic
extract
of
Malpighia
emarginata
bagasse:
phenolic
compounds
and
inhibitory
potential
on
digestive
enzymes
Tamara
R.
Marques
∗,
Aline
A.
Caetano,
Anderson
A.
Simão,
Flávia
Cíntia
de
O.
Castro,
Vinicius
de
Oliveira
Ramos,
Angelita
D.
Corrêa
LaboratóriodeBioquímica,DepartamentodeQuímica,UniversidadeFederaldeLavras,Lavras,MG,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received16May2015 Accepted18August2015 Availableonline29November2015
Keywords: Malpighiaemarginata
␣-Amylase
␣-Glucosidase Lipase Trypsin Inhibitor
a
b
s
t
r
a
c
t
Addingvaluetofruitresiduesisofgreatinterest,sincetheycanbepresentedasaviablesolution insearchofnewdrugsforthetreatmentofobesityandrelateddiseases,duetobioactivesubstances, especiallyphenoliccompounds.Thus,theobjectiveofthisstudywastopreparethemethanolextractof acerolabagasseflour,inordertoevaluateitspotentialasasourceofinhibitorsoftheenzymes␣-amylase, ␣-glucosidase,lipaseandtrypsin,anddeterminethecontentofphenoliccompoundsbyhighperformance liquidchromatography.Enzymaticinhibitionassayswereconductedinthepresenceorabsenceof sim-ulatedgastricfluid.Inthemethanolextractofacerolabagasseflour,thefollowingphenoliccompounds wereidentified:gallicacid,syringicandp-coumaricacid,catechin,epigallocatechingallate,epicatechin andquercetin;epicatechinwasthemajorcompound.Intheabsenceofgastricfluid,simulatedenzymes hadavariableinhibitionoftheacerolabagasseflourextract,exceptforlipase,whichwasnotinhibited. Inthepresenceofsimulatedgastricfluid,therewasaninhibitionof170.08IEU(InhibitedEnzymeUnit inmolmin−1g−1)for␣-amylaseand69.29IEUfor␣-glucosidase,indicatingthatthisextractshows potentialasanadjuvantinthetreatmentofobesityandotherdyslipidemia.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Obesityisadiseaseresultingfromtheexcessiveaccumulation ofbodyfat,andbringsmultipleoutcomesforhealth,suchasthe prevalenceandprogressionofcardiovasculardiseases(especially heartdiseasesandstroke),whichwerethemajorcausesofdeath in2012;Sometypesofcancer(endometrium,breastandcolon); skeletalmuscledisturbs(speciallyosteoarthritis–ahighly inca-pacitatingdegenerativedisease);hypertensionandtype2diabetes mellitus(WanderleyandFerreira,2010;WHO,2015).
Between1980and2014,theworld’sobesityprevalency dou-bled.DatafromtheWorldHealthOrganizationreportthat,in2014, morethan1.9billionadultswereoverweightand,amongthem, morethan600millionwereobese(WHO,2015).
One way to fight this epidemic disease is drug treatment. Medicinetofightweightgain,whichhastheobjectivetorestrict energy absorption and cause weight loss, is widely available (Boniglia et al., 2008). However,these drugs cause side effects andareprohibitedbyAnvisasince2011(Abeso, 2014).Another
∗ Correspondingauthor.
E-mail:angelita@dqi.ufla.br(T.R.Marques).
alternativebroadlyemployedistheuseofplantextracts.Overthe lastyears,therewasasubstantialincreaseinitsuse,bythefact thatthepopulationbelievesitsintakeisharmless,withalowcost, andmayinhibitdigestiveenzymes,leadingtobeneficialchangesin metabolism(Simãoetal.,2012).Howevernotallnaturalproducts arebeneficialandfurtherstudiesarenecessarytoevaluatetheir effectsontheorganism.
Enzymes like ␣-amylase and ␣-glycosidase, responsible for processingdietarycarbohydrates,actonstarchbreakdown, result-inginmonosaccharideabsorptionbyenterocytes.Therefore,their inhibitionoffersapromisingstrategyforthepreventionofobesity, aswellastype2diabetesassociatedtohyperglycemia,by inhibi-tingstarchbreakdownandglucoseabsorptioninthesmallintestine (Kwonetal.,2008;Balasubramaniametal.,2013).
Lipase,involvedinfatmetabolism,isalsoanimportanttarget forinhibitors,sinceitsinhibitionlimitstriacylglycerolabsorption, leadingtoadecreaseincaloricyieldandweightloss.Ontheother hand,trypsininhibition,involvedinproteindigestion,hasamalefic effect,onceitimpairsthecompleteaminoacidabsorptioninfood, essentialfortheorganism.
Research has been carried out for evaluating the effects of naturalproductsonthetreatmentofobesityandassociated comor-bidities, reinforcing theneed for the search of new sources of
http://dx.doi.org/10.1016/j.bjp.2015.08.015
amylase, glycosidase and lipase inhibitors (Souza et al., 2011; Pereira et al., 2011a; Simão et al., 2012). Therefore, digestive inhibitorswhoassistinreducingfatandcarbohydrateabsorption inthesmallintestinemaybeusefulhelpersinthetreatmentof obesity.
Naturalproductshavebeengainingspaceandimportancein thepharmaceuticalindustry,sincetheyhavebioactivesubstances capableofinspiringnewphytomedicinesandphytotherapic prod-ucts. Phenolic compounds are among those substances. These compoundspresentchemicalstructureswithhydroxylsand aro-maticrings,whichcanbesimplestructuresorpolymers,originated from plant secondary metabolism and largely found in fruits (AngeloandJorge,2007).Manystudiesreportthebenefitsof phe-noliccompoundsasanadjunctinthetreatmentofobesity(Klaus etal.,2005;Henetal.,2006;Alterioetal.,2007;Santiago-Mora etal.,2011;Vogeletal.,2015;Zhangetal.,2015).
Alterioetal. (2007)andKlaus etal. (2005)reportthat phe-nolic compoundsact in theprevention of obesitydue totheir thermogeniceffects,abilitytooxidizebodyfatandbydecreasing intestinalabsorptionoffatsandcarbohydratescausedbythe inhi-bition of digestive enzymes, resulting in weight loss. Phenolic compounds,suchastannins,havetheabilityofcombiningwith digestiveenzymes,proteinsandotherpolymers(suchas carbo-hydrates),formingstable complexes, impairingabsorption and, therefore,makingthempossibleinhibitorsofsomeofthese diges-tiveenzymes(Wonetal.,2007;Gholamhoseinianetal.,2010).
In this context, the useof agro industrial residues of fruits ispromising fortheextractionofactive principlesthatmaybe employedasanalternativetothetreatmentofobesityand corre-lateddiseases.Bydiscardingtheseresidues,secondarymetabolites ofgreataggregatedvaluewithpossibleapplicationsin pharma-ceutical and food industries, are also eliminated. For example, theacerolabagasseoriginatedinjuiceprocessingis,accordingto
Marquesetal.(2013),richin phenoliccompounds,withrecord contentsof10.82g100g−1 drymatter;however,thesephenolic
compoundswerenotyetidentified.
Given the above,the objective of thepresent study wasto preparethemethanolextractofacerolabagasseflour(ABF), eval-uateitspotentialasasourceof␣-amylase,␣-glycosidase,lipase andtrypsininhibitors,anddeterminethephenoliccompoundsby highperformanceliquidchromatography(HPLC),aimingtouseit asanauxiliaryinthetreatmentofobesityandcorrelateddiseases, aggregatingvaluetothisresidue.
Materialandmethods
Preparationofacerolabagasseflour
AcerolaMalpighiaemarginataDC.,Malpighiaceae(BRS238 Fru-tacor)bagassewasobtainedfromplantsgrowninthemunicipality ofPerdões,MG,Brazil(21◦05′27′′S;45◦05′27′′W,848maltitude);
thelocalclimateaccordingtotheKöppensystemisclassifiedas Cwa:mildandrainysummerswithmoderatetemperatures,annual averagetemperaturebelow21◦C,averageannualprecipitationof
1529.7mm,andrelativehumidityof76%(Emater,2002).Acerola fruitswereusedforpulpextraction,andtheresidualbagassewas provided in threebatches by a fruitpulp plantfirm located in Perdões,MG,Brazil.
Acerolabagasse(4kg)wasfrozenat−18◦Candlyophilizedin
glasscontainersprotectedfromlight for7 daystoobtain450g drybagasse.Afterlyophilization,acerolabagassewashomogenized usingmortarandpestle,waspassedinsievesandmostflour parti-cleswereretainedonsievessized40mesh(0.425mm)to80mesh (0.180mm),thus,classifiedasfineandthenplacedinahermetically sealedflask,protectedfromlightinarefrigeratorat4◦C.
Obtentionoftheextract
Toobtainthemethanolextractofacerolabagasseflour(ABF),1g ofacerolabagasselyophilizedpowderwastransferredtoa250ml erlenmeyerandthenadded50mlof50%methanolsolutioninthree repetitions.Afterwards,itcoveredwithagroundglassjointand putonahotplateat80◦C.Afterboilingfor15min,theextractwas
filteredinfilterpaperandcollectedtoa250mlbecker.Theresidue wasonceagainputonanerlenmeyerandthisprocessrepeatedfor twomoretimes.Afterthethirdfiltration,thebeckerwastakento thehotplatetoevaporatethemethanoluntilthevolumereaches 16ml(AOAC,2012),andthensubmittedtoenzymaticinhibition analysis.
Forthechromatographyprocess,thebeckerwastakentothehot platetoevaporatethemethanol,posteriorlyfrozenandlyophilized (FreeZone®2.5literFreezeDrySustems).Lyophilyzedextract(1g)
wassolubilizedin16mlultrapurewaterobtainedfromaMilli-Q system(EMDMillipore,Billerica,MA,USA).
Identificationandquantificationofphenoliccompounds
HPLCwasperformedusingaShimadzuUHPLCchromatograph (ShimadzuCorporation,Kyoto,Japan)equippedwithtwoLC-20AT high-pressurepumps,anSPD-M20AUV–visdetector,aCTO-20AC oven,aCBM-20Ainterface,andanautomaticinjectorwithan SIL-20Aautosampler.SeparationswereperformedusingaShim-pack VP-ODS-C18 (250mm×4.6mm)column, connected toa
Shim-pack Column Holder (10mm×4.6mm)pre-column (Shimadzu,
Japan).
Themobilephaseconsistedofthefollowingsolutions:2%acetic acidinwater(A)andmethanol:water:aceticacid(70:28:2,v/v/v) (B).Analyseswereperformedforatotaltimeof65minat40◦C,flux
of1mlmin−1,wavelengthof280nm,andinjectionvolumeof20l
inagradient-typesystem(100%solventAfrom0.01to5min;70% solventAfrom5to25min;60%solventAfrom25to43min;55% solventAfrom43to50min;and0%solventAfor10min)untilthe endoftherun.SolventAwasincreasedto100%,seekingto main-taina balancedcolumn.Aceticacidandmethanol(HPLCgrade; Sigma–Aldrich,USA)wereusedinthepreparationofthemobile phase.
Addition of standards to the extracts was also used as an identificationparameter.Thephenolicstandardsusedwere gal-lic acid, catechin, epigallocatechin gallate, epicatechin, syringic acid,p-coumaric acid,ferulicacid,salicylicacid,resveratroland quercetinallobtainedfromSigma–Aldrich(St.Louis,MO,USA).The stockstandardsolutionswerepreparedinmethanol(HPLCgrade; Sigma–Aldrich,USA).
TheABFextractandthestandardswerefilteredthrougha
0.45-mnylonmembrane(EMDMillipore,USA)anddirectlyinjected intothechromatographicsystem,inthreereplicates.The pheno-liccompoundsintheextractwereidentifiedbycomparisonwith retention times of standards. Quantification wasperformed by theconstructionofanalyticalcurvesobtainedbylinearregression usingOrigin6.1computersoftware(OriginLab,Northampton,MA, USA)andconsideringthecoefficientofdetermination(R2)equalto 0.99.
Enzymeobtention
O
HO
HO
HO
HO HO
HO HO
OH
OH 500 000
400 000
300 000
200 000
Intensity (mV)
Time (min)
100 000
–100 000
–200 000 0
0 5 10 15 20 25 30 35 40 45 50 55 60 65
1 2
3 4
5
6 7
OH OH
OH
OH
OH OH
OH OH
OH OH
OH OH
OH OH
OH OH OH OH
OH
H3C
CH3
OH OH
OH
O
O
O O
O
O
O
O
O O
O
1
2
5
6
7
3
4
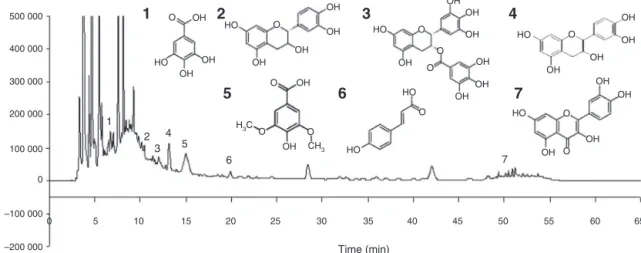
Fig.1.Chromatogramofacerolabagasseflourextract,withpeakidentification:(1)Gallicacid(time=6.541min);(2)catechin(time=10.419min);(3)epigallocatechingallate (time=11.987min);(4)epicatechin(time=13.139min);(5)syringicacid(time=14.988min);(6)p-coumaricacid(time=19.892min)and(7)quercetin(time=51.185min).
Activityof˛-amylase,˛-glucosidase,lipaseandtrypsin
The␣-amylaseactivitywasdeterminedusingthemethodology proposedbyNoeltingandBernfeld(1948).Thus,theextractand␣ -amylaseenzymewerepre-incubatedfor20min,inawaterbathat 37◦C.Thesubstratewasthe1%starchpreparedinTris0.05moll−1,
pH7.0bufferwith38mmoll−1NaCland0.1mmoll−1CaCl2.After
additionof100lofthesubstrate,themixturewasincubatedfor fourperiodsoftime.Thereactionwasinterruptedadding200lof 3.5dinitrosalicylicacidandtheproductreadinspectrophotometer at540nm.
The␣-glucosidaseactivitywasdeterminedaccordingtoKwon etal.(2008),using5mmoll−1p-nitrophenyl-␣-d-glucopyranoside
ina0.1moll−1pH7.0citrate–phosphatebufferassubstrate.Inthe
assay,extractand␣-glucosidaseenzymewereincubatedinawater bath,at37◦C,forfourperiodsoftime,afteradditionofthe
sub-strate.Thereactionwasinterruptedadding1.000lof0.05moll−1
NaOHandtheproductwasreadinaspectrophotometerat410nm. Thelipase activitywasdetermined accordingtoSouzaetal. (2011), using 8mmoll−1 p-nitrophenylpalmitate in Tris–HCl
0.05mmoll−1,pH8.0buffercontaining0.5%Triton-X100as
sub-strate.Intheassay,extractandlipaseenzymewasincubatedina waterbath,at37◦C,forfourperiodsoftime,afteradditionofthe
substrate.Thereactionwasstopped,transferringthetubestoan icebathandaddingTris–HCl0.05mmoll−1 pH8.0buffer.Thep
-nitrophenol,ofyellowcoloration, aproductof thelipase action onp-nitrophenylpalmitate,wasread in aspectrophotometer at 410nm.
Thetrypsinactivitywasdeterminedaccordingtothe method-ologyproposedbyErlangeretal.(1961).Thus,extractandtrypsin enzymewereincubatedinawaterbath,at37◦C,forfourperiods
oftime,afteradditionofp-benzoyl-dl-arginine-p-nitroanilide
sub-strate(BApNA),preparedinTris0.05moll−1,pH8.2.Thereaction
wasinterruptedadding200lof30%aceticacidandtheproduct
readinaspectrophotometerat410nm.
Foreachassayofenzymaticactivity,thevolumeofextractwas differentand its dilution rangedso that theenzyme inhibition rangedfrom50to80%,accordingtothemethodology.
Theinhibitionoftheenzymeswereobtainedfromthe deter-minationoftheslopesofthestraightlines(absorbance×time)of
thecontrolenzyme(withoutextract)andenzymes+inhibitor(with extract)activityassays.Theslopeofthestraightlineisduetothe speedofproductformationperminuteofreactionandthepresence oftheinhibitorcausesadecreaseinthatinclination.Fromthat incli-nation,theabsorbancevalueswereconvertedintomicromolesof productthroughastandardglucosecurvefortheamylaseandof
p-nitrophenolforglycosidaseandlipase,while,forthetrypsin,the ofBApNAmolarextinctioncoefficientdeterminedbyErlangeretal. (1961)wasused.
Preparationofsimulatedgastricfluid
Withtheobjectiveofsimulatingthedigestionprocessinthe stomachinvitro,enzymaticactivityassaysinthepresenceofa sim-ulatedgastricfluidwerealsocarriedout.Forsuch,theextractwas incubatedwiththesimulatedgastricfluidpreparedaccordingto
TheUnitedStatesandPharmacopeia,(2005),for1hinawaterbath at37◦C.Subsequently,wasneutralizedwithsodiumbicarbonate
salttopH7.2andonlythenrealizedtheactivityassays.
Resultsanddiscussion
Each100gABFyielded48goflyophilizedextract(48%yield). The following phenolic compounds were identified in the ABF extract,inmgl−1:gallicacid(3.32±0.23),catechin(11.33±0.33),
epigallocatechin gallate (9.13±0.89), epicatechin (91.86±1.49),
syringic acid (37.16±0.12), p-coumaric acid (2.41±0.13) and
quercetin(0.29±0.02)(Fig.1);gallicacidisahydrolyzabletannin
monomer,andepigallocatechingallate,catechinandepicatechin arecondensedtanninmonomers.Itwaspossibletoobservethat epicatechinhadthehighestcontent,followedbysyringicacid.The compoundsepicatechin,ferulicacid,salicylicacidandresveratrol werenotidentifiedintheABFextract.
LinandLin-Shiau(2006),Alterioetal.(2007),Choetal.(2010)
andRainsetal.(2011)reportedthatphenoliccompoundssuchas caffeicandchlorogenicacid,catechin,epigallocatechingallateand quercetinhavethermogeniceffect,abilitytooxidizefats,control appetite,regulatelevelsofhormonesrelatedtoobesityandinhibit digestiveenzymesinvolvedintheabsorptionofcarbohydratesand lipids.Thus,thisstudyshowsthattheacerolabagasseextracthas bioactivesubstancesandcanbeexploitedbythepharmaceutical industryinsearchofdrugstocontrolobesityandrelateddiseases. Theresultsforenzymatic inhibitionof ABFbeforethe expo-sure togastric fluids are shown in Table 1. The ABFmethanol extract inhibited theactivity of ␣-amylase, presenting an inhi-bition potential of 238.96molmin−1g−1 dry matter – DM.
Thispotential exceeds theone foundby Pereira et al.(2011b), who analyzed the white bean crude extract and detected an inhibition of 54.1molmin−1g−1. Simão et al. (2012),
study-ingaqueousextractsofmedicinalplants,observedaninhibition of2907.13molmin−1g−1 DMforTournefortiapaniculataCham.
Table1
Inhibitionofdigestiveenzymesbyacerolabagassepowderbeforeandafterthe exposuretosimulatedgastricfluid.
Enzyme Inhibition(IEUb)a
Beforeexposure Afterexposure
␣-Amylase 238.96±1.64 170.08±1.06
␣-Glycosidase 78.51±1.78 69.29±0.28
Lipase ndc ndc
Trypsin 227.52±3.59 84.73±5.41
Datafromthreerepetitions,withmean±standarddeviation.
aTheABFextractmeasuredforeachoftheenzymeswasdilutedtoprovidean
inhibitionbetween50%and80%,inordertoensureresultreliability.
b IEU=InhibitedEnzymeUnitinmolmin−1g−1drymatter–DM. c nd=inhibitionnotdetected.
relatedwiththedigestionofcarbohydratesand,consequently,with theelevationinglycemiclevelsafterameal.Highglycemiclevels leadtoserioushealthproblemsinthepopulation,suchastype2 diabetes.Theintakefoodrichin␣-amylaseposesasan interest-ingstrategyinthepreventionandtreatmentofhyperglycemia,by slowingpostprandialglucoselevelsinbloodaftertheingestionof carbohydrates(Vadiveletal.,2011).
Theinhibitionof␣-glucosidasebytheABFextractwasabout 78.51molmin−1g−1DM.TheinhibitorypotentialofABPfound
inthispapersurpasses theonesverifiedbySimão etal.(2012)
who,studyingaqueousextractsofmedicinalplantslikeAloevera
(L.) Burm.(Aloe), Simaba ferruginea St. Hil.(calunga), Baccharis trimera(Less.)DC (carqueja),Garcinia cambogiaDesr.(garcinia),
T.paniculataCham.(marmelinho),foundinhibitionsof0.58 and 35.46molmin−1g−1 DM, as well as those from Pereira et al. (2011a),who analyzedcommercialsamplesofHoodia gordonni, usedasanauxiliaryinthetreatmentofobesity,andfound inhi-bitionsof10.40e16.70molmin−1g−1DM.
Theinhibitionof␣-glucosidaseextendsgastricemptying,leads
tosatietyandweightloss,effectswhichcanbeusefulinthe treat-mentofobesity(Chenetal.,2008).
Therefore, theinhibition of ␣-amylase and ␣-glycosidaseby naturalproductscanprovideanalternativeforthetreatmentof obesityinsubstitutiontosyntheticdrugsnowavailableonthe mar-ket,besidescontrollingglucoselevelsinbloodintype2diabetes patients(McDougalletal.,2005a).
The ABF extract was not able to inhibit lipase, an enzyme involvedinlipidmetabolism,neitherbeforenoraftertheexposure tosimulatedgastricfluid. However,for trypsin,aninhibition of 227.52molmin−1g−1DMwasobserved.Whentrypsininhibitors
arepresentinthediet,thesemayleadtoareductioningrowthrate inanimals,followedbyadecreaseinproteindigestibility,leadingto weightlossandendogenousproteincatabolism(McDougalletal., 2005a).Therefore,thetrypsininhibitorisconsideredan antinutri-tionalfactor.
ThepassageoftheABFextractthroughthegastrointestinal cav-itymayleadtostructuralmodificationsontheinhibitorsbecause ofthepHofthegastricacid.Consideringtheexpressiveinhibition of␣-amylase,␣-glycosidaseandtrypsininthepresenceoftheABF extract,thisextractwassubmittedtoagastricfluidassay(Table1). Inthepresenceof simulatedgastricfluid, theABFmethanol extractstillmaintained71%ofitsinhibitoryactivityover␣-amylase and88%ofinhibitoryactivityover␣-glycosidase.Therefore,the extractdidnotshowaconsiderableinhibitoryactivityoverthese twoenzymesaftertheyweresubmittedtosimulatedgastricfluid. TheABFextractdecreasedtheinhibitionoftrypsinby63%in thepresenceofsimulatedgastricfluid.Thisreductionintrypsin inhibitionisconsideredpositivesince,wheninhibitionoccurs,it isconsideredantinutritional,impairingproteindigestion,which isthemainsourceofessentialaminoacids.However,aresidual inhibitoryactivityof37%wasstillobserved.Itisnotedthatthe
Table2
Contentofphenoliccompoundsinthemethanolextractofacerolabagasseflour, usedineachenzymaticassay.
Phenoliccompound(g) Enzymaticassays
␣-Amylasea ␣-Glycosidaseb Lipasec Trypsinc
Gallicacid 0.02 0.06 0.17 0.66 Catechin 0.07 0.19 0.57 2.26 Epigallocatechin-gallate 0.05 0.15 0.46 1.83 Epicatechin 0.54 1.53 4.60 18.4 Siringicacid 0.22 0.62 1.86 7.44
p-Cumaricacid 0.01 0.04 0.12 0.48 Quercetin 1.71×10−3 4.83
×10−3 0.01 0.06
Phenoliccompounds 0.91 2.59 7.79 31.13
aExtractdilution1:7.5. bExtractdilution1:2. c Crudeextract.
resistanceoftheinhibitortopassthroughthesimulatedgastric fluidisastrongindicativethattheseresultswillrepeatininvivo
assays.
Inthisstudy,theinhibitionofdigestiveenzymescanprobablybe explainedbythepresenceofphenoliccompoundsinthemethanol ABFextract,whoselevelsweredifferentforeachenzymaticassay assessed(Table2).␣-Amylasewastheone thathad the small-estcontentofphenoliccompounds,whichledtoaninhibitionof 0.91g.Ontheotherhand,thecontentofphenoliccompoundswas higher(31.13g)forthetrypsinassay,thatis,34timessuperior tothatfoundfor␣-amylase.Therefore,thissuggeststhatsmaller contentsofphenoliccompoundsmaynotleadtotrypsininhibition, whichwouldbebeneficial,sinceitcouldreducetheabsorptionof carbohydratesandallowproteindigestion.
Gallicacidisconsideredahydrolyzabletannin,whenfoundin theformofgallicacidesters,whilecatechin,epicatechingallateand epicatechin,whenfoundintheformofflavonoids,areconsidered condensed tannins. These compounds have strong interactions with metal ions and macromolecules such as polysaccharides, besidestheability toformsoluble complexeswithseveral pro-teins,asdigestivesenzymes(Wonetal.,2007;Gholamhoseinian etal.,2010).Thisabilitythattanninsexhibittointeractwith pro-teinsmakesthis classofsubstances powerfuldigestiveenzyme inhibitors.
McDougalletal.(2005b)reportthatredfruitextractsinphenolic compoundsinhibittwomainenzymesinvolvedinstarch diges-tion,␣-amylaseand␣-glycosidase,invitro.Inasimilarway,recent studieswithredfruitsreportedinhibitionof ␣-amylase and␣ -glycosidase,andmentionedthattanninswerethemosteffective compoundsininhibitingtheseenzymes(Boathetal.,2012).Kam etal.(2013),studyingtheeffectsofextractsfromdifferentparts ofpomegranate(pulp,peels,seedsandflower)overthedigestive enzymes␣-amylaseand␣-glycosidase,showedthatthemethanol extractfromthepomegranateflower,wherethephenolic com-pounds gallic acidandellagic acidarefound, exhibitsa potent inhibitoreffectontheseenzymes.
Klausetal.(2005)reportedthatratsfedepigallocatechingallate, purifiedfromgreentea,hadanobesitydecreaseinducedbythe diet,duetoareductioninenergyabsorptionandanincreasein lipidoxidation.Ontheotherhand,Bryansetal.(2007)reported thatblackteaisefficientinreducingpostprandialbloodglucose levelsandrelatedthisfacttothepresenceofphenoliccompounds suchasepigallocatechin,epigallocatechingallate,epicatechinand epicatechingallate.
Flavonoids like quercetin comprise a heterogeneous class of phenolic compounds present in plants, which can also act in the organism, inhibiting digestive enzymes. Wenzel (2013)
carbohydratedigestionandcontrollingpostprandialglucoselevels inblood,thusconfirmingtheresultobtainedbyTaderaetal.(2006), whoreportedtheinhibitoryactivityofquercetininthepresenceof
␣-amylase.
LinandLin-Shiau(2006),reportthatflavonoidshavetheability toactonthesympatheticnervoussystemthroughthemodulation ofnoradrenaline,thusincreasingthermogenesisandfatoxidation. Italsopreventstheincreaseinthesizeandnumberofadipocytes, thereforepreventingthedepositionoffatinthebodyand regulat-ingbodyweight.
Phenolicextractsoflentilscontainingp-hydroxybenzoicacid, syringicacid,trans-p-coumaricacid,epicatechingallate,quercetin andkaempferolwereshowntobegoodinhibitorsoflipaseand␣ -glycosidase,contributingtocontrolglucoselevelsinblood,aswell asobesity(Zhangetal.,2015).
Most phenols previously mentioned were foundin the ABF extract,which couldhaveledtoa complexationwithdigestive enzymesand,probably,contributedtoitsinhibition.Theinhibition ofdigestiveenzymesisapromisingalternativeforthetreatment ofobesityandtype2diabetes,especiallybythefacttheyactin thesmallintestine,withoutactinginthecentralnervoussystem, whereprescribedanorexigenicdrugsusuallyact.
Conclusion
The ABF methanol extract that contains the phenolic com-pounds gallic acid, catechin, epicatechin gallate, epicatechin, siringic acid, p-cumaricacidand quercetin, wasable toinhibit
in vitro digestive enzymes ␣-amylase and ␣-glucosidase. This shows that the ABF extract may represent a good source of inhibitors,andcanbeusedasanauxiliaryinthetreatmentof obe-sity,associatedcomorbiditiesandinthecontroloftype2diabetes.
Authors’contributions
TRM contributed in running the laboratory work, analysis of thedata and draftedthe paper.AAC contributed in running thelaboratorywork.AASparticipatedofenzymeactivityassays. FCOCcontributedtochromatographicanalysis.VORparticipatedof enzymeactivityassays.ADCdesignedthestudy,supervisedthe lab-oratoryworkandcontributedtocriticalreadingofthemanuscript. Alltheauthorshavereadthefinalmanuscriptandapprovedthe submission.
Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thatnoexperimentswereperformedonhumansoranimalsfor thisstudy.
Confidentialityofdata. Theauthorsdeclarethatnopatientdata
appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat
nopatientdataappearinthisarticle.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
TheauthorswouldliketothankFundac¸ãodeAmparoàPesquisa doEstadodeMinasGerais,CAPESandCNPqforthegrantsprovided.
References
Abeso, 2014. Associac¸ão Brasileira para o Estudo da Obesidade e da Sín-dromeMetabólica,http://www.abeso.org.br/lenoticia/1120/abeso+defende+o+ tratamento+completo+da+obesidade+com+acesso+a+medicamentos(accessed March2015).
Alterio,A.A.,Fava,D.A.F.,Navarro,F.,2007.Interactionofthedailyingestionofgreen tea(Camellasinensis)inthecellularmetabolismandtheadiposecellpromoting emagrecimento.Rev.Bras.Obes.Nut.Emag.1,27–37.
Angelo,P.M.,Jorge,N.,2007.Compostosfenólicosemalimentos–Umabreverevisão. Rev.Inst.AdolfoLutz66,1–9.
AssociationofOfficialAnalyticalChemistry,2012.OfficialMethodsofAnalysis, Gaithersburg,19thed,3000p.
Balasubramaniam,V.,Mustar,S.,Khalid,N.M.,Rashed,A.A.,Noh,M.F.M.,Wilcox, M.D., Peter, I.C.,Brownlee, I.A.,Pearson,J.P.,2013. Inhibitory activitiesof threeMalaysianedibleseaweedsonlipaseanda-amylase.J.Appl.Phycol.25, 1405–1412.
Boath,A.S.,Grussu,D.,Stewant,D.,McDougall,G.,2012.Berrypolyphenolsinhibit digestiveenzymes:asourceofpotentialhealthbenefits?FoodDig.3,1–7.
Boniglia,C.,Carratù,B.,DiStefano,S.,Giammarioli,S.,Mosca,M.,Sanzini,E.,2008.
Lectins,trypsinand␣-amylaseinhibitorsindietarysupplementscontaining Phaseolusvulgaris.Eur.FoodRes.Technol.227,689–693.
Bryans,J.A.,Judd,P.A.,Ellis,P.R.,2007.Theeffectofconsuminginstantblackteaon postprandialplasmaglucoseandinsulinconcentrationsinhealthyhumanos.J. Am.Coll.Nutr.26,471–477.
Chen,X.,Xu,G.,Li,X.,Li,Z.,Ying,H.,2008.Purificationofan␣-amylaseinhibitorin apolyethyleneglycol/fructose-1,6-bisphosphatetrisodiumsaltaqueous two-phasesystem.ProcessBiochem.43,765–768.
Cho,A.S.,Jeon,S.M.,Kim,M.J.,Yeo,J.,Seo,K.L.,Choi,M.S.,Lee,M.K.,2010.Chlorogenic acidexhibitsanti-obesitypropertyandimproveslipidmetabolisminhigh-fat diet-induced-obesemice.FoodChem.Toxicol.48,937–943.
Emater,2002.Áreadeprotec¸ãoambientaldoMunicípiodePerdões.Empresade AssistênciaTécnicaeExtensãoRural,UnidadedeConsultoriaeProjetos,Belo Horizonte,MinasGerais,Brasil.
Erlanger,B.F.,Kukowsky,N.,Cohen,W.,1961.Thepreparationandpropertiesoftwo newchromogenicsubstratesoftrypsin.Arch.Biochem.Biophys.95,271–278.
Gholamhoseinian,A.,Shahouzehi,B.,Sharifi-far,F.,2010.Inhibitoryeffectofsome plantextractsonpancreaticlipase.Int.J.Pharm.6,18–24.
Hen,Q.,Lv,Y.,Yao,K.,2006.Effectsofteapolyphenolsontheactivitiesof␣-amylase, pepsin,trypsinandlipase.FoodChem.101,1178–1182.
Kam,A.,Li,K.M.,Razmovshi-Naumovshi,V.,Nammi,S.,Shi,J.,Chan,K.,Li,G.Q.,2013.
Acomparativestudyontheinhibitoryeffectsofdifferentpartsandchemical constituentsofpomegranateon␣-amylaseand␣-glucosidase.Phytother.Res. 27,1614–1620.
Klaus,S.,Pultz,S.,Thone-Reineke,C.,Wolfram,S.,2005.Epigallocatechingallate attenuatesdiet-inducedobesityinmicebydecreasingenergyabsorptionand increasingfatoxidation.Int.J.Obes.29,615–623.
Kwon,Y.I.,Apostolidis,E.,Shetty,K.,2008.Inhibitorypotentialofwineandtea against␣-amylaseand␣-glucosidaseformanagementofhyperglycemialinked totype2diabetes.J.FoodBiochem.32,15–31.
Lin,J.K.,Lin-Shiau,S.Y.,2006.Mechanismsofhypolipidemicandanti-obesityeffects ofteapolyphenols.Mol.Nutr.FoodRes.50,211–217.
Marques, T.R.,Corrêa, A.D., Lino, J.B., dos, R., Abreu, C.M.P., de Simão,A.A., 2013.Chemicalcomponentsandfunctionalpropertiesofacerola(Malpighia emarginataDC.)residueflour.FoodSci.Technol.33,526–531.
McDougall,G.J.,Shpiro,F.,Dobson,P.,Smith,P.,Blake,A.,Stewart,D.,2005a.Different polyphenoliccomponentsofsoftfruitsinhibit␣-amylaseand␣-glucosidase.J. Agric.FoodChem.53,2760–2766.
McDougall,G.J.,Fiffe,S.,Dobson,P.,Stewart,D.,2005b.Anthocyaninsfromredwine –theirstabilityundersimulatedgastrointestinaldigestion.Phytochemistry66, 2540–2548.
Noelting,G.,Bernfeld,P.,1948.Surlesenzymesamylolytiques–III.La-amylase: dosaged’activitéetcontrôledel’absenced’␣-amylase.Helv.Chim.Acta31, 286–290.
Pereira,C.A.,Pereira,L.L.S.,Corrêa,A.D.,Chagas,P.M.B.,Souza,S.P.,Santos,C.D., 2011a.InhibitionofdigestiveenzymesbycommercialpowderextractsofHoodia gordonii.Rev.Bras.Biocienc.9,265–269.
Pereira,L.L.S.,Santos,C.D.,Sátiro,L.C.,Marcussi,S.,Pereira,C.A.,Souza,S.P.,2011b.
Inhibitoryactivityandstabilityofthewhitebeanflourextractondigestive enzymesinthepresenceofsimulatedgastricfluid.Rev.Bras.Farm.92,367–372.
Rains,T.M.,Agarwal,S.,Maki,K.C.,2011.Antiobesityeffectsofgreenteacatechins: amechanisticreview.J.Nutr.Biochem.22,1–7.
Santiago-Mora,R.,Casado-Díaz,A.,Castro,M.D.,Quesada-Gómez,J.M.,2011. Oleu-ropeinenhancesosteoblastogenesisandinhibitsadipogenesis:theeffecton differentiationinstemcellsderivedfrombonemarrow.Osteoporos.Int.22, 675–684.
Simão,A.A.,Corrêa,A.D.,Chagas,P.M.B.,2012.Inhibitionofdigestiveenzymesby medicinalplantaqueousextractsusedtoaidthetreatmentofobesity.J.Med. PlantsRes.6,5826–5830.
Souza,S.P.,Pereira,L.L.S.,Souza,A.A.,Santos,C.D.,2011.Inhibitionofpancreatic lipasebyextractsofBaccharistrimera(Less.)DC.Asteraceae:evaluationof antin-utrientsandeffectonglycosidases.Rev.Bras.Farm.21,450–455.
Tadera,K.,Minami,Y.,Takamatsu,K.,Matsuoka,T.,2006.Inhibitionofa-glucosidase anda-amylasebyflavonoids.J.Nutr.Sci.Vitaminol.52,149–153.
Vadivel,V.,Nandety,A.,BIesalski,H.K.,2011.Antioxidant,freeradical scaveng-ingandtypeIIdiabetes-relatedenzymeinhibitionpropertiesoftraditionally processedJequiritybean(Abruspecatorius L.).Int.J.FoodSci. Technol.46, 2505–2512.
Vogel,P.,Machado,I.K.,Garavaglia,J.,Zani,V.T.,Souza,D.,DalBosco,S.M.,2015.
Polyphenolsbenefitsofoliveleaf(OleaeuropaeaL.)tohumanhealth.Nutr.Hosp. 31,1427–1433.
Wanderley,E.M.,Ferreira,V.A.,2010.Obesity:apluralperspective.Cienc.Saúde Coletiva15,185–194.
Wenzel,U.,2013.Flavonoidsasdrugsatthesmallintestinallevel.Curr. Opin. Pharmacol.13,864–868.
WHO,2015.ObesityandOverweight.WorldHealthOrganization,http://www.who. int/mediacentre/factsheets/fs311/en/(accessedMarch2015).
Won,S.,Kim,S.,Kim,Y.,2007.LicochalconeA:alipaseinhibitorfromtherootsof Glycyrrhizauralensis.FoodRes.Int.40,1046–1050.
Zhang,B.,Deng,Z.,Ramdath,D.D.,Tang,Y.,Chen,P.X.,Liu,R.,Liu,Q.,Tsão,R.,2015.