SOCIEDADE BRASILEIRA DE ORTOPEDIA E TRAUMATOLOGIA
w w w . r b o . o r g . b r
Original
Article
Adverse
effect
of
beta-tricalcium
phosphate
with
zeta
potential
control
in
repairing
critical
defects
in
rats’
calvaria
夽
Daniel
Falbo
Martins
de
Souza,
Luciana
Correa,
Daniel
Isaac
Sendyk,
Rafael
Augusto
Burim,
Maria
da
Grac¸a
Naclério-Homem,
Maria
Cristina
Zindel
Deboni
∗FacultyofDentistry,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received22June2015 Accepted7July2015 Availableonline26April2016
Keywords:
Boneregeneration Wistarrats
Biocompatiblematerials Zetapotential
a
b
s
t
r
a
c
t
Objective:Toevaluatewhetheranewbiphasiccementcomposedofcalciumsulfateandbeta tricalciumphosphatewithzetapotentialcontrolcouldinduceorleadtoboneneoformation incriticaldefects.
Methods:Acriticaldefectofdiameter8mmwasmadeinthecalvariaoffortymaleWistar rats.IntheTestGroup(n=20),thedefectswerefilledwithcement.IntheControlGroup (n=20),thedefectwasnotfilledandonlycoagulumwaspresent.Theanimalswere sac-rificed7, 14,21 and42 daysafterthe operation.Calvariaspecimensweresubjectedto microtomographyandwerethenpreparedforhistologicalanalysis.Theanalysesincluded morphologicalassessmentonthehistopathologyoftherepair;comparativemorphometric evaluationoftheareaofformationofbonetrabeculae betweenthegroups;and histo-chemicalstainingbymeansoftartrate-resistantphosphatase(TRAP)inordertoidentify osteoclasts.
Results:Microtomographicimagesofthedefectsfilledbythecement didnot showany decreaseinareaoverthecourseofpostoperativeevolution.IntheTestGroup,thematerial continuedtopresentaforeign-bodyresponseuntilthelastobservationalperiods. Histomor-phologicalanalysisshowedthatthereweremoresignificantgroupingsofgiantcellsinthe TestGroupandgreatermaturityofneoformedboneintheControlGroup.Exogenous mate-rialwasalsopresent.HistomorphometricanalysisshowedthatintheControlGroup,the totalareaofboneneoformationwassignificantlygreater(p=0.009)andgrewprogressively. ThegiantcellspresentedapositivereactiontoTRAPbutnoosteoclastswereobserved.
夽
StudycarriedoutattheDepartmentofSurgery,ProsthesisandOralandMaxillofacialTraumatology,FaculdadedeOdontologia, UniversidadedeSãoPaulo(USP),SãoPaulo,SP,Brazil.
∗ Correspondingauthor.
E-mail:mczdebon@usp.br(M.C.Z.Deboni).
http://dx.doi.org/10.1016/j.rboe.2015.07.010
Conclusion: Theceramiccementdidnotinduceorleadtoboneneoformationfromthe microtomographicorhistologicalpointofview.
©2015SociedadeBrasileiradeOrtopediaeTraumatologia.PublishedbyElsevierEditora Ltda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http://
creativecommons.org/licenses/by-nc-nd/4.0/).
Efeito
adverso
do
beta-fosfato
tricálcico
com
controle
de
potencial
zeta
no
reparo
de
defeitos
críticos
em
calvária
de
ratos
Palavras-chave:
Regenerac¸ãoóssea RatosWistar
Materiaisbiocompatíveis Potencialzeta
r
e
s
u
m
o
Objetivo: Avaliarseumnovocimentobifásicocompostoporsulfatodecálcioebetafosfato tricálcicocomcontroledepotencialzetapoderiainduzirouconduziraneoformac¸ãoóssea emdefeitoscríticos.
Métodos: Foifeitoumdefeitocríticode8mmdediâmetronacalváriade40ratosWistar machos. Nogrupo teste(n=20)os defeitosforampreenchidospelo cimento.Nogrupo controle(n=20)osdefeitosnãoforampreenchidos,permaneceuapenasocoágulo.Os ani-maissofrerameutanásiaem7,14,21e42diasdopós-operatório.Espécimesdacalvária forammicrotomografadoseposteriormentepreparadosparaanálisehistológica.Asanálises incluíramaavaliac¸ãomorfológicadahistopatologiadoreparoeaavaliac¸ãomorfométrica daáreadeformac¸ãodastrabéculasósseascomparativamenteentreosgruposecolorac¸ão histoquímicapormeiodafosfatasetartrato-resistente(TRAP)paraidentificac¸ãode osteo-clastos.
Resultados:Asimagensmicrotomográficasdosdefeitospreenchidospelocimentonão apre-sentaramdiminuic¸ãodaáreadeacordocomaprogressãodosperíodospós-operatórios.No grupotestehouvepermanênciadomaterialerespostacorpoestranhoatéosúltimos perío-dosdeobservac¸ão.Ahistomorfologiamostrouagrupamentosmaisexpressivosdecélulas gigantesnogrupotesteeossoneoformadomaismaduronogrupocontroleecomprovoua presenc¸adematerialexógeno.Nahistomorfometria,aáreatotaldeneoformac¸ãoósseafoi significativamentemaior(p=0,009)ecrescentenogrupocontrole.Ascélulasgigantes apre-sentaramexpressãohistoquímicapositivaparaTRAPenãoforamobservadososteoclatos.
Conclusão: Ocimentocerâmiconãoinduziuouconduziuaneoformac¸ãoósseasoboponto devistamicrotomográficoehistológico.
©2015SociedadeBrasileiradeOrtopediaeTraumatologia.PublicadoporElsevier EditoraLtda.Este ´eumartigoOpenAccesssobumalicenc¸aCCBY-NC-ND(http://
creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Autogenous graft is still the material of choice for the reconstructionofbonetissuelossinorthopedicand maxillo-facialsurgery.However,increaseinoperationtime,surgical traumaandpossiblecomplicationsinherenttothedonorarea approachdoes notalwaysmakeitfeasible.New biomateri-alsandsubstancesthatcanmimicthecharacteristicsofthe autogenousbonetissuehavebeenaconstantpursuitof bio-engineering.
Amongthealloplasticmaterialsmostoftenusednowadays arebioceramics,mainlyhydroxyapatiteandbeta-tricalcium phosphate(-TCP).Thelattershowsamorerapid biodegrada-tionthanhydroxyapatiteandinsomesituationsthismaybe amoreadvantageouscharacteristicforabiomaterial,mainly whenthere is noneed formechanical strength. Moreover, beta-tricalciumphosphatehasbeenwidelyusedasacarrier orscaffoldintissueengineering.
Recently,abiphasicceramicmaterialconsistingofcalcium sulfateand beta-tricalciumphosphate withanegative sur-facecharge,calledzetapotentialcontrol,waslaunchedinthe
international market with the proposal of making beta-tricalciumphosphateaninductivebonesubstituteandthus, promoteboneregeneration.1
According to the manufacturer,2 this ceramic is fully synthetic and has what they called “intelligent porosity”, whichfacilitatescellgrowthandnutrientdistributioninthe extracellularmatrixinternallyinthemacroporositiesofthis compound. Someauthors havedemonstratedintensebone regenerationcapacityinvertebraldefectsinsheep.1
Theosteogenicpotentialofthiscompound,however,has beenquestioned.Someauthorshaveshownconcernaboutthe biologicalsafetyoftheproduct.3Otherresearchers4 discontin-uedearlyclinicaltrialsduetotheappearanceofunexpected adverse effects, suchas aseptic inflammationand delayed repair.Theliteratureisscarceontheanalysisofthe biolog-icalbehaviorofthisnewbiomaterialinbonerepairininvivo
studies.
Material
and
methods
Experimentalprocedure
ThisresearchprojectwasapprovedbytheInstitutionalEthics Committee on Animal Use (CEUA) under protocol number 006/2014 and in accordance with the ethical principles of animalexperimentationadoptedbytheBrazilianSocietyfor LaboratoryAnimalScience(SBCAL).
Forty (40) male Wistar rats (Rattus norvegicus albinus), weighingbetween 200gand 250g and agedapproximately 45 days, were operated on under general anesthesia by intramuscular injection in the right rear paw of each ani-malof ketaminehydrochloride (Dopalen®, Vetbrands) at a
doseof0.8mg/kgassociatedwithmusclerelaxantxylazine (Rompum®, Bayer) at a dose of 0.3mg/kg. All animals
receivedantibioticprophylaxisthroughintramuscular injec-tion of benzathine benzylpenicillin (Roche®) at a dose of
150,000IU/kg.
After trichotomy, followed by skin antisepsis with 2% chlorhexidine digluconate,anaccess wasmade tothe cal-varia througha 2cm-rectilinear incision in the skinofthe medialregionof theskull, extendingfrom the nasofrontal area tothe occipitalprotuberance. Theskin, subcutaneous tissue,temporal muscle and theperiosteum were divulsed laterally.
A bone defect was created in the central region ofthe animalcalvariausingan8mm-diametersteeltrephinedrill (Sistemas de Implantes Nacionais – SIN®), adapted to a
counter-angleimplant motor (Driller® – Carapicuíba – São
Paulo) under low speed and constant irrigation with 0.9% salinesolution.
Theanimalswererandomlydividedintotwogroups:Test Group(n=20),whichreceivedcementconsistingofbeta tri-calciumphosphate(-TCP)andcalciumsulphateataratioof 1:1(Genex–Biocomposites®–Staffordshire–England)tofill
thecriticaldefectinthecalvariaattheamountof25mm3per
defect,andtheControlGroup(n=20),whichremainedwith thecriticaldefectfilledonlybyaclot.
Synthesisoftheskinwasperformedwith3/0silkthread (Ethicon®).Throughout thestudy period, theanimals were
keptinventilated polypropylenecages,coveredwith steril-izedwoodshavings,withday/nightcyclesof12/12h,andwere fedrodentchow(LabinaforRodents,Purina®)andwaterad
libitum.
Fiveanimalsfromeach groupwereeuthanizedinaCO2
chamberafter 7, 14, 21 and 42 days postoperatively. After euthanasia,theskullsweredissectedandrepresentative frag-mentsofthecalvariawiththedefectareawerefixedin10% bufferedformalin.
Priortotheprocessingandhistologicalanalysis,a speci-menfromaratintheTestGroupandfromaratintheControl Group,ineachofthestudyperiods,weresubmittedto micro-tomographyina100kVto100ASkyScanmicrotomographer intheDepartmentofAnimalPhysiologyoftheBiology Insti-tuteofUniversidadedeSãoPaulo.
Thesampleswereplacedatandfixedtotheequipmentcell, whichwassetforaresolutionof2000×2000pixelsand16m sections.Thetimeofimageacquisitionwasapproximately2h
persample.Imageswereanalyzedandreconstructedintwo andthreedimensions,usingtheInVesaliussofware(Division ofThree-DimensionalTechnologiesofCentrodeTecnologia daInformac¸ãodeCampinas–SãoPaulo).
Thebiomaterialvolumewasquantifiedincubic millime-ters(mm3).Inthetwo-dimensionalimages,thesagittaland
axialdistancesofthebonedefectandthree-dimensionalaxial distanceweremeasuredinmillimeters(mm).
For the histologicalanalysis, the specimens(n=5) from each group and preoperative observation period were immersed in10% EDTAsolution (pH7.4) for sixweeksfor bone tissue decalcification. The defect area was sectioned inhalf, exactlyatthe mediansagittalplaneofthe rat cal-varia, which resulted in two parts of the wound: the left sideand theright side(S1and S2).Eachsideofthe defect wasembeddedinparaffinandsubsequently,four4.5cm-thick parasagittalsectionswereobtainedandstainedwithMasson trichrome.Histologicalsectionswereassessedusinga conven-tionallightmicroscope(Olimpus®CH2,OlympusOpticalCo.
Ltd.,Japan)inablindedfashionbyanexperienced patholo-gist.Thehistomorphologicalaspectsconsideredthepresence ofthefollowingrepaircharacteristics:edema,inflammatory infiltrate,granulationtissue,bleeding/bloodclot,bone neofor-mation,foreignbodyreactionandthepresenceofexogenous material. Theparameters were weighed usingan arbitrary scalebythefollowingscores:0=absent;1=mild;2=mildto moderate;3=moderatetointenseand4=intense.
Thehistomorphometricanalysiswasusedtoquantifythe neoformedbonetissueareainrandomlyselectedsectionson bothhalves(leftandright)inthreeregionsofthewound,the anteriorregion(closetothenasalbone),thecentralregionand theposteriorregion(closetotheoccipitalbone).Histological imagesofeachregionwereobtainedwitha40×magnification usingaCCDcamera(Sony®)coupledtoamicroscope(Jeol®)
andanimagecaptureboard(Captivator®).
Then,theseimagesweretransferredtoadigital morphom-etry softwareprogram(ImageJ,NIH– NationalInstitutesof Health). The formation ofneoformed trabecular bone was identifiedanddelimitedusingahandfreesoftwaretool. Mea-surements were performed in four semi-serial histological sectionsofeachslide.
Thetotalareaofboneneoformationwasobtainedateach fieldandaddedtothevalueobtainedontheoppositesideof thewound.Attheend,meanwascalculatedforthetotalarea ofneoformedbonetissueforeachgroupandperiod.
Inordertoidentifyosteoclasticcells,tworandomizedcuts oneachsideofthewoundfromtwoanimalsfromeachgroup wererandomlychosenandsubmittedtohistochemical stain-ingtodemonstratetartarphosphatase-resistantacid(TRAP). Histologicalsectionswereincubatedfor3hatroom tempera-ture,insolutioncontainingsodiumtartrate,tartaricacidand disodiumsalt(SigmaTM).
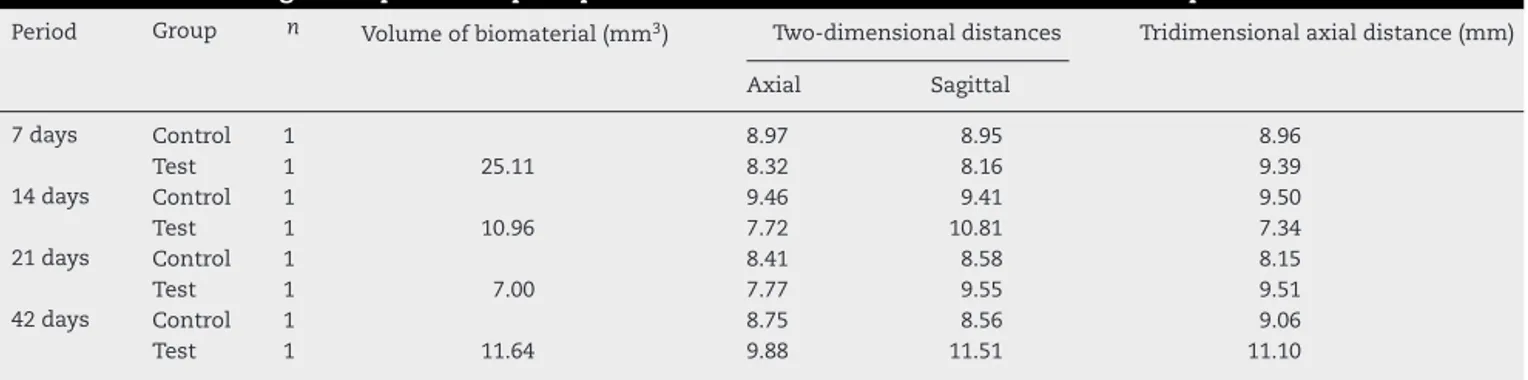
Table1–Volumeofremainingmaterialandmicrotomographictwo-dimensional,three-dimensionalmeasurementsof bonedefectsaccordingtotheperiodsofpostoperativeobservationintheTestandControlGroups.
Period Group n Volumeofbiomaterial(mm3) Two-dimensionaldistances Tridimensionalaxialdistance(mm)
Axial Sagittal
7days Control 1 8.97 8.95 8.96
Test 1 25.11 8.32 8.16 9.39
14days Control 1 9.46 9.41 9.50
Test 1 10.96 7.72 10.81 7.34
21days Control 1 8.41 8.58 8.15
Test 1 7.00 7.77 9.55 9.51
42days Control 1 8.75 8.56 9.06
Test 1 11.64 9.88 11.51 11.10
Histologicaldatawereanalyzedbynonparametric
inferen-tialstatisticsusingtheMann–WhitneytestwiththeBiostat
5.0softwarewithasignificancelevelof5%.
Results
Theresultsofmicrotomographicimageanalysisareshownin
Table1andFig.1.Theintensityingraylevelsofthe
bioma-terialwasclosetothatofbone,butthebiomaterialshowed granularity,allowingittobedifferentiatedfrombone.
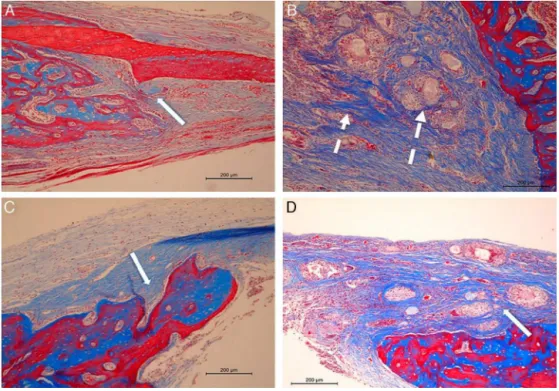
Histologicalexaminationregardingrepairmorphology is showninTable 2andmainhistologicalaspectsare shown inFig. 2. Fig.3 shows the resultofstainingfor TRAP (tar-trate-resistantacidphosphatase)intheTestGroup42days postoperatively.
Resultsofthehistomorphometricanalysisofbone neofor-mationareaareshowninFig.4.
Discussion
Theliteraturedescribesthat calcium phosphatesand their derivatives, including beta-tricalcium phosphate, act more directlyonosteoblastsandhavebeenextensivelystudiedas bonereplacementformorethan twodecades,asthey pro-moteboneneoformationbyosteoconductionandhavegood biocompatibility.5–7
Itseffectsasinorganicscaffoldforbonetissueregeneration arealreadywelldocumented,includingdentallesions,8–11in spiteofstillcontroversialfindings,rangingfrombone neo-formationthatissuperiortotheoneobservedinautogenous grafts12andanorganicbovinebonegraft,13torobustevidence ofinsufficientosteogenesis.14,15
Thebiomaterialusedinthisstudy (GeneX®)isrelatively
newanditisaceramiccompound,whichhasnegative sur-face charge control. Investigations about its efficacy have
Table2–Valuesofthemedians[max–min]ofthescoreintensitiesforthehistologicalvariablesofthebonedefect.
Variables 7days 14days 21days 42days
Control Test p Control Test p Control Test p Control Test p
Edema 2[2-1] 1[2-1] 0.22 2[2-1] 1[1-0] 0.075 1[1-0] 1[1-0] 1 0[2-0] 0[1-0] 0.916 Inflammatoryinfiltrate 1[2-1] 4[4-4] 0.004 1[3-1] 4[4-3] 0.008 1[1-0] 4[4-4] 0.003 1[1-0] 4[4-3] 0.006 Granulationtissue 3[4-3] 3[3-2] 0.212 2[3-1] 4[4-3] 0.011 1[1-1] 2[3-2] 0.004 1[2-1] 1[1-1] 0.317 Hemorrhage/thrombus 3[3-1] 2[3-1] 0.496 2[3-2] 1[4-0] 0.193 3[3-1] 0[4-0] 0.106 2[3-0] 1[3-0] 0.335 Boneneoformation 1[2-1] 1[1-1] 0.317 2[2-1] 2[3-1] 0.605 2[3-1] 2[2-1] 0.339 2[3-1] 1[2-1] 0.418 Foreignbodyreaction 0[1-0] 4[4-4] 0.005 1[2-1] 4[4-4] 0.005 0[2-0] 4[4-3] 0.007 1[2-0] 4[4-3] 0.006 Exogenousmaterial 0[0-0] 4[4-3] 0.007 0[0-0] 4[4-4] 0.005 0[0-0] 2[3-2] 0.005 0[0-0] 3[4-2] 0.008
0,absent;1,mild;2,mildtomoderate;3,moderatetointense;4,intense. PvaluesforKruskall–Wallistest.Significantwhenp<0.05.
Mann–Whitneytestissignificantwhenp<0.05.
most oftenfocused on repair defects of the spine or long
bones.16Yangetal.1demonstratedinaninvivostudythatthis
ceramicmaterialpromotedsuperiorboneformationin verte-braldefectsineightweekswhencomparedwiththeuseofa polymercement(polymethylmethacrylate).However,intheir conclusions,theyindicatethatthe-TCPceramicneedstobe furtherassessed.
Asthemanufacturer’sproposalwastoaddasurface treat-mentontheceramicparticlesthatallowedosteoinduction,a criticalbonedefectmodelwouldprovideimportant informa-tioniftheboneneoformationoccurredwhereitwouldnotbe expected.
Thistypeofmodelisquiteoftendescribed inthe litera-tureanditis,inaway,acknowledgedforthistypeofinvivo
assay.17–24 Weusedthecriticaldefect modelinrat calvaria becauseit iseasy tocreate,reproduce andthefactthatan 8mm-extensiondefect would allowusto verify the occur-rence ofosteoconductionor inductionofthe materialina standardizedandreliablemanner.
Contrary tothefavorableoutcome describedbyGerman researchers,25 our resultsdidnotshow boneneoformation after insertionofthis ceramiccompoundin filling defects. Cement withzetapotentialcontrol didnotstimulatebone neoformation in critical defects created in rat calvaria or showedrapidabsorptionbythevolumeofexogenousmaterial observedinmicrotomographicimagesinallstudyperiods.
Recently,Saadounetal.3andFriesenbichleretal.4showed significant complications ofthis materialon tissues when
Fig.2–Histologicalsectionsrepresentativeofcriticaldefectsineachgroupintheperiodsof21and42dayspostoperatively. In(A)ControlGroup–21days,showsdepositionofcollagenandosteoidmatrixadjacenttothedefectborder(arrow).In(B) TestGroup–21days,showsexogenousmaterialcluster(longarrow)encapsulatedbyintensedepositionoffibrous
Fig.3–Positiveexpressionoftartrate-resistantacid phosphatase(TRAP)ofmultinucleatedgiantcells(large arrows)adjacenttotheexogenousmaterialclusters(thin arrows)–TestGroupperiodof42days(TRAPstaining, magnification40×).
usedtofillbonedefectsinclinical trials,althoughLaycock andCooper26attributedtheseadverseeffectstothestill inad-equateuseofthisbiomaterial.
Obviously,thepresenceofasignificantlyhigheramountof inflammatoryinfiltrateintheTestGroupisdirectlyrelatedto thegreaterresponsetothepresenceofaforeignbodywhen comparedtotheControlGroup.Thefactthattheforeignbody responseremainedfortheentiredurationoftheexperiment canleadtotheformationofmaterialclusters,whichshould beexpelledfromthewoundareaintheencapsulatedform. Thisfactmayhelptoexplainthedescriptionof“softtissue cysts”madebyFriesenbichleretal.4
Theclinicalrelevanceofthisstudyshouldbeemphasized fromthehistopathologicalpointofview,becausetherewere
7 days 14 days 21 days
Periods of observation
Kruskall-Wallis test – Significant when P<.05 Area of bone neoformation
42 days 1 600 000
P=.009
P=.009
Test group Control group
P=.009
P=.0283
µ
m 2
1 400 000
1 200 000
1 000 000
800 000
600 000
400 000
200 000
0
Fig.4–Histomorphometricanalysisforthearea(m2)of boneneoformationrelatedtothegroupsandperiods. Means(±standarddeviation).
nostandardizedexperimentalpreclinicalstudiestodisclose the behaviorofthis materialontherepairofhealthybone tissueyet.
Accordingtothehistomorphometricfindings,theproposal ofamoresignificantboneneoformationintheTestGroupwas notdemonstratedeither.Theresultsshowthatbone forma-tionwasmoreintenseandsignificantlyhigherintheControl Groupanddeniedourhypothesisoftryingtodemonstratethe osteoinductivepotentialpromisedbythemanufacturer.
Resultsshowedthatthesuperiorityinboneformationin controlsled ustobelievethat this ceramiccomposite pre-vented the small bone formation that would occur in the defect.
Ourresultsarealsoinaccordancewiththosefromother authors,27,28accordingtowhomtheapplicationofothertypes ofbeta-tricalciumphosphate forbonelossrepair,inminor defectsandcrossoverstudiesinthesameanimaldidnot influ-encetheamountofboneformation.
Toassesswhethertherewasincreasedosteoclastogenesis andincreasedboneresorption,ahistochemicalreactionwas carriedoutbyTRAPstaininginthewoundarea.However,the foreignbodyresponsegiantcellsweretheonesthatshowed positivityforthisbiochemicalmarker.Thismayleadtothe hypothesisthatthematerialinthetissuemightpossiblyhave inducedspecificbiochemicalsignals.
Thus, giant cells, probably in an attempt to absorb exogenous material, might alsohave resorbed bone. Stud-ies demonstratingthe expressionofother specificmarkers relatedtorepairandinductionofbonetissueformationcould becarriedouttobetterexplainthisfact.
Bythetimethisstudywasplanned,weexpectedtofind beneficialeffectsofGenex®inboneregeneration,especially
consideringthatthismaterialhadfavorablereportsinclinical use.Finally,ourresultsarerelevant,astheyconfirmthatthis typeofceramicmustbereconsideredasabonesubstitute.
Conclusion
Within the limitations of this study, the biphasic ceramic cement consisting of calcium sulfate and beta-tricalcium phosphate andzetapotentialdidnotinduceorresultedin boneneoformationof8mmcriticalbonedefectscreatedinrat calvariafromthemicrotomographicandhistologicalpointsof view.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
r
e
f
e
r
e
n
c
e
s
1. YangHL,ZhuXS,ChenL,ChenCM,ManghamDC,Coulton
LA,etal.Bonehealingresponsetoasyntheticcalcium
sulfate/-tricalciumphosphategraftmaterialinasheep
vertebralbodydefectmodel.JBiomedMaterResBAppl
Biomater.2012;100(7):1911–21.
2. BiocompositesL.GeneX®.InjectablebonegraftwithZPC.
Biocomposites,Ltda.;2014.Availableat:http://www.
biocomposites.com/ortho/Genex2.asp[cited15.02.14].
3. SaadounS,MacdonaldC,BellBA,PapadopoulosMC.Dangers
ofbonegraftsubstitutes:lessonsfromusingGeneX.JNeurol
NeurosurgPsychiatry.2011;82(8):e3.
4. FriesenbichlerJ,Maurer-ErtlW,SadoghiP,Pirker-FruehaufU,
BodoK,LeithnerA.Adversereactionsofartificialbonegraft
substitutes:lessonslearnedfromusingtricalciumphosphate
geneX®.ClinOrthopRelatRes.2014;472(3):976–82.
5. PiolettiDP,TakeiH,LinT,VanLanduytP,MaQJ,KwonSY,
etal.Theeffectsofcalciumphosphatecementparticleson
osteoblastfunctions.Biomaterials.2000;21(11):1103–14.
6. HandschelJ,WiesmannHP,StratmannU,KleinheinzJ,Meyer
U,JoosU.TCPishardlyresorbedandnotosteoconductiveina
non-loadingcalvarialmodel.Biomaterials.2002;23(7):1689–95.
7. MatsumotoG,OmiY,KubotaE,OzonoS,TsuzukiH,Kinoshita
Y,etal.Enhancedregenerationofcriticalbonedefectsusinga
biodegradablegelatinspongeandbeta-tricalciumphosphate
withbonemorphogeneticprotein-2.JBiomaterAppl.
2009;24(4):327–42.
8. AguirreZorzanoLA,RodríguezTojoMJ,AguirreUrizarJM.
Maxillarysinusliftwithintraoralautologousboneand
B-tricalciumphosphate:histologicalandhistomorphometric
clinicalstudy.MedOralPatolOralCirBucal.2007;12(7):
E532–6.
9. OrtegaEV,MoureloJP,EgeaJJS,PerezOP,SoterasRM.La
utilizacióndelbetafosfatotricálcicocomobiomaterialen
implantologíaoral.RevPeriodoncia.2007;19(3):141–9.
10.MoureloJP,GuerraAJ,GuilLM,EgeaJJS,OrtegaEV.
Regeneraciónóseaguiadaconimplanteunitariocon
nanosuperficieybetafosfatotricálcico.RevPeriodoncia.
2010;22(3):127–37.
11.ZavagliaFC.Síntese,caracterizac¸ãoeprocessamentodebeta
fosfatotricálcicoparaamanufaturadeimplantes
personalizados[tese].Campinas:UniversidadeEstadualde
Campinas,FaculdadedeEngenhariaQuímica;2011.
12.LuvizutoER,TanglS,ZanoniG,OkamotoT,SonodaCK,
GruberR,etal.TheeffectofBMP-2ontheosteoconductive
propertiesof-tricalciumphosphateinratcalvariadefects.
Biomaterials.2011;32(15):3855–61.
13.VelascoOrtegaE,PatoMoureloJ,SeguraEgeaJJ,PérezPérezO,
MedelSoterasR.Lautilizacióndelbeta-fosfatotricálcico
comobiomaterialenimplantologiaoral.AvPeriodoncia.
2007;19(3):141–50.
14.OriiH,SotomeS,ChenJ,WangJ,ShinomiyaK.
Beta-tricalciumphosphate(beta-TCP)graftcombinedwith
bonemarrowstromalcells(MSCs)forposterolateralspine
fusion.JMedDentSci.2005;52(1):51–7.
15.GuptaMC,TheerajunyapornT,MaitraS,SchmidtMB,HolyCE,
KadiyalaS,etal.Efficacyofmesenchymalstemcellenriched
graftsinanovineposterolaterallumbarspinemodel.Spine
(PhilaPa1976).2007;32(7):720–6.
16.LiuB,LunDX.Currentapplicationof-tricalciumphosphate
compositesinorthopaedics.OrthopSurg.2012;4(3):139–44.
17.EinhornTA.Clinicallyappliedmodelsofboneregenerationin
tissueengineeringresearch.ClinOrthopRelatRes.1999;367
Suppl:S59–67.
18.CacchioliA,SpaggiariB,RavanettiF,MartiniFM,BorghettiP,
GabbiC.Thecriticalsizedbonedefect:morphologicalstudy
ofbonehealing.AnnFacMedVetParma.2006;26:97–110.
19.BoddeEW,BoermanOC,RusselFG,MikosAG,SpauwenPH,
JansenJA.Thekineticandbiologicalactivityofdifferent
loadedrhBMP-2calciumphosphatecementimplantsinrats.J
BiomedMaterResA.2008;87(3):780–91.
20.MessoraMR,NagataMJ,DornellesRC,BomfimSR,Furlaneto
FA,deMeloLG,etal.Bonehealingincritical-sizedefects
treatedwithplatelet-richplasmaactivatedbytwodifferent
methods.Ahistologicandhistometricstudyinratcalvaria.J
PeriodontalRes.2008;43(6):723–9.
21.EfeogluC,BurkeJL,ParsonsAJ,AitchisonGA,ScotchfordC,
RuddC,etal.Analysisofcalvarialbonedefectsinratsusing
microcomputedtomography:potentialforanovelcomposite
materialandanewquantitativemeasurement.BrJOral
MaxillofacSurg.2009;47(8):616–21.
22.PauloAdeO,Castro-SilvaII,OliveiraDF,MachadoME,
Bonetti-FilhoI,GranjeiroJM.Repairofcritical-sizedefects
withautogenousperiosteum-derivedcellscombinedwith
bovineanorganicapatite/collagen:anexperimentalstudyin
ratcalvaria.BrazDentJ.2011;22(4):322–8.
23.ScotchfordCA,ShahtaheriM,ChenPS,EvansM,ParsonsAJ,
AitchisonGA,etal.Repairofcalvarialdefectsinratsby
prefabricated,degradable,longfibrecompositeimplants.J
BiomedMaterResA.2011;96(1):230–8.
24.SpicerPP,KretlowJD,YoungS,JansenJA,KasperFK,Mikos
AG.Evaluationofboneregenerationusingtheratcriticalsize
calvarialdefect.NatProtoc.2012;7(10):1918–29.
25.SmeetsR,KolkA,GerressenM,DriemelO,MaciejewskiO,
Hermanns-SachwehB,etal.Anewbiphasicosteoinductive
calciumcompositematerialwithanegativeZetapotentialfor
boneaugmentation.HeadFaceMed.2009;5:13.
26.LaycockPA,CooperJJ.Adversereactionsofartificialbone
graftsubstitutes:lessonslearnedfromusingtricalcium
phosphategeneX®.ClinOrthopRelatRes.2014;472(2):765–6.
27.GeraI,DöriF,KeglevichT,AntonS,SzilágyiE,WindischP.
Experiencewiththeclinicaluseofbeta-tri-calcium
phosphate(Cerasorb)asabonereplacementgraftmaterialin
humanperiodontalosseousdefects.FogorvSz.
2002;95(4):143–7.
28.FrotaR,DaSilva-JúniorVA,TeixeiraM,SobralAP,
Emanuel-Dias-deOliveiraeSilva,DaSilveiraMM,etal.
Histologicalevaluationofbonerepairusing-tricalcium