ww w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Original
article
Unbalanced
expression
of
aryl
hydrocarbon
receptor
in
peripheral
blood
CCR6
+
CD4
+
and
CD4
+
CD25
+
T
cells
of
rheumatoid
arthritis
夽
Lin
Cheng
a,
Long
Qian
a,∗,
Yue
Tan
a,
Guo-Sheng
Wang
b,
Xiao-Mei
Li
b,
Xiang-Pei
Li
b,
Chao-Yin
Luo
aaAnhuiMedicalUniversity,TheSecondAffiliatedHospital,DepartmentofRheumatologyandImmunology,Hefei,China
bAnhuiMedicalUniversity,AffiliatedAnhuiProvincialHospital,DepartmentofRheumatologyandImmunology,Hefei,China
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received24August2015 Accepted17April2016 Availableonline22July2016
Keywords:
Rheumatoidarthritis Arylhydrocarbonreceptor CD4+CD25+Tcells
CCR6+CD4+Tcells
a
b
s
t
r
a
c
t
Objective:Thegoalofthisstudywastoanalyzetheroleofarylhydrocarbonreceptorin peripheralbloodCCR6+CD4+andCD4+CD25+Tcellsofpatientswithrheumatoidarthritis. Methods:FlowcytometrywasappliedtodeterminetheproportionofAhRpositivecellsin CCR6+CD4+T,CD4+CD25+Tandperipheralbloodperipheralmononuclearcellsfromeach
subject.AhRmRNAandCYP1A1mRNArelativeexpressionlevelsweretestedbyreal-time PCR.
Results:ThepercentageofAhRpositivecellsinperipheralbloodmononuclearcellswas higherinRAgroupthanthatinhealthycases[(35.23±10.71)%vs.(18.83±7.32)%,p<0.01]. TheexpressionlevelsofAhRandCYP1A1werebothincreasedinpatientswithRAwhile comparedtocontrols[(3.71±1.63)vs. (2.00±1.27),p=0.002; (2.62±2.08) vs. (0.62±0.29), p<0.01,respectively].InRApatients,thepercentageofAhRpositivecellsinCD4+CD25+T
cellswassignificantlylowerthanthatfromcontrols[17.90(6.10±80.10)%vs.(52.49±19.18)%, p<0.01];Inhealthycontrols,thepercentageofAhRpositivecellsinCD4+CD25+Tcellswas
significantlyhigherthanthatinCCR6+CD4+Tcells,andwasalsosignificantlyhigherthan
thatinPBMCs[(52.49±19.18)%vs.(23.18±5.62)%vs.(18.06±7.80)%,X2=24.03,p<0.01];in
RApatients,thepercentageofAhRpositivecellsinCCR6+CD4+T cellswassignificantly
increasedthanthatinCD4+CD25+TcellsandPBMCs[(46.02±14.68)%vs.17.90(6.10±80.10)% vs.(34.22±10.33)%,X2=38.29,p<0.01];Nevertheless,nostatisticallysignificantrelationship
wasfoundbetweenclinicaldataandAhRpositivecellsinCCR6+CD4+TandCD4+CD25+T
cells.
Conclusion:AhRmayparticipateinthepathologicalprogressofRAbycontrollingthe differ-entiationofTh17andTregcellsinperipheralblood.
©2016ElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
夽
StudyoriginatedatDepartmentofRheumatologyandImmunology,TheSecondAffiliatedHospital,AnhuiMedicalUniversity,Hefei, China.
∗ Correspondingauthor.
E-mail:longqian0551@hotmail.com(L.Qian). http://dx.doi.org/10.1016/j.rbre.2016.07.002
Expressão
não
equilibrada
do
receptor
de
hidrocarboneto
arílico
nos
linfócitos
T
CCR6+
CD4+
e
CD4+
CD25+
do
sangue
periférico
na
artrite
reumatoide
Palavras-chave: Artritereumatoide
Receptoresdehidrocarboneto arílico
LinfócitosTCD4+CD25+ LinfócitosTCCR6+CD4+
r
e
s
u
m
o
Objetivo:Analisaropapeldoreceptordehidrocarbonetoarílico(AhR)noslinfócitosTCCR6+ CD4+eCD4+CD25+nosangueperiféricodepacientescomartritereumatoide(AR). Métodos: Foiaplicadacitometriadefluxoparadeterminaraproporc¸ãodecélulasAhR posi-tivasemlinfócitosCCR6+CD4+eCD4+CD25+dosangueperiféricoecélulasmononucleares periféricasdecadaindivíduo.Osníveisdeexpressãorelativadeácidoribonucleico men-sageiro(doinglêsribonucleicacid,RNAm,)deAhReRNAmdeenzimadeprimeiroestágio essencialparaoAhR(CYP1A1)foramtestadosporreac¸ãoemcadeiadepolimerase(doinglês polymerasechainreaction,PCR,)emtemporeal.
Resultados: ApercentagemdecélulasAhRpositivasnascélulasmononuclearesdosangue periféricofoimaiornogrupocomARdoquenosindivíduossaudáveis[(35,23±10,71)%vs. (18,83±7,32)%,(p<0,01)].OsníveisdeexpressãodeAhReCYP1A1estavamaumentados empacientescomARquandocomparadoscomoscontroles[(3,71±1,63)vs.(2,00±1,27), p=0,002;(2,62±2,08)vs.(0,62±0,29),p<0,01,respectivamente].EmpacientescomAR,a percentagemdecélulasAhRpositivasnoslinfócitosTCD4+CD25+foisignificativamente inferioràdos controles[17,90(6,10±80,10)]%vs.(52,49±19,18)%,p<0,01]; emcontroles saudáveis,apercentagemdecélulasAhRpositivasnoslinfócitosTCD4+CD25+foi significa-tivamentemaiselevadadoquenoslinfócitosTCCR6+CD4+etambémfoisignificativamente maiordoquenascélulasmononuclearesdosangueperiférico(doinglêsperipheralblood mononuclearcells,PBMC,)[(52,49±19,18)%vs.(23,18±5,62)%vs.(18,06±7,80)%,X2=24,03,
p<0,01];empacientescomAR,apercentagemdecélulasAHRpositivasnoslinfócitosT CCR6+CD4+erasignificativamentemaioremcomparac¸ãocomoslinfócitosTCD4+CD25+ ePBMC(46,02±14,68)%vs.[17,90(6,10±80.10)]%vs.(34,22±10,33)%,X2=38,29,p<0,01];no entanto,nãofoiencontradacorrelac¸ãoestatisticamentesignificativaentreosdadosclínicos ecélulasAhRpositivasemlinfócitosTCCR6+CD4+eCD4+CD25+.
Conclusão:OAhrpodeparticipardoprogressopatológicodaARaocontrolaradiferenciac¸ão delinfócitosTh17eTregnosangueperiférico.
©2016ElsevierEditoraLtda.Este ´eumartigoOpenAccesssobumalicenc¸aCC BY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Rheumatoid arthritis (RA) is a systemic chronic inflam-matory disease that affects about 1% of the population.1
The aryl hydrocarbon receptor (AhR), consisted of 806 amino acids, is a transcription factor that belongs to the bHLH (basic Helix-Loop-Helix)-PAS (Per-ARNT-Sim) family.2
AhR combines with chaperone proteins in the cytoplasm and maintains an inactive formin the absence of its lig-andssuchas2,3,7,8-tetrachlorodibenzo-p-dioxin(TCDD)and 6-formylindolo[3,2-b]carbazole (FICZ). Once activated, AhR dissociatesfromtheproteinsandtransferstothenucleusto inducetheexpressionofdownstreamgenes.
Thelper(Th)17cellsandregulatoryTcells(Treg)aregroups oftheCD4+Tcellsfamily.Interleukin(IL)17–producingTh17 cells predominantly express CC chemokine receptor (CCR) 6which isa crucialmarkerfor Th17 cells inautoimmune diseases3; previous study has suggested that rheumatoid
arthritisinanimalmodelscould beimprovedwhentreated withanti-CCR6monoclonalantibody,3 whichmayindicate
CCR6expressionplaysaprimaryroleinautoimmunediseases. CCR6+CD4+TcellisthemaincomponentofTh17cells,3–6and
meanwhileCCR6isregardedasabiomarkerofhumanTh17 cells.5,6 TheCD4+CD25+ lineage ofTreg is an immunosup-pressive cell which playsa vital characterin immune and autoimmuneresponsesandthetranscriptionfactorforkhead boxproteinP3(Foxp3)hasbeenthoughttodeterminetheTreg cells lineage.7 Recently,studieshavefoundAhRwashighly
expressedinTh17andTregcells,8,9 whichcouldactivateits
signalingpathways,controlthedifferentiationofthecells,and furtherinvolveinthepathogenesisofautoimmunediseases inaligand-specificmanner.8,10,11
Patients
and
methods
Studysubjects
Thirty-fivepatientswithRA(meanage49.22±8.94,8males and 27 females) according to the 1987 revised criteria of AmericanRheumatismAssociation12wererecruitedfromthe
DepartmentofRheumatology,TheSecondAffiliated Hospi-talofAnhuiMedicalUniversity.Theinclusioncriteriaofthe study(a) adefinitediagnosis,(b)freeofotherautoimmune diseases,(c)undergoingnoinfectiousdiseases,(d)treatment withsamedrugsforRApatients.Meanwhile,fourteenageand sexmatchedhealthycontrolswere randomlyselectedfrom healthcenter(meanage46.5±7.5,3malesand11females). Theclinicalparametersandthediseaseactivityscorein28 joints(DAS28)werecalculatedindetail.TheEthics Commit-tee of the Hospital approved the study. Informed consent wasobtainedfromthepatientsorfromtheirrelativesifthe patientswereincapableofconsent.
Cellisolation
5-mL venous peripheral blood was stored in anticoagula-tiontubecontainingEDTAastheanticoagulant.PBMCswere separatedusingdensity-gradientcentrifugation(Beijing Solar-bioScienceandTechnologyCompany,Beijing, China).Total PBMCsnumberofeachsamplewasnotlessthan1×106.
Flowcytometry
Cellsurfacemarkers
5LCD25-PEand2LCD4-PerCP-Cy5.5werebothaddedto
themarkedflowtubes(a,b),atthesametime5LCCR6-PE
and2LCD4-PerCP-Cy5.5wereaddedtotheothertwotubes
(c,d).Aftertheliquidinfourtubeswasmixed,weplacedthem inarefrigeratorat4◦Cfor20min.Then2-mLbufferwas pre-paredtowashthemforonce.Theabove-mentionedantibodies werepurchasedfromBecton,DickinsonandCompany(BD)of American.
Fixationandperforation
100-LPBMCswasmixedwith500Lfixatives(Becton,
Dickin-sonandCompany,American)infourtubestogiveincubation avoidinglightat4◦Ctemperatureandafterthat,1-mLbuffer wastowashthemfortwotimes.
TheindirectmethodofmarkingAhR
Eachtubewasaddedthemixtureof2Lfirstantibody(the
antibodyofanti-arylhydrocarbonreceptor,AbcamCompany, England)and2Lphosphatebufferedsaline(PBS).1-mLbuffer
wasappliedtowashthemafterputthemixtureatroom tem-peraturetoavoidlightfor1handthen2Lsecondantibody
(mouse monoclonal(2A9)secondary antibodytorabbit IgG heavychain(FITC),AbcamCompany,England)wasaddedto thefourtubesandplacedtheminchamberatroom temper-aturetoavoidlightforhalfanhour.2Lbufferwasusedto
washthemfortwotimesandatlast200LPBSwasaddedto
thetubes.
Table1–Primersusedforthequantitativereal-time RT-PCR.
Genes Primer
AhR
Forwardprimer 5′-ATACCGAAGACCGAGCTGAAT-3′
Reverseprimer 5′-CCAGCAGACACCTTAGACGAC-3′
ˇ-Action
Forwardprimer 5′-AGCGAGCATCCCCCAAAGTT-3′
Reverseprimer 5′-GGGCACGAAGGCTCATCATT-3′
CYP1A1
Forwardprimer 5′-CATCCCCCACAGCACAACAAGAGA-3′
Reverseprimer 5′-GCAGCAGGATAGCCAGGAAGAGAA-3′
AhR,arylhydrocarbonreceptor;CYP1A1,vitalphaseIenzymeof AhR.
Flow-cytometricanalysis
The percentage of CD4+CD25+ and CCR6+CD4+ T cells in PBMCs and the proportionof AhRpositive cells inPBMCs, CD4+CD25+TandCCR6+CD4+Tcellswerealldetectedby Cel-lQuestsoftware(FACSaclibur,BDCompany).Thenumberof cellswastestedatleast100,000atatimeandFlowJo7.6.1was appliedtoanalyzethedata.
IsolationandreversetranscriptionoftotalRNA
TotalRNAfromRAperipheralbloodmononuclearcellswas separated usingTrizol reagent(Invitrogen, California,USA). PrimersweredesignedandsynthesizedbyTakara Biotechnol-ogy(Tokyo,Japan).Thespecificprimersequenceswereshown inTable1. ThePrimeScriptRT reagentKit(Takara Biotech-nology,Japan) wasused toperformthe synthesis ofcDNA accordingtothemanufacturer’sinstructions.
Real-timequantitativePCR
ThePCRwasperformedina20Lreactionsystem
contain-ing1.6Lprimers(0.8Lforwardprimerandreverseprimer,
respectively), 2L cDNA, 0.4L ROX Reference dye II, 6L
dH2O,and10LSYBRPremixExTaqTMII.Allthesampleswere
amplifiedatthesamesiteonABI7500real-timepolymerase chainreactionsystem(AppliedBiosystems,FosterCity,CA). 2−ctwasusedtocomputetheexpressionvalueofAhRand CYP1A1.
Laboratorymeasurement
ForalltheRApatients,erythrocytesedimentationrate(ESR), Creactiveprotein(CRP)andanti-cycliccitrullinatedpeptide antibody(A-CCP)weremeasured.
Statistical
analysis
60
40
20
0
Control
RA
AhR, %
P<.01
Fig.1–ThepercentageofAHRinperipheralblood mononuclearcellsfromRApatientsandhealthycontrols.
datainthreegroups,andthecomparisonbetweentwogroups inthethreegroupswasanalyzedbyLSD-torMann–WhitneyU test.Spearmancorrelationanalysiswasappliedinourstudy. p<0.05wasthoughttobestatisticallysignificant.
Results
ThepercentagesofAhRinPBMCsfrompatientswithRA andcontrols
AsshowninFig.1,thepercentageofAhRpositivecellswas decreased in healthy controls (18.83±7.32)% than that in patientswithRA(35.23±10.71)%(p<0.01).
TheincreasedmRNAlevelsofAhRandCYP1A1inPBMCs fromRApatients
AsshowninFig.2,AhRandCYP1A1mRNAexpressionlevels werebothincreasedinpatientswithRAwhencomparedwith controls[(3.71±1.63)vs.(2.00±1.27),p=0.002;(2.62±2.08)vs. (0.62±0.29),p<0.01,respectively].
ThepercentageofAhRinCCR6+CD4+TandCD4+CD25+T
cellsfromRAcasesandhealthysubjects
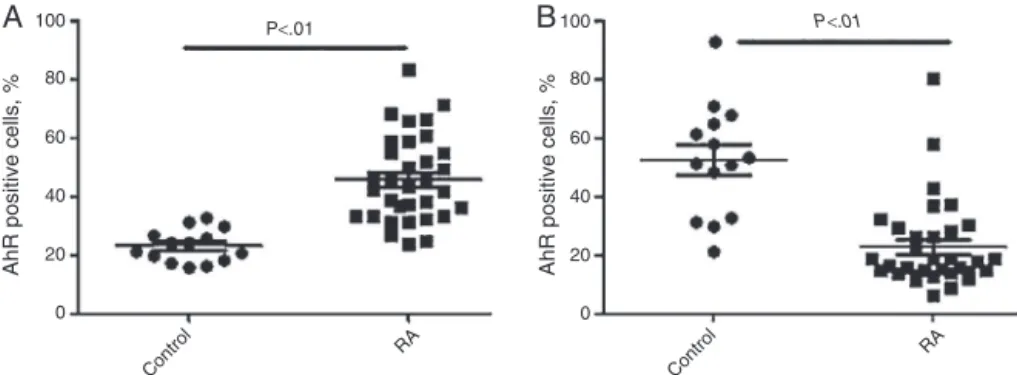
As shown in Fig. 3, the percentage of AhR positive cells in CCR6+CD4+T cells was clearly higher in patients (46.02±14.68)%thanthatinhealthysubjects(23.18±5.62)%
(p<0.01)(Fig.3A).Incontrast,thepercentageofAhRpositive cellsinCD4+CD25+TcellswasdecreasedinRApatients[17.90 (6.10,80.10)]%thanhealthygroup(52.49±19.18)%(Fig.3B). Sig-nificantdifferenceswereexistedbetweentwogroups(p<0.01).
ThecomparisonaboutthepercentageofAhRpositivecells inperipheralbloodCD4+CD25+T,CCR6+CD4+Tcellsand
PBMCsfromNormalcontrolandRAgroup
In the healthy group, the percentage ofAhR positive cells in CD4+CD25+T cells was significantly higher than that in CCR6+CD4+Tcells, and wasalso statisticallyhigher than in PBMCs[(52.49±19.18)%vs.(23.18±5.62)%vs.(18.06±7.80)%, X2=24.03, p<0.01] (Fig. 4A). Nevertheless, the percentage ofAhRpositivecells inCCR6+CD4+Tcells wassignificantly increasedthanthatinCD4+CD25+Tcells,andwasalso sig-nificantlyhigherthanthatinPBMCs[(46.02±14.68)%vs.17.90 (6.10,80.10)%vs.(34.22±10.33)%,X2=38.29,p<0.01](Fig.4B).
CorrelationbetweentheAhRpositivecellsinperipheral bloodCCR6+CD4+T,CD4+CD25+Tcellsandmajorclinical
parametersfromRApatients
WeconductedSpearman’scorrelationtoassesstheassociation betweenAhR/CYP1A1mRNAexpressionlevels,AhRpositive cellsinperipheralbloodCCR6+CD4+T,CD4+CD25+Tcellsand majorclinicalparameters.However,nosignificantcorrelation wasfoundbetweenthem,andthedetailsweresummarized inTable2.
Discussion
RAisasystemicautoimmunediseaseprimarilymanifestas polyarthritis,especiallythesmalljointsofhandsandfeet,that ischaracterizedbyjointdestructionandchronicdisability.13
However,themechanismsabouttheimmunepathwaysinRA havenotbeenidentifiedyet.Forthepastfewyears,awealthy ofdatahasshowedself-toleranceplaysadominantrolein autoimmunityincludingRAasit erroneouslywasactivated in response to its self-antigen. The self-tolerance in RA patientsmayberegulatedbyvariousfactorssuchascytokine, environmentalrisksandlifestylefactors.14 Currently,itwas
believed thataberrantT-cellhomeostasisplaysagreatpart inthepathogenesis.Moreover,Tregcellsdysfunctionand/or Th17 cells dysregulation are thought to contribute to the
10
8
6
4
2
0
10
8
6
4
2
0 P<.05
P<.01
AhR mRNA relative expression
CYP1A1 mRNA relative
expression
RA
Control Control
RA
A
B
100
80
60
40
20
AhR positive cells, % AhR positive cells, %
0
100
80
60
40
20
0
P<.01 P<.01
RA Control
RA Control
A
B
Fig.3–ThepercentagesofAHRpositivecellsinCCR6+CD4+T(A)andCD4+CD25+T(B)cellsfromhealthycontrolsandRA
patients.
100
80
60
40
20
0
100
80
60
40
20
0
AhR positive cells, %
AhR positive cells, %
PBMCs
CCR6+CD4+T CD4+CD25+T
PBMCS
CCR6+CD4+T CD4+CD25+T
P<.01
P<.01
P<.01 P
<.01
P>.05
P<.01
A
B
Fig.4–ThepercentagesofAHRpositivecellsinPBMCs,CCR6+CD4+T,CD4+CD25+Tcellsfromnormalcontrol(A)andRA
group(B).
development of autoimmune disorders. Similarly, accu-mulated evidence indicates AhR plays a critical place in regulatingthedifferentiationofTh17andTregcells, affect-ingthe developmentofanumber ofautoimmunediseases includingRA.11,15–20
AhRislocatedinthecytoplasmandmaintainsaninactive formintheabsenceofitsligandssuchasTCDDandFICZ.Once
activated,AhRcomplexcombineswithAhRnuclear transloca-torprotein(Arnt)tobeheterodimerinthenucleuswherethey bindtospecificDNAsequencesknownasxenobiotic-response elements(XRE),andthisbindingwillproducesomereaction. Duringthisactivatedprocess,avitalphaseIenzymeofAhR (CYP1A1)thatisamostwidelystudiedresponsivegenehada higherexpression.21Severalgroupspreviouslyhaveshowna
Table2–ThecorrelationbetweenpercentagesofAhRpositivecellsinCCR6+CD4+T/CD4+CD25+Tcells,
AhR/CYP1A1mRNAexpressionfromRApatientsandclinicalparameters.
Clinicalparameters ESR CRP Anti-CCP DAS28scores Course
AhRmRNA
r-Value 0.115 0.014 0.003 0.068 −0.107
p-Value 0.511 0.936 0.987 0.698 0.541
CYP1A1mRNA
r-Value 0.007 0.149 −0.035 0.143 0.031
p-Value 0.967 0.392 0.842 0.411 0.860
CCR6+CD4+T
r-Value −0.262 −0.273 0.055 −0.187 −0.309
p-Value 0.14 0.124 0.761 0.296 0.08
CD4+CD25+T
r-Value −0.129 0.013 0.140 −0.045 −0.081
p-Value 0.475 0.943 0.436 0.804 0.653
stateAhRwashighlyexpressedinubiquitouscellsincluding Th17,Treg,dendriticcells(DCs).8,9,22
Interestingly, the essential factor of the pathogenesis ontraditional view inautoimmune diseases ismainlydue to Th1 and Th2 cells ratio imbalance. However, recent researchers had a tendency to focus more on the bal-ancebetween Th17 and Treg cells belong tothe family of CD4+Tcells.Quintanaetal.17showedthatAhRactivationby
its ligand2,3,7,8-tetrachlorodibenzo-p-dioxininduced func-tionalTregcellsthatsuppressedexperimentalautoimmune encephalomyelitis(EAE), butboostedTh17 cells differentia-tionandfurthermoreincreasedtheriskofEAEiftheligand wasFICZinanimalmodels. Thisstudy furtherilluminated theimportanceofthebalanceonTh17andTregcells.Astudy bydeKleeretal.23showedasignificantlyhighernumberof
CD4+CD25+Tregcellswasfoundinjuvenileidiopathic arthri-tis(JIA)thanthatinextendedoligoarticularJIA(ext-OAJIA), andsuggestedthehigherfrequenciesofCD4+CD25+Tregcells insynovialtissueofpatientswithJIA,thelowerseverityofthe disease.IthasbeenreportedCD4+CD25+Treg-deficiencymight exacerbatesymptomsinpatientswithRA.24,25Inthe
mean-time,Nguyenetal.26showedthediseaseprogressioncouldnot
bepreventedbyCD4+CD25+Tregcells,buttheseveritywould beloweredforitsimmunosuppressivefunction.
Todate,thestudy aboutAhRinperipheralblood aswell asinTh17and TregofRApatientsismuchless.Basedon the mentioned studies and the notion, we conducted the experiment.Consistentwithpreviousfindings,8,10,11,17firstly,
we found a statistically higher frequency of AhR positive cellsinRApatientswhen comparedwithhealthycontrols. Inaddition,wealsogot AhRand CYP1A1mRNAwere both overexpressedinpatientswithRA.Giventheresult,wemight speculateAhRwasactivatedandplayedasignificantrolein RApatients.Secondly,ourstudyshowednotonlythe percent-ageofAhRinCCR6+CD4+Tcellswas enrichedbut alsothe percentageinCD4+CD25+TcellswasreducedinRApatients comparedtohealthysubjects.Moreover,inpresentstudy,the highestfrequencyofAhRpositivecellsinCD4+CD25+Twas testedofCCR6+CD4+TandPBMCsinhealthypersons, how-everitsproportioninCCR6+CD4+Tcellswasincreasedthan PBMCsandbothofthemwere higherthan inCD4+CD25+T cells.Allthesefindingscollectivelysupportedtheviewthat AhRplaysanessentialroleindifferentiationandfunctionof CD4+CD25+TregandCCR6+CD4+Th17,controllingthebalance ofTh17andTregcells,andwhatcouldprovidemore thera-peuticapproachesinRA.However,nosignificantcorrelations betweenAhR/CYP1A1mRNAexpressionlevels,theAhR posi-tivecellsinCD4+CD25+TaswellasCCR6+CD4+TcellsandCRP, ESR,A-CCP,DAS28,courseofdiseasewerefound.Itpotentially toldustheycouldnotreflectthediseaseactivityorinsufficient samplesizemightbeoneofthereasons.
Inconclusion,ourfindingsfirstdemonstratean associa-tionbetweenAhRandCD4+CD25+Treg,CCR6+CD4+Th17cells inperipheralbloodfromRApatients,whichmaybroadenour horizonsintheetiologyofRAandprovidenewinsightsinto thepathogenesisofRA.OurresultssuggestthatAhRmayplay acentralroleincontrollingthebalanceofTh17andTregforits unbalancedexpressioninperipheralbloodandfurther partic-ipateinthepathogenesisofRA.Ofcourse,theseresultsmay beneededtoelucidateinafurtherresearch.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
Thisworkwassupportedbyallthedonorsforfriendly provid-ingthebloodsamples.
r
e
f
e
r
e
n
c
e
s
1.SanghaO.Epidemiologyofrheumaticdisease.Rheumatology. 2000;39Suppl.2:3–12.
2.BurbachKM,PolandA,BradfieldCA.Cloningofthe Ah-receptorcDNArevealsadistinctiveligand-activated transcriptionfactor.ProcNatlAcadSciUSA.1992;89:8185–9. 3.HirotaK,YoshitomiH,HashimotoM,MaedaS,TeradairaS,
SugimotoN,etal.Preferentialrecruitmentof
CCR6-expressingTh17cellstoinflamedjointsviaCCL20in rheumatoidarthritisanditsanimalmodel.JExpMed. 2007;204:2803–12.
4.Acosta-RodriguezEV,RivinoL,GeginatJ,JarrossayD,Gattorno M,LanzavecchiaA,etal.Surfacephenotypeandantigenic specificityofhumaninterleukin17-producingThelper memorycells.NatImmunol.2007;8:639–46.
5.AnnunziatoF,CosmiL,SantarlasciV,MaggiL,LiottaF, MazzinghiB,etal.Phenotypicandfunctionalfeaturesof humanTh17cells.JExpMed.2007;204:1849–61.
6.SinghSP,ZhangHH,FoleyJF,HedrickMN,FarberJM.HumanT cellsthatareabletoproduceIL-17expressthechemokine receptorCCR6.JImmunol.2008;180:214–21.
7.HillJA,FeuererM,TashK,HaxhinastoS,PerezJ,MelamedR, etal.Foxp3transcription-factor-dependentand-independent regulationoftheregulatoryTcelltranscriptionalsignature. Immunity.2007;27:786–800.
8.VeldhoenM,HirotaK,WestendorfAM,BuerJ,DumoutierL, RenauldJC,etal.TheArylhydrocarbonreceptorlinks Th17-mediatedautoimmunitytoenvironmentaltoxins. Nature.2008;453:106–9.
9.NakahamaT,KimuraA,NguyenNT,ChinenI,HaniehH, NoharaK,etal.ArylhydrocarbonreceptordeficiencyinT cellssuppressesthedevelopmentofcollagen-induced arthritis.ProcNatlAcadSciUSA.2011;108:14222–7. 10.SakaguchiS,YamaguchiT,NomuraT,OnoM.RegulatoryT
cellsandimmunetolerance.Cell.2008;133:775–87. 11.SinghNP,SinghUP,SinghB,PriceRL,NagarkattiM,
NagarkattiPS.Activationofarylhydrocarbonreceptor(AhR) leadstoreciprocalepigeneticregulationofFoxP3andIL-17 expressionandameliorationofexperimentalcolitis.PLoS ONE.2011;6:e23522.
12.AraeaFC,EdworthySM,BlochDA,McShaneDJ,FriesJF, CooperNS,etal.TheAmericanRheumatismAssociation 1987revisedcriteriafortheclassificationofrheumatoid arthritis.ArthritisRheum.1988;31:315–24.
13.FiresteinGS.Immunologicmechanismsinthepathogenesis ofrheumatoidarthritis.JClinRheumatol.2005;113 Suppl.:S39–44.
14.OliverJE,SilmanAJ.Riskfactorsforthedevelopmentof rheumatoidarthritis.ScandJRheumatol.2006;35:169–74. 15.NguyenNT,NakahamaT,KishimotoT.Arylhydrocarbon receptorandexperimentalautoimmunearthritis.Semin Immunopathol.2013;35:637–44.
andthedioxinTCDDinrheumatoidarthritis.Rheumatology. 2008;47:1317–22.
17.QuintanaFJ,BassoAS,IglesiasAH,KornT,FarezMF,BettelliE, etal.ControlofTregandTH17celldifferentiationbythearyl hydrocarbonreceptor.Nature.2008;453:65–71.
18.TamakiA,HayashiH,NakajimaH,TakiiT,KatagiriD, MiyazawaK,etal.Polycyclicaromatichydrocarbonincreases mRNAlevelforinterleukin1betainhumanfibroblast-like aynoviocytelineviaarylhydrocarbonreceptor.BiolPharm Bull.2004;27:407–10.
19.LahotiTS,JohnK,HughesJM,KusnadiA,MurrayIA,
KrishnegowdaG,etal.Arylhydrocarbonreceptorantagonism mitigatescytokine-mediatedinflammatorysignallingin primaryhumanfibroblast-likesynoviocytes.AnnRheumDis. 2013;72:1708–16.
20.NakahamaT,KimuraA,NguyenNT,ChinenI,HaniehH, NoharaK,etal.ArylhydrocarbonreceptordeficiencyinT cellssuppressesthedevelopmentofcollageninduced arthritis.ProcNatlAcadSciUSA.2011;108:14222–7. 21.DenisonMS,NagySR.Activationofthearylhydrocarbon
receptorbystructurallydiverseexogenousandendogenous
chemicals.AnnuRevPharmacolToxicol.2003;43: 309–34.
22.FrericksM,MeissnerM,EsserC.Microarrayanalysisofthe AHRsystem:tissue-specificflexibilityinandtargetgenes. ToxicolApplPharmacol.2007;220:320–32.
23.deKleerIM,WedderburnLR,TaamsLS,PatelA,VarsaniH, KleinM,etal.CD4+CD25brightregulatoryTcellsactively regulateinflammationinthejointsofpatientswiththe remittingformofjuvenileidiopathicarthritis.JImmunol. 2004;172:6435–43.
24.EhrensteinMR,EvansJG,SinghA,MooreS,WarnesG, IsenbergDA,etal.CompromisedfunctionofregulatoryT cellsinrheumatoidarthritisandreversalbyanti-TNFalpha therapy.JExpMed.2004;200:277–85.
25.ValenciaX,StephensG,Goldbach-ManskyR,WilsonM, ShevachEM,LipskyPE.TNFdown-modulatesthefunctionof humanCD4+CD25hiT-regulatorycells.Blood.2006;108:253–61. 26.NguyenLT,JacobsJ,MathisD,BenoistC.Where