w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Original
article
Th17
cells
and
CD4
+
multifunctional
T
cells
in
patients
with
systemic
lupus
erythematosus
Júlio
Antônio
Pereira
Araújo
a,
Danilo
Mesquita
Jr
a,
Wilson
de
Melo
Cruvinel
a,b,
Karina
Inácio
Salmazi
c,
Esper
Georges
Kallás
c,
Luis
Eduardo
Coelho
Andrade
a,∗aDepartmentofRheumatology,UniversidadeFederaldeSãoPaulo(UNIFESP),SãoPaulo,SP,Brazil
bDepartmentofBiomedicine,PontifíciaUniversidadeCatólicadeGoiás(PUC-GO),Goiânia,GO,Brazil
cDepartmentofClinicalImmunologyandAllergy,UniversidadedeSãoPaulo(USP),SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received4April2014 Accepted22August2015
Availableonline19November2015
Keywords:
Systemiclupuserythematosus Tlymphocytes
Th17
MultifunctionalTcells
a
b
s
t
r
a
c
t
Introduction/Objective:RecentevidencesuggeststhatabnormalitiesinvolvingTh17
lympho-cytesareassociatedwiththepathophysiologyofsystemiclupuserythematosus(SLE).In addition,multifunctionalTcells(MFT),i.e.,thoseproducingmultiplecytokines simulta-neously,arepresentintheinflammatorymilieuandmaybeimplicatedintheautoimmune processobservedinSLE.Inthepresentstudy,weaimedtocharacterizethefunctionalstatus ofCD4+TcellsinSLEbysimultaneouslydeterminingtheconcentrationofIL-2,
IFN-␥and
IL-17inlymphocyteculturesunderexogenousandself-antigenicstimuli.
Patientsandmethods:Eighteenpatientswithactivedisease,18withinactivedisease,and14
healthycontrolshadfunctionalstatusofCD4+Tcellsanalyzed.
Results:WefoundthatSLEpatientspresentedadecreasednumberoftotalCD4+cells,an
increasednumberofactivatedTcells,andanincreasedfrequencyofTh17cellscompared tohealthycontrols(HC).MFTcellshadincreasedfrequencyinSLEpatientsandtherewasan increasedfrequencyoftri-functionalMFTinpatientswithactiveSLEcomparedwiththose withinactiveSLE.Interestingly,MTFcellsproducedlargeramountsofIFN␥than mono-functionalTcellsinpatientsandcontrols.
Conclusion:TakentogetherthesedataindicatetheparticipationofrecentlyactivatedTh17
cellsandMTFcellsintheSLEpathophysiology.
©2015ElsevierEditoraLtda.Allrightsreserved.
Linfócitos
Th17
e
linfócitos
T
CD4
+multifuncionais
em
pacientes
com
lúpus
eritematoso
sistêmico
Palavras-chave:
Lúpuseritematososistêmico LinfócitosT
r
e
s
u
m
o
Introduc¸ão/Objetivo:Evidênciasrecentessugeremqueanormalidadesqueenvolvemos
lin-fócitosTh17estãoassociadasàfisiopatologiadolúpuseritematososistêmico(LES).Além disso, os linfócitos T multifuncionais (LTM), ouseja, aqueles que produzem múltiplas
∗
Correspondingauthor.
E-mail:luis.andrade@unifesp.br(L.E.C.Andrade).
http://dx.doi.org/10.1016/j.rbre.2015.10.003
Th17
LinfócitosTmultifuncionais
citocinassimultaneamente,estãopresentesnomeioinflamatórioepodemestarimplicados noprocessoautoimuneobservadonoLES.Nopresenteestudo,objetiva-secaracterizaro estadofuncionaldoslinfócitosTCD4+noLESedeterminarsimultaneamenteaconcentrac¸ão
deIL-2,IFN-␥eIL-17emculturasdelinfócitossobestímulosexógenoseautoantigênicos.
Pacientesemétodos:Dezoitopacientescomdoenc¸aativa,18comdoenc¸ainativae14controles
saudáveisforamsubmetidosàanálisedoestadofuncionaldoslinfócitosTCD4+.
Resultados:Encontrou-sequeospacientescomLESapresentaramumadiminuic¸ãona
quan-tidadetotaldecélulasCD4+,um aumentonaquantidadedelinfócitosT ativadoseum
aumentonafrequênciadelinfócitosTh17emcomparac¸ãocomcontrolessaudáveis(HC). AscélulasLTMtinhafrequênciaaumentadaempacientescomLESehouveumaumentona frequênciadeLTMtrifuncionaisempacientescomLESativoemcomparac¸ãocomaqueles comLESinativo.Curiosamente,ascélulasMTFproduziramquantidadesmaioresdeIFN-␥ doqueoslinfócitosTmonofuncionaisempacientesecontroles.
Conclusão: Analisadosemconjunto,essesdadosindicamaparticipac¸ãodoslinfócitosTh17
recentementeativadosecélulasMTFnafisiopatologiadoLES.
©2015ElsevierEditoraLtda.Todososdireitosreservados.
Introduction
Effector CD4+ T cells have been initially categorized into
two subsets based onthe cytokines theyproduce.1–3 Th17 cells are part of this new T cell scenario and display an activatedCD4+ T cell phenotype characterizedby the
pro-duction of high amounts of IL-17.4–6 Th17 cells seem to playa crucial role in the development of awide range of autoimmuneandchronicinflammatorydisorders.7–9 Infact, ithasbeensuggestedthatinappropriateregulationofTh17 cells may be a key event in the pathogenesis of rheuma-toid arthritis and systemic lupus erythematosus (SLE).10–14 Several studies reported significantly higher serum levels ofIL-17andhigherfrequencyofIL-17-producingperipheral blood mononuclear cells (PBMC) in SLE patients as com-paredtonormal individuals.13,15–18 Itwas alsoshown that Th17 response is correlated with disease activity in SLE patients.17
Anovel strategyforthe evaluationof Tcell functional-ity is based on the simultaneous determination ofseveral cytokinesexpressedbysubtypes ofcells.According tothis approach T cells that produce multiple cytokines simulta-neouslyaretermedmultifunctionalTcells.Multifunctional T cell responseshave been documented in HIV-119 and in the immune response to vaccination against Hepatitis B virus20 and HIV.19,20 This novel approach has been largely possible due to technological advances in flow cytometry that nowadays allows for the simultaneous detection of numerousfunctional,phenotypic,andlineagemarkersonT cells.21
Inthepresentstudy,wesoughttofurthercharacterizethe functionalstatusofCD4+TcellsinSLEpatientswithactive
andinactivediseasesbysimultaneouslydeterminingthe con-centrationofseveralcytokinesinlymphocyteculturesunder different antigenic stimuli. Wealso quantified the propor-tionofTh17andmultifunctionalTcellsandcorrelatedthem withSLEDAI(SystemicLupusErythematosusDiseaseActivity Index),andwiththefrequencyofactivatedTcellsandTREG
cells.
Materials
and
methods
Thirty-six adultpatients (33women and3 men,aged40± 7.2years)withadiagnosisofSLEbasedontheAmerican Col-legeofRheumatologycriteria22wereconsecutivelyenrolledin thestudyafterprovidinginformedconsent.Allpatientswere referredfromtheoutpatientclinicattheDivisionof Rheuma-tology of the Universidade Federal de São Paulo (Federal UniversityofSãoPaulo).Patientsweredividedintotwogroups basedontheirSLEDiseaseActivityIndex(SLEDAI)score.23,24 TheactiveSLEgroup(A-SLE)comprised18patients(SLEDAI score≥6;17womenand1man,aged36.7±10.2years)and theinactiveSLEgroup(I-SLE)comprised18patients(SLEDAI score=0;16womenand2men,aged39.2±13.9years).Table1
depicts the demographic and clinical characteristics of all patientsenrolledinthestudy.Asacontrol,14healthy labora-toryworkerswereenrolledinthestudy(13womenand1man, aged33.9±10.4years)aftergivinginformedconsent.Patients and healthycontrolsunderwentastructuredquestionnaire anddonated60mLofvenousblood.Thestudyprotocolwas reviewed and approvedby the institution’s research ethics committee.
Peripheralbloodmononuclearcells(PBMC)wereisolated bydensity-gradientsedimentationoverFicoll–Paque (Pharma-cia Biotech,Uppsala,Sweden) andwashed twiceinHank’s balanced salt solution (Gibco,Grand Island, NY). For cryo-preservation,cellswereslowlyfrozenin90%offetalbovine serum(FBS;HyCloneLaboratories,Logan,UT),andstoredin liquidnitrogen.Atthetimeoftheassay,PBMCwererapidly thawedina37◦Cwaterbathandwashedinpre-heatedRPMI
1640supplementedwith10%fetalcalfserum,100U/mL peni-cillin, 100g/mLstreptomycin and 20mM glutamine (R10). Cellswere counted,checkedforviability,and suspendedin R10ataconcentrationof1×106viablecells/mL.
Thawed PBMC were incubated in 96-well plates
Table1–Demographiccharacteristicsofcontrolsandpatientswithsystemiclupuserythematosus.
Controls(n=14) A-SLE(n=18) I-SLE(n=18) pValue
Age 33.9±10.4 36.7±10.2 39.2±13.9 0.624
Female(%) 92.8% 94.4% 88.9% 0.987
SD,standarddeviation;A-SLE,activesystemiclupuserythematosus;I-SLE,inactivesystemiclupuserythematosus;SLEDAI,systemiclupus
erythematosusdiseaseactivityindex;ns,notsignificant.
1500gfor5min,suspendedinMacsbuffer and transferred
into V-bottom 96-wellplates (Nunc,Roskilde,Denmark) in
100L of staining buffer [phosphate-buffered saline (PBS)
supplementedwith0.1%sodiumazide(Sigma)and1%FBS,
pH 7.4] with the panel of surface monoclonal antibodies
(CD4–PerCP, CD3–APC-CY7 and CD69–PE-Cy7). Cells were
incubatedat4◦Cinthedarkfor30min,washedtwicewith
PBS and then suspended in 100L of fixation buffer [1%
paraformaldehyde(Polysciences,Warrington,PA)inPBS,pH
7.4].Forintracellularstaining,cellsalreadylabeledforsurface
markerswereincubatedwith100Lof4%fixationbufferand
washedwithpermeabilizationbuffer(PBSsupplementedwith
0.1%sodium azide,1%FBSand0.1%saponin;Sigma). Each
samplewassuspendedin100Lofpermeabilizationbuffer,
incubatedfor15minatroomtemperatureinthedark,washed
with100Lofstainingbufferandincubatedfor30minat4◦C
inthedarkwitheithernoantibody(unstainedtube)orwith
a pool of anti-IL-2–PE, anti-IFN-␥–APC, and anti-IL-17–FITC
monoclonal antibodies in 50L of staining buffer.25 Cells
werewashedwith200Lofstainingbufferandsuspendedin 100Loffreshlyprepared1%paraformaldehyde(PFA)pH7.4. TREG cells and effector CD25+ T cells were identified
according to a previously established protocol.26 Briefly,
PBMCs were washed in PBS and 0.5×106 viable cells
wereincubatedwithfluoresceinisothiocyanate(FITC)-labeled anti-CD127,allophycocyanin-Cy3(APC-Cy3)-labeledanti-CD3, PerCP-labeled anti-CD4, and phycoerythrin (PE)-Cy7-labeled anti-CD25 (Becton Dickinson, San Jose, CA, USA) antibod-ies, according to the manufacturer’s instructions. After 30minofincubation at4◦Cinthedark,cells werewashed
withMacsbuffer, fixedandpermeabilizedwithFoxP3 fixa-tion/permeabilizationbuffer(eBioscience,SanDiego,CA,USA) andthenprocessedforFoxP3stainingusingtheFoxP3 stain-ingkitandAPC-labeledanti-FoxP3(eBioscience)accordingto themanufacturer’sinstructions.
Samples were processedon a FACSCanto flow cytometer, usingFACSDIVAsoftware(BDBiosciences),andtheacquired data were analyzed with FLOWJO software (Tree Star, San Carlo, CA). Fluorescence voltages were determined using matchedunstainedcells.Compensationwascarriedoutusing CompBeads(BDBiosciences)singlestainedwithCD3–PerCP,
CD4–FITC,CD8–APC–CY7,CD4–PE–CY7,CD3–PEorCD3–APC,
respectively. Samples were acquired until at least 500,000 eventsinalivecellgatewereobtained.
Data were reported as median and interquartile range (IQR). Comparisons among groups were carried out using the Kruskall–Wallisnon-parametric test, followed by inter-group comparisons by the Dunnet test. Correlations were
performed using the Spearman’s non-parametric method.
Statisticalinferencelevelwasestablishedat5%(p<0.05).
Results
DecreasedfrequencyofCD4+Tcellsandincreased
frequencyofnewlyactivatedcellsinSLE
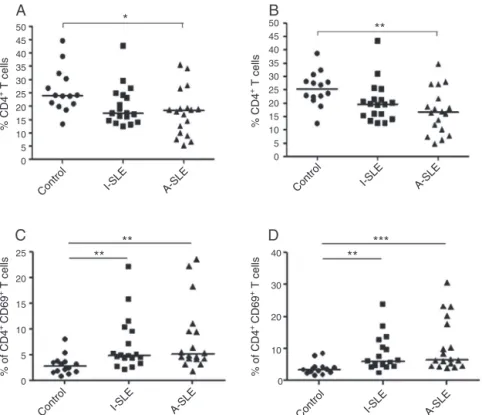
TherelativefrequencyofCD4+ cellsovertotalCD3+cellsin
PBMCculturesstimulatedwithHEp-2cellextractwas signif-icantlylowerinsamplesfrompatientswithA-SLE(18.4±8.9) and I-SLE (17.3±7.6) as compared to controls (24.0±8.2) (Fig.1A).Likewise,PBMCculturesstimulatedwithPMA/Io pre-sentedlowerrelativefrequencyofCD4+cellsovertotalCD3+
cellsinsamplesfrompatientswithA-SLE(16.6±8.4)and I-SLE(19.6±7.6) ascomparedtocontrols(25.3±6.4) (Fig.1B). The same wasobserved fornon-stimulated PBMCcultures (data not shown). In contrast, when we evaluated newly activatedcells,characterizedbytheexpressionoftheCD69 molecule,higherrelativefrequencyofCD4+CD69+ cellsover
totalCD4+cellswasobservedinpatientswithA-SLE(5.1±6.6)
andI-SLE(4.9±5.2)whencomparedtocontrols(2.8±1.9)in culturesstimulatedwithHEp-2cell extract(Fig.1C)aswell aswithPMA/Io:A-SLE(6.6±8.3),I-SLE(5.9±5.5),andcontrols (3.5±2.0)(Fig.1D).Again,asimilarbehaviorwasobservedin non-stimulatedPBMCcultures(datanotshown).
MultifunctionalprofileofCD4+TcellsinSLE
Next, we investigated the functional profile of CD4+ cells
after auto-antigen-specific(HEp-2 extract)and non-specific (PMA/Io)stimuli.Thiswas accomplishedbysimultaneously determiningintracellularIL-2,IL-17andINF-␥byflow cytom-etryafterinvitrostimulationwithHEp-2extractandPMA/Io, respectively.Fig.2Ashowsthegatingstrategyandthe multi-parametriccharacterizationofmono-,bi-,andtri-functional responsepatternsinarepresentativepatientwithactiveSLE. The frequencyofthese Tcell functional subsets was then exploredinhealthycontrolsandinpatientswithactiveand inactiveSLE.
50
*
45A
B
C
D
40 35 30 25 20 15 10 5 0
0 4 5
2
30
20
10
0 20
15
10
5
0
50
**
**
**
**
***
4540 35 30 25 20 15 10 5 0
% CD4
+ T cells
Control I-SLE A-SLE
Control I-SLE A-SLE
Control I-SLE A-SLE
Control I-SLE A-SLE
% of CD4
+ CD69 + T cells
% of CD4
+ CD69 + T cells
% CD4
+ T cells
Fig.1–RelativefrequencyofCD4+TcellsinpatientswithactiveSLE,inactiveSLEandcontrols(A)andrelativefrequencyof CD4+CD69+TcellsinpatientswithactiveSLE,inactiveSLEandcontrolsinstimulationwithHEp-2(A,C)andstimulated withPMA/Io(B,D).Thebarsrepresentthemedianforeachgroup.*p<0.05;**p<0.01;***p<0.001.
frequencyinsamplesfromSLEpatientsandcontrols. How-ever,samplesfrompatientswithA-SLEandI-SLEpresented higherfrequencyofIL-17producingcellsincultures stimu-latedwithPMA/IobutnotinthosestimulatedwithHEp-2cell extract(Fig.2DandTable2).Inaddition,bi-functionalcells producingIL-17andINF-␥showedastrongtrendforhigher frequencyinPMA-stimulatedculturesfromA-SLEandI-SLE patientsascomparedwiththosefromnormalcontrols(Fig.2D andTable2).AscanbeappreciatedinFig. 2D,therewas a broadvariationinthefrequencyofmono-functionalcellsin stimulatedculturesfromSLEpatients,withseveralpatients presentingveryhighfrequencyofcellsexpressingIL-17and INF-␥.
Bi-functionalIL-2+
+CD4+TcellsproducemoreINF thanmono-functionalINF-+CD4+Tcells
Nextweaskediftheamountofcytokinesproducedby mul-tifunctionalandmono-functionalTcellswasequivalent.To evaluatethisparameterweassessedthemeanfluorescence intensity(MFI)foreachofthethreecytokinesevaluated.As canbeseeninexperimentswithPMA/Io-stimulatedsamples from controls, IFN-␥MFI in bi-functional IL-2+/INF-␥+ cells wassignificantlyhigherthaninotherTcellsubpopulations, eventhosethatproduceonlyINF-␥(Fig.3AandB).This fea-turewasequallyobservedinsamplesfromSLEpatientsand controls(Fig.3C–E).Thisphenomenonwasnotobservedin thosecultureswithoutstimulationorstimulatedwithHEp-2 cellsextracts(datanotshown).Thisbehaviorwasobserved inall36 SLEpatients and14 healthyvolunteers examined.
SuchabehaviorwasnotobservedforIL-17andIL-2inCD4+
T cells in any ofthe three groupsof individuals (datanot shown).
CorrelationbetweenthefrequencyofTh17cellsandnewly activatedcellsinpatientswithinactiveSLE
andwithCD4+TeffectorcellsinactiveSLE
There was apositive correlation betweenthe frequencyof Th17cellsandrecentlyactivatedcellsinPBMCsamplesfrom patients with I-SLE innon-stimulated cultures(Fig. 4A) or under stimulus with HEp-2 cells extract (Fig. 4B), but not whencellswerestimulatedwithPMA/Io(Fig.4C).Therewas also a positive correlation between the frequency of Th17 lymphocytes and CD4+CD25+Foxp3∅ cells in samples from
patients with active SLE either without or under different stimuli(Fig.4D–F).Incontrolsamples,therewasalsoapositive correlationbetweenthefrequencyofTh17andCD25+Foxp3∅
cells when stimulated withHEp-2 cell extract and in non-stimulatedcultures,butnotinthosestimulatedwithPMA/Io (datanotshown).Therewasnosuchcorrelationincultures ofsamplesfrompatientswithinactiveSLE(datanotshown). Therewasnocorrelationbetweentherelativefrequencyof Th17cellsandTreg cells(CD25highCD127∅Foxp3+)insamples
IL-17
IL-2 IL-17 IFN-γ
% of total CD4+ T cells that produce
cytokines
Boolean gate % of total CD4+ T cells that produce
cytokines
% of total CD4
+ T cells that produce cytokines
A-SLE
Aa
B
Ab
I-SLE
Control
A-SLE
A-SLE I-SLE
I-SLE
4%
2% 2% p < 0.5 3%
86%
86% 86%
88%
82% 85%
4% 4%
Control
Control HEp-2
PMA/lo
A-SLE I-SLE Control 0 5 10
40
30
20
10
0
15 20 0 10 20 30 40
*** *
***
* *
**
A
B
D
C
3 functions
3 functions 2 functions
2 functions
1 function
1 function
SSC CD4
CD3
CD4 FSC
R1
R3 R4 R5
R2
IL-2
IL-17 + + +
+ + –
– + +
+ – +
+ + +
+ + –
+ – +
– + +
+ – –
– + +
– – + – + + – + – + –
– + – + +
– – + +
– – + +
– – + +
– – + +
– –
–
– +
–
+ – IL-2
INF-γ
INF-γ
IL-2
Hep-2
PMA/lo
IL-17 IFN-γ
Fig.2–(A)Multi-parameterstrategyofanalysistoidentifyTh17cellsandpolyfunctionalcellsinPBMCofHCstimulated withPMA/Io.Initiallywasestablishedagatedinlymphocytepopulation(R1),thenonlythepopulationCD3+CD4+was selected(R2).FromthispopulationwereobtainedcellsexpressingIL-2(R3)IL-17(R4)andINF-␥(R5).Relativefrequencyof
CD4+Tcellsthatproducecytokines(IL-2,IL-17or
INF-␥)bystimulationwithHEp-2(B)andPMA/Io(C).Thebarsrepresent
thestandarderrorforeachgroup.(D)DiagramillustratingthebehavioroffunctionalsubsetsofCD4+TcellsfromA-SLE, I-SLEandcontrols.TheboxplotgraphrepresentstherelativefrequencyofeachCD4+Tfunctionalsubsetinthethree groupsunderthetwostimulusconditions(extractofHEp-2andPMA/Io,respectively).Theresponsepatternsaregrouped
andcolor-codedbynumberofpositivefunctionsandsummarizedinpiechartformwhereeachpieslicerepresentsthe
meanproportionofthetotalCD4+Tcellresponsecontributedbyresponsepatternsthathaveallthree(green)orany combinationoftwo(red)orone(blue)ofthemeasuredfunctions.*p<0.05;**p<0.01;***p<0.001.
Discussion
Thepurposeofthepresentstudywastoevaluatetheimmune systemofpatientswithSLE,regardingeffectorpathwaysof CD4+TcellsproducingIL-17(Th17)andmultifunctionalCD4+
Tcells.Therefore, we stimulatedPBMC cultureswith HEp-2cellextract,whichexpressesmostself-antigensofclinical importanceinSLE,includingSm/RNP,SS-A/Ro,andSS-B/La polypeptides,aswell aschromatin antigens.Thisstimulus allowedustoevaluate,amongthepoolofCD4+Tcells,those
withself-reactivecapacity,comparedtonon-stimulatedcells andwithcells stimulated withPMA/Io(polyclonal stimula-tion).TheseexperimentsshowedthatPBMCfromSLEpatients didrespondtostimulationwithauto-antigens.Furthermore, weobservedthateveninhealthyindividuals,therewereCD4+
TcellssensitivetostimulationwithHEp-2cell-derived self-antigens, but in smaller proportions compared topatients withSLE.
Inthis context,weobservedthat culturesfrom patients withactiveSLEpresentedlowerrelativefrequencyofCD4+T
cells,aspreviouslyreportedbyWoutersetal.27Ontheother hand,samplesfrompatientswithactiveandinactiveSLEhad anincreasedfrequencyofnewlyactivatedTcells(CD4+CD69+).
ThereductioninCD4+cellrelativefrequencymayberelated
totheimmunosuppressant therapyeffects,sinceseveralof the studied patientswere under immunosuppressant ther-apy.Incontrast,theincreasedfrequencyofCD4+CD69+Tcells
possiblycorrespondstothe fractionofrecentlyactivatedT cellsthatmaycontributetothemaintenanceofexacerbated autoimmuneresponseanddiseaseactivitydespitetreatment. TherewasanincreasedrelativefrequencyofCD4+Tcells
T able 2 – Rela ti v e fr equenc y of cells pr oducing cytokines in rela tion to total CD4+ T cells in cultur es stim ula ted with HEp-2 cell extr act or PMA/Io. Subpopulations Stim ulus HEp-2 cell e xtr act PMA/Io Contr ols A-SLE I-SLE p Contr ols A-SLE I-SLE p IL-2 + 0.70% (0.32–1.03) 0.99% (0.75–1.54) 1.07% (0.79–1.61) 0.245 1.09% (0.89–1.69) 1.65% (1.41–2.20) 1.90% (1.62–2.44) 0.388 IL-17 + 2.47% (0.73–5.88) 4.37% (0.63–20.90) 3.71% (0.73–25.80) 0.132 3.60% (1.09–9.66) 6.25% (1.83–25.62) 7.20% (2.51–33.20) 0.010 INF-␥ + 1.67% (0.35–6.69) 3.61% (2.46–5.55) 3.16% (2.27–9.61) 0.653 4.96% (3.56–6.37) 6.25% (5.10–8.19) 11.47% (10.58–17.92) 0.156 IL-2 +lL17 + INF-␥ + 0.17% (0.05–0.54) 0.42% (0.27–1.08) 0.48% (0.30–0.80) 0.074 0.26% (0.11–0.52) 0.47% (0.32–1.13) 0.54% (0.36–0.86) 0.526 IL-2 +lL17 + 0.27% (0.09–0.75) 0.47% (0.023–1.28) 0.49% (0.27–0.91) 0.179 0.33% (0.15–0.80) 0.71% (0.47–1.52) 0.85% (0.63–1.27) 0.422 IL-2 + INF-␥ + 0.12% (0.07–0.20) 0.16% (0.11–0.21) 0.14% (0.08–0.37) 0.279 0.54% (0.48–0.61) 0.60% (0.54–0.64) 0.94% (0.88–1.17) 0.944 IL-17 + INF-␥ + 0.17% (0.09–0.77) 0.41% (0.20–2.02) 0.94% (0.76–1.50) 0.166 0.23% (0.16–0.83) 0.66% (0.45–2.27) 1.19% (1.01–1.75) 0.087 Bold v alue is highlight significant p v alue .
takeoneffector function.Itispossiblethat thisextendsto inflammatorysitesoftargetorgans,therebycontributingto theperpetuationofinflammationatthesesites.
Thenextstepwastoevaluatetherelativefrequencyofcells abletoproduceeach cytokineindividuallyandcellsableto takeamultifunctionalphenotypebyproducingmorethanone cytokine.Infact,wefoundanincreasedrelativefrequencyof CD4+ IL-17-producingTcells(Th17lymphocytes)in
PMA/Io-stimulatedculturesfrompatientswithA-SLEandI-SLE.This findingcorroboratespreviousreportsintheliterature, show-inganincreasedfrequencyofTh17cellsandincreasedserum levelsofIL-17inSLEpatients.10,15,16,18,28–31However,we can-not exclude the participation of Th1cells and the related cytokine INF-␥intheinflammatoryprocess ofSLE.Infact, our data showthat the frequency ofIFN-␥-producing cells was consistentlyhigherinsamplesfrom SLEpatientsthan incontrolsamples,underPMA/IoorHEp-2stimulus.
Tothebestofourknowledge,thisisthefirststudyassessing therelativefrequencyofmultifunctionalTcellsinSLE.This typeofanalysisallowsaflexibledefinitionoffunctional sub-typesofTcellsthatcontrastswiththetraditionalpolarized classificationofsubtypesdefinedbysurfacemarkers.Based onthisconcept weanalyzedthemultifunctionalactivityof CD4+Tcells.Therewasnodifferenceintherelativefrequency
ofbi-functional(INF-␥+/IL-2+;IL-2+/IL-17+;IFN-␥+/IL-17+)and tri-functionalcells(INF-␥+IL-2+IL-17+)CD4+cellsinpatients withA-SLE,I-SLEandcontrols.However,whenweanalyzed thefrequencyoftri-functionalcellsrelativetoallcells produc-inganyoneoftheanalyzedcytokines,wedidfindasignificant differenceinculturesstimulatedwithPMA/Io,wheresamples frompatientswithA-SLEhadsignificantlyhigherrelative fre-quencyofmultifunctionalTcellsthanthosefromI-SLE.This findingsuggeststhatpatientswithA-SLEshowanincreased subpopulationofThelpercellsabletoproduceabroad spec-trumofpro-inflammatorycytokinesafterastrongstimulus, suchasPMA/Io.Thisincreasedsubpopulationmaycontribute totheimmunedisordersinSLEautoimmuneprocess.Another interestingfindingconcerningmultifunctionalTcellswasthe observationthatCD4+TcellsexpressingIL-2andINF-␥
pro-ducedmoreIFN-␥thandidcells thatexpressedonlyIFN-␥. Thiswasequallyobservedinsamplesfrompatientsand con-trols.Ananalogous phenomenonhasbeenshown byBetts et al.,19 who observedan increasedproductionofINF-␥by tri-functionalcellsinHIV-infectedpatients.
Therewasasignificantcorrelationbetweenthefrequency of Th17 cells and recently activated cells (CD4+CD69+) in
patients with I-SLE as well as a correlation between the frequency of Th17 cells and CD4+CD25+Foxp3∅ in patients
with A-SLE. These resultssuggest that IL-17 productionis predominantly associatedwithrecentlyactivatedTcellsin inactive-stageSLEandwithregularactivatedeffectorTcellsin active-stageSLE.ThisconceptisillustratedinFig.5.Wefound nocorrelationbetweenSLEactivity,measuredbySLEDAI,and thefrequencyofIL17-producingTcellsinpatientswithA-SLE, asalsoobservedbyWongetal.(2000).However,other stud-iesshowedsignificantcorrelationbetweenSLEDAIandTh17 frequency.16,29
INF-γ+ 386 323 564 1150 CD4 Functionality
A
B
C
D
E
INF-γ MFI INF-γ MFI INF-γ MFI INF-γ MFI
CD4 Functionality CD4 Functionality CD4 Functionality
INF-γ+IL-2+IL-17+
INF-γ+ IL-2+IL-17+ INF-γ+ IL-17+ INF-γ+ IL-2+
INF-γ
INF -γ+IL-2+IL-17+C
INF -γ+IL-2+IL-17+C
INF -γ+IL-2+IL-17+I
INF -γ+IL-2+IL-17+A
INF -γ+IL-2+I
INF -γ+IL-2+A
INF -γ+IL-17+I
INF -γ+IL-17+A
INF+IINF+A
INF -γ+IL-2+IL-17+IINF
-γ+IL-2+C
INF -γ+IL-2+I
INF -γ+IL-17+C
INF -γ+IL-17+IINF
-γ+C
INF -γ+I
INF -γ+IL-2+IL-17+AINF
-γ+IL-2+C
INF -γ+IL-2+A
INF -γ+IL-17+C
IINF -γ+IL-17+A
INF +C
INF+A
INF -γ+IL-2+
INF
-γ+IL-17+ INF
+
100 80
60 40
% of cells
20 8000 *** ** *** 6000 4000 2000 8000 6000 4000 2000 0 8000 5500 5000 4500 4000 3500 3000 2500 2000 1500 1000 500 0 6000 4000 2000 0 0 0
0 102 103 104 105
Fig.3–(A)Histogramrepresentingthemeanfluorescenceintensity(MFI)ofINF-␥afterstimulationwithPMA/Iointhe
differentfunctionalsubpopulationsofCD4+Tcellsfromarepresentativesampleofnormalcontrol.Thebluelinerepresents theMFIofcellswithonefunction,thegreenlinethreefunctionscellsandrowsoforangeandredtwofunctionscellsto INF-␥+IL-17andINF-␥+IL-2,respectively.(B)GraphrepresentingthedifferencebetweentheMFIofIFN-␥betweendifferent
functionalsubpopulationsofCD4+TcellsincontrolsbystimulationwithPMA/Io.ComparisonoftheMFIofIFN-␥by differenttypesofCD4+cellsafterstimulationwithPMA/IoamongthecontrolandA-SLE(C),controlandI-SLE(D)andI-SLE andA-SLE(E).**p<0.01;***p<0.001.
SLEandopensperspectivestothedevelopmentofalternative therapiesforthisdisease.Webelievethattheimmunological patternobservedinthepresentstudyoccursinSLEpatientsin general,butthereisconsiderableheterogeneityinthegroup.
The present study did not aim to investigate the associa-tionofthisphenomenonwiththediversemanifestationsof SLE. Thisis arelevant pointto beinvestigatedina future study speciallydesignedforthisaim. Itisconceivablethat
% of CD4+ CD69+ T cells
15 40 30 20 10 0 40 30 20 10 0 30 20 10 0 40 30 20 10 0 40 30 20 10 0 10 P=.0002
A
B
C
F
E
D
5 0 0 5 0 5 0 50 5 0
0 10 15
10 15
10 15 20 25
10 15 20 25 10 20 30
10 20 30
% of CD4+ CD69+ T cells % of CD4+ CD69+ T cells
% of Th17 cells
% of CD25
+ Foxp3
. T cells
% of CD25
+ Foxp3 . T cells
% of CD25
+ Foxp3
. T cells
% of Th17 cells
% of Th17 cells % of Th17 cells % of Th17 cells
% of Th17 cells
R=.7665 P=.006 R=.619 P=.100 R=.399 P=.010 R=.732 P=.006 R=.763 P=.023 R=.674
Fig.4–CorrelationamongtherelativefrequencyofTh17cellsandCD4+CD69+TcellsinculturesofPBMCofpatientswith I-SLEwithoutstimulation(A),culturestimulatedwithHEp-2cellsextract(B)andculturestimulatedwithPMA/Io(C)n=18.
CorrelationamongtherelativefrequencyofTh17cellsandCD4+CD25+Foxp3−inculturesofPBMCofpatientswithA-SLE
Th17 cells
Th17 cells CD69+
T cells
CD69+ T cells CD25+Foxp3φ
T cells
CD25+Foxp3φ
T cells
Active SLE Inactive SLE
Fig.5–ProposedconceptionofcorrelationamongtherelativefrequencyofTh17cellsandCD4+CD69+Tcellsinpatients withI-SLEandcorrelationamongtherelativefrequencyofTh17cellsandCD4+CD25+Foxp3−inpatientswithA-SLE.
ShowingthatthemajorityofcellsresponsibleforproductionofIL-17arethosenewlyactivatedinpatientswithI-SLE,while inpatientswithSLE-A,mostoftheIL-17producingcellsarethosechronicallyactivated.
personalizedcytokineblockingtherapyspecificallydesigned accordingtothepredominantprofileofmultifunctional effec-torTcellswillbeeffectiveinhelpingrestoretheimmunologic balanceineachpatient.IL-17-producingTlymphocytesseem toplayaprominentroleinSLEpathophysiologyandmay rep-resentapotentialtargetfortherapy.Infact,itispossiblethat cytokine-targetedandpersonalizedtherapymaycontributeto improvingthebalance ofeffectorimmuneresponse,
avoid-ing orminimizing the damagecausedbythe autoimmune
response.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. MosmannTR,CherwinskiH,BondMW,GiedlinMA,Coffman RL.TwotypesofmurinehelperTcellclone.I.Definition accordingtoprofilesoflymphokineactivitiesandsecreted proteins.JImmunol.1986;136:2348–57.
2. MosmannTR,CoffmanRL.TH1andTH2cells:different patternsoflymphokinesecretionleadtodifferentfunctional properties.AnnuRevImmunol.1989;7:145–73.
3. NakayamadaS,TakahashiH,KannoY,O’SheaJJ.HelperTcell diversityandplasticity.CurrOpinImmunol.2012;24:297–302.
4. Infante-DuarteC,HortonHF,ByrneMC,KamradtT.Microbial lipopeptidesinducetheproductionofIL-17inThcells.J Immunol.2000;165:6107–15.
5. ShinMS,LeeN,KangI.EffectorT-cellsubsetsinsystemic lupuserythematosus:updatefocusingonTh17cells.Curr OpinRheumatol.2011;23:444–8.
6. HarringtonLE,HattonRD,ManganPR,TurnerH,MurphyTL, MurphyKM,etal.Interleukin17-producingCD4+effectorT cellsdevelopviaalineagedistinctfromtheThelpertype1 and2lineages.NatImmunol.2005;6:1123–32.
7. ParkH,LiZ,YangXO,ChangSH,NurievaR,WangYH,etal.A distinctlineageofCD4Tcellsregulatestissueinflammation byproducinginterleukin17.NatImmunol.2005;6:
1133–41.
8.TesmerLA,LundySK,SarkarS,FoxDA.Th17cellsinhuman disease.ImmunolRev.2008;223:87–113.
9.CrispínJC,TsokosGC.Interleukin-17-producingTcellsin lupus.CurrOpinRheumatol.2010;22:499–503.
10.Garrett-SinhaLA,JohnS,GaffenSL.IL-17andtheTh17 lineageinsystemiclupuserythematosus.CurrOpin Rheumatol.2008;20:519–25.
11.LubbertsE.IL-17/Th17targeting:ontheroadtoprevent chronicdestructivearthritis?Cytokine.2008;41:84–91.
12.MaJ,YuJ,TaoX,CaiL,WangJ,ZhengSG.Theimbalance betweenregulatoryandIL-17-secretingCD4+Tcellsinlupus patients.ClinRheumatol.2010;29:1251–8.
13.B ˘al ˘anescuP,B ˘al ˘anescuE,T ˘an ˘asescuC,NicolauA,T ˘an ˘asescu R,GranceaC,etal.Thelper17cellpopulationinlupus erythematosus.RomJInternMed.2010;48:255–9.
14.PradoC,dePazB,GómezJ,LópezP,Rodríguez-CarrioJ,Suárez A.GlucocorticoidsenhanceTh17/Th1imbalanceandsignal transducerandactivatoroftranscription3expressionin systemiclupuserythematosuspatients.Rheumatology(Oxf). 2011;50:1794–801.
15.WongCK,HoCY,LiEK,LamCW.Elevationof
proinflammatorycytokine(IL-18,IL-17,IL-12)andTh2 cytokine(IL-4)concentrationsinpatientswithsystemiclupus erythematosus.Lupus.2000;9:589–93.
16.WongCK,LitLC,TamLS,LiEK,WongPT,LamCW.
HyperproductionofIL-23andIL-17inpatientswithsystemic lupuserythematosus:implicationsforTh17-mediated inflammationinauto-immunity.ClinImmunol. 2008;127:385–93.
17.ShahK,LeeWW,LeeSH,KimSH,KangSW,CraftJ,etal. DysregulatedbalanceofTh17andTh1cellsinsystemiclupus erythematosus.ArthritisResTher.2010;12:R53.
18.DongG,YeR,ShiW,LiuS,WangT,YangX,etal.IL-17induces autoantibodyoverproductionandperipheralblood
mononuclearcelloverexpressionofIL-6inlupusnephritis patients.ChinMedJ(Engl).2003;116:543–8.
19.BettsMR,NasonMC,WestSM,DeRosaSC,MiguelesSA, AbrahamJ,etal.HIVnonprogressorspreferentiallymaintain highlyfunctionalHIV-specificCD8+Tcells.Blood.
2006;107:4781–9.
20.DeRosaSC,LuFX,YuJ,PerfettoSP,FalloonJ,MoserS,etal. VaccinationinhumansgeneratesbroadTcellcytokine responses.JImmunol.2004;173:5372–80.
immunophenotypingbypolychromaticflowcytometry.Nat Med.2006;12:972–7.
22.HochbergMC.UpdatingtheAmericanCollegeof Rheumatologyrevisedcriteriafortheclassificationof systemiclupuserythematosus.ArthritisRheum. 1997;40:1725.
23.BombardierC,GladmanDD,UrowitzMB,CaronD,ChangCH. DerivationoftheSLEDAI.Adiseaseactivityindexforlupus patients.TheCommitteeonPrognosisStudiesinSLE. ArthritisRheum.1992;35:630–40.
24.CookRJ,GladmanDD,PericakD,UrowitzMB.Predictionof shorttermmortalityinsystemiclupuserythematosuswith timedependentmeasuresofdiseaseactivity.JRheumatol. 2000;27:1892–5.
25.KallasEG,GibbonsDC,SoucierH,FitzgeraldT,TreanorJJ, EvansTG.Detectionofintracellularantigen-specificcytokines inhumanTcellpopulations.JInfectDis.1999;179:1124–31.
26.MesquitaD,deMeloCruvinelW,AraujoJ,PucciF,SalmaziK, KallasE,etal.Systemiclupuserythematosusexhibitsa dynamicandcontinuumspectrumofeffector/regulatoryT cells.ScandJRheumatol.2011;40:41–50.
27.WoutersCH,DiegenantC,CeuppensJL,DegreefH,Stevens EA.Thecirculatinglymphocyteprofilesinpatientswith discoidlupuserythematosusandsystemiclupus erythematosussuggestapathogeneticrelationship.BrJ Dermatol.2004;150:693–700.
28.YuJJ,GaffenSL.Interleukin-17:anovelinflammatory cytokinethatbridgesinnateandadaptiveimmunity.Front Biosci.2008;13:170–7.
29.YangJ,YangX,ZouH,ChuY,LiM.Recoveryoftheimmune balancebetweenTh17andregulatoryTcellsasatreatment forsystemiclupuserythematosus.Rheumatology(Oxf). 2011;50:1366–72.
30.MokMY,WuHJ,LoY,LauCS.Therelationofinterleukin17 (IL-17)andIL-23toTh1/Th2cytokinesanddiseaseactivityin systemiclupuserythematosus.JRheumatol.2010;37: 2046–52.