w w w . r e u m a t o l o g i a . c o m . b r
REVISTA
BRASILEIRA
DE
REUMATOLOGIA
Original
article
Polymorphisms
in
NAT2
(N-acetyltransferase
2)
gene
in
patients
with
systemic
lupus
erythematosus
Elaine
Cristina
Lima
dos
Santos
a,
Amanda
Chaves
Pinto
a,
Evandro
Mendes
Klumb
b,
Jacyara
Maria
Brito
Macedo
a,∗aUniversidadedoEstadodoRiodeJaneiro(UERJ),DepartamentodeBioquímica,RiodeJaneiro,RJ,Brazil
bUniversidadedoEstadodoRiodeJaneiro(UERJ),DisciplinadeReumatologia,RiodeJaneiro,RJ,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received10June2015 Accepted23July2016
Availableonline25October2016
Keywords:
Systemiclupuserythematosus N-acetyltransferase2
Geneticpolymorphism Acetylatorphenotype Clinicalphenotypes
a
b
s
t
r
a
c
t
Objective:ToinvestigatepotentialassociationsoffoursubstitutionsinNAT2geneandof acetylatorphenotypeofNAT2withsystemiclupuserythematosus(SLE)andclinical phen-otypes.
Methods:Molecular analysis of 481C>T, 590G>A, 857G>A, and 191G>A substitutions in theNAT2genewasperformedbypolymerasechainreaction-restrictionfragmentlength polymorphism(PCR-RFLP)technique,fromDNAextractedfromperipheralbloodsamples obtainedfrompatientswithSLE(n=91)andcontrols(n=97).
Results and conclusions:The 857GA genotypewas moreprevalent among nonwhite SLE patients(OR=4.01,95%CI=1.18–13.59).The481Talleleshowedapositiveassociationwith hematologicaldisordersthatinvolveautoimmunemechanisms,specificallyautoimmune hemolyticanemiaorautoimmunethrombocytopenia(OR=1.97;95%CI=1.01–3.81).
©2016ElsevierEditoraLtda.ThisisanopenaccessarticleundertheCCBY-NC-ND license(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Polimorfismos
no
gene
NAT2
(N-acetiltransferase
2)
em
pacientes
com
lúpus
eritematoso
sistêmico
Palavras-chave:
Lúpuseritematososistêmico N-acetiltransferase2 Polimorfismogenético Fenótipoacetilador Fenótiposclínicos
r
e
s
u
m
o
Objetivo:Investigar potenciais associac¸ões de quatro substituic¸ões do gene NAT2 (N-acetiltransferase2)edofenótipoacetiladordeNAT2comolúpuseritematososistêmico (LES)eosfenótiposclínicos.
Métodos:Aanálisemoleculardassubstituic¸ões481C>T,590G>A,857G>Ae191G>Adogene NAT2foifeitacomatécnicadePCR-RFLP,usandoDNAextraídodeamostrasdesangue periféricoobtidasdepacientescomLES(n=91)econtroles(n=97).
∗ Correspondingauthor.
E-mails:jacyara@uerj.br,jacyarauerj@gmail.com(J.M.Macedo).
http://dx.doi.org/10.1016/j.rbre.2016.09.015
Resultadoseconclusões: Ogenótipo857GAfoimaisprevalenteentrepacientescomLESnão brancas(OR=4,01,IC95%=1,18-13,59).Oalelo481Tapresentouassociac¸ãopositivacomas alterac¸õeshematológicasqueenvolvemmecanismosautoimunes,especificamenteanemia hemolíticaautoimuneoutrombocitopeniaautoimune(OR=1,97;IC95%=1,01-3,81).
©2016ElsevierEditoraLtda.Este ´eumartigoOpenAccesssobumalicenc¸aCC BY-NC-ND(http://creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Systemic lupus erythematosus (SLE) is an autoimmune, chronicandsystemicsyndrome.Theinvolvementofvarious tissuesandorgansistheresultofchronicinflammatory pro-cesses generatedby the depositionof immunecomplexes, resulting from an increased production of autoantibodies. AlthoughtheetiologyofSLEisnotfullyunderstood,studies pointtoacomplexinterplaybetweengeneticand environ-mentaldeterminants.1Ultravioletradiation,drugs,infectious
agents, smoking, and various chemical agents have been describedaspotentialriskfactorsforthisdisease.2Studiesin
differentpopulationshaveshownthatsmokinghabitshave apositiveassociationwithSLE,withoddsratiosbetween1.6 and6.6.2–7InadditiontoinfluencingthedevelopmentofSLE,
thesmokinghabithasalsobeenlinkedtothemaintenance ofdiseaseactivityandtoalowerresponsetotreatmentwith antimalarialdrugs.4,8
Theassociationbetweengeneticfactorsandlupus, espe-ciallyhydralazine-inducedlupus,wasfirstassessedin1970. ArelationshipbetweenN-acetyltransferase2enzyme(NAT2), capableofacetylatingthedrug,andlupuswasobserved,with a greater proportion of slow acetylator phenotype among patientswiththedisease.9 Alowercumulativedoseof
pro-cainamidewasalsoabletoinducelupusinpatientswithslow acetylatorphenotypewhencomparedtopatients withfast acetylatorphenotype.10Inanotherstudy,inwhichthe
smok-ing habit and the phenotypeofNAT2 enzyme,involved in theacetylationofcigarettesmokecomponents,were consid-ered,thehigherrisk(2.26times)developingofSLEattributed tosmokersversusnonsmokerswasevenhigher(6.44times) whentheslowphenotypeofNAT2enzymewasobserved.2
The N-acetyltransferase enzymes (NATs) participate in the second phase of metabolism of xenobiotics, and the acetylationreactioncanoccurbytwodistinctpathways:by O-acetylation,aftertheactionofthefirst-phaseenzymeP450 (CYP450),andbyN-acetylation,whentheenzymeactsonthe compoundinaprevioussteptothe actionofCYP450.Both pathwaysmayresultintheformationofaDNAadduct.11
There are two isoforms of human N-acetyltransferase enzymes,NAT1and NAT2, which are differently expressed according to the tissue and have specificity for differ-entsubstrates.11–13 Acetylationofaromaticcompoundsand
heterocyclic amines, such as 4-aminobiphenyl, present in cigarettesmoke,14throughthetransferofacetylgroupofa
moleculeofacetyl-CoAtothefree-aminogroupofthe com-pound,ispreferablypromotedbyNAT2.14
TheNAT2isoform is encodedbythe NAT2 gene,which islocatedonchromosome8(8p22),together withtheNAT1 gene and the pseudogene NATP. The NAT2 gene is highly
polymorphicandthediversityofthedescribedallelesresults from thecombination ofpointmutationsofselectedbases (SNPs–singlenucleotidepolymorphisms).15Thephenotype
of the enzymecan bedetermined by existing SNPs in the coding regionofthe gene,whichinfluencestheaffinityfor the substrate, the catalytic activity and/or stability of the resultingprotein.16Amongthenucleotidechangescommonly
used in order to infer the acetylator phenotype of NAT2, 481C>T(rs1799929),590G>A(rs1799930),857G>A(rs1799931), and191G>A(rs1801279)standout.2,17,18
Duetotheimportanceofthetopicinquestionandthelack ofBrazilianresearch,inthisstudyweperformedan evalua-tion ofthepresenceoffoursubstitutionsintheNAT2gene (481C>T,590G>A,857G>Aand191G>A)andthephenotypeof NAT2enzymeinpatientswithsystemiclupuserythematosus residentsinthecityofRiodeJaneiro.Ourstudypopulation wasstratifiedaccordingtosmokingstatusandethnic charac-teristics.Someclinicalfeaturesofthediseasewerealsotaken intoaccountinouranalysis.
Materials
and
methods
Studypopulation
Thestudypopulationwasselectedfromagroupofwomen participatinginapreviousstudy,whichsequentiallyincluded SLEpatients(accordingtotheAmericanCollegeof Rheuma-tology classification criteria), regularly followed in the RheumatologyUnit,UERJ,andwomenwithoutlupuswhohad attendedforroutinegynecologicalcareinthesameuniversity (UERJ).19,20
Clinical and socio-demographicaspects, including infor-mation on race/ethnicity of the female participant and of their parents, by self-declaration, and smoking habits were obtainedbyapplyingasemi-structuredquestionnaire. Patients and controlswere classified aswhite-colored sub-jectswhentheindividualandhisparentswerewhite-colored byself-declaration,andasnonwhite-colorediftheindividual and/oratleastoneofherparentswasbrown-orblack-colored byself-declaration.Specificclinicaldatawereobtainedfrom medicalchartreview.
TheprojectwasapprovedbytheResearchEthics Commit-teeofHUPE/UERJ(HUPE/UERJ#321and#909),andallpatients were includedonlyaftersigninganinformedconsentform previouslyapproved.
MolecularanalysisofpolymorphismsinNAT2
481C>T, 590G>A, 857G>A and 191G>A in the NAT2 gene was carried out with the use of PCR-RFLP (Polymerase ChainReaction-RestrictionFragmentLengthPolymorphism) technique,accordingtoHuangetal.,17withsome
modifica-tions.Amplification ofgenomic DNA was performedusing the following primers: 5′-GGAACAAATTGGACTTGG-3′ and 5′-TCTAGCATGAATCACTCTGC-3′ (Life Technologies®). After
amplification, aliquots ofPCR productwere digested sepa-ratelywiththerestrictionenzymesKpnI(481C>T)andBamHI (590G>A), followed by electrophoretic analysis in agarose gel,and withMspI(857G>A) andTaqI(191G>A), followedby analysis by polyacrylamide gel electrophoresis. After elec-trophoresis,thegelswerestainedwithethidiumbromideand theproductsofdigestionwerevisualizedunderUVlight.To evaluatethereproducibilityofthetechnique,10%ofsamples wererandomlyselectedandreevaluated.
Phenotypicclassification
In this study, the bimodal classification,17 in which
inter-mediate and fast phenotypes are grouped into a single category,wasused. Thus,thepresenceand absenceofthe WTallele (absenceofthe four NAT2substitutions, 481C>T, 590G>A,857G>A,and191G>A)definethefastandslow phen-otypes,respectively.Inpractice,sampleslackingoneofthe fourchanges,or samplesheterozygousforonlyoneofthe substitutions,wereconsideredashavingfastacetylator phen-otypes.Ontheotherhand,thehomozygoussamplesinonly oneof the substitutions were classified as slow acetylator phenotype.Thedefinitionofphenotypesofsampleshaving twoormorechangeshasbeenmadeconsideringallpossible combinationsofallelesandverifyingtheirfrequencyinour population,accordingtothehaplotypes’estimationanalysis carriedoutbasedonthefrequenciesofindividual substitut-ions,asdescribedlater.Combinationscontainingatleastone rarehaplotypewithanestimatedfrequency<0.01were con-sideredunlikelyandwerediscarded.
Thesampleswithoutaresultforoneofthefour substitut-ionsanalyzedwhosemissinggenotypewouldnotinterferein thephenotypedefinedbytheotherthreesubstitutionswere includedinthestudy.
Statisticalanalysis
Student’st-testwasusedtoanalyzethedifferencesbetween meansandstandarddeviationsandwasperformedusingthe GraphPadPrism software version 6.05 (GraphPadSoftware, Inc.,SanDiego,CA).
The study population was tested for deviations from Hardy–WeinbergusingtheChi-squaredtest(2),assuminga
degreeoffreedom=1,withtheuseofSNPStatssoftware (Insti-tutCatalàd’Oncologia,Barcelona,Spain).22
Thedifferencesbetweengenotypeandallelefrequencies of groups of cases and controls were evaluated by the 2 test or Fisher’s exact test. The odds ratios (OR) and confi-denceintervals(95%CI)weredeterminedinordertoestimate the magnitudeof the association betweenNAT2 substitut-ions with the presence of systemic lupus erythematosus, using,wherepossible,co-dominant,dominant,andrecessive geneticmodels.Themostfrequentalleleandthehomozygous
genotype of this allele were considered as references. These tests were performed using GraphPad Prism ver-sion 6.05 software (GraphPad Software, Inc., San Diego, CA).
Theanalysesofinteractionsbetweengenotypesor phen-otypesofNAT2,smoking status,ethnic characteristics,and diagnosisofSLE,potentialassociationsbetweengenotypesor phenotypesofNAT2,andseveralclinicalfeaturesofSLE,as wellastheestimationofhaplotypesfromthefrequenciesof genotypescorrespondingtothefoursubstitutions,were per-formedusingSNPStatssoftware(InstitutCatalàd’Oncologia, Barcelona, Spain).22 p-Values <0.05 were considered
signifi-cant.
Results
Our study population consistedof188 participants,91 SLE patients (the casegroup) and 97 women without SLE (the controlgroup). Themean age (±standarddeviation)ofSLE patients (40.6±11.1 years; 20–69 years) and of controls (36.9±10.8years;17–66years)atthetimeofinclusioninthe studywassignificantlydifferent(p=0.0214).Althoughthe dis-tributionsbyageinbothgroupsalsohavebeenshowntobe different(p=0.0168),approximately60%ofwomenbelonging tothetwogroupswereagedbetween30and49years.The per-centagesofnon-whitewomenweredifferentbetweenthetwo studygroups,withamarginalp-value(p=0.0539).Themost importantclinicalfeaturesobservedinpatientswithSLEare showninTable1.
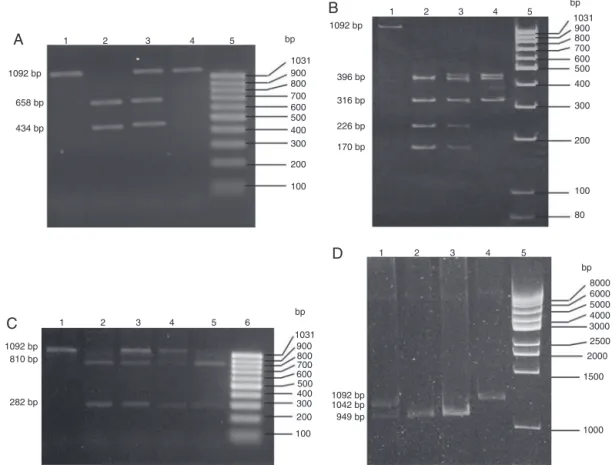
Atotalof188samplesofDNA,correspondingtoourstudy population, were assessed with respect to the four NAT2 substitutions. The digestion profiles representative ofeach analysiscanbeseeninFig.1.
ThecontrolgroupfollowedtheHardy–Weinbergprinciple with respect to the analyzed substitutions (Table 2). The genotype andallele distributionscorrespondingtothe four
Table1–Clinicalcharacteristicsofpatientswith systemiclupuserythematosusincludedinthisstudy.
Clinicaldata
Ageatonsetofsymptoms(mean±SD)(years) 27.8±10.9 Elapsedtimebetweenonsetofsymptomsand
diagnosisofSLE(mean±SD)(months)
20.0±28.3
AmericanCollegeofRheumatology(ACR)criteria(%)
Malarrash 78.0
Discoidlesions 15.4
Photosensitivity 72.5
Oralulcers 53.8
Arthritis 84.6
Pleuritis 33.0
Pericarditis 28.6
Hematologicalabnormalitiesa 39.6
Hemolyticanemia 44.6
Psychosis 14.3
Seizures 5.5
Renalimpairment(proteinuria) 78.0
ANA,positive 60.4
a Reductions of platelets, lymphocytes and leukocytes were
1
A
B
D
C
1092 bp
bp
bp bp
1031
1092 bp
396 bp
316 bp
226 bp
170 bp 900
800 700 600 500 400 300
200
100
bp
1031 900 800 700 600 500 400 300 200
100
8000 6000 5000 4000 3000 2500
2000
1500
1000 1031
900 800 700 600 500 400
300
200
100
80 658 bp
434 bp
1092 bp 1042 bp 949 bp 1092 bp
810 bp
282 bp
2 3 4 5
1 2 3 4 5
1 2 3 4 5
1 2 3 4 5 6
Fig.1–MolecularanalysisoffoursubstitutionsintheNAT2gene.(A)Photographofa2.0%agarosegelstainedwith ethidiumbromideforanalysisofthesubstitution481C>T.Lane1–negativecontrolofthedigestionreaction(intact amplicon);Lane2–representativesampleoftheNAT2genotype481CC(twoalleleswithKpnIrestrictionsite);Lane3– representativesampleoftheNAT2genotype481CT(onlyoneofthealleleswithKpnIrestrictionsite);Lane4–representative sampleoftheNAT2genotype481TT(twoalleleswithoutKpnIrestrictionsite);Lane5–molecularweightpattern
(GeneRulerTM100bpDNALadder,readytouse–Fermentas).(B)Photographofa6.0%polyacrylamidegelstainedwith ethidiumbromideforanalysisofthe590G>Asubstitution.Lane1–negativereactioncontrol(intactamplicon);Lane2– representativesampleoftheNAT2genotype590GG(twoalleleswithanadditionalTaqIrestrictionsite);Lane3–
representativesampleoftheNAT2genotype590GA(onlyoneofthealleleswithanadditionalTaqIrestrictionsite);Lane4– representativesampleoftheNAT2genotype590AA(twoalleleswithoutanadditionalTaqIrestrictionsite);Lane5– molecularweightpattern(GeneRulerTM100bpDNALadder,readytouse–Fermentas).(C)Photographofa1%agarosegel stainedwithethidiumbromideforanalysisofthe857G>Asubstitution.Lane1–negativecontrolofdigestionreaction (intactamplicon);Lanes2and5–representativesamplesoftheNAT2genotype857GG(twoalleleswithanadditionalBamHI restrictionsite);Lanes3and4–representativesamplesoftheNAT2genotype857GA(onlyoneallelewithanadditional
BamHIrestrictionsite);Lane6–molecularweightpattern(GeneRulerTM100bpDNAladder,readytouse–Fermentas).(D) Photographofa5%polyacrylamidegelstainedwithethidiumbromideforanalysisofthe191G>Asubstitution.Lane1– representativesampleoftheNAT2genotype191GA(onlyoneofthealleleswithaMsplrestrictionsite);Lanes2and3– representativesamplesoftheNAT2genotype191GG(twoallelesofanadditionalMspIrestrictionsite);Lane4–negative controlfordigestionreaction(intactamplicon);Lane5–molecularweightpattern(GeneRulerTM1kpDNALadder,readyto use–Fermentas).
substitutions of the NAT2 gene, as well as the phenotype distributions of NAT2 (Table 2) and haplotype frequencies (Table 3) showed no statistically significant differences betweencasesandcontrols.
Thestudypopulationwasdividedaccordingtoethnic char-acteristicsintwosubgroups, white andnonwhite subjects. Therewasnosignificantdifferenceintheanalysisrelatedto 481C>T,590G>A,and191G>Asubstitutions,ortothe pheno-typeofNAT2.However,withregardtothe857G>Asubstitution, the GA genotype was moreprevalent in patientswith SLE
comparedtocontrols,butonlyamongnon-whiteparticipants (OR=4.01,95%CI=1.18–13.59;p=0.023)(Table4).
Thestudy populationwasfurtherstratifiedaccordingto smokingstatus(smokersorformersmokers,and nonsmok-ers),andnoassociationofthisvariablewasfound,alongwith NAT2genotypesortheacetylatorphenotypeoftheenzyme, withthediagnosisofdisease(datanotshown).
Table2–GenotypeandalleledistributionsforeachofthefoursubstitutionsoftheNAT2geneandacetylatorphenotype incase-(patientswithSLE)andcontrolgroups.
Substitution/acetylatorphenotype Genotype/allele/phenotype Cases(n=91) Controlsa(n=97) Casesvs.controls
n(%)or(f) n(%)or(f) OR(95%CI)
NAT2481C>T CC 40(44) 34(35) 1.00
CT 39(43) 46(48) 0.72(0.39–1.35)
TT 12(13) 16(17) 0.64(0.27–1.53)
CT+TT 51(56) 62(65) 0.70(0.39–1.26)
C 119(0.65) 114(0.59) 1.00
T 63(0.35) 78(0.41) 0.77(0.51–1.18)
NAT2590G>A GG 53(59) 66(68) 1.00
GA 32(36) 27(28) 1.48(0.79–2.76)
AA 5(6) 4(4) 1.56(0.40–6.09)
GA+AA 37(41) 31(32) 1.49(0.82–2.71)
G 138(0.77) 159(0.82) 1.00
A 42(0.23) 35(0.18) 1.38(0.84–2.29)
NAT2857G>A GG 70(84) 88(93) 1.00
GA 13(16) 7(7) 2.34(0.88–6.17)
AA 0 0 N/A
GA+AA 13(16) 7(7) 2.34(0.88–6.17)
G 153(0.92) 183(0.96) 1.00
A 13(0.08) 7(0.04) 2.22(0.86–5.71)
NAT2191G>A GG 87(96) 81(91) 1.00
GA 4(4) 7(8) 0.53(0.15–1.89)
AA 0 1(1) N/A
GA+AA 4(4) 8(9) 0.47(0.14–1.61)
G 178(0.98) 169(0.95) 1.00
A 4(0.02) 9(0.05) 0.42(0.13–1.40)
Phenotype Fast 45(53) 48(53) 1.00
Slow 40(47) 43(47) 1.00(0.56–1.82)
n,numberofpatients;f,allelefrequency;N/A,notapplicable.
ThetotalsampleswithdatarelatingtothefourNAT2polymorphismsandtotheacetylatorphenotypemaydifferfromthetotalofanalyzed samples,duetotheexistenceofunsatisfactoryresults.
a Hardy–Weinbergequilibriumtest:NAT2481C>T(p=1.00);NAT2590G>A(p=0.50);NAT2857G>A(p=1.00);NAT2191G>A(p=0.19)(SNPStats).
Table3–DistributionsofhaplotypesoftheNAT2geneingroupsofcases(lupusSLEpatients)andcontrols.
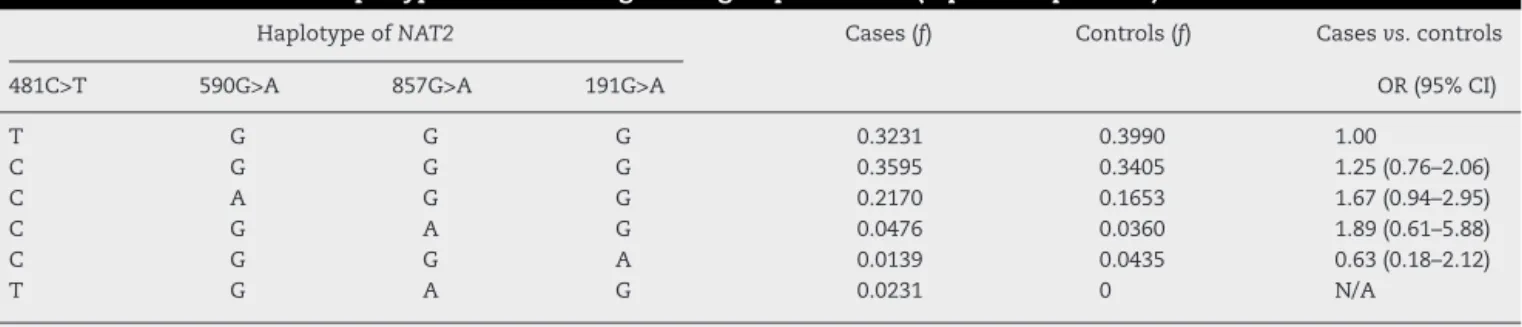
HaplotypeofNAT2 Cases(f) Controls(f) Casesvs.controls
481C>T 590G>A 857G>A 191G>A OR(95%CI)
T G G G 0.3231 0.3990 1.00
C G G G 0.3595 0.3405 1.25(0.76–2.06)
C A G G 0.2170 0.1653 1.67(0.94–2.95)
C G A G 0.0476 0.0360 1.89(0.61–5.88)
C G G A 0.0139 0.0435 0.63(0.18–2.12)
T G A G 0.0231 0 N/A
f,haplotypefrequency;N/A,notapplicable.
Haplotypeswithfrequency<0.01werenotconsidered.
lesions, photosensitivity, oral ulcers, arthritis, pleuritis, pericarditis, autoimmune hemolytic anemia, autoimmune thrombocytopenia,psychosis,seizures,proteinuria,and pos-itive ANA. The481T allele was more prevalent inpatients withhematological disorders (autoimmunehemolytic ane-mia or autoimmune thrombocytopenia), when compared to those who did not show this feature (OR=1.97; 95% CI=1.02–3.82;p=0.0477).Therewasnoassociation between the other NAT2 substitutions, or NAT2 phenotype, and clinical characteristics of the patients (data not shown).
Table4–AnalysisofinteractionbetweengenotypesrelatedtosubstitutionsintheNAT2geneorNAT2phenotype andethnicorigin,incase-(SLEpatients)andcontrolgroups.
Substitution/acetylator phenotype
Skincolor/ethnicity Genotype/phenotype Casesn(%) Controlsn(%) Casesvs.controls
OR(95%CI)
NAT2481C>T White C/C 12(36) 6(27) 1.00
C/T 19(58) 11(50) 0.86(0.25–2.95)
T/T 2(6) 5(23) 0.20(0.03–1.35)
Nonwhite C/C 28(48) 28(38) 1.00
C/T 20(35) 35(47) 0.57(0.27–1.22)
T/T 10(17) 11(15) 0.91(0.33–2.48)
NAT2590G>A White G/G 18(55) 17(77) 1.00
G/A 14(42) 4(18) 3.31(0.91–12.06)
A/A 1(3) 1(5) 0.94(0.05–16.34)
Nonwhite G/G 35(61) 49(65) 1.00
G/A 18(32) 23(31) 1.10(0.52–2.33)
A/A 4(7) 3(4) 1.87(0.39–8.87)
NAT2857G>A White G/G 27(90) 19(86) 1.00
G/A 3(10) 3(14) 0.70(0.13–3.87)
A/A 0 0 N/A
Nonwhite G/G 43(81) 69(95) 1.00
G/A 10(19) 4(5) 4.01(1.18–13.59)a
A/A 0 0 N/A
NAT2191G>A White G/G 32(97) 20(95) 1.00
G/A 1(3) 1(5) 0.62(0.04–10.57)
A/A 0 0 NA
Nonwhite G/G 55(95) 61(90) 1.00
G/A 3(5) 6(9) 0.55(0.13–2.32)
A/A 0 1(1) N/A
Phenotype White Fast 17(55) 11(52) 1.00
Slow 14(45) 11(48) 1.21(0.41–3.63)
Nonwhite Fast 28(52) 37(54) 1.00
Slow 26(48) 32(46) 0.93(0.46–1.90)
White,individualandtheparentswhite-coloredbyself-declaration;Nonwhite,anindividualand/oratleastoneoftheparentsbrownor black-coloredbyself-declaration;n,numberofpatients;N/A,notapplicable.
Significantresultisinboldletters. a Fisher’stest:p=0.0230.
Discussion
and
conclusion
N-acetyltransferase2(NAT2)variantshavebeenwidely stud-iedinordertoassessitsdistributionamongdifferentethnic groups,whichcanalsoberelatedtotheexposuretodifferent environmentalfactors;anditsassociationwithawiderangeof diseases,includingthoseofautoimmuneorigin.Inthispaper, weevaluatethepossibleexistenceofanassociationbetween the481C>T,590G>A,857G>Aand191G>Apolymorphismsin theNAT2geneandsystemiclupuserythematosus.
The studies of an association between NAT2 and SLE describedintheliterature,ingeneral,considertheacetylator phenotypeand notNAT2 polymorphisms inisolation. Tak-inginto accountthatthesubstitutionshere discussedmay interfere withthe levels of gene expressionthrough post-transcriptionalregulationmechanisms(481C>T),changethe stabilityoftheenzyme(590G>Aand191G>A),ormodifythe selectivityand catalytic activity (857G>A),16 an association
analysiswasalsoperformedwitheach ofthesubstitutions inisolation.However,ourresultsshowedanabsenceofan associationbetweenthefourNAT2substitutions,ortheNAT2 acetylatorphenotypeandthedisease(Table1).Theestimateof
haplotypesalsoshowednoassociationwithSLE(Table2),even thoughaidinginthephenotypicclassificationofthesamples, asmentionedabove.Recentstudieshavealsousedthisfeature toinferNAT2phenotypesbasedongenotypingdata.23,24
ThepolymorphismsanalyzedinNAT2genehavedistinct allelic distributions among different populations. Sabbagh etal.conductedaworldwidesurveyofthefrequencyof sev-eralNAT2polymorphismsandalsooftheirphenotype.25The
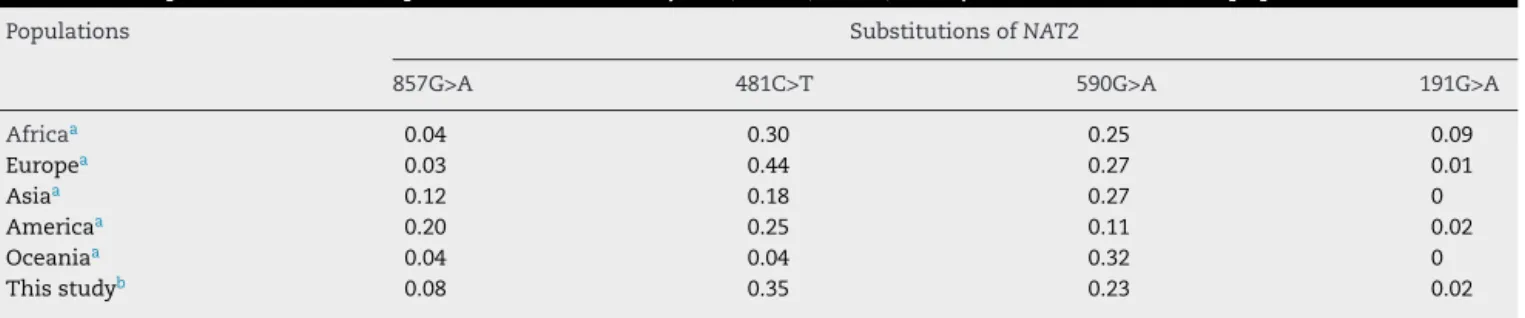
Table5–FrequenciesoflessfrequentallelesofNAT2(481T,590A,857A,191A)observedinvariouspopulations.
Populations SubstitutionsofNAT2
857G>A 481C>T 590G>A 191G>A
Africaa 0.04 0.30 0.25 0.09
Europea 0.03 0.44 0.27 0.01
Asiaa 0.12 0.18 0.27 0
Americaa 0.20 0.25 0.11 0.02
Oceaniaa 0.04 0.04 0.32 0
Thisstudyb 0.08 0.35 0.23 0.02
a Sabbaghetal.,2011.Controldataspecificforeachofdiseasemodelsincludedinthecasegroup.
b Patientsfromcontrolgroup.
thissamepopulation,itwasobservedthatwhiteindividuals, throughself-declaration,had86%ofEuropeanancestryand 13%ofAfricanorAmerindianancestry.Ontheotherhand,the patientsself-declaredasnonwhites,ofbrownorblackcolor, had24%and49%ofAfricanancestryand67%and43%of Euro-peanancestry, respectively.27 These datarevealthe greater
degreeofmiscegenationpresentinthoseself-declaredasof brownandblackcolor.Thehighdegreeofethnic heterogene-ityofourstudypopulationcouldexplaintheclose,but not identical,valuestothoseobservedinotherpopulations.
Inthe literature,thereare reportson theassociation of NAT2 polymorphisms with various diseases, such as dif-ferent types of cancer,28 periodontitis,29 and autoimmune
diseases,3,30,31 whoadoptthesmokinghabitasan
environ-mentalriskfactor.29,32Anassociationbetweentheacetylator
phenotypeofNAT2,smokingandSLEwasalreadyobserved.2
However,in the Brazilianpopulation, there isno study on the association of polymorphisms in NAT2 gene and/or of acetylatorphenotypewiththisdisease,especiallyin smok-ers.Althoughseveralauthorsconsiderthattheexposureto tobaccocomponentsmayconstitutea“trigger”forthe devel-opmentofSLE,2–7 wehadaccesstoinformationonsmoking
habitduringthecollectionofbiologicalsamples,andnotat thetimeofdiseaseonset.Therefore,ourstudypopulationwas subdividedintotwogroups,accordingtothesmokinghabit,as smokersorex-smokersandnonsmokers.Ourresultsshowed noassociationbetweenthesevariablesandthedisease(data notshown).Itisworthnotingthat,atthetimeapatientis diag-nosed,shereceivesguidancetoabstainfromsmoking,which mayhavecreatedabiasintheanalysis.Infact,fewersmokers andformersmokerswereobservedinthegroupofcases,a factthatwasnotrelatedtoaprotectionofsmokinghabitsin relationtodiseasedevelopment.Itshouldbenotedthat addi-tionalsmokingdata,includingexposuretimeandthenumber ofpacks ofcigarettes/day,atthefirst indicationofdisease manifestation,couldcontribute totheanalysiscarried out, takingintoaccountthepossibilityofthedifferential metabo-lizationofvariouscomponentsofcigarettesmoke,depending ontheacetylatorphenotype.However,thetimeofonsetofthe diseaseisacomplexaspecttobeestablished.Itisfrequentthe occurrenceofaperiodofseveralmonthsbetweentheonset ofsymptomsanddiseasediagnosis.
BesidesthedifferenceinthefrequencyofNAT2 polymor-phismsindifferentpopulations,theincidenceofSLEishigher inethnicallymixedpatients.5Thus,inthisstudy,weevaluated
whethertherewasanassociationbetweenthedistributionof
NAT2polymorphismsandSLEpatientswiththisethnic fea-ture.Withthisinmind,self-declarationsandinformationon theparent(fatherandmother)skincolorwereusedtodefine theethnicoriginofthepatients,asreportedintheliterature,21
butweacknowledgethatothermethodscouldbemore appro-priatetothis end.Significantresultswereobservedonlyin relationtotheNAT2857G>Apolymorphism,withthegenotype 857GA representingapotentialriskforthe diseasein non-whitewomen(Table4).TheNAT2857G>Asubstitutionhasas aconsequence,intheprotein,thechangeoftheaminoacid glycinebyglutamate(G286E).Thischangemodifiestheaccess totheactivesiteoftheenzyme,whichresultsinreduced selec-tivity and catalyticcapacity.16 Therefore, the NAT2857G>A
polymorphismisrelatedtothereductioninthemetabolismof aromaticandheterocyclicamines.16Thesubstitution857G>A
ispresentin11NAT2alleles,twoofwhichhaveaslow acety-lator phenotype,and theotheroneshavenotyethadtheir phenotypesdefined.33 Theslowacetylatorphenotypecould
conferanincreasedriskforSLE,sincethebodywouldremain exposed fora longer time, forexample, tocomponents of cigarette smoke. It is worth noting that the variables eth-nicoriginandNAT2857G>Agenotypeswerenotindividually associatedwithSLE.Nevertheless,theliteraturepointstoa higherincidenceofSLEinethnicallymixedindividuals,1and,
inapopulationofthecityofIlheus(Bahia/Brazil),the857GA genotype wasmoreprevalent inAfrican-Brazilian subjects, comparedtowhitesandAmerindians.34Theancestryofthe
RiodeJaneiropopulationissimilartothoseestimatedvalues forthepopulationofBrazilianNortheast.26,35However,ithas
beensuggestedthatlargeurbancenters,likeRiodeJaneiro, presentmorediversepatternsofethnicmixture.26
Theorgansaffectedandtheintensityofclinical manifes-tationsinSLEpatientscharacterizetheseverityofthedisease and somehowguidethe therapeuticschemeselection.The only significant resultin the analysis ofthe interaction of theNAT2polymorphismsoroftheacetylatorphenotypewith clinical features of SLE was the greater prevalence of the allele481Tinpatientswithhematologicdiseases,specifically autoimmunehemolyticanemiaorautoimmune thrombocy-topenia(datanotshown).Thereductionofthesecelltypes may beassociatedwiththe immunosuppressioncausedby theseverityofthediseaseorbytheuseof immunomodula-torydrugs.36 Sofar,thereisnoevidenceastothewaythe
However,when consideringonlythe smoking habit, we found a correlation with the presence of discoid lupus (OR=8.62, 95% CI 2.40–30.96; p=0.0011) and proteinuria (OR=0.17, 95% CI 0.05–0.59; p=0.0056). In several studies, dermatologic alterationspresent inpatients withSLEwere associatedwithsmokingand,aspreviouslymentioned,the treatmentoftheselesionsinsmokersseemstorequirehigher dosesofantimalarials,comparedtononsmokers.37–39Inour
study,thesmall numberofpatientswithSLEand smokers (n=7)didnotallowustoinferifsmokinghabitreallyhadan impactonthepharmacologicalapproach.Thesmokinghabit isalsoassociatedwithrenaldisorders,suchas proteinuria andnephropathy40;however,inthisstudy,from71patients
withproteinuria,only7are currentsmokers,13areformer smokers,andmostwerelifetimenonsmokers.
IntheBrazilianpopulation,therearefewstudieson geno-typeandalleledistributionsofNAT2,andtherearenoreports abouttheassociation ofNAT2polymorphisms andSLE.We believethatourworkhasaleadingroleinthisarea,andwe understandit asaformulatorofthehypothesisofa poten-tialroleofNAT2polymorphismsasafactorassociatedwith thediseaseinourcountry,andofapossibleassociationwith specificclinicalphenotypes.However,furtherstudies involv-inglargerpopulationsareneeded,toconfirmtheassociation betweentheNAT2857G>Apolymorphismandsystemiclupus erythematosusinself-declarednon-whiteBrazilianwomen, ideally including ethnic profiling with the use ofancestry markers.
Funding
ThisstudyreceivedfinancialsupportfromCNPq–National Council for Scientific and Technological Development (476116/2009-0), FAPERJ – Research Support Foundation of the State of Rio de Janeiro (E-26/170.922/2004 and E-26/111.334/2011),UERJ–StateUniversityofRiodeJaneiro,and CERPE–CentreofStudiesinRheumatologyPedroErnesto,Rio deJaneiro.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
1. SatoEI.Lúpuseritematososistêmico;2008.Availableat:
http://www.fmrp.usp.br/cg/novo/images/pdf/conteudo disciplinas/lupuseritematoso[accessed11.09.13]. 2. KiyoharaC,WashioM,HonuchiT,TadaY,AsamiT,IdeS,
etal.Cigarettesmoking,N-acetyltransferase2
polymorphismsandsystemiclupuserythematosusina Japanesepopulation.Lupus.2009;18:630–8.
3. KiyoharaC,WashioM,HoriuchiT,AsamiT,IdeS,AtsumiT, etal.Cigarettesmoking,alcoholconsumption,andriskof systemiclupuserythematosus:acase–controlstudyina Japanesepopulation.JRheumatol.2012;39:1363–70.
4. WallaceDJ.Principlesoftherapyandlocalmeasures.In: WallaceDJ,HahnBH,editors.Dubois’lupuserythematosus.
7thed.Philadelphia:LippincottWilliams&Wilkins;2007.p. 1132–44.
5.CostenbaderKH,KimDJ,PeerzadaJ,LockmanSL,
Nobles-KnightD,PetriM,etal.Cigarettesmokingandtherisk ofsystemiclupuserythematosus:ameta-analysis.Arthritis Rheum.2004;50:849–57.
6.FreemerMM,KingTEJr,CriswellLA.Associationofsmoking withdsDNAautoantibodyproductioninsystemiclupus erythematosus.AnnRheumDis.2006;65:581–4.
7.WashioM,HoriuchiT,KiyoharaC,KodamaH,TadaY,Asami T,etal.Smoking,drinking,sleepinghabits,andotherlifestyle factorsandtheriskofsystemiclupuserythematosusin Japanesefemales:findingsfromtheKYSSstudy.Mod Rheumatol.2006;16:143–50.
8.EzraN,JorizzoJ.Hydroxychloroquineandsmokinginpatients withcutaneouslupuserythematosus.ClinExpDermatol. 2012;37:327–34.
9.PerryHMJr,TanEM,CarmodyS,SakamotoA.Relationshipof acetyltransferaseactivitytoantinuclearantibodiesandtoxic symptomsinhypertensivepatientstreatedwithhydralazine. JLabClinMed.1970;76:114–25.
10.WoosleyRL,DrayerDE,ReidenbergMM,NiesAS,CarrK, OatesJA.Effectofacetylatorphenotypeontherateatwhich procainamideinducesantinuclearantibodiesandthelupus syndrome.NEnglJMed.1978;298:1157–9.
11.WindmillKF,GaedigkA,HallPM,SamaratungaH,GrantDM, McManusME.LocalizationofN-acetyltransferasesNAT1and NAT2inhumantissues.ToxicolSci.2000;54:19–29.
12.MeiselP,GiebelJ,PetersM,FoersterK,CascorbiI,WulffK, etal.ExpressionofN-acetyltransferasesinperiodontal granulationtissue.JDentRes.2002;81:349–53.
13.MitchellKR,WarshawskyD.Xenobioticinducibleregionsof thehumanarylamineN-acetyltransferase1and2genes. ToxicolLett.2003;139:11–23.
14.WuH,DombrovskyL,TempelW,MartinF,LoppnauP, GoodfellowGH,etal.Structuralbasisofsubstrate-binding specificityofhumanarylamineN-acetyltransferases.JBiol Chem.2007;282:30189–97.
15.García-MartinE.Interethnicandintraethnicvariabilityof
NAT2singlenucleotidepolymorphisms.CurrDrugMetab. 2008;9:487–97.
16.RajasekaranM,AbiramiS,ChenC.Effectsofsinglenucleotide polymorphismsonhumanN-acetyltransferase2structure anddynamicsbymoleculardynamicssimulation.PLoSONE. 2011;6:e25801.
17.HuangC,ChernH,ShenC,HsuS,ChangK.Association betweenN-acetyltransferase2(NAT2)geneticpolymorphism anddevelopmentofbreastcancerinpost-menopausal ChinesewomenTaiwan,anareaofgreatincreaseinbreast cancerincidence.IntJCancer.1999;82:175–9.
18.HeinDW,DollM,FretlandAJ,LeffMA,WebbSJ,XiaoGH,etal. MoleculargeneticsandepidemiologyoftheNAT1andNAT2
acetylationpolymorphisms.CancerEpidemiolBiomarkers Prev.2000;9:29–42.
19.KlumbEM,AraújoMLJr,JesusGR,SantosDB,OliveiraAV, AlbuquerqueEM,etal.Ishigherprevalenceofcervical intraepithelialneoplasiainwomenwithlupusdueto immunosuppression?JClinRheumatol.2010;16:153–7.
20.KlumbEM,PintoAC,JesusGR,AraujoMJr,JasconeL,Gayer CR,etal.ArewomenwithlupusathigherriskofHPV infection.Lupus.2010;19:1485–91.
21.Vargas-TorresSL,PortariEA,KlumbEM,GuillobelHCR, CamargoMJ,RussomanoFB,etal.AssociationofCDKN2A polymorphismswiththeseverityofcervicalneoplasiaina Brazilianpopulation.Biomarkers.2014;19:121–7.
22.SoléX,GuinóE,VallsJ,IniestaR.,MorenoV.SNPStats:your webtoolforSNPanalysis.Availableat:
23.SinglaN,GuptaD,BirbianN,SinghJ.AssociationofNAT2,
GSTandCYP2E1polymorphismsandanti-tuberculosis drug-inducedhepatotoxicity.Tuberculosis.2014;94:293–8.
24.SelinskiS,BlaszkewiczM,IckstadtK,HengstlerJG,GolkaK. RefinementofthepredictionofN-acetyltransferase2(NAT2) phenotypeswithrespecttoenzymeactivityandurinary bladdercancerrisk.ArchToxicol.2013;87:2129–39.
25.SabbaghA,DarluP,Crouau-RoyB,PoloniES.Arylamine N-acetyltransferase2(NAT2)geneticdiversityandtraditional subsistence:aworldwidepopulationsurvey.PLoSONE. 2011;6:e18507.
26.MantaFSN,PereiraR,ViannaR,deAraújoARB,GitaíDLG, SilvaDA,etal.RevisitingthegeneticancestryofBrazilians usingautosomalAIM-Indels.PLoSONE.2013;8:e75145.
27.PenaSDJ,DiPietroG,Fuchshuber-MoraesM,GenroJP,Hutz MH,KehdyFSG,etal.Thegenomicancestryofindividuals fromdifferentgeographicalregionsofBrazilismoreuniform thanexpected.PLoSONE.2011;6:e17063.
28.HeinDW.N-acetyltransferase2geneticpolymorphism: effectsofcarcinogenandhaplotypeonurinarybladder cancerrisk.Oncogene.2006;25:1649–58.
29.MeiselP,TimmR,SawafH,FanghãnelJ,SiegmundW,Kocher T.PolymorphismoftheN-acetyltransferase(NAT2),smoking andthepotentialriskofperiodontaldisease.ArchToxicol. 2000;74:343–8.
30.KlareskogL,PadyukovL,AlfredssonL.Smokingasatrigger forinflammatoryrheumaticdiseases.CurrOpinRheumatol. 2007;19:49–54.
31.MikulsTR,LevanT,GouldKA,YuF,ThieleGM,BynoteKK, etal.ImpactofinteractionsofcigarettesmokingwithNAT2
polymorphismsonrheumatoidarthritisriskinAfrican Americans.ArthritisRheum.2012;64:655–64.
32.RouissiK,OuerhaniS,MarrakchiR,BenSlamaMR,SfaxiM, AyedM,etal.Combinedeffectofsmokingandinherited
polymorphismsinarylamineN-acetyltransferase2, glutathioneS-transferasesM1andT1onbladdercancerina Tunisianpopulation.CancerGenetCytogenet.2009;190:101–7.
33.BoukouvalaS,HeinDW,GrantDM,SimE,MinchinRF, AgúndezJAG,etal.ThearylamineN-acetyltransferasegene nomenclaturecommittee.DemocritusUniversityofThrace. Availableat:
http://nat.mbg.duth.gr/Human%20NAT2%20alleles2013.htm
[accessed26.11.14].
34.TalbotJ,MagnoLAV,SantanaCVN,SousaSMB,MeloPR, CorreaRX,etal.InterethnicdiversityofNAT2polymorphisms inBrazilianadmixedpopulations.BMCGenet.2010;11:87.
35.MagalhãesdaSilvaT,SandhyaRaniMR,deOliveiraCostaGN, FigueiredoMA,MeloPS,NascimentoJF,etal.Thecorrelation betweenancestryandcolorintwocitiesofNortheastBrazil withcontrastingethniccompositions.EurJHumGenet. 2015;23:984–9.
36.BashalF.Hematologicaldisordersinpatientswithsystemic lupuserythematosus.OpenRheumatolJ.2013;7:87–95.
37.DutzJ,WerthVP.Cigarettesmokingandresponseto antimalarialsincutaneouslupuserythematosuspatients: evolutionofadogma.JInvestDermatol.2011;131:1968–70.
38.PietteEW,FoeringKP,ChangAY,OkawaJ,TenHaveTR,Feng R,etal.Impactofsmokingincutaneouslupus
erythematosus.ArchDermatol.2011;148:317–22.
39.Bourré-TessierJ,PeschkenCA,BernatskyS,JosephL,Clarke AE,FortinPR,etal.Associationofsmokingwithcutaneous manifestationsinsystemiclupuserythematosus.Arthritis CareRes(Hoboken).2013;65:1275–80.