Marco Alexandre Figueira Rodrigues Guerreiro
Licenciatura em Biotecnologia
Unveiling the mating system and genetic variability
in the yeast Kwoniella mangroviensis
using molecular approaches
Dissertação para obtenção do Grau de Mestre em Genética Molecular e Biomedicina
Orientador: Prof. Doutor Álvaro Luís A. M. R. Fonseca,
Professor Auxiliar, FCT/UNL
Júri:
Presidente: Prof. Doutor José Paulo Nunes de Sousa Sampaio Arguente: Prof. Doutor Artur Jorge da Costa Peixoto Alves
Marco Alexandre Figueira Rodrigues Guerreiro
Licenciatura em Biotecnologia
Unveiling the mating system and genetic variability
in the yeast Kwoniella mangroviensis
using molecular approaches
Dissertação para obtenção do Grau de Mestre em Genética Molecular e Biomedicina
Orientador: Prof. Doutor Álvaro Luís A. M. R. Fonseca,
Professor Auxiliar, FCT/UNL
Júri:
Presidente: Prof. Doutor José Paulo Nunes de Sousa Sampaio Arguente: Prof. Doutor Artur Jorge da Costa Peixoto Alves
Unveiling the mating system and genetic variability in the yeast Kwoniella mangroviensis using molecular approaches
Copyright Marco Alexandre Figueira Rodrigues Guerreiro, FCT/UNL, UNL
Part of the results discussed in this thesis was presented in:
i
ACKNOWLEDGMENTS
I wish to express my deep gratitude to my supervisor, Prof. Álvaro Fonseca, for accepting me as a master student; the opportunity to work with him; the knowledge shared over these months; for the guidance, the wise advice, the support, the trust, the patience, the motivation and for everything else.
I would also like to thank: Prof. João Almeida (CREM) for support in bioinformatics issues and advice during this work; Dr. Deborah Springer (Duke University) for providing strains crucial for this work and some DNA sequences; and CREM for providing the technical support and conditions required for the practical work.
I would like to thank Cláudia Carvalho (PYCC/CREM) for the friendship and companionship, all the technical support, all the conversations, advice, laughter, kindness and for helping me in every single difficulty that I had.
A special thanks is owed to Dr. Andrey Yurkov (CREM) for the friendship, for all the advice, tips and suggestions, support in bioinformatics analyses, the endless discussions, the knowledge and all the stories shared during coffee breaks.
I would like to thank my lab colleagues at CREM for technical advice and for providing a healthy and joyful environment to work in.
I must also mention my family for all the support over the years, specially my mother for all the education and values transmitted. My friends who always supported me, Neuza Sousa, the drama solver and for making everything more bearable, Pedro Penedo, for everything done and also Miguel Lopes and Hélio Fazenda for making life a little bit more funnier and glamorous.
iii
ABSTRACT
In fungi, sexual reproduction is orchestrated by genomic regions known as mating type loci (MAT), which can be defined by two physically unlinked sex determining regions (tetrapolar mating system) or a single locus (bipolar system). Kwoniella mangroviensis is a saprobic basidiomycetous yeast, belonging to the Kwoniella clade of the order Tremellales, which was described as possessing a bipolar mating-system, similar to the related pathogenic species Cryptococcus neoformans and
Cryptococcus gattii of the sister Filobasidiella clade. Studies aimed at elucidating the evolution of the
MAT locus of these two Cryptococcus species of clinical importance, targeted several related saprobic
species in the Kwoniella and Filobasidiella clades. An evolutionary model ensuing from those studies suggests that the tetrapolar mating-systems found in most species are ancestral and gave rise to the bipolar mating-system of C. neoformans by chromosomal rearrangements and fusion events.
The present study comprised strains from the original work describing K. mangroviensis, as well as additional isolates from plant substrates in Europe and Africa. A multilocus sequence typing approach revealed genetic variability among those strains and led to the identification of two novel species closely related to K. mangroviensis: Kwoniella sp. A and Kwoniella sp. B. The mating system of K. mangroviensis and sibling species was further explored by a genetic approach based on sequencing of two MAT genes: STE20 and the divergently transcribed genes SXI1 and SXI2. The results obtained demonstrated tetrapolar mating systems in K. mangroviensis, as well as in Kwoniella sp. A and Kwoniella sp. B. Additionally, the MAT locus structure of K. mangroviensis was unveiled by sequencing a 43 kb genomic region containing the STE20 gene. Twelve genes also present in the MAT loci of related species were identified, and full synteny was found between K. mangroviensis and
Cryptococcus heveanensis, a distant member of the Kwoniella clade.These findings provided novel
insights into the evolution of MAT loci in basidiomycetous yeasts in the Tremellales.
v
RESUMO
A reprodução sexuada em fungos é regulada por regiões genómicas conhecidas como loci
MAT, que podem ser definidos por duas regiões fisicamente separadas (sistema de compatibilidade
tetrapolar) ou um único locus (sistema bipolar). Kwoniella mangroviensis é uma levedura basidiomiceta saprófita, pertencente ao clado Kwoniella da ordem Tremellales, descrita originalmente como possuindo um sistema bipolar, similar ao das espécies patogénicas afins Cryptococcus
neoformans e Cryptococcus gattii do clado Filobasidiella. Devido à relevância clínica destas duas
espécies de Cryptococcus, foram realizados estudos para compreender o processo evolutivo das suas regiões MAT em espécies saprófitas afins. Foram encontrados sistemas tetrapolares em todas as espécies estudadas o que sugere que estes sistemas são ancestrais e terão dado origem ao sistema bipolar de C. neoformans através de rearranjos cromossómicos e fusões.
O estudo descrito nesta tese compreendeu estirpes do trabalho original que descreveu K.
mangroviensis bem como novos isolados de substratos vegetais na Europa e África. Uma abordagem
de sequenciação multilocus demonstrou variabilidade genética entre as estirpes estudadas e levou à identificação de duas novas espécies próximas de K. mangroviensis: Kwoniella sp. A e Kwoniella sp. B. O sistema de compatibilidade de K. mangroviensis foi estudado com base na sequencição de dois genes MAT: STE20 e o par SXI1 e SXI2. Os resultados obtidos demonstraram a existência de sistemas tetrapolares em K. mangroviensis bem como em Kwoniella sp. A e Kwoniella sp. B. A estrutura do
locusMAT de K. mangroviensis foi parcialmente revelada por sequenciação dum fragmento genómico
de 43 kb contendo o gene STE20. Foram identificados 12 genes também presentes nos loci MAT de espécies afins e foi encontrada uma sintenia quase completa entre K. mangroviensis e Cryptococcus
heveanensis, membro mais distante do clado Kwoniella. Estes resultados forneceram novas pistas
valiosas sobre a evolução dos lociMAT em leveduras basidiomicetes da ordem Tremellales.
vii
TABLE OF CONTENTS
INDEX OF FIGURES ... ix
INDEX OF TABLES ... xi
LIST OF ABBREVIATIONS ... xiii
1 - INTRODUCTION ... 1
1.1 - Fungi and Basidiomycetes ... 1
1.2 - Looking for sexual reproduction in Fungi ... 1
1.3 - Basidiomycete life-cycle and Heterothallism vs. Homothallism ... 3
1.4 - Bipolar and Tetrapolar Mating Systems ... 4
1.5 - Mating type loci in the Basidiomycota ... 6
1.5.1 - Pheromone and Pheromone Receptors ... 6
1.5.2 - Homeodomain Transcription Factors ... 7
1.6 - Cryptococcus species complex ... 8
1.7 - Evolution of MAT in the Filobasidiella clade ... 12
1.7.1 - Cryptococcus amylolentus and Tsuchiyaeawingfieldii ... 12
1.7.2 - Filobasidielladepauperata ... 14
1.8 - Evolution of MAT in the Kwoniella clade ... 15
1.8.1 - Cryptococcus heveanensis... 15
1.9 - Evolution of MAT in the Tremella clade ... 17
1.9.1 - Tremella mesenterica ... 17
1.10 - MAT gene content and organization comparison ... 18
1.11 - Evolutionary model revision ... 22
1.12 - Kwoniella mangroviensis ... 23
1.13 - Unveiling the mating system and genetic variability in Kwoniella mangroviensis using molecular approaches... 24
2 - MATERIALS AND METHODS ... 25
2.1 - Yeast cultures ... 25
2.2 - Physiological Tests... 25
2.3 - Mating Assays ... 25
2.4 - DNA Extraction ... 27
2.5 - Fosmid Extraction ... 27
2.6 - PCR Fingerprinting ... 28
2.7 - Primers and Primer design ... 28
2.8 - PCR amplification ... 30
2.9 - Electrophoresis ... 32
2.10 - Amplification Product Purification and Sequencing ... 32
2.11 - Sequence data analysis ... 33
3 - RESULTS ... 35
3.1 - Molecular identification and phylogenetic diversity in Kwoniella mangroviensis ... 35
3.2 - Mating experiments ... 39
3.3 - M13 PCR fingerprinting ... 40
3.4 - Physiological Tests... 41
3.5 - Multilocus Phylogenetic Analyses ... 42
viii
3.5.2 - RPB2 phylogenetic analysis ... 44
3.5.3 - TEF1α phylogenetic analysis ... 46
3.5.4 - MCM7 phylogenetic analysis ... 48
3.5.5 - Concatenated sequence analyses ... 50
3.6 - Distance Analysis ... 52
3.7 - MAT gene analyses ... 53
3.7.1 - STE20 gene (P/R locus) ... 53
3.7.2 - SXI genes (HD locus) ... 55
3.7.3 - Molecular mating-types... 55
3.8 - Gene content and organization of the P/R region of K. mangroviensis ... 57
3.8.1 - K1 fosmid sequencing ... 57
3.8.2 - Synteny analysis ... 57
3.8.3 - Pheromone precursor analysis ... 58
3.8.4 - Phylogenetic patterns of genes within the P/R region ... 59
4 - DISCUSSION ... 61
4.1 - Reassessment of species boundaries of Kwoniella mangroviensis... 61
4.2 - Phylogeny and taxonomic status of species in the Kwoniella and Filobasidiella clades ... 62
4.3 - Mating experiments and molecular mating-types ... 63
4.4 - Tetrapolar mating-system in K. mangroviensis and sibling species ... 64
4.5 - Strain K9 ... 65
4.6 - MAT evolution in the Tremellales ... 66
4.7 - Conclusions and future perspectives ... 67
5 - REFERENCES ... 69
ix
INDEX OF FIGURES
Figure 1.1 - Life cycle of the basidiomycetous yeast Cryptococcus neoformans ... 4
Figure 1.2 - Fungal MAT Locus Paradigms ... 5
Figure 1.3 - Pheromone-activated MAPK transduction pathway in C. neoformans ... 7
Figure 1.4 - Phylogenetic relationships among selected members of the Tremellales ... 8
Figure 1.5 - MAT structure of C. neoformans var. neoformans, C. neoformans var. grubii and C. gattii ... 9
Figure 1.6 - Original model of MAT evolution ... 11
Figure 1.7 - MAT loci of Cryptococcus amylolentus CBS 6039 ... 12
Figure 1.8 - MAT loci of Tsuchiyaea wingfieldii CBS 7118 ... 12
Figure 1.9 - Fragment of MAT locus of Filobasidiella depauperata CBS 7855 ... 14
Figure 1.10 - MAT loci of Cryptococcus heveanensis CBS 569 ... 15
Figure 1.11 - Percent sequence identity plots of SXI1 and SXI2 ... 16
Figure 1.12 - MAT loci of Tremella mesenterica ATCC 24925 ... 17
Figure 3.1 - Phylogenetic tree of K. mangroviensis strains and related species in the Kwoniella and Filobasidiella clades based on LSU sequences ... 37
Figure 3.2 - Phylogenetic tree of K. mangroviensis strains and related species in the Kwoniella and Filobasidiella clades based on concatenated LSU and ITS sequences ... 38
Figure 3.3 - Fingerprinting profiles of the Kwoniella mangroviensis isolates used in the study ... 40
Figure 3.4 - Phylogenetic tree of K. mangroviensis strains and related species in the Kwoniella and Filobasidiella clades based on RPB1 sequences ... 43
Figure 3.5 - Phylogenetic tree of K. mangroviensis strains and related species in the Kwoniella and Filobasidiella clades based on RPB2 sequences ... 45
Figure 3.6 - Phylogenetic tree of K. mangroviensis strains and related species in the Kwoniella and Filobasidiella clades based on TEF1α sequences ... 47
Figure 3.7 - Phylogenetic tree of K. mangroviensis strains and related species in the Kwoniella and Filobasidiella clades based on MCM7 sequences ... 49
Figure 3.8 - Phylogenetic relationships among members of the Kwoniella and Filobasidiella clades based on a combined data set of concatenated sequences of six genomic loci (LSU, ITS, RPB1, RPB2, TEF1α and MCM7) ... 51
Figure 3.9 - Jukes-Cantor distances comparisons based on five concatenated gene sequences ... 52
Figure 3.10 - Phylogenetic tree of K. mangroviensis strains and related species in the Kwoniella and Filobasidiella clades based on STE20 partial sequence ... 54
Figure 3.11 - Phylogenetic tree of K. mangroviensis strains and related species in the Kwoniella and Filobasidiella clades based on SXI1, SXI2 partial sequences and their intergenic region ... 56
Figure 3.12 - Kwoniella mangroviensis P/R locus fragment ... 57
Figure 3.13 - Synteny analysis... 58
Figure 3.14 - Sequence analysis of the pheromone precursor protein ... 59
Figure 3.15 - Phylogenetic analyses of MAT genes ... 60
xi
INDEX OF TABLES
Table 1.1 - Summary of the MAT region gene content and phylogenetic pattern of MAT genes in the
studied species ... 19
Table 1.2 - Summary of previous mating experiments ... 24
Table 2.1 - Yeast cultures used in this study... 26
Table 2.2 - List of primers used in this study ... 29
Table 2.3 - PCR amplification conditions for each genomic locus ... 31
Table 2.4 - GenBank accession numbers of sequences of the listed loci used for MAT analyses ... 33
Table 2.5 - GenBank accession numbers of sequences of listed the loci used for MLST analyses ... 34
Table 3.1 - Accession numbers of nucleotide sequences of Kwoniella mangroviensis and related species ... 36
Table 3.2 - Nucleotide substitutions in ITS (top right) and LSU (bottom left) sequences of K. mangroviensis strains ... 39
Table 3.3 - Summary of mating assays performed with K. mangroviensis strains ... 40
Table 3.4 - Physiological tests results for K. mangroviensis strains ... 41
Table 3.5 - Nucleotide substitutions in RPB1 genomic (top right) and coding sequences (bottom left) of K. mangroviensis strains ... 44
Table 3.6 - Amino acid residue substitutions in RPB1 translated sequences of K. mangroviensis strains 44 Table 3.7 - Nucleotide Substitutions in RPB2 coding sequences (top right) and amino acid residue substitutions in translated sequences (bottom right) of K. mangroviensis strains ... 46
Table 3.8 - Nucleotide Substitutions in TEF1α genomic (top right) and coding sequences (bottom left) of K. mangroviensis strains ... 48
Table 3.9 - Amino acid residue substitutions in TEF1α translated sequences of K. mangroviensis strains ... 48
Table 3.10 - Nucleotide Substitutions in MCM7 coding sequences (top right) and amino acid residue substitutions in the translated sequences (bottom left) of K. mangroviensis strains ... 49
Table 3.11 - Jukes-Cantor distances ... 52
xiii
LIST OF ABBREVIATIONS
(T) –Type-strain
B.–Bullera
bp –Base pairs
C.–Cryptococcus
CDS –Coding Sequence
E. coli –Escherichia coli
F. –Filobasidiella
h –Hour(s)
HD –Homeodomain
ITS (Internal Transcriber Spacer) – ITS1 + 5.8S rRNA + ITS2
JGI –Joint Genome Initiative
K.–Kwoniella
kb –Kilobase
LSU –Large Subunitofribosomal RNA
MAPK –Mitogen-Activated Protein Kinase
MAT –Mating-type
min –Minute(s)
ML –Maximum Likelihood
MLST –Multilocus Sequence Typing
NJ –Neighbor Joining
P/R –Pheromone and pheromone receptor
PCR –Polymerase Chain Reaction
rpm –Revolutions per minute
SXI–SXI1, SXI2 and intergenic region
T. mesenterica–Tremella mesenterica
T. wingfieldii–Tsuchiyaeawingfieldii
var. –Variety
1
1 - INTRODUCTION
1.1 - Fungi and Basidiomycetes
The kingdom Fungi is a diverse group of eukaryotic microorganisms that includes mushrooms, molds and yeasts. The most recent classification of this kingdom (Hibbett et al., 2007) includes one subkingdom (Dikarya), seven phyla (Chytridiomycota, Neocallimastigomycota, Blastocladiomycota, Microsporidia, Glomeromycota, Ascomycota, Basidiomycota), ten subphyla, 35 classes, 12 subclasses and 129 orders. According to the Global Catalogue of Microorganisms, more than 77000 species of fungi have been described and some predictions suggest that 1.5 million species may exist (Hawksworth, 1991). The majority of known fungi are included in the subkingdom Dikarya, comprising the two sister phyla Ascomycota and Basidiomycota, which share a monophyletic origin (Hibbett et al., 2007). Yeasts are found in the latter two phyla and are mostly saprophytic but include some important human pathogenic fungi, namely Candida spp. (ascomycetes), Cryptococcus
neoformans and Cryptococcus gattii (basidiomycetes) (Cooper, 2011). Yeasts are generally
characterized by budding or fission as the primary mode of vegetative reproduction and have sexual states that are not enclosed in fruiting bodies (Kurtzman et al., 2011a).
The phylum Basidiomycota comprises economically, environmentally and medically important fungi that produce meiotic spores on the surface of often flask-shaped cells, termed basidia. This phylum is divided into three subphyla: the Pucciniomycotina (including rust fungi, anther smuts, some jelly-like fungi and many basidiomycetous yeasts), the Ustilaginomycotina (including the true smut fungi and some yeasts), and the Agaricomycotina (including the mushroom-forming species, jelly fungi and many yeasts in the class Tremellomycetes) (Hibbett et al., 2007; Kües et al., 2011).
1.2 - Looking for sexual reproduction in Fungi
In nature, there are many organisms that can reproduce asexually and/or sexually. Fungi can reproduce both ways, even though, some fungal species are only known by their asexual form.
2 Molecular approaches circumvent the need of testing strain compatibility under laboratory conditions and also allow to determine the sexual recombination versus clonal propagation ratio in a population (Campbell and Carter, 2006). Populations are defined as a group of organisms belonging to a single species, present in one place at one time. Sexual reproduction enables genetic exchange; therefore, the choice of the population is decisive for the recombination analysis. If the isolates are sampled too widely, they may belong to genetically isolated subpopulations and therefore, no sexual recombination will be found. If the isolates are sampled too narrowly, there might be an oversampling of clones. In both situations, clonality may be concluded by mistake due to inappropriate sampling. A molecular population genetic analysis requires the development of molecular markers and the analysis of their distribution among the population. For recombination analysis, the most important factor is that all loci arrange independently. This requirement excludes techniques such as DNA fingerprinting to identify sexual recombination, although this technique is useful to identify populations, discriminate and identify species and strains (Xu, 2006).
Multilocus sequence typing (MLST) is a method used for generating independent loci for recombination analyses (Campbell and Carter, 2006). This method, based on sequencing of independent and polymorphic loci, uses nucleotide sequences from several housekeeping genes to characterize genetic diversity. For phylogenetic analysis, the sequenced genes are chosen based on their degree of conservation within a species and among species. The ribosomal RNA (rRNA) gene cluster is the most common sequenced region for species identification (Xu, 2006). This region includes the internal transcribed spacers (ITS) 1 and 2, the intergenic spacer (IGS), 5.8S, 18S (SSU) and 26S (LSU) rRNA genes and it is chosen due to its multi-copy and high conservation within a species but high polymorphism among species along the spacers. Other genes besides the rRNA gene cluster are also used, such as mitochondrial ATPase subunits, beta-tubulin and elongation factor (Xu, 2006). For species to be identified and distinguished, single copy protein-coding genes, such as RNA polymerases (RPB1 and RPB2) (James et al., 2006) or DNA replication licensing factor (MCM7) (Schmitt et al., 2009) are useful, while for strains within a species, non-functional DNA regions are more informative than conserved genes due to evolutionary and selective pressure, whereas non-coding sequences are more variable (Xu, 2006). MLST has the advantage that it is highly reproducible, allowing data from different experiments and studies to be compiled together, continuously being improved and complemented (Campbell and Carter, 2006).
3
1.3 - Basidiomycete life-cycle and Heterothallism vs. Homothallism
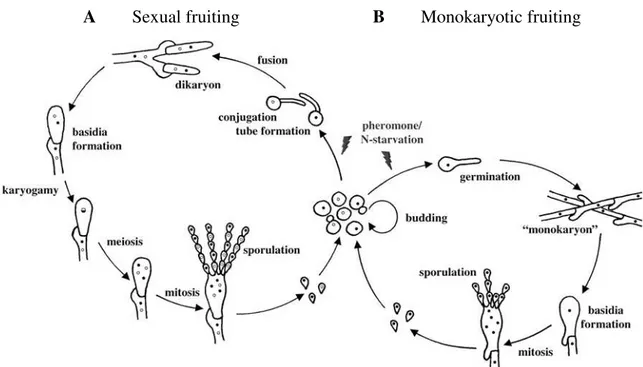
Basidiomycetes have a complex life-cycle, and undergo haploid, diploid and dikaryotic developmental pathways. Many fungi in the Basidiomycota have a dimorphic life-cycle, where monokaryotic yeasts alternate with dikaryotic filaments. Species that switch between single cell-yeasts and a filamentous growth form (most sexual basidiomycetous yeasts) are called “dimorphic”, in contrast with “monomorphic” species (such as mushrooms and the rusts) that only have one known growth form (Morrow and Fraser, 2009). Those dimorphic fungi can exist as yeasts that divide by budding, or as filaments that are involved in sexual or monokaryotic fruiting (Figure 1.1). Sexual reproduction is common among fungi and it can happen, usually under nutrient-limited conditions, in two different sexual systems: heterothallism (self-sterility) or homothallism (self-fertility).
Heterothallism requires cell-cell fusion between two morphologically identical cells with different and compatible sexual identities, while in homothallism cell-cell fusion occurs between cells of a single individual in solo culture (Kües et al., 2011; Ni et al., 2011). Some statistics suggest that approximately 90% of basidiomycetes are heterothallic and 10% are homothallic (Whitehouse, 1949), but sometimes, under some specific environmental conditions, some heterothallic fungi can undergo same-sex mating (for example, Cryptococcus neoformans) in the absence of a compatible mating partner (Ni et al., 2011).
In a typical heterothallic sexual-cycle of a dimorphic basidiomycete, two self-sterile haploid cells undergo chemoattraction, mediated by pheromones, and fuse (Figure 1.1.A). After cell-cell fusion, nuclear fusion is delayed and the dikaryon grows as hyphae, in most cases, with fused clamp connections. Nuclear migration is mediated also by pheromone signaling and regulated by a process involving clamp cell formation, fusion and the formation of a dikaryon with two different haploid nuclei. A single specialized cell, the basidium, is formed at the termini of the dikaryotic hyphae, where nuclear fusion (karyogamy) and meiosis occur to produce external haploid basidiospores (Kües et al., 2011). The reproduction in homothallic basidiomycetes may include uniparental reproduction without a partner (apomixis) or biparental reproduction (amphimixis). In this case mating can involve the fusion between two self-fertile strains (outcrossing) or the fusion of two cells/nuclei of the same strain (inbreeding) (Kües et al., 2011).
4
1.4 - Bipolar and Tetrapolar Mating Systems
Sexual identity is always established in the haploid phase and is determined by specialized genomic regions known as mating type loci (MAT), responsible for cell identity, cell-cell fusion and/or the viability of the zygote. In spite of the importance of this genomic region to the fungal cell cycle, species identity and fitness, it is extremely plastic and the genes within MAT differ considerably between fungal phyla and among species within phyla (Hsueh et al., 2011). In general, these regions can be arranged in two different sexual compatibility systems: tetrapolar and bipolar.
In the tetrapolar mating system, exclusive to the basidiomycetes (for example, Tremella
mesenterica and Ustilago maydis), the mating type is defined by two physically unlinked sex
determining loci. One locus contains genes encoding pheromones and pheromone receptors (P/R or A locus), and the other locus genes encoding homeodomain transcription factors (HD or B locus). The P/R locus is commonly biallelic (A1, A2), but there are some exceptions, while the HD locus is usually multiallelic (B1, B2, …, Bn) (Kües et al., 2011). Each allele combination (for example, A1B1,
Figure 1.1 - Life cycle of the basidiomycetous yeast Cryptococcus neoformans (adapted from Lengeler et al., 2000). Heterothallic sexual-cycle (A), two self-sterile haploid cells undergo chemoattraction, mediated by
pheromones, and fuse. After cell-cell fusion, nuclear fusion is delayed and the dikaryon grows as hyphae with fused clamp connections. The basidium is formed at the termini of the dikaryotic hyphae, where nuclear fusion and meiosis occur to produce haploid basidiospores. Different sexual identities are represented by different nucleus colors (black and white). Monokaryotic fruiting (B). Haploid cells with the same sexual
identity become diploid and cells with the same sexual identity fuse. The diploid monokaryotic hyphae form clamp connections that are not fused. The basidium is formed, meiosis occurs and haploid basidiospores are produced.
5 A1B3, A2B1) is a different mating-type, and in order to mate, individuals must have compatible mating-types, i.e. different alleles at both loci (Casselton and Challen, 2006). The shared regulation for these loci is to control the mating process by self and nonself recognition during early (cell fusion) and late (nuclear fusion) stages of sexual reproduction (Hsueh et al., 2011).
In the bipolar mating system, which is found in ascomycetes and basidiomycetes (for example,
Saccharomyces cerevisiae and Cryptococcus neoformans, respectively), a single locus, most of the
times biallelic (designated as, for example, aor α), contains genes encoding only transcription factors in ascomycetes or pheromone, pheromone receptors and homeodomain transcription factors in basidiomycetes (Kües et al., 2011).
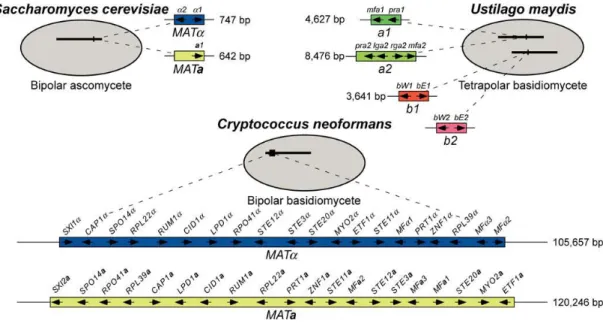
MAT loci vary considerably in size and gene content between mating systems and species. In
the basidiomycetous bipolar yeast Cryptococcus neoformans, this locus spans more than 100 kb and comprises more than 20 genes (Lengeler et al., 2002), while in the ascomycetous bipolar yeast
Saccharomyces cerevisiae it spans only 642 bp (MATa) or 747 bp (MATα) and comprises only one or
two genes (Figure 1.2) (Fraser et al., 2004). In the tetrapolar dimorphic basidiomycete Tremella
mesenterica, the P/R locus spans about 18 kb, encoding six genes, and the HD locus spans about 3 kb,
encoding two genes (SXI1 and SXI2) (Findley, 2010), while in the tetrapolar dimorphic basidiomycete
Ustilago maydis, the P/R locus spans 4.6 kb or 8.4 kb, encoding two or four genes, and the HD locus
spans about 3.6 kb, encoding 2 genes (bW and bE) as well (Figure 1.2) (Fraser et al., 2004).
Figure 1.2 - Fungal MAT Locus Paradigms (Fraser et al., 2004). MAT loci of the bipolar ascomycete Saccharomyces cerevisiae (upper left), the tetrapolar basidiomycete Ustilago maydis (upper right) and the
bipolar basidiomycete Cryptococcus neoformans (lower graphic). In U. maydis, the two MAT loci are
physically unlinked. One encodes for pheromone and pheromone receptors and the other for homeodomain transcription factors. In C. neoformans the same locus encodes both pheromone and pheromone receptors
and homeodomain transcription factors. The length is related to the extension of the non-recombining MAT
6
1.5 - Mating type loci in the Basidiomycota
In basidiomycetes, the mating type loci and their gene content differ between species; however, MAT genes comprise at least three classes: those encoding homeodomain transcription factors, pheromone precursors and cognate G protein-coupled 7-transmembrane pheromone receptors (Kües et al., 2011). These MAT genes are also found in self-fertile, homothallic species and in many anamorphic species. In some species, those genes are absent from the functional mating type loci, but present somewhere else in the genome (Kües et al., 2011).
1.5.1 - Pheromone and Pheromone Receptors
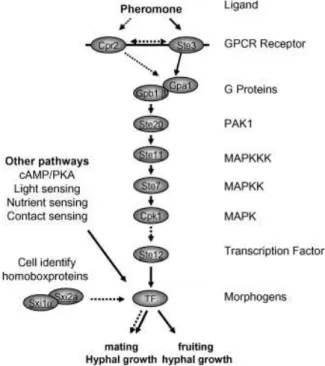
The first step of mating in most dimorphic basidiomycetes is the recognition of a compatible mating partner. This recognition occurs through export and sensing of pheromones. For different individuals to be sexually compatible, different pheromones and pheromone receptors must be produced. Pheromone and pheromone receptor genes are usually linked within MAT and differ when different alleles of the P/R locus are present in both individuals. The allele sequences of these genes from different mating types of the same species are generally highly divergent, as they regulate the mechanism by which self and non-self pheromones are recognized (Hsueh et al., 2011; Kües et al., 2011).
7 CPK1 also make up part of the pathway (Lin, 2009). The outcome response, generally involves
transcriptional activation/repression of many different genes responsible for regulation of filamentous growth during nitrogen starvation, response to osmotic shock and other stresses, cope with cell wall stress or initiation of sporulation. In mating, the final product of this cascade is involved in the regulation performed by the homeodomain transcription factors in the nucleus, and together they regulate the next stages of sexual reproduction (Figure 1.3).
1.5.2 - Homeodomain Transcription Factors
Mating type genes encoding homeodomain (HD) transcription factors are divided into two different classes, HD1 and HD2. These two genes classes are based on structural homologies and distinct sequences of the DNA-binding motifs of their deduced products (Kües et al., 2011). In basidiomycetes, these genes are generally arranged as adjacent and divergently transcribed genes within the HD locus. For a successful mating to occur, the products of both genes, but from different alleles, must interact with each other. Sequences of HD1 and HD2 alleles are usually highly divergent, and so are the resulting products. These differences are responsible for the mating type specificity of those proteins, and interaction between proteins from the same mating type is unsuccessful, being self-discriminatory. Interaction and dimerization between non-self proteins generates an active heterodimeric transcription factor (HD1-HD2) localized in the nucleus, which binds to specific sequences in the promoters of target genes and regulates the expression of genes involved in dikaryon development and sexual reproduction (Figure 1.3) (Kües et al., 2011).
8
1.6 - Cryptococcus species complex
The “Cryptococcus species complex” is a cluster of human pathogenic and dimorphic basidiomycetous yeasts, also known by the teleomorphic name Filobasidiella (based on morphological features of sexual state; Kwon-Chung, 2011), within the Filobasidiella clade, which belongs to the order Tremellales, class Tremellomycetes (Figure 1.4) (Findley et al., 2009). This clade comprises two sibling taxa, Cryptococcus neoformans, with two recognized varieties, C. neoformans var. neoformans and C. neoformans var. grubii, and Cryptococcus gattii (Campbell and Carter, 2006), which are responsible for respiratory diseases or neurological diseases in immunocompromised and immunocompetent patients, respectively (Casadevall and Perfect, 1998).
C. neoformans and C. gattii possess a biallelic bipolar mating system consisting of two
opposite mating types, aand α (MATa and MATα) (Lengeler et al., 2002). The length and gene content
Figure 1.4 - Phylogenetic relationships among selected members of the Tremellales (adapted from
Findley et al., 2009). Combined data set of six concatenated genomic loci (RPB1, RPB2, EF1α, mitSSU,
nucLSU (D1/D2), and ITS) shows the presence of 3 genetically different clades: Filobasidiella (C. amylolentus, T. wingfieldii, C. neoformans var. neoformans, C. neoformans var. grubii and C. gattii),
Kwoniella (B. dendrophila, C. bestiolae, C. dejecticola, K. mangroviensis and C. heveanensis) and Tremella
(T. mesenterica and T. globispora). C. humicola is the outgroup. The tree was built using Maximum
Likelihood method and the numbers on branches are bootstrap values from 1000 replicates.
Filobasidiella clade
Kwoniella clade
9 of MAT alleles from both species are similar, but the order of genes is highly rearranged (Fraser et al., 2004). MAT alleles are very distinct between each other, but also between the same mating type of different species within this complex (Figure 1.5).
Each MAT locus possesses only one gene encoding for homeodomain transcription factors, Sxi1 or Sxi2. Sxi1 is encoded by SXI1α gene present only in MATα strains and Sxi2 is encoded by
SXI2agene present only in MATa strains (Figure 1.5). The two compatible HD transcription factors,
form a heterodimeric complex, which, like HD1-HD2, regulates the fusion of a and α cells, the dikaryotic hyphal formation and the establishment of the a/α cell identity (Lengeler et al., 2002). In all other basidiomycetes studied both divergently oriented HD transcription factor genes are present in the same MAT allele (Kües et al., 2011; Casselton and Challen, 2006).
In the Cryptococcus species complex, the MAT locus also contains highly divergent alleles of genes for mating type specific Ste3-like pheromone receptors (STE3α and STE3a), three pheromone
precursor genes for MATa (MFa1, MFa2 and MFa3) or four pheromone precursor genes for MATα
Figure 1.5 - MAT structure of C. neoformans var. neoformans, C. neoformans var. grubii and C. gattii (Fraser et al., 2004). Synteny analysis of the MAT locus of C. neoformans var. neoformans, C. neoformans
var. grubii and C. gattii shows different gene organization and orientation between the same species and
variety. Each species MATα is represented in blue while MATa is represented in yellow. Pink and green
10 (MFα1, MFα2, MFα3 and MFα4), and alleles for other genes, predominantly mating type specific, such as: STE12aand STE12α, encoding Ste12-like transcription factors that regulate some aspects of
mating; STE20a and STE20α, for p21-activated protein kinases connected to the MAPK pathway;
STE11a and STE11α, for kinases of the MAPK cascade; and SPO14, RUM1 and BSP1 alleles, which may function in meiosis and sporulation (Figure 1.5 and Table 1.1). LPD1, CID1, RPO41, BSP2,
GEF1 are five genes within the Cryptococcus MAT locus that exhibit species-specific phylogenies
(Table 1.1), while highly conserving their synteny. Many other genes present within these extended loci do not have a known function in mating, namely BSP2, BSP3, CAP1, CID1, ETF1, FAO1, GEF1,
IKS1, LPD1, NOG2, PRT1 and ZNF1 (Hsueh et al., 2011).
Phylogenetic analyses of the MAT genes revealed that the genes within this locus can be classified into mating type-specific, intermediate mating type-specific and species-specific patterns (summarized in Table 1.1) (Fraser et al., 2004). Genes displaying mating type-specific phylogenies include those which are thought to have been most anciently acquired into this recombinationally suppressed region and are highly divergent between MAT alleles, namely those encoding for pheromones and pheromone receptors and genes involved in the MAPK pathway (Figure 1.3 and Figure 1.5, red and black arrows). Intermediate mating type-specific pattern genes have likely been contained within this region for a shorter period of time, and include those that cluster into a mating type-specific pattern, but for which different alleles share a higher percentage of similarity than the “ancient class” of genes (also represented as black arrows in Figure 1.5). Genes with a species-specific phylogenetic pattern are presumably the most recently acquired genes, and include genes that share a highly conversed sequence and nucleotide identity between alleles, similar to genes outside MAT (Figure 1.5, white arrows). The distinct phylogenetic patterns reflect the evolutionary origin of the different genes that compose the MAT locus, their relative acquisition into this genomic region and lastly, provide some clues about how this genomic structure evolved.
11 into a bipolar mating system. Finally, inversions produced more homogeneous bipolar structures and suppressed recombination between MAT alleles.
A recent multilocus sequence typing study (Findley et al., 2009) helped to clarify the phylogeny of taxa related to the Cryptococcus species complex. This study places this pathogenic complex phylogenetically closely related to saprobic basidiomycetous yeasts, namely, in the Filobasidiella, Kwoniella and Tremella clades (Figure 1.4). Comparative genomic approaches are useful to study and understand how the CryptococcusMAT gene cluster evolved and also to test and Figure 1.6 - Original model of MAT evolution (Fraser et al., 2004). This evolutionary model proposes that
the ancient P/R locus and the ancient HD locus were unlinked. These tetrapolar loci expanded twice and incorporated additional MAT-specific genes. In one mating type, chromosomal translocation events fused
both loci, creating an intermediate tripolar mating state. Chromosomes from the opposite mating type were eventually fused by dual recombination events, turning this intermediate tripolar mating state into a bipolar mating system. Inversions produced homogeneous bipolar structures and suppressed recombination between
12 improve the proposed evolutionary model. Due to their phylogenetic relatedness, the analysis of MAT structure was therefore extended to the several species included in those clades.
1.7 - Evolution of MAT in the Filobasidiella clade
1.7.1 - Cryptococcus amylolentus and Tsuchiyaeawingfieldii
Cryptococcus amylolentus and Tsuchiyaea wingfieldii are saprobic basidiomycetous yeasts,
phylogenetically closely related to the pathogenic Cryptococcus species complex (Figure 1.4). There are only two known strains of C. amylolentus and one of T. wingfieldii worldwide. The two available strains of C. amylolentus were found to be sexually compatible but T. wingfieldii lacks a known sexual cycle, maybe due to the lack of a compatible strain or sterility (Findley et al., 2012). Nevertheless, the
MAT locus of both species was sequenced and analyzed (Figures 1.7 and 1.8) (Findley et al., 2012).
The P/R and HD loci were found to be physically unlinked and located on different chromosomes in both species, suggesting a tetrapolar mating configuration for C. amylolentus and T.
wingfieldii. In C. amylolentus, the sequenced fragment of the P/R locus region spans about 60 kb,
comprising 21 genes and the HD locus region spans about 20 kb, comprising eight genes (Figure 1.7), while in T. wingfieldii, the P/R locus region spans about 70 kb, comprising 22 genes and the HD locus Figure 1.7 - MAT loci of Cryptococcus amylolentus CBS 6039 (Findley et al., 2012). Grey arrows
represent genes that either flank MAT or are hypothetical genes, black arrows represent MAT-specific genes
in pathogenic Cryptococcus species, and yellow indicates the genes more recently acquired into the
pathogenic Cryptococcus species MAT locus. Green bars represent gaps in the sequence.
Figure 1.8 - MAT loci of Tsuchiyaea wingfieldii CBS 7118 (Findley et al., 2012). Grey arrows represent
genes that either flank MAT or are hypothetical genes, black arrows represent MAT-specific genes in
13 region spans about 40 kb, comprising 14 genes (Figure 1.8). The gene content seems to be highly conserved between the two species and similar to the C. neoformans and C. gattii MAT alleles (Figure 1.5) (Hsueh et al., 2011).
The P/R locus of both species contained the two pheromone-signalling MAPK pathway genes (STE12 and STE20), the mating pheromone (MF) genes (two in C. amylolentus and three in T.
wingfieldii), the pheromone receptor gene (STE3), and the five genes (LPD1, RPO41, BSP2, CID1,
and GEF1) hypothesized to be the most recently acquired genes in C. neoformansMAT locus (Figure 1.6). In the pathogenic Cryptococcus species complex, STE11 is present within the P/R locus (Figure 1.5), however, in C. amylolentus and T. wingfieldii, STE11 gene is missing from the P/R locus region and located elsewhere in the genome. Additionally, NCP1 and NCP2 are duplicated genes in C.
amylolentus and T. wingfieldii while there is only one of these genes in the C. neoformans var.
neoformansMATa allele, suggesting that the other copy has been lost or translocated to another region
in the genome, while in the other mating-type or in C. neoformans var. grubii and C. gattiiNCP1 and
NCP2 are absent. In both C. amylolentus and T. wingfieldii HD locus region, two divergently
transcribed homeodomain transcription factors are present (SXI1 and SXI2).
Phylogenetic analyses were performed on several genes (CID1, ETF1, GEF1, LPD1, STE3,
STE12, STE20, SXI1, and SXI2) located within the MAT locus of C. amylolentus and T. wingfieldii that
were also in C. neoformansMAT (Findley et al., 2012). The results showed that CID1, GEF1, LPD1 and ETF1 exhibit a species-specific pattern, while STE3, STE12, STE20 exhibit a mating-type specific pattern and SXI1 and SXI2 are mating-type unique genes (see also Table 1.1, page 19). ETF1 is species-specific in these two sibling species and an intermediate class I gene in C. neoformans. Comparing sequences of both C. amylolentus strains led the authors to conclude that the P/R locus, in this species, is defined by the mating pheromone genes (MF), STE3, STE12 and STE20, among other genes, and that SXI1 and SXI2 span about 2 kb and define the HD locus. Due to the lack of additional
T. wingfieldii strains, it was not possible to determine which genes define the P/R and HD loci in this
species.
From synteny analysis between the two sibling species, C. amylolentus and T. wingfieldii, two major inversions were detected between the P/R regions of each species and a syntenic conservation of the HD regions (Findley et al., 2012). Synteny comparison of both species with C. neoformans and C.
gattii revealed extensive gene rearrangements and inversions and that the C. amylolentus and T.
wingfieldii MAT arrangement and gene content may correspond to an evolutionary intermediate in
MAT evolution, in which the MAT loci have expanded but not yet fused. Homologues of several
14 translocations may have occurred between these two chromosomes during the MAT evolution of the
Cryptococcus species complex.
1.7.2 - Filobasidielladepauperata
Filobasidiella depauperata is a strictly filamentous species that forms sexual structures
regardless of environmental conditions (Kwon-Chung, 2011). This species has aseptate basidia, monokaryotic hyphae without clamp connections and monokaryotic basidiospores. These morphological characteristics are similar to the modified form of sexual cycle of C. neoformans which involves monokaryotic fruiting (same-sex mating). A fragment of the putative MAT locus of F.
depauperata was sequenced and analyzed (Figure 1.9) (Rodriguez-Carres et al., 2010).
A contiguous sequence of about 70 kb was obtained, comprising 19 genes, corresponding to regions of the chromosome 4 of C. neoformans where MAT is located. Another sequence was obtained, comprising the STE11 gene, corresponding to regions of chromosome 4 and 5 of C.
neoformans. Nine (LPD1, CID1, RPO41, STE20, MYO2, PRT1, ZNF1, RPL39 and STE11) of the 20
genes present in the C. neoformans MAT locus were present in the sequenced regions of F.
depauperata (Figure 1.9, black arrows); however, pheromone, STE3, STE12 and SXI genes were
missing. None of the nine genes showed conserved synteny with C. neoformans MAT region. Phylogenetic analysis showed that STE11, CID1, LPD1, RPO41, RPL22 and ZNF1 had a species-specific pattern (Table 1.1). STE20 and MYO2 genes formed a monophyletic cluster between F.
depauperata and one of the C. neoformans alleles, suggesting that, at least, STE20 and MYO2 MAT
-genes, may have been acquired into MAT prior to the F. depauperata divergence (Rodriguez-Carres et
al., 2010). Three of the species-specific genes (LPD1, CID1 and RPO41), and five of the ancestral
MAT associated genes (STE20, MYO2, PRT1, ZNF1 and RPL39) were found in a contiguous cluster,
suggesting that at least three of the five recently acquired genes were closely located in the common ancestor of C. neoformans and F. depauperata.
Synteny analysis showed a higher number of translocations in F. depauperata corresponding to chromosomes 4 and 5 of C. neoformans (Rodriguez-Carres et al., 2010). One of the translocated
Figure 1.9 - Fragment of MAT locus of Filobasidiella depauperata CBS 7855 (Hsueh et al., 2011).Black
arrows represent genes with homologues in the MAT locus of the pathogenic Cryptococcus species, and
15 regions included STE11, which was translocated out of the MAT cluster, whereas it is part of the cluster associated with the pheromone and pheromone receptor genes in C. neoformans and C. gattii. From the evolutionary point of view, the results from synteny analysis between F. depauperata and C.
neoformans, showed that some genes linked to the MAT locus in C. neoformans are present in
different chromosomes in F. depauperata, suggesting that a higher number of chromosomal rearrangements occurred in the genes associated with MAT in C. neoformans, while the synteny in the other genomic regions was conserved. Chromosomal rearrangements appear, therefore, to be the most relevant event in speciation and sexual divergence in these closely related pathogenic and saprobic species (Hsueh et al., 2011; Rodriguez-Carres et al., 2010).
1.8 - Evolution of MAT in the Kwoniella clade
1.8.1 - Cryptococcus heveanensis
Cryptococcus heveanensis is a saprobic basidiomycetous yeast, located in the Kwoniella
clade, a sister clade to the Filobasidiella clade (Figure 1.4). A sexual cycle was discovered for this species - teleomorph name Kwoniella heveanensis (Sheng et al., 2011) - and the MAT locus was sequenced and analyzed (Figure 1.10) (Metin et al., 2010). Two apparently unlinked genomic loci were found, P/R and HD loci, indicating a tetrapolar MAT organization for C. heveanensis. The region containing the P/R locus spans about 180 kb, comprising 47 genes, and the region containing the HD locus spans about 80 kb, comprising 22 genes.
The P/R locus and flanking regions contained a pheromone gene, the pheromone receptor gene
STE3, the pheromone-signalling MAPK pathway genes STE11, STE12 and STE20, and many other
genes that are present in the MAT locus of C. neoformans and C. gattii (LPD1, GEF1, CID1, BSP1,
Figure 1.10 - MAT loci of Cryptococcus heveanensis CBS 569 (Metin et al., 2010). Black arrows represent
genes that are present in the MAT locus of the pathogenic Cryptococcus species and yellow represent genes
16 ETF1, RPO41, BSP2, ZNF1, PRT1, NCP1, MYO2, IKS1, RPL39, and BSP3) (Figure 1.10, black
arrows). There were additional genes that are not located within the MAT locus of C. neoformans and
C. gattii, but located elsewhere in the genome (Table 1.1) and also genes that do not have homologues
in these pathogenic species (An11g04720, UM00103, UM02602). This region contained also the five most recently acquired genes (LPD1, GEF1, CID1, RPO41, and BSP2) as hypothesized by the evolutionary model (Figure 1.6).
The HD locus region (Figure 1.10) contained two divergently transcribed HD genes (SXI1 and
SXI2), as well as FAO1, FCY1, UAP1, RPL22, SPO14 and CAP1 genes. FAO1, FCY1 and UAP1 are
located in the left boundary of C. neoformans and C. gattiiMAT locus, while RPL22, SPO14, CAP1 are contained within the MAT locus of these pathogenic Cryptococcus species (Figure 1.5).
Phylogenetic analyses of MAT genes from several C. heveanensis strains (Metin et al., 2010) showed that STE3 and STE12 were the only genes exhibiting a mating-type specific pattern, along with SXI1 and SXI2, in the sequenced regions (Table 1.1). Although, BSP1, SPO14, ETF1, STE20 and
STE11 exhibit a mating type-specific pattern in C. neoformans and C. gattii, these genes are
species-specific in C. heveanensis. CID1, GEF1 and LPD1 also exhibit a species-specific pattern. The P/R locus appeared to be at least biallelic and to contain at least the pheromone, STE3,STE12, CNB00600 and CNG04540 genes. The HD locus appeared to be multiallelic and defined by SXI1 and SXI2. Specificity is defined by a 2 kb spanning region, including the intergenic region between the two genes, and the 5’-end of both genes, where the sequence is highly divergent (Figure 1.11).
Figure 1.11 - Percent sequence identity plots of SXI1 and SXI2 (Metin et al., 2010). Comparison of the
same HD region, between several strains (left bold numbers) and alleles (B1-B6), shows a high variability
17 The P/R and HD gene clusters of C. heveanensis have several linked genes that are not present in the MAT locus of the pathogenic Cryptococcus species complex (Figure 1.10, yellow arrows). Furthermore, some of these genes do not have an apparent homolog in these pathogenic species (An11g04720, UM02602), suggesting that these genes might have been lost during the evolution of the
Cryptococcus MAT locus. The genes that have homologues in C. neoformans are located in
chromosome 4, which comprises the MAT locus, suggesting intrachromosomal rearrangements during
MAT evolution.
1.9 - Evolution of MAT in the Tremella clade
1.9.1 - Tremella mesenterica
Tremella mesenterica is a heterothallic basidiomycetous saprobic and dimorphic fungus, more
distantly related to C. neoformans (Figure 1.4), that produces fruiting body structures (Bandoni and Boekhout, 2011). The whole genome of one strain of this species was sequenced by the Department of Energy’s Joint Genome Initiative (JGI) (http://www.jgi.doe.gov/) and two unlinked MAT loci were determined and analyzed (Figure 1.12) (Findley, 2010; Metin et al., 2010).
Several hypothetical genes were found along the chromosomes, corresponding to homologues located in the chromosome containing MAT in C. neoformans. Homologues found in unrelated fungi are also located in the P/R locus (for example, An11g04720, UM02602 and UM04613). The authors hypothesize that, in C. neoformans, these genes have been lost from MAT and relocated to the Figure 1.12 - MAT loci of Tremella mesenterica ATCC 24925 (adapted from Findley, 2010). Black arrows
represent genes with homologs in C. neoformans and yellow indicates hypothetical genes. MAT-restricted
18 telomeric ends of the chromosome containing MAT (Metin et al., 2010). Two copies of the NCP1 gene (NPC1 and NCP2) are present also as found in C. amylolentus and T. wingfieldii.
The HD region contained two homeodomain transcription factors (SXI1 and SXI2) linked and divergently oriented. Additionally, there were two HD linked genes (RPL22 and SPO14) also present, while SPO14, a gene closely located to HD genes in C. neoformans, is not adjacent to the HD genes in this species. Also, the C. neoformans HD flanking genes (FAO1, UAP1, and FCY1) (Figure 1.5) are not linked to the HD genes and are located elsewhere in T. mesenterica genome.
The five most recently acquired genes (RPO41, BSP2, GEF1, CID1 and LPD1) in the proposed evolutionary model (Figure 1.6) are present, with RPO41 and BSP2 linked and located on the 5’ of the P/R locus region, while LPD1, CID1, and GEF1 are linked but located distantly from the P/R or HD loci (Figure 1.12).
Phylogenetic analyses for unlinked and MAT-linked genes (Findley, 2010; Findley et al., 2012) showed that STE3, STE12 and ETF1 exhibit a mating-type specific pattern, GEF1, RPO41,
CID1, and LPD1 a species-specific pattern and SXI1 and SXI2 were very divergent from their C.
neoformans and C. gattii homologues (Table 1.1). From these analyses, it was determined that, in T.
mesenterica, the P/R locus is biallelic and spans about 18 kb, defined by STE3, STE12, one pheromone
and two hypothetical genes (CNB00600 and CNB00610), while the HD locus is multiallelic and spans about 3 kb, and is defined by the two homeodomain transcription factors (SXI1 and SXI2). In contrast with the pathogenic Cryptococcus species, in T. mesenterica, STE11 is located outside the P/R locus, although it is within the same chromosome and relatively close to the MAT determining region.
1.10 - MAT gene content and organization comparison
From the gene content, phylogenetic pattern and synteny analyses performed on C.
amylolentus, T. wingfieldii, F. depauperata, C. heveanensis and T. mesenterica MAT regions,
comparisons were done with C. neoformans and C. gattii, in order to determine relevant similarities and differences that could provide some insight into this region’s evolutionary process (Hsueh et al., 2011) (see also Table 1.1).
The presence of a tetrapolar mating system in C. neoformans and C. gattii closest relatives, C.
amylolentus and T. wingfieldii, but also in more distant species, such as C. heveanensis and T.
mesenterica, allows to infer that the tetrapolar configuration represents the ancestral form of MAT and
19 Table 1.1 - Summary of the MAT region gene content and phylogenetic pattern of MAT genes in the studied species.
Species
HD region P/R region
HD genesa
Additional genes present (bold) or absent in C. neoformansMATα
Pheromone genesa
Pheromone
Receptor genea Additional genes present (bold) or absent in C.neoformansMATα*
C. neof. var. neoformans
(MATα)
SXI1α BSP1c, CAP1c, FAO1b, FCY1, RPL22c,
RUM1c,SPO14c, UAP1
MFα1, MFα2,
MFα3 STE3
BSP2b, BSP3b, CID1b, ETF1b, GEF1b, IKS1b, LPD1d, MYO2a, NCM1, NOG2b, PAN6, PRT1c, RPL39c, RPO41b, STE11a,
STE12a, STE20a, ZNF1c
C. neof. var. neoformans
(MATa)
SXI2a BSP2
b
, CAP1c, FAO1b, FCY1, RPL39c, RPO41b, SPO14c, UAP1
MFa1, MFa2,
MFa3 STE3
BSP1c, BSP3b, CID1b, ETF1b, GEF1b, IKS1b, LPD1d, MYO2a, NCM1, NOG2b, PAN6, PRT1c, RPL22c, RUM1c, STE11a,
STE12a, STE20a, ZNF1c, NCP1
C. neof. var. grubii
(MATα)
SXI1α CAP1c, FAO1b, FCY1, RPL22c, SPO14c,
UAP1, NADA4
MFα1, MFα2,
MFα3, MFα4 STE3
BSP1c, BSP2b, BSP3c, CID1b, ETF1c, GEF1b, IKS1a, LPD1d, MYO2a, NCM1, NOG2b, PAN6, PRT1c, RPL39c, RPO41b,
RUM1c, STE11a, STE12a, STE20a, ZNF1c
C. neof. var. grubii
(MATa)
SXI2a BSP3
c
, CAP1c, FAO1b, FCY1, NCM1, RPL39c, SPO14c, UAP1
MFa1, MFa2,
MFa3 STE3
BSP1c, BSP2b, CID1b, ETF1c, GEF1b, IKS1a, LPD1d, MYO2a, NOG2b, PAN6, PRT1c, RPL22c, RPO41b, RUM1c, STE11a,
STE12a, STE20a, ZNF1c
C. gattii
(MATα) SXI1α
BSP1c, CAP1c, CID1b, FAO1b, FCY1, GEF1b, LPD1b, RPL22c, RUM1c,
SPO14c, UAP1, VPS26
MFα1, MFα2,
MFα3, MFα4 STE3
BSP2b, BSP3c, ETF1c, IKS1a, MYO2a, NCM1, NOG2b, PAN6, PRT1c, RPL39c, RPO41b, STE11a, STE12a, STE20a, ZNF1c
C. gattii
(MATa) SXI2a
BSP3c, CAP1c, FAO1b, FCY1, NCM1, RPL39c, SPO14c, UAP1
MFa1, MFa2,
MFa3 STE3
BSP1c, BSP2b, CID1b, ETF1c, GEF1b, IKS1a, LPD1b, MYO2a, NOG2b, PAN6, PRT1c, RPL22c, RPO41b, RUM1c, STE11a,
STE12a, STE20a, ZNF1c
20 Table 1.1 (cont.) - Summary of the MAT region gene content and phylogenetic pattern of MAT genes in the studied species.
Species
HD region P/R region
HD genesa
Additional genes present (bold) or absent in C. neoformansMATα
Pheromone genesa
Pheromone Receptor
genea Additional genes present (bold) or absent in
C.neoformansMATα*
C. amylolentus SXI1α, SXI2a
CAP1, FCY1, UAP1 CND01650, CND06030,
CND06040
MFa1,
MFa2 STE3
BSP2, BSP3, CID1b, ETF1b, GEF1b, LPD1b, MYO2, PRT1, RPL39, RPO41, STE12a, STE20a, ZNF1, CNE02670, CNE02690, NCP1, NCP2, VPS26
T. wingfieldii SXI1α, SXI2a
CAP1, FCY1, RPL22, SPO14, UAP1,CNBE0480, CND01650,
CND06020, CND06030, CND06040, CNE04320,
NH75487
MFa1, MFa2, MFa3
STE3 BSP2, BSP3, CID1 b
, ETF1b, GEF1b, LPD1b, MYO2, PRT1, RPL39, RPO41, STE12a, STE20a, ZNF1, CNE02670, CNE02690, NCP1, NCP2, VSP26
F. depauperata - - - - CID1
b
, LPD1b, MYO2a, PRT1, RPL39b, RPO41b, STE20a, ZNF1b, CND01240, CND01430, CND01460
C. heveanensis SXI1α, SXI2a
CAP1, FAO1, FCY1, RPL22, SPO14b, UAP1, UM00103
MFA1
or
MFA2
STE3
BSP1b, BSP2, BSP3, CID1b, ETF1b, GEF1b, IKS1, LPD1b, MYO2, PRT1, RPL39, RPO41, STE11b, STE12a, STE20b, ZNF1, An11g04720, CNA03170,
CNB00600, CNB00610, CND01240, CND01250, CND01450, CND01460, CND01530, CND01540, CND01550, CND01560, CND01570, CND01580, CND01630, CND01640, CND05260, CND05390, CNE02190, CNE02670,
CNG04540, CNI00160, CNN00870, NCP1, NCP2, UM02602
T. mesenterica SXI1α,
SXI2a RPL22
b
, SPO14, UM00103
Tremerogen-a13 STE3
BSP1, BSP2, BSP3, CID1b, ETF1a, GEF1b, IKS1, LPD1b, MYO2, PAN6, PRT1, RPL39, RPO41b, RUM1, STE11, STE12a, STE20, ZNF1, An11g04720, CNA03170, CNB00600a, CNB00610a, CND01240, CND01250, CND01430,
CND01450, CND01460, CND01530, CND01540, CND01550, CND01560, CND01570b, CND01580, CND01630, CND01640, CND05260, CND05390, CNE02190, CNE02670, CNG04540b, CNI00160, CNN00870, NCP1, NCP2,
UM02602