Faculdade de Ciências
Departamento de Biologia Vegetal
Dissertação
Virna de Santiago Dutra
MESTRADO EM MICROBIOLOGIA APLICADA
2013
In vitro assessment of the ability of
selected lactic acid bacteria to
counteract foodborne pathogenic
In vitro assessment of the ability of
selected lactic acid bacteria to
counteract foodborne pathogenic
infections
Virna de Santiago Dutra
Master Thesis
2013
This thesis was fully performed at the Microbiology Lab of the Instituto Superior de
Agronomia (CBAA) under direct supervision of Prof. Drª. Luísa Brito in the acope of the
infections
Faculdade de Ciências
Departamento de Biologia Vegetal
In vitro assessment of the ability of
selected lactic acid bacteria to
counteract foodborne pathogenic
Dissertação Orientada por Prof. Doutora Luísa Brito (ISA/UTL)
e por Prof. Doutora Lélia Chambel (FCUL)
Virna de Santiago Dutra
MESTRADO EM MICROBIOLOGIA APLICADA
2013
i
Acknowledgments
Desafio tão grande quanto escrever essa tese, foi utilizar apenas uma página para agradecer a às pessoas que fizeram parte desta fase da minha vida.
Inicio os agradecimentos à minha família, aos meus pais, aos meus irmãos, que sempre primaram pela minha Educação, por sempre estarem presentes (apesar da distância física), por todo amor, dedicação e sou muito feliz por isso.
Ao meu Giulio, por estar o tempo todo ao meu lado. Nos momentos mais difíceis, que não foram raros neste último ano. Sou grata por cada gesto carinhoso, cada sorriso, obrigada meu amor.
Agradeço à minha excelente orientadora, Professora Doutora Luísa Brito, por me receber e me aceitar no ISA, obrigada pela oportunidade de me acompanhar neste ano, pela seriedade, pelos incentivos, aos ensinamentos de pensar criticamente, pela compreensão, pelas constantes demonstrações de sabedoria e humildade. Pela compreensão e disponibilidade das nossas reuniões em horários muitas vezes inconvenientes. Obrigada por acreditar em mim. À Mestre Ana Carla Silva, por me apresentar, por me ensinar e me acompanhar no cuidado das nossas células, pela amizade, pela simpatia, por toda paciência e eterna disponibilidade. Ao Professor Rogério Tenreiro, pela sua sensibilidade, disponibilidade, sabedoria e organização como coordenador do Mestrado.
Aos companheiros do laboratório: Inês, Vivi, Ana Telles, Ana Ramos, Susana, Rute, Sara, Miguel, Rita, João, em especial aos quase doutores: António e Paula, pelos ensinamentos, pelo convívio, pelo apoio, pela compreensão e amizade.
À D. Lena e D. Manuela por fazerem este laboratório brilhar, por deixar tudo impecavelmente preparado para o trabalho.
À Professora Lélia Chambell por me co-orientar neste trabalho.
Ao Instituto Superior de Agronomia, particularmente ao Laboratório de Microbiologia, por ter me acolhido e permitido a realização deste trabalho.
Finalmente, agradeço à Professora Cidália Peres e à Mestre Cátia Peres pelo interesse neste estudo e à FCT o apoio financeiro no âmbito projecto NEW PROTECTION: NativE, Wild
ii
Abstract
Listeria monocytogenes, Salmonella enterica and Escherichia coli O157:H7 are among the
most important foodborne pathogens worldwide due to the considerable human rates of illness reported.
The role of two selected Lactobacillus spp. strains (LABs), isolated from spontaneously fermenting olive brines, and two probiotic strains, L. casei Shirota and L. rhamnosus GG, in the attenuation of the virulence potential of these foodborne pathogenic bacteria were investigated in this work.
The ability of LABs in adhering to the intestinal mucosa and counteracting bacterial infections in the gut was evaluated. Different in vitro tests of virulence, based on animal cells, were used. For the competitive exclusion assays, intestinal human cells (HT-29) were used with L.
monocytogenes and Salmonella, in adhesion and invasion assays. With L. monocytogenes,
plaque-forming assays (PFAs) were further performed. The cytotoxicity of E. coli O157:H7 Shiga toxins was evaluated by using a cytotoxicity assay based on lactate dehydrogenase release from Vero cells.
In the present study, LAB strains showed strong inhibitory effects, with significative reduction (P<0.05) on the virulence potential of Salmonella and L. monocytogenes in adhesion and invasion of HT-29 cells. At the same time, it have been gathered initial evidences which indicating that cell free supernatants from LAB strains significantly reduced (P<0.05) the cytotoxicity produced by one E. coli O157:H7 strain.
In conclusion, the results obtained suggest that the Lactobacillus strains tested in this study, L.
plantarum and L. paraplantarum, are able to prevent infections by pathogens and can be
considered as potential strains for probiotic use with interesting potential in preventing enteric infections in humans.
Keywords:
iii
Resumo expandido
As infecções provocadas pela presença de microrganismos patogénicos, em alimentos e bebidas, são um problema generalizado de saúde pública. Os surtos de origem alimentar envolvem frequentemente Listeria monocytogenes, Escherichia coli e Salmonella enterica. Foram confirmados em 2011, um total de 9.485 casos de infecções de Escherichia coli verotoxinogénicas (VTEC). A maioria destes casos foi causada pelo serogrupo O157.
Salmonella foi o género bacteriano mais frequentemente relatado como causador de surtos de
origem alimentar em 2011, com 95.548 casos confirmados. Como nos anos anteriores, S. Enteritidis e S. Typhimurium foram os serovares mais frequentemente relatados (44,4% e 24,9%, respectivamente). O número de casos confirmados de listeriose foi 1.476. A listeriose representa a doença de origem alimentar humana mais grave em termos de hospitalização e casos fatais.
Escherichia coli é uma bactéria gram-negativa sendo um microrganismo competidor que faz
parte do grupo de anaeróbios facultativos mais abundantes da microbiota intestinal animal, colonizando o trato gastrointestinal humano logo após o nascimento. As estirpes pertencentes ao serótipo O157:H7 (EHEC – enterohemorragic E. coli) são responsáveis por inúmeros surtos mundiais. EHEC aderem, inicialmente, à superfície do epitélio intestinal na forma de microcolónias localizadas e, posteriormente, causam uma lesão típica nas células intestinais, denominada attaching and effacing lesion (A/E). Os genes bacterianos responsáveis pela atividade de transdução de sinal são codificados numa ilha de patogenicidade, denominada
locus of enterocyte effacement (LEE). Os genes associados com a virulência codificam um
sistema de secreção do tipo III (TTSS), promovendo a transferência de moléculas bacterianas efetoras para a célula receptora. Actuam ao nível do cólon e libertam a toxina Shiga (Stx), também designada por verotoxina (VT), cuja absorção sistémica coloca em risco a vida do hospedeiro. EHEC causa diarreia sanguinolenta, colite hemorrágica, síndroma hemolítica urémica e púrpura trombótica trombocitopénica.
Salmonella é um outro género da família Enterobacteriaceae. São bactérias gram-negativas,
anaeróbias facultativas e não formam esporos. O género Salmonella contém cerca de 2.600 linhagens diferentes, as quais são denominadas de serovares ou serótipos. São diferenciáveis pelos seus antigénios O, H e Vi, utilizando o esquema de Kaufmann-White. Os sintomas característicos de doenças de origem alimentar causadas por Salmonella incluem diarréia, náusea, dor abdominal, febre ligeira e calafrios e, algumas vezes, vómitos, dor de cabeça e fraqueza. Salmonella consegue invadir o intestino pelos mecanismos Zipper e Trigger. A enfermidade é causada pela passagem no lúmen e penetração de células de Salmonella no epitélio do intestino delgado, onde se multiplicam. A seguir, a bactéria invade o íleo e, inclusive, o cólon. A infecção propicia uma resposta inflamatória.
iv
Listeria monocytogenes é uma bactéria gram-positiva, não esporulada, não capsulada. É
responsável por infecções oportunistas, infectando, preferencialmente, indivíduos com o sistema imunológico comprometido, incluindo mulheres grávidas, recém-nascidos e idosos. L.
monocytogenes pode invadir o epitélio gastrointestinal. Uma vez entrando nos monócitos,
macrófagos ou leucócitos polimorfos nucleares do hospedeiro a bactéria pode disseminar-se pela corrente sanguínea, levando à septicemia. A sua presença intracelular em células fagocitárias também permite o acesso ao cérebro e provavelmente a migração da placenta para o feto em mulheres grávidas. A patogenicidade de L. monocytogenes baseia-se na habilidade em sobreviver e se multiplicar nas células fagocitárias dos seus hospedeiros. Probióticos são microrganismos vivos, não patogénicos que, quando administrados em quantidades apropriadas, conferem benefício à saúde do hospedeiro. Os mecanismos de acção dos probióticos incluem a aderência ao lúmen intestinal; competição com patogénios para, ligação a receptores, nutrientes e colonização; alteração do pH intestinal, inactivação de toxinas; aprimoramento da função de barreira da mucosa, promoção de respostas imune, inata e adaptativa e elaboração de bacteriocinas. Existe uma quantidade crescente de evidências indicando benefícios para a saúde através do consumo de alimentos contendo probióticos que podem ser usados na prevenção e tratamento de doenças gastrointestinais causadas por microrganismos patogénicos.
Este trabalho foi realizado no âmbito do projecto NEW PROTECTION: NativE, Wild
PRObioticsTrain EffecCT In Olives in brine (PTDC/AGRALI/117658/2010). O objectivo geral do
projecto consiste em confirmar a aplicabilidade de estirpes de Lactobacillus spp. Selecionadas para eventual criação de novos alimentos probióticos a partir de azeitonas.
Neste contexto, o objectivo deste trabalho foi investigar o efeito de duas estipes de
Lactobacillus spp. (isoladas da fermentação de azeitonas) e de dois probióticos de referência,
na atenuação do potencial de virulência de estirpes de Salmonella enterica, L. monocytogenes e E. coli O157:H7.
Avaliou-se a capacidade de quatro estirpes de Bactérias Ácido Lácticas (BALs) em aderir à mucosa intestinal e neutralizar infecções bacterianas em células do epitélio intestinal. Utilizaram-se diferentes ensaios de virulência in vitro com cultura de células animais: células de adenocarcinoma do cólon humano (HT-29) e células de rim de macaco verde africano (Vero). Nos ensaios de aderência e/ou invasão avaliou-se o efeito da exclusão competitiva por BALs, sobre três estirpes patogénicas pertencentes a duas espécies bacterianas (Listeria
monocytogenes e Salmonella enterica). L. monocytogenes foi também estudada em ensaios de
formação de placas de lise (PFA – Plaque Forming Assay). Nos ensaios destinados a investigar a capacidade das BALs na atenuação do potencial virulento das bactérias patogénicas, as células HT-29 foram pré- expostas às BALs por 3 horas.
v
A citotoxicidade das Shiga toxinas de E. coli O157:H7 foi avaliada por meio de um ensaio com base na libertação de lactato desidrogenase (LDH) a partir de células Vero. Avaliou-se o efeito da presença de sobrenadantes de co-culturas de BALs, com E. coli O157:H7 ou das misturas de sobrenadantes de culturas puras BALs com sobrenadante de culturas puras de E. coli O157:H7.
Das quatro estirpes estudadas de BALs, duas correspondem a probióticos de referência (Lactobacillus casei e Lactobacillus rhamnosus) e duas estirpes (LB95 e LB13) estão a ser avaliadas relativamente às suas características probióticas.
No presente estudo, as estirpes de Lactobacillus mostraram fortes efeitos inibidores, com redução significativa (P<0,05) no potencial de virulência de Salmonella e de L. monocytogenes em ensaios de exclusão competitiva usando células HT-29. Ao mesmo tempo, foram recolhidas evidências que indicam que os sobrenadantes de BALs reduziram significativamente (P<0,05) a citotoxicidade de uma estirpe de E. coli O157:H7.
Os resultados obtidos neste trabalho mostraram que as quatro estirpes de BALs reduzem a virulência das bactérias patogénicas testadas. Adicionalmente, as estirpes LB95 e LB13 apresentaram sempre características iguais ou superiores as dos probióticos de referência. Nos ensaios com L. monocytogenes e Salmonella spp., a estirpe LB95 apresentou os efeitos mais significativos na atenuação da virulência. Nos ensaios com E. coli O157:H7, a estirpe LB13 apresentou o efeito mais significativo na atenuação da virulência, e também a estirpe LB95 quando em co-cultura com a estirpe LB13 e a patogénica.
Em conclusão, os resultados obtidos sugerem que as estirpes de BALs testadas neste estudo,
L. plantarum (LB95) e L. paraplantarum (LB13) são capazes de prevenir as infecções por
agentes patogénicos. Logo, poderiam ser consideradas como estirpes com potenciais interessantes para a criação de novos alimentos probióticos de origem vegetal.
Palavras-chaves:
Probióticos; Lactobacillus spp.; bactérias patogénicas de origem alimentar; ensaios de virulência; cultura de células animais.
vi
Table of contents
ACKNOWLEDGEMENTS ... I ABSTRACT ... II RESUMO EXPANDIDO ... III FIGURE INDEX ... VIII
1. INTRODUCTION ... 1
1.1. FOODBORNE DISEASES ... 1
1.2. ESCHERICHIA COLI O157:H7 ... 1
1.2.1.History and epidemiology ... 1
1.2.2.Shiga toxins ... 2
1.3. SALMONELLA ... 4
1.3.1.Antigenic structure ... 4
1.3.2.Habitat ... 4
1.3.3.Human salmonellosis ... 4
1.3.4.Cell invasion pathways ... 5
1.4. LISTERIA MONOCYTOGENES ... 6
1.4.1.Listeriosis ... 6
1.4.2.Intracellular life cycle ... 7
1.5. PROBIOTICS ... 8
1.5.1.Description of probiotics ... 8
1.5.2.Beneficial properties of probiotics ... 8
1.5.3.Competitive exclusion ... 10
1.6. AIM AND FRAMEWORK OF THE STUDY ... 11
2. MATERIALS AND METHODS ... 12
2.1. BACTERIAL STRAINS ... 12
2.2. CULTURE MEDIA………..…………..12
2.3. CELL LINE AND CULTURE CONDITIONS ... 12
2.4. PLAQUE FORMING ASSAY (PFA) ... 13
2.5. PLAQUE FORMING ASSAY (PFA) IN THE PRESENCE OF PROBIOTICS ... 13
2.6. ADHESION AND INVASION ASSAY ... 14
2.6.1.Adhesion of the strains ... 14
2.6.2.Adhesion in the presence of probiotic bacteria ... 14
2.6.3.Invasion assay of pathogenic strains ... 14
2.6.4.Invasion assay in the presence of probiotic bacteria ... 15
2.7. CYTOTOXICITY ASSAY ... 15
vii
2.7.2.Cell free supernatant from co-cultures (CFSC) ... 16
2.7.3.Toxin preparation ... 16
2.7.4.Cytotoxicity assay with toxins from CFSC ... 17
2.7.5.Cytotoxicity assay with CFSs ... 17
2.8.EXPRESSION OF THE RESULTS AND DATA ANALYSIS ... 18
3. RESULTS AND DISCUSSION ... 19
3.1. ADHESION OF THE STRAINS TO HT-29 CELLS ... 19
3.2. INVASION CAPABILITY OF ENTEROPATHOGENIC STRAINS TO HT-29 CELLS... 20
3.3. THE EFFECT OF LABS ON THE VIRULENCE POTENTIAL OF SALMONELLA ... 21
3.4. THE EFFECT OF LAB ON THE VIRULENCE POTENTIAL OF L. MONOCYTOGENES ... 23
3.5. THE EFFECT OF LAB ON THE CYTOTOXIC EFFECTS OF E. COLI O157:H7 TO VERO CELLS ... 26
4. CONCLUSION... 29
viii
Figure Index
FIGURE 1-NEW WORKING MODEL FOR EHEC INFECTION IN HUMANS. ... 3
FIGURE 2-INVASION OF INTESTINAL MUCOSA BY SALMONELLA ... 5
FIGURE 3-TRIGGER AND ZIPPER MECHANISMS USED BY SALMONELLA TO ENTER CELLS. ... 6
FIGURE 4-SCHEMATIC REPRESENTATION OF LISTERIAINTRACELLULAR LIFE CYCLE ... 7
FIGURE 5-SCHEMATIC DIAGRAM ILLUSTRATING POTENTIAL OR KNOWN MECHANISMS WHEREBY PROBIOTIC BACTERIA MIGHT IMPACT ON THE MICROBIOTA. ... 9
FIGURE 7-BACTERIAL ADHESION TO HT-29 CELLS. ... 19
FIGURE 8- ENTEROPATHOGENIC INVASION OF HT-29 CELLS ... 20
FIGURE 9- ADHESION OF SALMONELLA STRAINS TO HT-29 CELLS. ... 21
FIGURE 10- INVASION OF SALMONELLA STRAINS TO HT-29 CELLS. ... 22
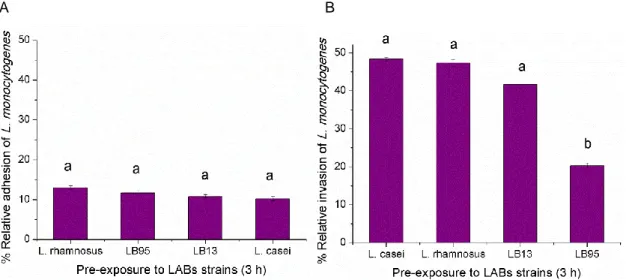
FIGURE 11-COMPETITIVE EXCLUSION OF LISTERIA MONOCYTOGENES (LM) TO HT-29 CELLS BY LAB STRAINS.(A)ADHESION; (B)INVASION. ... 24
FIGURE 12-THE VIRULENCE POTENTIAL OF L. MONOCYTOGENES STRAIN EGDE WITH AND WITHOUT THE PREVIOUS EXPOSURE OF THE HUMAN CELL MONOLAYERS TO LAB STRAINS FOR 3 H. ... 25
1
1. Introduction
1.1.
Foodborne diseases
Microbiological safety of foods and foodborne illnesses are complex issues since there are more than 200 known diseases that are transmitted through foods. Primary causative agents of foodborne illness are viruses, bacteria, parasites, microbial toxins, and prions. The symptoms of these foodborne illnesses range from mild gastroenteritis to life threatening neurologic, hepatic, and renal syndromes. The European Food Safety Authority (EFSA) and the European Centre for Disease Prevention and Control (ECDC) has estimated that a total of 5,648 foodborne outbreaks were reported in the European Union (EU), resulting in 69,553 human cases, 7,125 hospitalisations and 93 deaths in 2011 (EFSA and ECDC, 2013).
A total of 9,485 confirmed verotoxigenic Escherichia coli (VTEC) infections were reported in 2011. Most of these cases were caused by the serogroup O157. This represents an increase of 159.4% compared with 2010 as a result of the large STEC/VTEC outbreak that occurred in 2011 in the EU, primarily in Germany (EFSA and ECDC, 2013). Salmonella was the most frequently cause of reported foodborne outbreaks in 2011, with 95,548 cases confirmed. As in previous years, S. Enteritidis and S. Typhimurium were the most frequently reported serovars (44.4% and 24.9%, respectively), of all known reported serovars in human cases. Human S. Enteritidis cases are most commonly associated with the consumption of contaminated eggs and poultry meat, while S. Typhimurium cases are mostly associated with the consumption of contaminated pig, bovine and poultry meat (EFSA and ECDC, 2013). The number of confirmed human listeriosis cases was 1,476, in 2011. Listeriosis represents the most severe human foodborne disease in terms of hospitalisation and fatal cases (12.7%), reflecting the focus of the EU listeriosis surveillance on severe, systemic infections (EFSA and ECDC, 2013).
1.2.
Escherichia coli O157:H7
1.2.1. History and epidemiology
Escherichia coli was first discovered in 1885 by Theodore Escherich as a gram-negative,
facultative anaerobe, which typically inhabits the lower intestinal tract of many warm-blooded animals. In humans, the microorganism is acquired as a commensal bacterium in the colon shortly after weaning, and persists for life as part of the gut microbiota within the lumen and mucus layer of the large intestine (Kaper et al., 2004).
Although E. coli O157:H7 is a human pathogen responsible for numerous infectious outbreaks worldwide, it is also a resident commensal bacterium commonly found in the intestinal tract of ruminants such as cattle, sheep, goats, and deer (Karch et al., 2005). Human exposure to this pathogen is classically associated with the ingestion of undercooked ground beef, but infections
2 can also arise following the ingestion of fecally contaminated foodstuffs such as fruits, vegetables, drinking water, as well as person-to-person contact (Karch et al., 2005).
Pathogenic E. coli evolved from commensal E. coli through the acquisition of multiple virulence determinants, such as toxins, adhesins, and secreted effector proteins that modulate multiple host responses, through the acquisition of mobile virulence plasmids, phages, and pathogenicity islands (PAI). The combined effects of different virulence factors determine the extent of E. coli pathogenesis and the severity of human disease. Infectious strains resulting in common diseases are grouped as pathotypes, including: adherent-invasive E. coli (AIEC), diffusely adherent E. coli (DAEC), enteroaggregative E. coli (EAEC), enterohemorrhagic E. coli (EHEC), enteroinvasive E. coli (EIEC), enteropathogenic E. coli (EPEC), atypical enteropathogenic E.
coli (ATEC), enterotoxigenic E. coli (ETEC), sepsis/meningitis causing E. coli (MNEC), and
uropathogenic E. coli (UPEC) (Croxen and Finlay, 2010).Within each pathotype, E. coli strains are further characterized by antigenic variants including O-antigen (lipopolysaccharide), H-antigen (flagellar), and K-H-antigen(capsular) types (DebRoy et al., 2011). For instance, the most common serotype within EHEC is O157:H7 also definited VTEC (verotoxinogenic E. coli). EHEC causes acute renal failure in children, hemolytic uremic syndrome (HUS) that can also occur in adults. Characteristic features of the syndrome are microangiopathic anemia, thrombotic thrombocytopenia, and renal failure (Marshall et al., 2006). Long term complications of EHEC infection include irritable bowel syndrome (IBS).EHECs, including O157:H7, are non-invasive enteric bacterial pathogens that can cause both sporadic cases and outbreaks of diarrheal disease in humans, as well as hemorrhagic colitis and hemolytic-uremic syndrome (DuPont, 2009). The infective dose of EHEC O157:H7 is estimated to be very low, in the range of 10 to 100 cells (FDA, 2012).
1.2.2. Shiga toxins
Whitin the major virulence factors attributed to EHEC pathogenesis are Shiga toxins. The cytotoxic effects of these toxins were first documented on Vero cells (African green monkey kidney epithelial cells) in 1977 (Konowalchuk et al., 1977) hence the name VTEC, and later confirmed in 1983 from a patient with an E. coli O157:H7 infection and hemolytic-uremic syndrome (Karmali et al., 1983). Subsequent studies showed that multiple E. coli serotypes elicited the same cytotoxic effects (Karmali et al., 1985), and further studies showed that EHEC contains two Shiga toxins encoded by bacteriophages (Stx1 and Stx2) (Obrig, 2010).
Colonization of the large intestine by EHEC O157:H7 results in an ‘‘attaching-and-effacing’’ lesion characterized by an actin-rich pedestal formed by the host cell around the bacteria, destruction of brush border microvilli, and intimate adhesion of the pathogen to the enterocyte surface (Natarro and Kaper, 1998; LeBlanc, 2003). Two bacterial genes expressed from a chromosomal pathogenicity island termed the locus for enterocyte effacement (LEE) contribute to this pattern of adherence. Intimin, the product of the eae gene, is a major outer membrane
3 surface adhesin (Jerse and Kaper, 1991). The translocated intimin receptor (Tir) is a transmembrane protein secreted through the type III secretion system of the LEE locus that is inserted into the host cell membrane to serve as a receptor for intimin (Natarro and Kaper, 1998; LeBlanc, 2003)
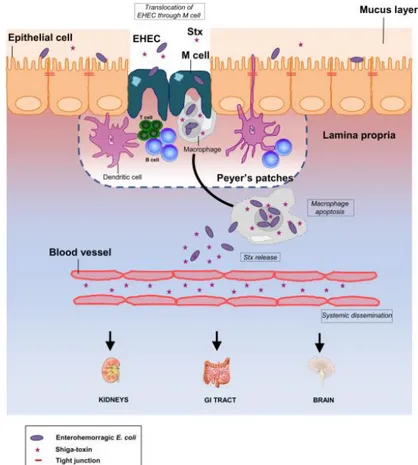
Etienne and co-workers (2011) proposed a new model for EHEC infection in humans (Fig. 1) and suggested that bacteria cross the intestinal barrier through M cells overlying Peyer’s patches (PPs). In the lamina propria, bacteria could enter, survive, and produce Stx within resident macrophages, inducing host cell apoptosis. In this case, however, bacteria would be eventually killed without causing bacteremia. Subsequently, released Stx would cross the downstream blood vessels to reach the kidneys, intestine, and brain leading to severe disease in humans. The study of the relationship between interactions with M cells and the development of the disease could help in designing novel therapeutic approaches to EHEC infection (Etienne
et al., 2011).
Figure 1 - New working model for EHEC infection in humans.
The diagram shows a monolayer of intestinal epithelial cells with EHEC infection in the lumen. Stx production occurs in the intestine. The bacteria cross the intestinal barrier through M cells. In the lamina propria, bacteria enter, survive, and produce Stx within resident macrophages. Following replication of bacteria in macrophages, extensive Stx production induces host cell death. Subsequently, released Stx could cross the downstream blood vessels to reach the kidneys, intestine, and brain. Damage to these organs results in serious life-threatening complications in humans (Etienne et al., 2011).
4
1.3.
Salmonella enterica
Salmonella is another member of the Enterobacteriaceae family, a large group of
gram-negative, facultative anaerobic and nonspore-forming bacilli (Guibourdenche et al., 2010).
1.3.1. Antigenic structure
The genus Salmonella is divided into two species: S. enterica and S. bongori. S. enterica is further divided into six subspecies, and most zoonotic Salmonella belong to the subspecies S.
enterica subsp. enteric. The agglutinating properties of the somatic O, flagellar H, and capsular
Vi antigens are used to differentiate more than 2600 serologically distinct Salmonella (Guibourdenche et al., 2010). Salmonella nomenclature is now based on the name of serotypes belonging to subspecies. For example, Salmonella enterica subsp. enterica serotype Typhimurium is shortened to Salmonella Typhimurium (Brenner et al., 2000).
1.3.2. Habitat
The common reservoir of Salmonella is the intestinal tract of a wide range of domestic and wild animals, which may result in a variety of foodstuffs of both animal and plant origin becoming contaminated with faecal organisms, either directly or indirectly. Transmission often occurs when organisms are introduced in food preparation areas and are allowed to multiply in food,
e.g. due to inadequate storage temperatures, inadequate cooking or cross contamination of
ready-to-eat (RTE)food. The organism may also be transmitted through direct contact with infected animals, or humans, or faecally contaminated environments. Infected food handlers may also act as a source of contamination for foodstuffs (Todar, 2005).
1.3.3. Human salmonellosis
Human salmonellosis is usually characterized by acute onset of fever, abdominal pain, nausea, and sometimes vomiting, after an incubation period of 12-36 hours. Symptoms are often mild and most infections are self-limiting, lasting a few days. However, in some patients, the infection may be more serious and the associated dehydration can be life threatening. When Salmonella causes systemic infections, such as septicaemia, effective antimicrobials are essential for treatment. Salmonellosis has also been associated with long-term and sometimes chronic sequelae, e.g. reactive arthritis. Mortality is usually low, and less than 1% of reported
Salmonella cases have been fatal (EFSA and ECDC, 2013). The infective dose is estimated to
be as low as one cell, depending on age and health of host and strain differences among members of the genus (FDA, 2012).
5
1.3.4. Cell invasion pathways
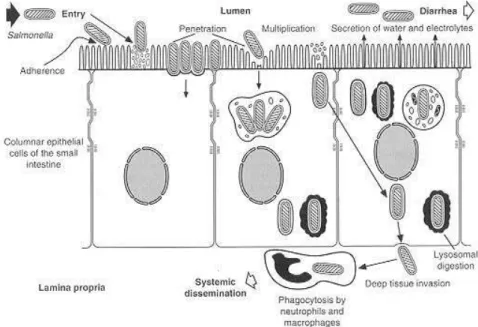
The ability to invade cells, the ability to replicate intracellularly, the elaboration of toxins and a complete lipopolyssacharide coat are a variation of attributes know as virulence factors needed by Salmonella to be fully pathogenic. The organisms colonise the ileum and colon after ingestion; then they invade the intestinal epithelium and reproduce within the epithelium and lymphoid follicles. This mechanism occurs after binding to specific receptors on the epithelial cell surface (Fig. 2) (Giannella, 1996).
Figure 2 - Invasion of intestinal mucosa by Salmonella (Giannella, 1996).
An essential feature of the pathogenicity of Salmonella is its interaction with phagocytic and nonphagocytic cells. Salmonella entry into host cells is known to be critical for bacterial survival and establishment of disease in a host. In general, intracellular bacterial pathogens enter nonphagocytic eukaryotic cells via two mechanisms, which are initially differentiated according to morphological criteria based on membrane remodeling. The “Trigger” mechanism involves dramatic cytoskeletal rearrangements known as “membrane ruffles” (Fig. 3A). In contrast, in the “Zipper” mechanism, or “receptor-mediated entry,” the invading bacteria are tightly bound to the host cell membrane, and only minor cytoskeletal protein rearrangements are initiated by specific contact between bacterial ligands (invasin) and host cell surface receptors (Fig. 3B) (Velge et
6
Figure 3 - Trigger and Zipper mechanisms used by Salmonella to enter cells.
(A) Scanning electron microscopy of Salmonella entering into cells via a Trigger mechanism, which is characterized by the apparition of large membrane ruffles at the bacterial entry site. (B) Scanning electron microscopy of Salmonella entering into cells via a Zipper mechanism, this is characterized by weak membrane rearrangements (Velge et al., 2012).
An important mechanistic difference between the Trigger and Zipper modes of entry is that the former is triggered from “inside” via the action of bacterial effectors delivered by secretion systems, whereas the latter is promoted from “outside” through activation of host cell receptors. However, in both cases, bacteria hijack the cell’s physiological processes through the modulation of existing cell signaling cascades. It has recently been reported that Salmonella is the first bacteria shown to be able to enter cells using both these mechanisms (Rosselin et al., 2010).
1.4.
Listeria monocytogenes
L. monocytogenes is a gram-positive, motile, non-spore forming, rod shaped bacterium. It can
multiply at temperatures around 0 °C and it can survive even at freezing temperatures. It is facultative anaerobic and it can grow in the presence of 10-12% sodium chloride. Certain strains may grow at water activity as low as 0.90. Heat resistance of L. monocytogenes increases as the water activity of the food matrix decreases. Therefore, it creates problems for food manufacturers who use low water activity and heat treatments to maintain food safety.
1.4.1. Listeriosis
The bacterial genus Listeria currently comprises 10 species, with two new species described in 2013 (Bertsch et al., 2013; Lang Halter et al., 2013), but human cases of listeriosis are almost exclusively caused by the species L. monocytogenes.
L. monocytogenes causes disease in certain high-risk groups including, pregnant women,
neonates, the elderly and immunocompromised in general. Occasionally, listeriosis occurs in healthy adults. A person infected with L. monocytogenes usually shows flu-like symptoms
7 initially; i.e., fever, muscle aches, and sometimes gastrointestinal symptoms such as nausea or diarrhea. In non-pregnant adults, L. monocytogenes can also enter in the bloodstream and cause septicemia. If the infection spreads to the nervous system, then it can cause meningitis and meningoencephalitis. The mortality rate of L. monocytogenes infection is 20-25%. Infected pregnant women generally only display mild flu-like symptoms. However, infection during pregnancy can lead to miscarriage, premature delivery, infection of the newborn with serious long-term consequences, or even stillbirth (Vazquez-Boland et al., 2001). The infective dose of
L. monocytogenes is undetermined, but is believed to vary with the strain and susceptibility of
the host, and the food matrix involved also may affect the dose-response relationship. In cases associated with raw or inadequately pasteurized milk, for example, it is likely that fewer than 1,000 cells may cause disease in susceptible individuals. As noted, however, the infective dose may vary widely and depends on a variety of factors (FDA, 2012).
1.4.2. Intracellular life cycle
In addition to multiplying within macrophages, L. monocytogenes organisms are invasive pathogens that can induce their own internalization in various types of cells that are not normally phagocytic. These include epithelial cells, fibroblasts, hepatocytes, endothelial cells, and various types of nerve cell, including neurons. The cycle begins with adhesion to the surface of the eukaryote cell and subsequent penetration of the bacterium into the host cell (Fig. 4) (Hamon et al., 2012). The invasion of nonphagocytic cells involves a Zipper mechanism, in that the bacterium gradually sinks into dip like structures of the host cell surface until it is finally engulfed (Vazquez-Boland et al., 2001).The multisystemic nature of listerial infection indicates that L. monocytogenes probably recognizes a number of different eukaryotic receptors.
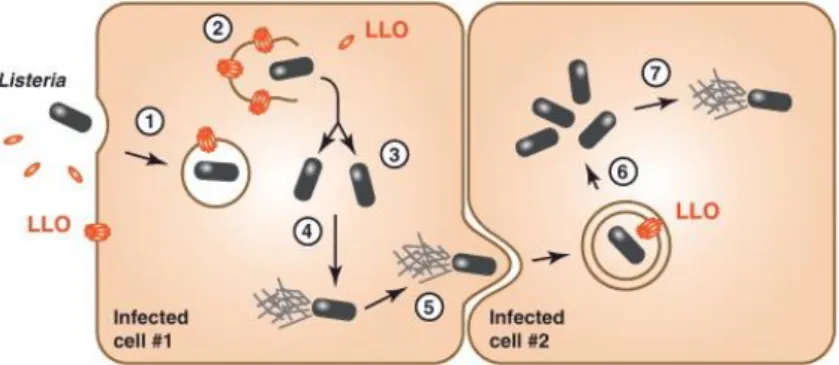
Figure 4 - Schematic representation of Listeria intracellular life cycle.
After inducing its own uptake by receptor-mediated phagocytosis, Listeria is entrapped in a phagosome (1), which it destabilizes by expressing listeriolysin O (LLO) and two broad-range phospholipases (PLC), PC-PLC and PI-PLC, allowing bacterial escape (2). Intracellular LLO seems to be rapidly degraded to avoid inside-out damaging of host cell membranes. Cytosolic bacteria replicate (3) and express ActA, inducing host cell actin polymerization to propel the bacterium across the cytosol allowing spread to neighboring cells (5). There, Listeria is entrapped in a double-membrane vacuole, employing LLO and PLC expression and possibly additional molecular mechanisms to disrupt it (6) and start the intracellular replication cycle again (7) (Hamon et al., 2012).
8
L. monocytogenes specifically targets its receptor on intestinal villi and crosses the intestinal
epithelium to disseminate systemically. Nikitas and co-workers (2011) demonstrated that receptor E-cadherin (Ecad) is luminally accessible around mucus-expelling goblet cells (GCs), around extruding enterocytes at the tip and lateral sides of villi, and in villus epithelial folds. It has been shown that upon preferential adherence to accessible Ecad on GCs, L.
monocytogenes is internalized, rapidly transcytosed across the intestinal epithelium, and
released in the lamina propria by exocytosis from where it disseminates systemically.
1.5.
Probiotics
There is an increasing amount of evidence indicating health benefits by consumption of food containing probiotics which can be use in the prevention and treatment of gastrointestinal diseases caused by pathogenic microorganisms or by disturbances in the normal microbiota (O’Sullivan et al., 2002).
1.5.1. Description of probiotics
Probiotics are defined as live, non-pathogenic microorganisms that confer health benefits to the host, and are increasingly being employed as an option for preventing and treating bacterial infections (Gareau et al., 2010). Probiotics include multiple bacterial species, from different genera such as Lactobacilli, Bifidobacteria, and Streptococci which have been found to generally promote and maintain a balanced intestinal microenvironment, and may prevent active viral and bacterial infections (Gareau et al., 2010).Health promoting skills of these microbes were first documented in 1908 by Elie Metchinikoff in his book “Prolongation of Life” (reviewed by Gareau et al., 2010).
Lactic acid bacteria (LAB) form a phylogenetically diverse group, widely distributed in nature and defined as gram-positive, non-sporulating, catalase-negative, devoid of cytochromes, aerotolerant, fastidious, acid tolerant and strictly fermentative bacteria that secrete lactic acid as their major end product of sugar fermentation (Mitsuoka, 1992; Axelsson, 1998).
Requirements of LAB strains as probiotics include their tolerance to the acid and bile conditions in host gastrointestinal tract, their capabilities to adhere to the host intestinal epithelium, and also their antagonistic effect against pathogenic bacteria as well as their immunomodulating activity in hosts (Delgado et al., 2007; Wu et al., 2007)
1.5.2. Beneficial properties of probiotics
Mechanisms of probiotic action described to date include adhesion to the intestinal-lumen interface; competition with pathogens for receptor binding, nutrients and colonization; enhancement of mucosal barrier function; promotion of innate and adaptive immune responses;
9 elaboration of bacteriocins; and modulation of cell kinetics, with further mechanisms of action likely to be identified (Howarth et al., 2010).
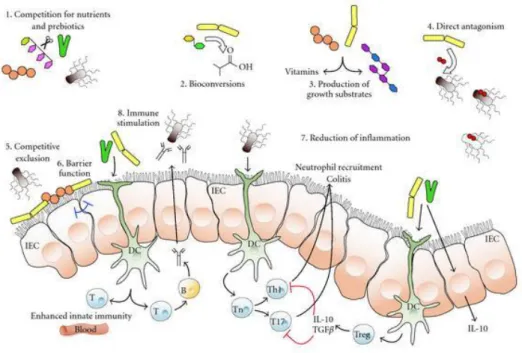
Figure 5 shows a schematic overview of the potential mechanisms whereby probiotic microorganisms might influence the intestinal microbiota (Toole and Cooney, 2008).
Figure 5 - Schematic diagram illustrating potential or known mechanisms whereby probiotic bacteria might impact on the microbiota.
These mechanisms include (1) competition for dietary ingredients as growth substrates, (2) bioconversion of, for example, sugars into fermentation products with inhibitory properties, (3) production of growth substrates, for example, EPS or vitamins, for other bacteria, (4) direct antagonism by bacteriocins, (5) competitive exclusion for binding sites, (6) improved barrier function, (7) reduction of inflammation, thus altering intestinal properties for colonization and persistence within, and (8) stimulation of innate immune response (by unknown mechanisms).
IEC: epithelial cells; DC: dendritic cells; T:T-cells (Toole et al., 2008).
Probiotics elicit their beneficial effects through a diverse array of mechanisms, ranging from secreting antimicrobial products to modulating host immune pathways. As reviewed by Sherman et al. (2009), certain probiotics secrete antibacterial products, such as bacteriocins, that inhibit the growth and virulence of pathogenic bacteria (Corr et al., 2007), while other lactic acid producing probiotics suppress pathogen growth by altering the pH in the local microenvironment (Fayol-Messaoudi et al., 2005), or inhibit enteropathogenic infection through acetate production (Fukuda et al., 2011).
10 In several cases, ingestion of these bacteria was found effective in preventing and treating diarrhea, lactose intolerance, constipation, acute gastritis, food allergies, atopic dermatitis, Crohn’s disease, rheumatoid arthritis, pelvic radiotherapy treatment, intestinal inflammation, colon cancer, small bowel bacterial over growth, immune modulation, cholesterol control, antihypertensive effect and urogenital infections (Salminen et al., 1998; Sanders and Huisin’t Veld, 1999). Adhesion to intestinal epithelium is one of the most important selection criteria for probiotics (Salminen et al., 1998). Gorbach (2000) stated that the ability of the microbe to attach to the intestinal enterocytes enhances their therapeutic activity. This may lead to competitive displacement of intestinal pathogens, and engagement of cell membrane receptors, which activate signaling events that lead to cytokine synthesis, including interferons, and cell resistance to viral attack (O’Sullivan, 2001).
Probiotic organisms also alter the composition of the microbiota by production of lactic acid, and bacteriocins, which are active against pathogens. Probiotic bacteria have shown antibacterial activity against several pathogens including Escherichia coli, Clostridium spp., Bacteroides,
Salmonella enterica, etc. (Khedkar et al., 1990; Collado et al., 2005). Recently, Archambaud
and co-workers (2012) demonstrated that probiotcs were able to limit the L. monocytogenes dissemination in a gnotobiotic humanized mouse model. Other benefits of probiotics include production of mucosal micronutrients, elimination of toxins and reduction of fecal ammonia (Bezkorovainy, 2001; Ouwehand et al., 2002).Adhesion helps in increasing the residence period of probiotic bacteria in the Gastrointestinal (GI) tract, resisting peristaltic stress and stimulating immune system (Granato et al., 1999; Kalliomaki and Isolauri, 2004; Kinoshita et al., 2007).
1.5.3. Competitive exclusion
The competition for space to adhere between indigenous bacteria and exogenous pathogens result in the competitive exclusion of pathogenic bacteria (Ohashi, 2009). Competitive exclusion refers to physical blocking of pathogenic bacteria colonization by probiotic bacteria from their favorite site such as intestinal villus, goblet cells and colonic crypts (Chichlowski et al., 2007). The probiotic bacteria alter the physical environment of the intestines in such a way that pathogenic bacteria cannot survive. Probiotic bacteria exclude the opportunistic bacteria in two ways. First, the probiotic bacteria compete with pathogenic bacteria for nutrients and energy source thus, preventing them from acquiring energy required for growth and proliferation of pathogenic bacteria in the gut environment (Cummings and Macfarlane, 1997).Second, probiotics produce several organic acid and Volatile Fatty Acids (VFA) as a result of their metabolism and fermentation. Consequently, the pH of the gut is lowered below that essential for survival of pathogenic bacteria such as E. coli and Salmonella (Marteu et al., 2004; Chichlowski et al., 2007). Probiotic bacteria also eject the colonization of pathogenic bacteria by attaching themselves to the surface of the gut thus preventing the adhesion of the pathogenic bacteria to gastrointestinal epithelium.
11
1.6.
Aim and framework of the study
The aim of this work was to investigate the role of two selected Lactobacillus spp. strains (LABs), isolated from spontaneously fermenting olive brines, in the attenuation of the virulence potential of foodborne pathogenic bacteria (Salmonella enterica, L. monocytogenes and E. coli O157:H7). Two probiotic strains, L. casei Shirota and L. rhamnosus GG, were used as references. This work was performed under the scope of FCT – project NEW PROTECTION: NativE, Wild PRObiotics Train EffecCT In Olives in brine (PTDC/AGRALI/117658/2010). The overall objective of the project is to generate fundamental knowledge on, and confirm applicability of selected Lactobacillus spp. strains for eventual rational design of probiotic brined olives and olive pastes.
The work presented here focused on in vitro assessment of the ability of LABs to adhere to the intestinal mucosa, and to counteract bacterial infections in the gut. The competitive exclusion properties of the LAB strains, by blocking of adhesion sites of Salmonella and L.
monocytogenes, to a HT-29 intestinal cell line, as well as the effect of their metabolites on the
12
2. Materials and Methods
2.1.
Bacterial strains
The two Lactobacillus strains used as probiotics reference strains were: Lactobacillus casei Shirota (ACA-DC 6002) and Lactobacillus rhamnosus GG, provided by Laboratory of Microbiology and Biotechnology of Food at the Agricultural University of Athens (Iera Odos, Greece). The two selected LAB used in this study came from industrial and homemade fermented table olives of Portuguese cultivars: Lactobacillus paraplantarum (LB13) and
Lactobacillus plantarum (LB95), provided by Institute de Tecnologia Química e Biológica
(ITQB). The pathogenic strains used: Listeria monocytogenes EGDe (wild-type serovar 1/2a strain) obtained from Institut National de la Recherche Agronomique, Tours, Escherichia coli
O157:H7 from American Type Culture Collection (ATCC43895), E. coli B, from Coleção de Bactérias do Instituto Superior de Agronomia, Salmonella enterica subsp. enterica serovar Typhimurium (ATCC 14028) and Salmonella enterica subsp. enterica serovar Enteritidis from Instituto de Ciência Aplicada e Tecnologia (ICAT).
2.2.
Culture media
All strains were grown at 37 °C in the following media: E. coli strains were grown with shaking in brain heart infusion (BHI) broth or onto BHI plates [(BHI supplemented with 1.5% (w/v) agar)] or onto Tryptone Soya Yeast Extract Agar [(TSA-YE with 1.5% (w/v) agar)] or onto Tryptone Bile X-Glucuronide (TBX agar). L. monocytogenes strains were grown onto TSA-YE or onto PALCAM. Salmonella strains were grown onto TSA-YE or onto Xylose Lysine Deoxycholate agar (XLD agar). The strains L. casei Shirota, L. rhamnosus GG, LB13 and LB95 were grown in Man Rogosa Sharpe broth (MRS) or onto MRS plates [(MRS supplemented with 1.5% (w/v) agar)]. Afterwards, strains were grown in the condition described for the virulence assays.
2.3.
Cell line and culture conditions
The human adenocarcinoma cell line HT-29 (ECACC nº91072201) was used between passages 34 and 64 and the Vero cell line (ATCC Cell Biology Collection nºCCL-81) was used between passages 68 and 71.
Cells were routinely grown in 75 cm2 flasks (Orange Scientifique, Braine-l’Alleud, Belgium) in RPMI 1640 (Roswell Park Memorial Institute) (Sigma/DE) with fetal bovine serum (FBS) [(10% (v/v)] and L-glutamine (2 mM) (complete medium). Penicillin (100 IU mL-1) and streptomycin (100 μg mL-1
) were always added to the culture medium, except to the medium used 24 h prior to the virulence assays. Cells were maintained in a humidified atmosphere using an incubator at 37 ˚C under 5% (v/v) CO2 in air.
13
2.4.
Plaque Forming Assay (PFA)
HT-29 cells were trypsinized from the 75 cm2 flasks and 3 x 104 cells were deposited per well in a 96-well tissue culture plate. To obtain confluent monolayers, the plates were incubated for 3 days with antibiotics followed by incubation for 24 h without antibiotics.
The overnight grown L. monocytogenes (TSA-YE, 37 ˚C) were suspended in buffered saline to a concentration of 4 x 108CFU mL-1 and serial dilutions were made in complete medium. HT-29 cell monolayers were infected with dilution series of 102 to 107 L. monocytogenes CFU per well,
and incubated for 2 h at 37 ˚C. The inocula concentration was confirmed by duplicate plating of the appropriate dilutions onto TSA-YE and incubation at 37 ˚C for 24 h, before counting colonies.
After 2 h incubation with the bacterial cells, the suspensions were removed and the monolayer washed twice with phosphate buffer saline (PBS), pH 7.2. Cell monolayers were then incubated for 1.5 h with complete RPMI 1640 medium containing 100 μg gentamicin mL-1
. The wells were then covered with complete RPMI medium supplemented with 10 μg gentamicin mL-1
and 2.5% (w/v) agarose. Once the agar media was solidified, complete medium was added to the top of the agar media to prevent cell starvation. Culture plates were incubated for 24 to 48 h at 37 ˚C under 5% (v/v) CO2 in air. Formed plaques were counted 24 h after bacteria deposition on the
HT-29 cell monolayer, and confirmed after 48 h of incubation. Enumeration of the plaques was done using an inverted microscope (Roche et al., 2001; Neves et al., 2008).
2.5.
Plaque Forming Assay (PFA) in the presence of probiotics
The probiotic strains cultured in MRS medium were harvested by centrifugation at8123 g for 5 min. The bacterial pellet was resuspended in 10 mL of PBS and centrifuged as before. After removing the supernatant, the bacterial suspension was resuspended to 4 × 109CFU mL-1of complete RPMI 1640 without any antibiotic and 25 μL aliquots of these suspensions of each probiotic bacteria were added to each well of the tissue culture plate with the HT-29 monolayers. The microplates were incubated for 3 h at 37°C in 5% (v/v) CO2 in air. Afterwards,
the RPMI was removed and the monolayers were washed two times with PBS to remove non-attached bacterial cells. After this, the infection assay proceeded with the addition of L.
monocytogenes into the respective wells, in order to reach bacterial concentrations of 102 to 107
CFU/well. The microplates were subsequently incubated for 2 h at 37 °C with 5% CO2 in air,
and the assay proceeded as described before. The concentration of L. monocytogenes and probiotics were confirmed by duplicate plating of the appropriate dilutions onto TSA-YE and MRS, and incubation at 37 ˚C for 24 and 48 h, respectively.
14
2.6.
Adhesion and invasion assay
For adhesion and invasion assays, HT-29 cells were seeded in 24-well tissue culture plates for 3 days. Cells cultures were replenished with complete RPMI1640 without antibiotics, 24 h before the assays were performed. The adhesion and invasion of bacteria strains to epithelial cells were evaluated both separately and in a competitive exclusion assay.
In all the assays, LAB strains (108 CFU/well) and pathogenic strains (107 CFU/well) were used with HT-29 cells using multiplicity of exposure (MOE) of 100:1 and 10:1, respectively, in all assays.
2.6.1. Adhesion of the strains
Fresh overnight culture of each pathogenic strain was suspended in complete RPMI1640 medium. HT-29 monolayers in 24-well plates were then inoculated with 300 μl of suspension of a single strain per well in duplicate. Plates were then incubated for 1.5 h at 37 ºC with 5% CO2in
air. After this, the free bacteria were eliminated by washing the monolayer twice with 500 μl of PBS. Cells with adherent bacteria were harvested with 500 μl of Active-Trypsin-Versene (ATV) (0.5% Trypsin; 0.2% ETDA) and incubated for 15 min under the same conditions above. Adherent LAB were enumerated onto MRS, L. monocytogenes onto PALCAM, E. coli B onto TBX and Salmonella enterica. onto XLD agar plates and incubated for 24 to 48 h at 37ºC. The adhesion ability of the strains was expressed as the number of adherent bacteria divided by the total number of bacteria added, multiplied by 100.
2.6.2. Adhesion in the presence of probiotic bacteria
LAB strains were added to the wells with the HT-29 cell monolayers and incubated for 3 h. Nonattached bacteria were removed by washing with PBS as described above, and L.
monocytogenes or Salmonella strains were then added, separately, and incubated for 1 h. The
HT-29 monolayers were then washed and adherent bacteria were released with ATV and plated, as described above. The inhibition of the adhesion of the pathogenic bacteria in a competitive exclusion assay with the LAB strains was expressed as a relative percentage of the adhesion of the pathogen to HT-29 cells in the absence of previously incubation of HT-29 with LAB bacteria.
2.6.3. Invasion assay of pathogenic strains
Bacterial suspensions of pathogenic strains were added to HT-29 monolayers and incubated for 1.5 h at 37 ºC with 5% CO2 in air. After, the monolayers were washed twice with PBS. Complete
RPMI1640 medium containing 100 µg mL-1 gentamicin was added, followed by 1 h of incubation. Afterword, the medium was removed, the monolayer was washed with PBS and treated with ATV and bacteria were plated for enumeration of internalized bacteria, as described
15 above. The invasion ability of the strain was expressed as the number of invading bacteria divided by the total number of bacteria added, multiplied by 100.
2.6.4. Invasion assay in the presence of probio tic bacteria
LAB strains were added 3 h before the infections of HT-29 monolayers with the pathogenic strains, as described above for the adhesion assay. After, nonattached bacteria were removed by washing with PBS. Bacterial suspensions of pathogenic strains were then added to cell monolayers, and incubated for 1.5 h at 37 ºC with 5% CO2 in air.
After, the monolayers were washed twice with PBS, and RPMI 1640 containing 100 µg mL-1 gentamicin was added followed by 1 h of incubation. Afterword, the medium was removed, the monolayer was washed with PBS and treated with ATV and bacteria were plated for enumeration of internalized bacteria. The inhibition of the invasion of the pathogenic bacteria in a competitive exclusion assay with the LAB strains was expressed as a relative percentage of the invasion of the pathogen to HT-29 cells in the absence of previously incubation of HT-29 with LAB bacteria.
2.7.
Cytotoxicity assay
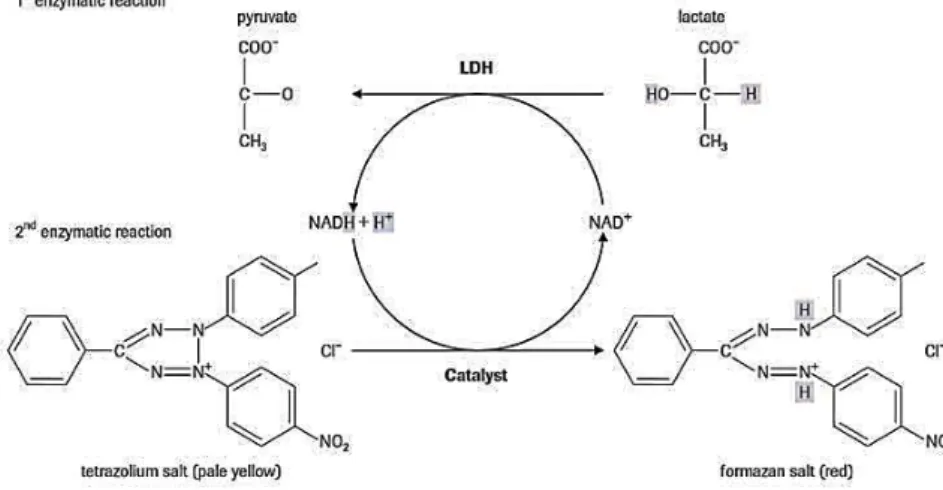
The cytotoxic potential of E. coli O157:H7 strain was evaluated based on the method described by Maldonado et al. (2005) with some modifications. This cytotoxicity test is based on the release of lactate dehydrogenase (LDH) from Vero cells and was assessed using Cytotoxicity Detection KitPLUS (Roche, Indianapolis, IN, EUA). The principle of the method is illustrated in Figure 6.
Figure 6 - Cytotoxicity test principle.
The cell-free culture supernatant is collected and incubated with the reaction mixture from the kit. The LDH activity is determined in an enzymatic test: In the first step, NAD+ is reduced to NADH/H+ by the LDH-catalyzed conversion of lactate to pyruvate. In the second step, the catalyst (diaphorase) transfers H/H+ from NADH/H+ to the tetrazolium salt INT which is reduced to formazan (Roche, Indianapolis, IN, EUA).
16 For the purpose of calculating percent cytotoxicity values, background control (LDH contained in the assay medium), low control (spontaneous LDH released) and the treated cells as high control (maximum LDH released) were considered (Roberts et al., 2001; Roche, 2006), as follows:
<
The effect of different LAB, on the attenuation of the cytotoxicity of E. coli O157:H7 strain on Vero cell monolayers was tested using bacterial cell free supernatant (CFS), or bacterial cell free supernatants from co-cultures (CFSC). In all the assays, E. coli O157:H7 was used as the positive and for the negative control was used the nonpathogenic E. coli B.
2.7.1. Cell free supernatant from pure cultures (CFS)
Lactobacillus spp. and E. coli cultures were grown separately under the conditions described
above. Aliquots (1.5 ml) from the cultures were centrifuged (8160 g, 3 min). Cell-free supernatants (CFS) were stored at 4°C.
2.7.2. Cell free supernatant from co-cultures (CFSC)
Pure cultures of each LAB strain and of the E. coli O157:H7 strains, grown as described before, were used to produce co-cultures of each LAB strain with E. coli O157:H7, in 20 ml of BHI. For the negative control, the LAB strain was replaced by E. coli B, in the co-culture with E. coli O157:H7. Incubation proceeded at 37 ºC with shaking for 24 h. After this period, the concentration of each strain in the co-culture ranged from 1.32 to 7.75 x 108 CFU mL-1 for the LAB strains, and from 2.43 to 9.75 x 108 CFU mL-1 for the E. coli strains. Aliquots (1.5 mL) from each co-culture were centrifuged (8160 g, 3 min). Cell-free supernatants from the co-cultures (CFSC) were stored at 4 °C
2.7.3. Toxin preparation
Each cell pellet (from 2.7.1. and 2.7.2.) was resuspended in 75 μL of a polymyxin B sulfate solution (2 mg mL-1) in order to release cell-bound toxins (Donohue-Rolfe and Keusch, 1983) and was incubated at 37°C for 30 min in a shaker incubator. Samples were centrifuged (8160 g, 5 min) and the supernatant combined with the original cell-free supernatants. Toxin preparations were then filtered with 0.2 μm pore size syringe filters (Millipore, Massachusetts, EUA), and the filtrates were, either used immediately or kept at 4 °C for a maximum of 24 h. % Cytotoxicity = {[(A490 nm -650 nm – (A background control) (A low control)]/Ahigh control – Alow control} × 100
17
2.7.4. Cytotoxicity assay with toxins from CFSC
For the cytotoxicity assay, Vero cells were used to inoculate 96-well plates (3 x 104 cells/well) and incubation proceeded for 24 h, at 37°C with 5% CO2 in air, until confluent monolayer.
After washing the cell monolayer twice with RPMI1640 (serum-antibiotics-free), the Vero cells monolayers were exposed to 50 μL of each bacterial toxin preparation and 50 μL of serum-antibiotics-free RPMI1640. For the background and low control, 100 μL serum-serum-antibiotics-free RPMI1640 was added. For high control, 95 μL of serum-antibiotics-free RPMI1640 was added. Each sample was tested in triplicate. After 12 h of incubation at 37°C in the presence of 5% CO2
in air, 5 μL of lysis solution was added to the well for high control, and incubated at 37°C for 15 min in an orbital incubator. The release of endogenous LDH from the cytosol of damaged cells was measured by dispensing 100 μL of reaction mixture [diaphorase NAD+, iodotetrazolium
chloride (INT) and sodium lactate], freshly prepared, per well. The microplate was incubated in
the dark, for 5 min at room temperature, according to the instructions provided in the kit. After this incubation, 50 μL of stop solution was added to the wells and shacked for 10 s. The absorbance at 490 and at 650 nm was measured in a microplate reader (model 680, BIO-RAD, Philadelphia, EUA).
2.7.5. Cytotoxicity assay with CFSs
For the cytotoxicity assay with CFSs, the Vero cell monolayers were washed twice with RPMI1640 medium (serum-antibiotics-free) and exposed to 25 μL of the E. coli toxin with 25 μL of CFS from each probiotic and 50 μL of serum-antibiotics-free RPMI1640. For the negative control, 25 μL of the E. coli toxin was mixed with 25 μL of CFS of E. coli B and 50 μL of serum-antibiotics-free RPMI1640. The rest of the procedure was as described in 2.7.4.
18
2.8. Expression of the results and data analysis
All the assays were performed with at least three independent trials. For the PFA, the pathogenic potential of the isolates was expressed as the mean log of the number of plaques formed (for 107 Listeria cells per well) (log PFA), in duplicate. For the adhesion and invasion
assays, the results were expressed as a percentage or a relative percentage. For the cytotoxicity assays, the obtained differences of absorbance’s (A490 - 650 nm) were submitted to a
rank transformation for normalizing the data. Conformance to the normality of the data generated by the PFA, adhesion and invasion and cytotoxicity assays was checked using the Anderson-Darling test, and conformance to the homogeneity of variance was determined using Levene’s test. The comparison between, the log PFA values, the percentages of adhesion and the percentages of invasion values, respectively, was then performed by analysis of variance (ANOVA) Tukey's multiple comparison tests. The comparison between the rank transformation cytotoxicity values was performed using Schoeffer comparison test.
Those values whose probability of occurrence were greater than 95% (p<0.05) were considered as significant values. All the statistical analysis, were performed by using the software Statistica (version 7.0; Statsoft, Tulsa, OK).
19
3. Results and discussion
3.1.
Adhesion of the strains to HT-29 cells
The ability to adhere to, or to colonize epithelial cells is essential and a prerequisite trait for probiotic bacteria (FAO/WHO, 2002; Kaushik et al., 2009). At the same time, adhesion of pathogens to the intestinal epithelial surface is a key first step in pathogenesis and is central to its colonization of the intestine (Finlay, 1997).
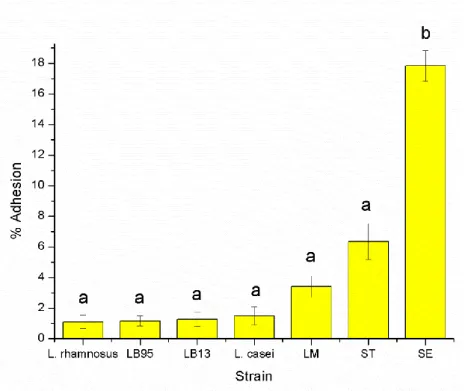
The attachment profiles, to HT-29 cells, of the lactic acid bacteria (LAB) strains, including two well-characterized probiotics (Lactobacillus casei Shirota and L. rhamnosus GG) and the enteropathogenic strains were investigated. Attachment of LAB varied from 0.68% to 1.8%.
Salmonella Enteritidis (SE) showed the highest rate of adhesion (17.84%) followed by Salmonella Typhimurium (ST) (6.35%) and Listeria monocytogenes (LM) (3.4%) (Fig. 7).
Figure 7 - Bacterial adhesion to HT-29 cells.
The percentage of adhesion was calculated relatively to the number of CFU initially added to the HT-29 cell monolayer. The data are average values of three independent experiments, performed in triplicate. In the columns, different letters indicate significative differences (P<0.05) between average values. The error bars represent standard deviations.
L. casei Shirota, L. rhamnosus GG, LB13, LB95, S. Typhimurium and L. monocytogenes
attached to HT-29 cells in significantly lower numbers (P<0.05) than those of S. Enteritidis. The obtained results suggest that there was not a significant difference (P>0.05) among adhesion of LAB strains to HT-29 cells. Tuomola and Salminen (1998) studied adhesion of 12
20 different Lactobacillus strains using Caco-2 cell line, as an in vitro model for intestinal epithelium, and also reported no significant differences in the adhesion of the strains.
The adhesion of S. Enteritidis was significantly higher (P<0.05) than the adhesion of S. Typhimurium. These in vitro findings suggest that S. Enteritidis has a higher ability to adhere to the intestinal epithelium than S. Typhimurium. These results could explain more cases reported of S. Enteritidis (44.4%) than of S. Typhimurium (24.9%) in 2011, in the EU (EFSA, 2013).
3.2.
Invasion capability of enteropathogenic strains to HT-29 cells
The abilities of one L. monocytogenes (EGDE) and two Salmonella strains (S. Enteritidis and S. Typhimurium) to invade HT-29 cells were compared. The three strains showed average invasion efficiencies relative to the initial number of bacteria inoculated in the well, ranging from 11.2 to 32.3% (Fig. 8). When the percentage of invasion of the Salmonella strains were compared, the results showed a significative difference (P<0.05) between the two strains. The results also showed that there was no significant difference (P>0.05) between the percentage of invasion displayed by L. monocytogenes and S. Typhimurium. These two strains were significantly more invasive (P<0.05) than S. Enteritidis.
Although, S. Typhimurium strain was able to invade HT-29 cells more efficiently than S. Enteritidis, its ability to adhere to the same cell line was significantly lower than the one displayed by S. Enteritidis (See 3.1.). This may suggest the importance of the adhesion ability of
Salmonella strains in the infection of the host.
Figure 8 - Enteropathogenic invasion of HT-29 cells.
The percentage of invasion was calculated relatively to the number of CFU initially added to the HT-29 cells. The data are average values of three independent experiments, performed in triplicate. In the columns, different letters indicate significative differences (P<0.05) between average values. The error bars represent standard deviations.
21
3.3.
The effect of LABs on the virulence potential of Salmonella
Anti-adhesiveness of the pathogens by probiotics can be caused by secretion of antimicrobial substances (organic acids, bacteriocin or hydrogen peroxide) by the probiotic strains, degradation of carbohydrate receptors by secretion of proteins, establishment of a biofilm, and production of receptor analogs and biosurfactants (Oelschlaeger, 2010). The inhibition of the pathogens could also be related with specific receptors that probiotics and pathogens are competing for, or with other factors such as co-aggregation of both strains.
Using a competitive exclusion assay, the effect of the pre-exposure on HT-29 cells to LABs, in the reduction of Salmonella adhesion was investigated. LAB strains were, separately, added to the HT-29 monolayers for a 3 h period of contact. After this, LABs were removed and
Salmonella was used to infect the HT-29 monolayers.
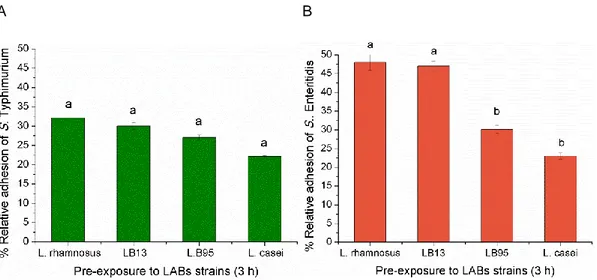
The results showed that the four tested LAB strains (L. rhamnosus, LB13, LB95 and L. casei) reduced the initial S. Typhimurium adhesion to 32, 30, 27, and 22%, respectively. Nevertheless, no significant (P>0.05) differences were detected among the effect of the LAB strains (Fig 9A). LAB pre-exposure of HT-29 cells also reduced S. Enteritidis adhesion from 48 to 23% and the results showed a significative difference (P<0.05) among LABs (Fig 9B). When the HT-29 cells were pre-exposure to LB95 and to L. casei, the relative percentage of Salmonella Enteriditis adhesion reduced significantly (P<0.05) (Fig 9B).
A B
Figure 9 - Adhesion of Salmonella strains to HT-29 cells.
The percentage of adhesion was calculated relatively to the number of CFU initially added to the HT-29 cells. The data are average values of three independent experiments, performed in triplicate. In the columns, different letters indicate significative differences (P<0.05) between average values. The error bars represent standard deviations.
22 Recently, in vitro and in vivo studies reported the antagonistic effect of certain LAB strains from human, animals and milk products, such as L. acidophilus, L. rhamnosus GG, L. casei,
Bifidobacterium globosum, B. breve and Enterococcus faecium, against invasion by S.
Typhimurium (Bernet et al., 1994; Hudault et al., 1997; Audisio et al., 1999; Gill et al., 2001; Tsai et al., 2005).
On the other hand, numerous studies have shown that adherent LAB strains are able to protect intestinal cells from infection by pathogenic bacteria (Naidu et al., 1999; Coconier et al., 2000). Apart from competitive exclusion, several other mechanisms responsible for inhibition of
Salmonella invasion, by LAB, have also been suggested. For example, organic acids, especially
lactic acid, or bacteriocin produced by viable LAB cells may also play roles in inhibiting the growth of pathogens in the gastrointestinal tract of animals or humans (Casey et al., 2004; Keersmaecker et al., 2006; Lima et al., 2007).
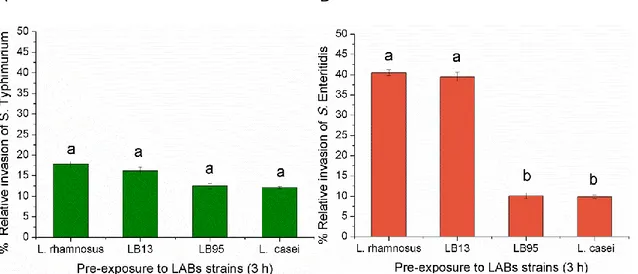
In the present study, all the LAB strains effectively (P<0.05) inhibited invasion of HT-29 cells by the two Salmonella strains. The relative percentage of invasion of S. Typhimurium ranged from 12 to 18%. Nevertheless, no significant (P>0.05) differences were detected among the effect of the LAB strains (Fig 10A). LAB pre-exposure of HT-29 cells also reduced the initial S. Enteritidis percentage invasion by 10 to 40% and with significant (P<0.05) differences among LABs (Fig 10B). In fact, when the HT-29 cells were pre-exposure with LB95 and L. casei, the relative percentage of Salmonella Enteriditis invasion reduced significantly (P<0.05) (Fig 10B). A B
Figure 10 - Invasion of Salmonella strains to HT-29 cells.
The percentage of invasion was calculated relatively to the number of CFU initially added to the HT-29 cells. The data are average values of three independent experiments, performed in triplicate. In the columns, different letters indicate significative differences (P<0.05) between average values. The error bars represent standard deviations.