UNIVERSIDADE DE TRÁS-OS-MONTES E ALTO DOURO
PAROXYSMAL DYSKINESIA IN DOGS
DISSERTAÇÃO DE MESTRADO INTEGRADO EM MEDICINA VETERINÁRIA
MELANIE JESUS ALMEIDA
ORIENTADOR:
UNIVERSIDADE DE TRÁS-OS-MONTES E ALTO DOURO
PAROXYSMAL DYSKINESIA IN DOGS
DISSERTAÇÃO DE MESTRADO INTEGRADO EM MEDICINA VETERINÁRIA
MELANIE JESUS ALMEIDA
ORIENTADOR:
Professor Doutor Artur Severo Proença Varejão
DECLARAÇÃO
NOME: Melanie Jesus Almeida
C.C.: 14051488
TELEMÓVEL: (+351) 910 566 948
CORREIO ELETRÓNICO: melaniejalmeida@gmail.com
DESIGNAÇÃO DO MESTRADO: Mestrado Integrado em Medicina Veterinária
TÍTULO DA DISSERTAÇÃO DE MESTRADO EM MEDICINA VETERINÁRIA: Paroxysmal Dyskinesia in Dogs
ORIENTADOR: Professor Doutor Artur Severo Proença Varejão
ANO DE CONCLUSÃO: 2016
Declaro que esta dissertação de mestrado é resultado da minha pesquisa e trabalho pessoal e das orientações dos meus supervisores. O conteúdo é original e as fontes consultadas estão devidamente mencionadas no texto e na bibliografia final. Declaro ainda que este trabalho não foi apresentado em nenhuma outra instituição parra obtenção de qualquer grau académico.
Vila Real, Outubro de 2016 Melanie Jesus Almeida
Acknowledgements
Acknowledgements
Ao meu orientador, Professor Doutor Artur Varejão, por ter aceite orientar este trabalho. Confesso que, inicialmente me encontrava reticente relativamente a este tema, mas, com o decorrer da elaboração desta dissertação, fiquei a conhecer toda uma nova faceta da neurologia que aprecio bastante. Não posso deixar de agradecer por toda a ajuda prestada, toda a disponibilidade e todas as palavras de incentivo.
A toda a equipa do Hospital Veterinário da Universidade do Tennessee, em especial ao Dr. Mike Nystrom e à Dra. Amy Hodshon (DACVIM, Neurologia), por me fazerem gostar ainda mais de neurologia e por me fazerem crescer no mundo da prática clinica, mesmo no meio da loucura daquelas duas semanas no serviço de neurologia.
Um grande obrigado à minha segunda família, àquela que criei em Vila Real. Quero agradecer por todos os momentos, impossíveis de enumerar, que nunca irei esquecer. Sem vocês, a minha vida não seria, sem dúvida nenhuma, a mesma coisa.
Quero agradecer-te a ti, Zé Miguel, porque ao longo destes 6 anos foste uma pessoa importantíssima na minha vida e sei que, felizmente, continuarás a ser. Obrigada por todo o incentivo e todo o apoio incondicional.
Não posso deixar de agradecer ao meu irmão, Sam, por todo o tempo e toda a ajuda que disponibilizou na elaboração e correção desta dissertação. Mesmo quando estavas demasiado ocupado ou cansado, sei que fizeste um esforço por me ajudar!
Em último lugar, mas sem dúvida não menos importante, quero agradecer aos meus pais, porque sem eles, nada disto seria possível. Foram os vossos sacrifícios que permitiram que eu atingisse os meus objetivos e realizasse os meus sonhos! Obrigada do fundo do coração.
Abstract
Abstract
Movement disorders, although characterized by dramatic presentations, are infrequently recognized and reported in veterinary medicine. The etiology of involuntary movement disorders has not yet been determined and classification systems remain a controversy. Included in the involuntary movement disorders is dyskinesia which is characterized by impairment of voluntary movements. Dyskinesia is often considered and named as paroxysmal due to the fact that they occur discontinuously, without repetition and in a normally functioning animal. Several forms of paroxysmal dyskinesia are currently recognized in veterinary medicine. They can be primary (i.e. hereditary) or secondary and they can also be classified based on their trigger factors, duration, frequency and age of onset into paroxysmal kinesigenic dyskinesia, paroxysmal non-kinesigenic dyskinesia and paroxysmal exertion induced dyskinesia.
The goal of this dissertation was to better understand each type of paroxysmal dyskinesia reported to date in the literature and attempt to properly classify cases that presented to the Veterinary Teaching Hospital of the University of Trás-os-Montes e Alto Douro.
Three cases are included and described in this dissertation. First, a Bichon Frise that presented with signs that relate to the non-kinesigenic form of paroxysmal dyskinesia, very similar to another case reported in a Bichon Frise. Second, an English Bulldog that manifests with head tremors in a vertical and horizontal manner and is one of the main breeds affected with idiopathic head tremors. Finally, a Yorkshire Terrier that presents with canine epileptoid cramping syndrome, with signs similar to another Yorkshire Terrier with the same disorder both resolving with changes in diet. The cases presented are, therefore, consistent with what has been reported in the literature to date.
Resumo
Resumo
As desordens de movimento, embora caracterizadas por apresentações dramáticas, são raramente reconhecidas e reportadas em medicina veterinária. A etiologia das desordens de movimento involuntário ainda não foi determinada e os sistemas de classificação permanecem uma controvérsia.
Incluído nas desordens de movimento involuntário encontra-se a discinesia que é caracterizada por impedimento de movimentos voluntários. A discinesia é frequentemente considerada e denominada de paroxística uma vez que esta forma de movimento involuntário ocorre de forma descontinuada, sem repetição e num animal completamente normal. Várias formas de discinesia paroxística são reconhecidas atualmente em medicina veterinária. Podem ser primárias (hereditárias) ou secundárias e podem também ser classificadas em discinesia cinesiogénica, discinesia não cinesiogénica e discinesia induzida pelo esforço de acordo com os fatores desencadeantes, a duração, a frequência e a idade com que surgem os primeiros sinais.
O objetivo desta dissertação é o de melhor entender cada forma de discinesia paroxística reportada até à data na literatura e classificar os casos que foram apresentados ao HV-UTAD. Três casos são incluídos e descritos nesta dissertação. Primeiro, um Bichon Frise que apresenta sinais que se relacionam com a forma não cinesiogénica de discinesia paroxística, muito semelhante a outro caso também reportado num Bichon Frise. Segundo, um Bulldogue Inglês que se manifesta com tremores da cabeça num plano vertical e horizontal e é uma das raças mais afetadas pelos tremores idiopáticos da cabeça. Finalmente, um Yorkshire Terrier, que apresenta síndrome epileptoide canina com cãimbras com sinais similares a outro Yorkshire Terrier com a mesma desordem, ambos resolvendo com alterações na dieta. Os casos reportados são, portanto, consistentes com o que está descrito na literatura até à data. Palavras-chave: desordens de movimento, movimentos involuntários, discinesia paroxística, cão.
Index
Index
Acknowledgements ... v
Abstract ... vii
Resumo ... ix
Index of Figures ... xiii
Acronyms ... xv
1
Introduction ... 1
2
State of the Art: Forms of Involuntary Movements ... 3
2.1 Myoclonus ... 3
2.2 Tremors ... 4
2.3 Dyskinesia ... 5
2.3.1 Primary Dyskinesia ... 8
2.3.1.1 Familial/Hereditary ... 8
2.3.1.1.1 Scottie Cramp in the Scottish Terrier ... 8
2.3.1.1.2 Episodic Falling in the Cavalier King Charles Spaniel ... 9
2.3.1.1.3 Canine Epileptoid Cramping Syndrome in the Border Terrier ... 11
2.3.1.1.4 Episodic/Idiopathic Head Tremor Syndrome ... 13
2.3.1.1.5 Paroxysmal Dyskinesia in Chinooks ... 16
2.3.1.1.6 Wheaten Paroxysmal Dyskinesia... 17
2.3.1.1.7 Startle Disease in the Irish Wolfhound ... 17
2.3.1.2 Sporadic Reports ... 18
2.3.1.2.1 Paroxysmal Dyskinesia in a Bichon Frise ... 18
2.3.1.2.2 Paroxysmal Dyskinesia in the Boxer ... 19
2.3.2 Secondary Paroxysmal Dyskinesia ...20
2.3.2.1 Phenobarbital-Induced PD in the Chow Chow ... 20
2.3.2.2 Anticonvulsant-Responsive PD ... 21
3
Study objectives ... 23
4
Case Presentation ... 25
4.1 Material and Methods ...25
4.2 Clinical Cases ...26 4.2.1 Clinical Case #1 ...26 4.2.2 Clinical Case #2 ...29 4.2.3 Clinical Case #3 ...31
5
Discussion ... 35
6
Conclusion ... 41
7
References ... 43
Index of Figures
Index of Figures
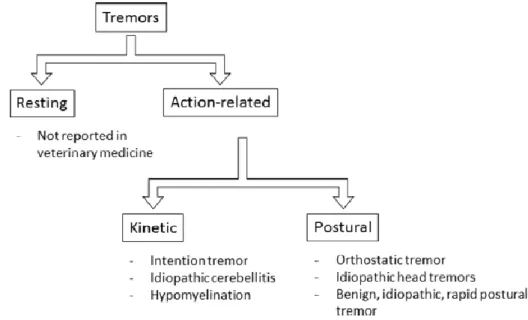
Figure 1. Algorithm for classification of tremors according to moment of occurrence. (Adapted from Lowrie & Garosi 2016a) ... 4 Figure 2. Clinical signs of EF in a female CKCS. (a) hypertonicity, (b) “deer-stalking”, (c)
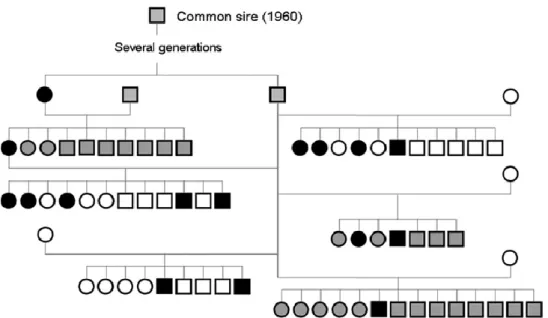
eventual falling. (Adapted from Gill et al., 2012) ...10 Figure 3. Clinical signs of CECS. (A) contraction of the muscles of the hind limbs, (B) progression to forelimbs, (C) Kyphosis. (Adapted from Park et al., 2014) ...12 Figure 4. Schematic genealogical representation of a single common sire among a
subpopulation of Doberman pinschers with 16 affected dogs with idiopathic head tremor syndrome. (Adapted from Wolf et al., 2011) ...15 Figure 5. A kyphotic posture in a Bichon Frise dog with dystonia of the right hind limb
simultaneously. (Adapted from Penderis & Franklin, 2001) ...19 Figure 6. 8-year-old, male intact, Bichon Frise of clinical case # 1. ...26 Figure 7. Bichon Frise with (A) flexion of the left thoracic limb that progresses to the (B) right thoracic limb and (C) finally advances to the pelvic limbs, in this case the right side. Note that throughout the whole episode the animal adopts a kyphotic posture. ...27 Figure 8. MRI images of different planes where no abnormalities are detected. (A) T1 sagital (B) T2 sagital (C) T1 Axial (D) T2 Axial (E) T1 Coronal (F) T2 Coronal. ...28 Figure 9. 3-year-old male intact English Bulldog – case # 2...29 Figure 10. 7-year-old, male intact, Yorkshire Terrier – clinical case # 3. ...31 Figure 11. Yorkshire Terrier of clinical case # 3 showing signs of (A) inability to walk and stand
(B) kyphotic posture with contraction and flexion of pelvic limb; (C) continued contraction of right pelvic limb...32 Figure 12. MRI images in (A) T1 and (B) T2 of the brain of the Yorkshire Terrier obtained in a sagital plane that reveal no abnormalities. ...32 Figure 13. MRI images of clinical case # 3 retrieved in coronal planes in (A) T1 and (B) T2 that
show no abnormalities. ...33 Figure 14. MRI images in an axial plane in (A) T1 and (B) T2 from the same animal of the previous images that also do not reveal any changes. ...33
Acronyms
Acronyms
BCAN Brevican gene
CECS Canine epileptoid cramping syndrome CSF Cerebrospinal fluid
CT Computed tomography EEG Electroencephalography EF Episodic Falling
GLUT-1 Glucose transporter 1
GlyR Postsynaptic glycine receptor GlyT2 Presynaptic glycine receptor GLRA1 Glycine receptor - 1 subunit gene GLRB Glycine receptor - subunit gene IHTS Idiopathic head tremor syndrome IM Involuntary movement(s)
LMN Lower motor neuron
KCNMA1 Potassium Calcium-Activated Channel Subfamily M Alpha 1 gene MD Movement disorders
MR-1 Major Histocompatibility Complex, Class I-Related gene MRI Magnetic resonance imaging
PCR Polymerase-chain reaction PD Paroxysmal dyskinesias
PIGN Phosphatidylinositol glycan anchor biosynthesis class N gene PKD Paroxysmal kinesigenic dyskinesia
PNKD Paroxysmal non-kinesigenic dyskinesia PED Paroxysmal exertion-induced dyskinesias PRRT Proline Rich Transmembrane Protein 2 gene SLC6A5 GlyT2 encoder
Introduction
1 Introduction
Movement disorders (MDs) are seldom recognized in veterinary medicine, possibly due to lack of knowledge and reports of cases on behalf of veterinarians. Despite this, MDs are often characterized by a dramatic clinical presentation occurring frequently in periods of rest (Bagley, 2004; Platt, 2016; Strain, 2016).
In human neurology, many involuntary movement (IM) disorders are considered a disarray of the extrapyramidal system nuclei. Veterinary medicine has not yet been able to recognize these abnormal movements as a consequence of the same etiology, but it is thought that a disruption in the extrapyramidal system nuclei is involved in the establishment of these disorders (De Lahunta & Glass, 2009; De Lahunta et al., 2015). For this reason, seizures are not included in these types of IMs, though they do cause them, since they are a result of abnormal hyperexcitability of the forebrain (Thomas, 2010).
In veterinary medicine, it is important to first determine the type of IM that is present by means of pattern recognition. Attempting to establish the source of the disorder is obtained as a second step in the diagnostic approach (Bagley, 2004; Platt, 2016).
Some authors consider IMs to be the result of involuntary skeletal muscle contractions. Some IMs manifest in a very characteristic manner, allowing to identify a specific cause, while others only reflect a dysfunction in the nervous (more specifically the lower motor neuron) or musculoskeletal systems (De Lahunta, et al., 2006; De Lahunta et al., 2015; Platt, 2016). Platt (2016) believes IMs are represented by atypical movements of the trunk, head and limbs and are therefore distinguished from other movements such as twitches, fasciculations, shivering, tics and shuddering, as these are usually associated with a contraction or movement of a muscle or group of muscles without motion of a segment of the body.
Bagley (2004) considers that there are seven different types of IM and include seizures in their description. Although, not all seven will be approached and detailed, as the main goal of this dissertation is in regards to paroxysmal MDs and also because, as mentioned previously, much controversy revolves around determination and definition of IMs. Therefore, a brief description of some IMs will be presented with more detail of paroxysmal dyskinesias (PDs).
State of the Art: Forms of Involuntary Movements
2 State of the Art: Forms of Involuntary Movements
2.1 Myoclonus
Myoclonus is an IM characterized by a sudden contraction of a muscle or a group of muscles followed by instant relaxation (Kent, 2012), generated within the central nervous system (Lanska, 2009). Myoclonus tends to occur repeatedly and manifests rhythmically in a form that is analogous to that of the heart beat (Bagley, 2004; Platt, 2016). A good way to think of this IM is to consider a single electric depolarization or shock that stimulates the nerve or nerves that innervate the affected muscle, in a way that is enough to produce a shock-like contraction of it, i.e., a jerking motion. Regardless of its definition, in human neurology, myoclonus can be “positive” in nature (i.e. when a muscle contraction occurs) or “negative”, the latter corresponding to loss of muscle tone. Positive myoclonus is, in humans, more frequently seen (Lanska, 2009; Abdo et al., 2010).
Myoclonus is different from myokymia and fasciculations, as it occurs normally with movement of the section of the body affected by it. In the other two IMs, there is no other affliction on a segment of the body either than the IM itself (Abdo et al., 2010).
According to De Lahunta et al. (2006) and De Lahunta et al. (2015) myoclonus manifests differently, and includes sporadic and repetitive types. The latter includes constant, action-related (congenital and acquired), postural, episodic nonpostural and resting forms. Also, in regard to the repetitive type, these authors consider that the action-related repetitive form can be considered as tremors. However, tremors will be approached separately in this dissertation, as a type of IM by itself.
In regard to how myoclonus affects the muscles, this IM can be classified into focal, segmental, multifocal or generalized if it affects an individual muscle, contiguous muscles, several different muscles or several muscles in a global form, respectively. Another classification of myoclonus is based on clinical presentation, the pathophysiology and/or cause (Levy & Chen, 2016). In dogs, myoclonus is a neurological sign associated with distemper and was once practically considered pathognomonic (Breazile et al., 1966) of the disease, although it has already manifested in other neurological disorders, namely inflammatory (Tipold et al., 1994; Bagley,
State of the Art: Forms of Involuntary Movements
4
1981). In canine distemper, myoclonus develops in one or more limbs as a repetitive jerking motion, which can be present even during sleep (Podell, 2004b).
2.2 Tremors
It is common to misdiagnose or even include tremors as a form of myoclonus as they are defined as an involuntary rhythmic contraction of a part or the whole body, similar to the definition of myoclonus. In fact, this was considered in human medicine. However, when considered that tremors occur with a fixed frequency (i.e., they are rhythmic) and are a consequence of alternate or simultaneous contraction of antagonist muscles that are innervated mutually, giving this IM a biphasic property (a unique feature of tremors), that idea was dismissed (Bagley, 1992; Deuschl et al., 1998; Platt, 2016).
Tremors can be classified based on the part of the body it affects (focal or generalized), its amplitude and frequency. Classification based on amplitude and frequency gives rise to resting and action-related types of tremor (Figure 1). Action tremors are further divided into postural (when a part of the body is held against gravity voluntarily), kinetic or intentional (when movement is purposely performed with a goal – associated frequently with cerebellar disease), isometric and task-specific - these two occurring mainly in primates that are able to apprehend objects with their hands, the location where the tremor occurs (Podell, 2004b).
Figure 1. Algorithm for classification of tremors according to moment of occurrence. (Adapted from Lowrie & Garosi 2016a)
State of the Art: Forms of Involuntary Movements
According to some authors (Lowrie & Garosi, 2016a), it is difficult to accurately determine and achieve a correct classification for tremors agreeing with etiology, as tremors themselves are classified differently and have many causes. These authors believe that a classification system for human tremors developed in 1998 (Deuschl et al., 1998) based on occurrence is an important method of classification. Lowrie & Garosi (2016a) classify tremors according to clinical evidences, an aspect used frequently in clinical neurology, and, therefore, believe that tremors are categorized into syndromes distinguished by clinical presentation. Their classification is adapted from another developed for human medicine (Abdo et al., 2010). According to the classification proposed by Lowrie & Garosi (2016a), some IMs that are thought to be paroxysmal are included in the postural form. Regardless of this classification, some authors (Urkasemsin & Olby, 2014) presume idiopathic head tremors to be paroxysmal in nature. Therefore, these will be discussed independently as a form of PD.
In human medicine, tremors often occur as a result of disturbances in the extrapyramidal system, namely the substantia nigra and the basal nuclei. Although, this does not translate to dogs, or has not been yet proven (Bagley, 1992).
2.3 Dyskinesia
Dyskinesia is defined as an IM characterized by impairment of voluntary movements, causing disintegrated ones, as a result of a reversible onset of neurological dysfunction (Urkasemsin & Olby, 2014; Platt, 2016). This type of IM is portrayed as being episodic, with a sudden onset in particular groups of muscles that can occur over a period of time in an awake and fully conscious animal, either at rest or while in activity (Kent, 2012). Due to the fact that they do not occur continuously, without repetition and in a normally functioning animal, they are commonly designated as paroxysmal.
In practice, it is difficult to distinguish these MDs from seizures due to their similar presentation (Richter et al., 2015). Some authors (Podell, 2004b) even consider that PDs can be classified as seizure disorders with changes in brain activity with hyperexcitability (epileptic seizures) or without these changes (nonepileptic seizures). However, several other authors consider that seizures should be classified differently from PDs. This is because they are a result of an abnormal activity in the forebrain and PDs, although without a known and confirmed pathophysiology, have a suspicion of a dysfunction in the connection between the motor cortex, the extrapyramidal system nuclei and the thalamus. Nevertheless, features that help separate the two include the fact that PDs do not manifest with autonomic signs
State of the Art: Forms of Involuntary Movements
6
no abnormalities are detected in the electroencephalography (EEG) between episodes. It Is very common for seizures to also present different events, including the aura, the ictal phase and the post-ictal phase (Thomas, 2010). These aspects help, to some degree, to differentiate PDs from seizures. However, it still becomes difficult to differentiate PDs from the simple partial type of seizures, as these do occur in a conscious animal. Nevertheless, they do become less likely, as the duration of the these seizures is much less than that of PDs (Lowrie & Garosi, 2016b).
In human medicine, it is considered that PDs involve different, non-stereotypical IMs, including: Chorea – sudden contraction of distinctive collections of muscles in a
discontinued manner.
Dystonia – a contraction of a group of muscles in a persistent fashion often affecting the hind limbs, clinically appearing as increased extensor tone. Athetosis – extended contraction of muscles related to the trunk which often
leads to a kyphotic posture or writhing movement.
Ballism – sudden contractions of muscles pertaining to the limbs originating a thrashing twisting movement.
In some cases, PDs can be termed as paroxysmal dystonia, when dystonia is the core of the movement disorder.
Some authors (De Lahunta et al., 2006; De Lahunta & Glass, 2009; De Lahunta et al., 2015) consider tetany a form of paroxysmal movement, as it is defined as a contraction of muscles responsible for extension in an occasionally inconstant manner.
Several classification systems have been attempted and developed throughout the years relating veterinary and human neurology cases (Meyers et al., 1969; Woods, 1977; Herrtage & Palmer, 1983; Nakahata et al., 1992; Ramsey, et. al, 1999; Jankovic and Demirkiran, 2002). According to several authors (Erro et al., 2014; Urkasemsin & Olby, 2014; Richter et al., 2015; Waln & Jankovic, 2015), PDs can be primary, secondary or even be part of other chronic neurological syndromes. Primary dyskinesias are those where the animal remains neurologically normal between episodes and can be further classified into sporadic – with no apparent cause – or familial – where an autosomal recessive mode of inheritance is present. Secondary PDs can result from other diseases, that can be neurological in nature or not, and, therefore, affected animals may show neurological abnormalities in between episodes reflecting the underlying disease. Although rare, secondary PDs have been reported in
State of the Art: Forms of Involuntary Movements
veterinary medicine, as a consequence of drug administrations (Erro et al., 2014; Kube et al., 2006; Richter et al., 2015; Urkasemsin & Olby, 2014; Waln & Jankovic, 2015).
Waln & Jankovic (2015) proposed a sub-classification for human PDs that divides them into four different categories: (1) paroxysmal kinesigenic dyskinesia (PKD), (2) paroxysmal non-kinesigenic dyskinesia (PNKD), (3) paroxysmal exertion-induced dyskinesia (PED) and (4) paroxysmal hypnogenic dyskinesia. Other authors (Raina et al., 2015) consider paroxysmal hypnogenic dyskinesia a form of non-REM epilepsy rather than a form of PD. This classification system was established based on the triggers, the age of onset and the length of the episodes. Some authors (Black et al., 2014; Urkasemsin & Olby, 2014; Richter et al., 2015) believe the frequency of the episodes is also important. Raina et al. (2015) and Richter et al. (2015) also consider that response to therapy is significant in classifying PD.
PKD occurs as a result of abrupt movements, while PNKD occurs spontaneously at rest (in humans it is associated with stress, caffeine or alcohol). PED results from prolonged exercise (Urkasemsin & Olby, 2014; Waln & Jankovic, 2015). In veterinary medicine, these MDs have not yet gained a classification for themselves, and the classification scheme proposed for human medicine has been adapted to the many cases reported to date.
According to the classification systems used in veterinary literature which link human medicine literature (Urkasemsin & Olby, 2014; Waln & Jankovic, 2015), PKD typically has a duration of less than 2 minutes, while PNKD usually has a duration of 10 minutes to 10 hours (generally less than 4 hours) and PED has a mean duration time of 2-5 minutes.
Regarding treatment options, it is a frequent feature for cases which present as PKD to respond to treatment, specifically antiepileptic drugs, as previously reported in other dogs (Geiger & Klopp, 2009; Harcourt-Brown, 2008; Herrtage & Palmer, 1983; Shelton, 2004). PED typically also responds to other treatment managements, such as a ketogenic diets or gabapentin (Weber et al., 2008) or even gluten-free diets (Lowrie et al., 2015). PNKD patients do not respond as well to treatments and prevention is based on avoiding trigger factors (Waln & Jankovic, 2015).
In veterinary medicine, the cases reported to date seem to be mainly consistent with PNKD (Ramsey et al., 1999; Penderis & Franklin, 2001; Packer et al., 2010). However, there has been a report of a PD compatible with PKD in a German shorthaired pointer (Harcourt-Brown, 2008) and it is thought, by some authors that Episodic Falling (EF) in the Cavalier King Charles Spaniel (Herrtage & Palmer, 1983) and Scottie cramp in the Scottish Terrier (Meyers et al.,
State of the Art: Forms of Involuntary Movements
8
Although veterinary medicine has not yet evolved on this subject as much as human medicine, due to the increase in number of cases reported and the repetition of certain breeds, an interest and concern arose towards the familial form of PD. This led to further authors stating that mutations in certain genes can be responsible for the episodes (Meyers et. al, 1970; Gill et al., 2011; Forman et al., 2012; James et al., 2012; Black et al., 2014). Studies demonstrate that clinical categorization is important, but genetic classification should not be forgotten, as some crossover of clinical types have been established by the genetic differentiation. To date, it is known that PKD is associated with the Proline Rich Transmembrane Protein 2 gene (PRRT2), while PNKD is related to the PRRT2, Major Histocompatibility Complex, Class I-Related (MR-1) and Potassium Calcium-Activated Channel Subfamily M Alpha 1
(
KCNMA1) genes and PED is linked to the MR-1 and glucose transporter 1 (GLUT-1) genes (Erro et al., 2014).Genetic testing is available, although, as discussed before, there isn’t always a correlation between the genetics and the clinical signs. Erro and colleagues (2014) have proposed an algorithm to determine when genetic testing should be performed in human medicine.
It is important to remember that this classification system proposed initially in 1995 (Demirkiran & Jankovic, 1995) does not lead to or identify a true cause for these PDs. However, it is thought that these MDs result from an issue in the neurotransmission that can be due to any part pertaining to this mechanism (Black et al., 2014).
2.3.1 Primary Dyskinesia
2.3.1.1 Familial/Hereditary
2.3.1.1.1 Scottie Cramp in the Scottish Terrier
According to Meyers (1971), paroxysmal hypertonicity, one form of PNKD, was reported as early as 1942 by Klarenbeek et al. in Scottish Terriers, which gives it the name of Scottie cramp in this breed (Klarenbeek, et al., 1942 apud Meyers et al., 1971). In 1969, an association between serotonin and this disease was determined. However, the underlying defect has not yet been determined and it is yet to be established if the hormone plays a primary role in the pathophysiology. A pharmacological study was performed which determined that a certain substance that reduced the serotonin concentration would lead to an increase in severity of the signs, while another substance that augmented the concentration of the hormone was able to do the exact opposite or even avoid prompting of signs (Meyers et al., 1969). A year later, it was established that this neurological disease was genetic and could be inherited as an autosomal recessive feature (Meyers et al., 1970). In 2015, a study was performed to elaborate the clinical characteristics of this disease and 74% of the animals included in the study were
State of the Art: Forms of Involuntary Movements
female, which led the authors to suspect that there could be an X-linked type of mode of inheritance (Urkasemsin & Olby, 2015).
As the name suggests, there is a progressive abnormal increase in muscle tone and, as a result, the gait and posture of the affected animal are impaired without any kind of pain (Shelton, 2004). Signs suggestive of Scottie cramp include abduction of the front limbs or the animal may adopt a kyphotic posture in the lumbar region and hyperflexion of the hind limbs (Geiger & Klopp, 2009). With progression of signs, the hind limbs become severely compromised, and affected dogs develop stiffness with goose stepping, “bunny-hopping” and eventually, even falling (Urkasemsin & Olby, 2014; Urkasemsin & Olby, 2015). Signs appear with exercise or excitement and are absent during rest (Meyers et al., 1970; Urkasemsin & Olby, 2014). These signs do tend to progress, but then reach a peak where they become stable. Age of onset varies from 1 month (signs appear as early as 6 months) to 7 years of age. Signs tend to improve with age, which can be an outcome of changes in behavior or the levels of activity of the animal, or signs can even improve on their own (Urkasemsin & Olby, 2015). In some animals, medications, such as diazepam and fluoxetine, have seemed to help improve signs (Geiger & Klopp 2009; Urkasemsin & Olby, 2015).
Diagnostically, routine and non-routine laboratory tests are unremarkable as is the electromyogram. No significant changes have been seen in the nervous system or muscles of these dogs, either macro or microscopically. Having a very characteristic clinical appearance, diagnosis can be made presumptively based on this, associated to the fact that they occur in this breed, and after exclusion of other possible causes (Shelton, 2004; Urkasemsin & Olby, 2014)
2.3.1.1.2 Episodic Falling in the Cavalier King Charles Spaniel
Initially thought to be a myopathy, when reported in the 1980’s, EF of the Cavalier King Charles Spaniel (CKCS) is considered by some authors (Gill et al., 2012; Forman et al., 2012) a form of PED, as it appears to be triggered mainly by exercise, while, to others (Herrtage and Palmer, 1983) it is thought to be a form of PKD. The age on onset varies among several authors, ranging from a few months to several years of age (Gill et al., 2012; Forman et al., 2012; Urkasemsin & Olby, 2014).
State of the Art: Forms of Involuntary Movements
10
According to De Lahunta et al. (2015) it is a paroxysmal form of tetany. Others (Kent, 2012; Gill et al., 2012) consider it to be a hypertonicity that rapidly progresses in the thoracic and pelvic limbs leading to immobilization of the affected dog in a “deer-stalking” or “praying” position, which may eventually result in falling (Figure 2).
Other signs that may be associated include kyphosis and a “bunny-hopping” gait. It is important to state the clinical signs are variable among different cases. Episodes are of short duration, with length ranging from seconds to numerous minutes and recovery can last up to 10 minutes. It is also important to not forget that during the episodes the affected dog does not lose consciousness and between episodes it is neurologically normal (Shelton & Engvall, 2002; Forman et al., 2012; Gill et al., 2012).
This IM has been compared to startle or hyperekplexia disease in humans (which will be discussed further on) due to the fact that some cases have responded well to treatment with diazepam and clonazepam, which is used to successfully treat human hyperekplexia. It is also thought that acetazolamide, anecdotally a carbonic anhydrase inhibitor, may play a role in aiding with resolution of clinical signs in this IM (Garosi et al., 2002; Gill et al., 2012).
Due to suspicion of this IM being a result of an autosomal recessive mode of inheritance, in 2012, Forman et al. (2012) and Gill et al. (2012) decided to pursue genetic testing to determine the mutations associated with EF in the CKCS, having discovered a Brevican (BCAN) microdeletion. BCAN, is a proteoglycan which is highly expressed in the central nervous system. It is present at the nodes of Ranvier of myelinated axons of large diameter and is responsible for cation mobilization during conduction of action potentials. Thus, interruption of this mechanism is thought to be responsible for EFS in CKCS (Forman et al., 2012; Gill et al., 2012). Included in the study conducted by Forman et al. (2012) were 341 dogs from 34 different breeds. In their study, no mutations in the BCAN where noticed, which leads to a high suspicion
Figure 2. Clinical signs of EF in a female CKCS. (a) hypertonicity, (b) “deer-stalking”, (c) eventual falling. (Adapted
State of the Art: Forms of Involuntary Movements
that this mutation is limited to the CKCS. Though, the suspicion of this mutation being exclusive to the CKCS cannot be confirmed because not all breeds where included in the study. Another conclusion of this study was that the degree of activity that the different CKCS dogs undergo and the environment they are in may influence the phenotype of the disease as several dogs in the study where homozygous for the mutation and did not develop EF.
In some cases, dogs affected for several months or even years can show improvement and signs of this IM can even subside, with this condition being self-limiting. It is speculated that this may be a consequence of a compensatory effect on behalf of other proteoglycans from the same family as the brevican. It can also be due to changes in behavior in the animal, in activity or even the fact that the triggers have simply been avoided (Forman et al., 2012). Like Scottie cramp in the Scottish Terrier, the diagnostic tests in this type of IM are unremarkable and, therefore, diagnosis is based on exclusion of other causes and also observation of clinical signs characteristic of this IM in this breed (Platt, 2016).
2.3.1.1.3 Canine Epileptoid Cramping Syndrome in the Border Terrier
Also known as Spike’s Disease, this PD has been studied by veterinarians for many years (Shelton, 2004; De Lahunta et al., 2006). Suspicions that this was breed-related, several authors (Black et al., 2014) proposed to phenotypically characterize this disorder. According to this study, age of onset appeared to be before three years of age, ranging from 2 months to 7 years of age. The duration of episodes was found to be variable, but usually under 30 minutes (episodes could sometimes last hours). Periods of clusters, with 3 or more episodes in 24 hours were also noted as were periods of several months without a single episode, which could give this PD a wax and waning status (Black et al., 2014).
Clinically, the limbs and neck are commonly affected, with rare but possible involvement of the back, tail and abdomen. Limbs present extensor rigidity, associated with involuntary contraction of groups of muscles and kyphosis, which, when severe, leads to difficulty walking and eventually much effort to remain standing. Other signs noticed are air licking and stretching in an exaggerated manner. Interestingly, episodes are also sometimes associated with borborygmi, which result from cramping of the muscles of the intestines in severe cases (Black et al., 2014; Park et al., 2014; Lowrie et al., 2015; Marioni-henry et al., 2015).
This form of PD is thought to be analogous to the non-kinesigenic form of PD as episodes are triggered by stress or even excitement and do not occur with movement or only during rest or sleep. Duration of episodes is also compatible with PNKD and, therefore, the other proposed
State of the Art: Forms of Involuntary Movements
12
The etiology of this PD is unknown and attempts to treat the affected dogs with medication commonly used to treat seizure episodes, such as phenobarbital, diazepam and potassium bromide, were unsuccessful (Black et al., 2014). As a possible cause, hepatic microvascular dysplasia and other metabolic diseases have been suspected, because in some affected individuals, high bile acids levels are detected. Nonetheless, it is thought to be primarily hereditary in Border Terriers (Shelton, 2004) and for this reason, concern in seeking a DNA marker responsible for this PD is ongoing (Garosi & Harvey, 2012).
In the study conducted by Black et al. (2014), it was stated that the owners were able to predict when an episode would occur since affected Border Terriers would begin to seek attention from the owners, become quieter or even vomit and have diarrhea. Similar behavior would frequently be seen also after the episodes. However, one must take into consideration these signs along with the characteristic signs of Canine Epileptoid Cramping Syndrome (CECS) when evaluating the animal and trying to determine a cause, as they may resemble the pre and post-ictal stages of epileptic seizures, which leads to erroneous diagnosis. It is not possible to completely rule out seizures as a cause, although it must be considered that these pre and post-ictal behaviors may be a result of abdominal pain and distress that the animals undergo during an episode (Thomas, 2010; Black et al., 2014; Urkasemsin & Olby, 2014).
Recently, a suspected case of CECS was reported in a Yorkshire Terrier (Figure 3). Initially thought to be a partial seizure, opinions changed when characteristic signs of this IM, such as hypertonic muscle tone and continued involuntary contractions of the extremities that would lead to flexing of the joints, were noticed along with a conscious and responsive dog during the episode. Also, several diagnostic tests were performed which were all unremarkable and, in between episodes, the affected dog was neurologically normal, with no changes in gait. During episodes, the affected dog did not show any autonomic signs and there was no pre-ictal or post-pre-ictal phase behavior was prominent. Alongside the episodes, periods of anorexia and borborygmi were also present (Park et al., 2014).
Figure 3. Clinical signs of CECS. (A) contraction of the muscles of the hind limbs, (B) progression to forelimbs, (C) Kyphosis. (Adapted from Park et al., 2014)
State of the Art: Forms of Involuntary Movements
As mentioned previously, several cases reported in the study performed by Black et al. (2014) manifested with audible borborygmi, as did the case reported in the Yorkshire Terrier (Park et al., 2014). Also, in these studies, changes of diet to gluten-free or hypoallergenic diets led to a significant decrease in severity and frequency of the episodes. This could be of great significance as there could be an association between the intestinal signs and CECS with a possible food allergy or intolerance involvement (Black et al., 2014; Park et al., 2014). Similarly, in human medicine, there has been a connection between celiac disease and involuntary MDs (Hall et al., 2007) and there has been increasing evidence that there is influence of the brain-gut-microbiome alliance and enteric glial cells are responsible not only for many gastrointestinal diseases but also for Parkinson’s (Clairembault, et al., 2015; Hadjivassiliou, et al., 2015; O’Mahony et al., 2015). Despite this conceivable association, at this time it was not possible to rule out a coincidental concurrent disease process and it was known that waxing and waning processes could respond to placebos (Roberts et al., 1993), as well as some reports of canine epilepsy (Muñana et al., 2010).
In an attempt to determine a definitive connection between a gluten sensitivity and CECS, Lowrie et al. (2015) performed a study that would resolve this matter. Interestingly, their investigation supported the association between the MD and a gluten sensitivity stating that this PD is the first serologically tied to gluten. Gliadin, a protein that is part of gluten, is believed to be the fraction responsible for the process of sensitivity. Evidence of this sensitivity is based on response to treatment with a gluten-free diet and the presence of anti-gliadin and TG2 in many serological samples obtained from affected dogs. Amusingly, the reintroduction of gluten in diets led to reoccurrence of CECS in 2 dogs (Lowrie et al., 2015).
It is, therefore, possible to say that in an attempt to correctly diagnose CECS and avoid misdiagnosis, careful evaluation of characteristic signs in a suggestive breed should be taken into consideration along with diet trials that are gluten-free.
2.3.1.1.4 Episodic/Idiopathic Head Tremor Syndrome
According to several authors, episodic head tremor syndrome, idiopathic head tremor syndrome (IHTS) or even head bobbing (as some describe it), is not a true MD, but rather a form of myoclonus, specifically a postural repetitive type (or tremor as some may categorize) due to the fact that these tremors have not been portrayed in human forms of PD (De Lahunta et al., 2006; Urkasemsin & Olby, 2014). In spite of this, this syndrome can be considered a form of PD as they are episodic and can even presumably be due to a channelopathy (Wolf et al., 2011).
State of the Art: Forms of Involuntary Movements
14
If idiopathic head tremors are considered a form of myoclonus, its name occurs as a result of the fact that this syndrome occurs when there is a certain degree of tension in the muscles pertaining to the neck region and the animal is in a weight bearing position. IHTS is a benign syndrome which occurs episodically and can start and stop spontaneously. It is characterized by quick tremors of the neck and head of the affected dog, when it is less active or asleep. These IMs disappear hastily as the dog changes the position in which it was, such as lying down its head on a surface, or even if the dog becomes active (De Lahunta et al., 2015; Lowrie & Garosi, 2016a).
Although pathophysiology is unknown, the fact that the tremors cease with a change in the position of the neck and head supports the idea that there may be a stretch reflex mechanism involved (De Lahunta & Glass, 2009; Kent, 2012; De Lahunta et al., 2015). It seems almost as if the affected dog can stop the tremors if it desires to do so or if it is focused on a task of some sort (Platt, 2016).
This type of MD commonly occurs in breeds such as the Doberman Pinscher and English Bulldog, although other breeds can also be affected (Guevar et al., 2014). In the Doberman Pinscher breed, a study performed by Wolf et al. (2011) found that all affected dogs where traceable to a single common ancestry, suggesting a mode of inheritance (Figure 4).
Signs characteristic of this syndrome include an abrupt onset of head and neck tremors with variable duration (from seconds to hours with a average duration of 3 minutes) which occur in a vertical (“yes”) or horizontal (“no”) direction. In a recent study, some cases were reported as having a “rotational” head tremor, which could be compatible with the cases described by Wolf
et al. (2011) where there was vertical head tremor as well as horizontal (Shell et al., 2015).
Episodes seem to occur at least once per day, ranging up to 20 and occur for several weeks with another several in between of absence (De Lahunta et al., 2006). The frequency of the tremors has already been reported as being between approximately 5 and 8 Hz (Urkasemsin & Olby, 2014; Platt, 2016). This syndrome typically occurs in young adults, with age of onset usually between 6 months to 3 years of age. However, some cases were reported to occur at 3 months or even at 12 years of age (Wolf et al., 2011; Shell et al., 2015). It seems that the episodes may also resolve spontaneously in the course of the dogs life (Guevar et al., 2014)
State of the Art: Forms of Involuntary Movements
Figure 4. Schematic genealogical representation of a single common sire among a subpopulation of Doberman pinschers with 16 affected dogs with idiopathic head tremor syndrome. (Adapted from Wolf
et al., 2011)
Diagnosis of this condition is based on signalment, history, the typical signs noticed of the head and neck and their frequency, along with an alert and conscious state of the animal. Physical and neurological exams are unremarkable as well as other diagnostic tests that have been performed to date, such as cerebrospinal fluid (CSF) analysis and magnetic resonance imaging (MRI) (De Lahunta et al., 2015; Shell et al., 2015; Lowrie & Garosi, 2016a). In regards to treatment, it appears that anti-epileptic drugs are ineffective as well as corticosteroids, although it is hard to assess efficacy of these medications due to short duration of episodes and their sporadic nature (Lorenz, 2011; De Lahunta et al., 2015; Shell et al., 2015).
In regard to triggers, some owners state that these tremors occur when the dog is relaxing after prolonged periods of exercise, although some studies do not support this finding (Lorenz, 2011; Guevar et al., 2014). In fact, in the study performed by Shell et al. (2015), they found that about one fifth of their population of dogs presented with stressful incidents or simultaneous maladies that had occurred in a time frame of about one week from the onset of the tremors. These findings lead to suspicion that there could be stressors involved as triggers and their role should be further investigated (Shell et al., 2015).
Recently, a study performed by James et al. (2012) found that four of the dogs belonging to its population presented with abnormalities in EEG recordings. One could suspect that this would mean that this syndrome is in fact compatible with a form of epilepsy. Although, this is not necessarily true. In fact, other features should be taken into consideration such as the fact that
State of the Art: Forms of Involuntary Movements
16
IHTS does not present with those aspects (Wolf et al., 2011; Guevar et al., 2014; Shell et al., 2015)
It is important to not confuse IHTS with intention tremors when diagnosing this syndrome,. Intention tremors are frequently associated with a cerebellar affliction and are typically present when dogs are performing a goal-oriented task such as eating or drinking water. In these cases, it is common for the affected animal to show other signs of cerebellar disease such as head tilt, ataxia, dysmetria and nystagmus (Podell, 2004b).
2.3.1.1.5 Paroxysmal Dyskinesia in Chinooks
Also known as Chinook seizures, this form of PD is thought to be consistent with the PNKD form of the classification scheme for human PD, as episodes are not induced by prolonged exercise, are of short duration and do not occur during sleep (Packer et al., 2010). Some breeders and owners state that stress and fatigue contribute to the onset of these episodes, although, Packer et al. (2010) did not find that consistent with the findings in their study and triggers are to date uncertain.
Phenomenology seems to be similar among the different dogs affected with episodes characterized by dystonia, chorea and even ballism. All 4 limbs are typically affected or, sometimes, only ipsilateral limbs are disturbed, with variation of the side affected between episodes and an inability to walk or even stand. Some dogs have been reported to show signs of head tremor. Episodes can last from minutes to an hour and befall variably with several per day to many months or even years with not one occurrence (Packer et al., 2010; Platt, 2016). Age of onset of this PD in this breed seems to be from months (that can range from 2 to 6) up to 5 years of age, with most dogs affected before they were 3 years old (Packer et al., 2010; Richter et al., 2015).
EEG recordings have been performed in some dogs between episodes and have shown no abnormalities consistent with epileptic activity. However, 3 dogs in one family of Chinooks did manifest tonic-clonic types of seizures concurrent with the PD (Packer et al., 2010; Richter et al., 2015). Some specific mutations in humans can lead to PD associated with epilepsy in members of a same family (Du et al., 2005; Suls et al., 2008). For this reason, Packer et al. (2010) found that it could be possible for this to also occur in this breed and therefore, result from a channelopathy.
According to the study performed by Packer et al. (2010) PD in this breed is inherited as an autosomal recessive trait.
State of the Art: Forms of Involuntary Movements
2.3.1.1.6 Wheaten Paroxysmal Dyskinesia
Recent studies performed by Kolicheski et al. (2014) and O’Brien and colleagues (2015) have discovered a form of PD in Soft Coated Wheaten Terriers.
The age of onset of episodes seemed to be from as early as 8 months to as old as 3 years. Affected animals would present with dystonia or quick irregular IMs that would affect the limbs. In some cases, the dog may only show an exaggerated flexion of one of the limbs while walking, in others it can have a stiff gait and a kyphotic posture and there can even be inability to stand (O’Brien et al., 2015; Orthopedic Foundation for Animals, 2016).
Duration of episodes ranged from a few minutes to several hours and the intensity of the episodes as well as their frequency seemed to increase over time. No clear triggers were identified, although episodes do occur while the animal is awake and alert. Treatment was attempted with anticonvulsives (acetazolamide) and in some cases, helped reduce the episodes and in others even resolved (O’Brien et al., 2015).
The authors of the studies recognize this form of PD to be inherited in an autosomal recessive manner. Whole genome sequencing was performed in two dogs affected with paroxysmal dyskinesia in the studies conducted by Kolicheski et al. (2014) and O’Brien et al. (2015) and
revealed a variant of the Phosphatidylinositol Glycan Anchor Biosynthesis Class N (PIGN) gene. This gene is involved in mechanisms in which carbonic anhydrase partakes which can explain the effect of acetazolamide in treating these cases. Further sequencing was performed on the other affected dogs and revealed the same information (O’Brien et al., 2015).
2.3.1.1.7 Startle Disease in the Irish Wolfhound
Startle disease or hereditary hyperekplexia is very well known and reported in human medicine as non-epileptic seizures that are occur due to tactile or auditory triggers resulting in an exaggerated startle response and apnea with generalized muscle rigidity. It affects neonates and is a rare disease that can cause severe consequences including brain damage and even death (Eulenburg et al., 2006; Gill et al., 2011; Carta et al., 2012; Platt, 2016).
This disease is known to be caused by defects in the inhibitory glycinergic transmission. Initial studies revealed that this was due to defects mainly in the subunit 1 gene (GLRA1) of the inhibitory postsynaptic glycine receptor (GlyR) (Eulenburg et al., 2006; Platt, 2016). However, in one study (Vergouwe et al., 1997) it was noticed that 50% of the patients that manifested startle disease did not have a mutation in the GLRA1 gene and, therefore, a concern in
State of the Art: Forms of Involuntary Movements
18
GlyR (GLRB) could also lead to signs of startle disease and in 2012 it was similarly found (Carta et al., 2012; James et al., 2012) that a mutation in the gene that encodes the presynaptic glycine transporter GlyT2 (SLC6A5) could additionally be responsible.
In veterinary medicine, similar disorders to hyperekplexia have already been reported in calves (Harper et al., 1986; Gundlach et al., 1988; Gundlach, 1990; Lummis et al., 1990; Pierce et al., 2001; Charlier et al., 2008; Gill et al., 2011), horses (Gundlach et al., 1993), and dogs (Fox et al., 1984; Gill et al., 2011), with few having underlying genetic impairments identified. The ocurrences that have been identified match to those that have been reported in human medicine (Pierce et al., 2001; Charlier et al., 2008; Gill et al., 2011)
In the Irish Wolfhound breed of dogs, this disease is inherited in an autosomal recessive manner (Gill et al., 2011). The study performed by Gill et al. (2011) was actually the first to diagnose a microdeletion in the SLC6A5 gene. This disease occurred after handling puppies when they were sleeping or simply relaxed and onset would happen around 5-7 days of age. Signs were consistent with startle disease and were mainly difficulty standing due to stiffness in all 4 limbs and generalized tremors was noticed. Over time, the difficulty in managing the pups in regards to feeding increased and humane euthanasia was performed as it was thought that eventually they would not be able to eat on their own. Diagnostic testing is now available through polymerase-chain reactions (PCRs) to identify carriers of the disease (Gill et al., 2011; Platt, 2016).
2.3.1.2 Sporadic Reports
2.3.1.2.1 Paroxysmal Dyskinesia in a Bichon Frise
PD has been described in a Bichon Frise with suspicions of the episodes to be compatible with the PNKD form observed in humans due to the fact that episodes would occur either at rest, when the dog was excited or during exercise. Onset of the episodes was variable and random, with periods of several weeks of absence to a single day dominated by numerous attacks (Penderis & Franklin, 2001; Kent, 2012; Lorenz et al., 2011).
The dog affected in this case was 4 years old and had an on-going six-week history of attacks which were characterized by an onset of hyperflexion of any limb along with the spine, causing a kyphotic posture (Figure 5). In the midst of the episodes, the hyperflexion could be partial or complete with or without progression to any other limb (if signs progressed, resolution would occur in the previously affected limb) or there could be rapid extension and flexion of affected limbs. Occasionally, contraction of unilateral muscles of the facial area would also occur leading to a grimacing look. Throughout the episodes, the animal would remain fully conscious
State of the Art: Forms of Involuntary Movements
and would not exhibit any autonomic signs, with a rapid recovery afterwards (Penderis & Franklin, 2001; Kent, 2012; Urkasemsin & Olby, 2014; Platt, 2016).
Figure 5. A kyphotic posture in a Bichon Frise dog with dystonia of the right hind limb simultaneously. (Adapted from Penderis & Franklin, 2001)
Diagnostically, physical and neurological exams, as well as MRI and CSF analysis were performed which were all unremarkable. Treatment was also attempted with oral phenobarbital and, despite reaching therapeutic levels, no changes in frequency or severity of episodes was observed (Penderis & Franklin, 2001; Urkasemsin & Olby, 2014).
Amusingly, a case similar to the one occurred in the Bichon Frise has been observed in another Bichon Frise. This could suggest a possible genetic component involved, similar to those reported to date in other breeds of dogs.
2.3.1.2.2 Paroxysmal Dyskinesia in the Boxer
Similar to what has already been observed in the Bichon Frise, episodes of PD have been witnessed in two litters of boxer pups.
Occurrence of episodes was very irregular between members of the same litter and even in the same dog. It seemed that episodes could be triggered by excitement or other incentives, but no specific trigger was identified. Age of onset ranged from 2 to 3 months and curiously, it appeared that males were affected more frequently and more severely than females. With increase in age, the frequency of the episodes would gradually decrease (Ramsey et al., 1999; Urkasemsin & Olby, 2014).
Although frequency of episodes was inconsistent within the litter and even a single dog, signs were normally very consistent and involved the limbs, neck and facial area. Regarding the limbs, observed signs included continued hyperflexion, elevation of a limb in extension that
State of the Art: Forms of Involuntary Movements
20
ground. If the episodes arose during movement, it could lead to an ataxic gait and even eventual falling. A torticollis posture could also occur due to unilateral contraction of extensor muscles of the neck area. Unilateral contraction of the facial musculature could also occur, leading to a grimacing expression (Ramsey et al., 1999; Lorenz et al., 2011).
Duration of the episodes ranged from one to five minutes and affected dogs remained conscious throughout. Owners reported that stroking and talking to the pup would reduce the duration of the episode. Recovery from episodes was fairly quick and no neurological abnormalities were noticed (Ramsey et al., 1999).
Diagnostically, nothing relevant in MRI or other diagnostic tests were found. Treatment with phenobarbital was endeavored and unsuccessful in changing frequency, duration or severity of episodes (Ramsey et al., 1999).
Pedigree of both litters was investigated and a great grandsire common to both litters was found which also showed cases of inbreeding (Ramsey et al., 1999).
2.3.2 Secondary Paroxysmal Dyskinesia
2.3.2.1 Phenobarbital-Induced PD in the Chow Chow
Reports of acquired or secondary PD are very uncommon in veterinary medicine. Despite the low number of cases, one was reported in a six-year-old male Chow Chow (Kube et al., 2006). The initial reason for presentation of the dog to the teaching hospital was the fact that he had been suffering cluster seizures. For that reason, anticonvulsant therapy with phenobarbital was attempted and eight weeks later, the owner reported that the dog began to manifest excessive twitching, was unable to sleep and very anxious. A week afterwards, more seizures occurred and potassium bromide was added as a loading dose. With passing of time, the twitches and anxiety did not subside and the twitching got to a point where sometimes the dog would even fall. At that time, physical and neurological exams were performed and intermittent twitches of the face, ears, neck and shoulders were noticed as was the falling of the affected dog with increase in severity of the twitches. Diagnostically, and EEG was performed which did not reveal any abnormalities (Kube et al., 2006; Lorenz et al., 2011; Urkasemsin & Olby, 2014). Therapeutically, felbamate was added and the dose of phenobarbital was reduced gradually over the period of a month with no changes in the potassium bromide dosage. Interestingly, the number of episodes of twitching and their severity decreased. Due to an episode of status epilepticus, the dosage of phenobarbital was increased and upturn in the PD episodes was noticed. For this reason, felbamate dosage was increased and phenobarbital was gradually
State of the Art: Forms of Involuntary Movements
decreased again until completely discontinued. Afterwards, no episodes of twitching were noticed (Kube et al., 2006).
Episodes of PD after anticonvulsant therapy in humans has been previously reported. Anticonvulsants associated with PD in humans include phenobarbital, the anticonvulsant reported in the case of the Chow Chow (Wiznitner, et al., 1984; Burd, et al., 1986; Kube et al., 2006). Despite a number of theories, the pathophysiology of this mechanism is unknown. One theory claims that drugs can interfere with neurotransmission and patients treated with phenobarbital have been found to have increased concentrations of homovanillic acid and 5-hydroxyindoleacetic acid in their CSF. This suggests that this anticonvulsant may have dopamine agonistic properties, altering gamma-aminobutyric acid (GABA) functions as well as acetylcholine leading to disruption of neurotransmission in the brain which can cause dyskinesia (Chadwick et al., 1976; Sandyk, 1986).
2.3.2.2 Anticonvulsant-Responsive PD
A PD responsive to phenobarbital therapy was identified in a seventeen-month-old German Shorthaired Pointer (Harcourt-Brown, 2008).
Episodes in the affected dog were characterized by a kyphotic posture initially, followed by a progressive increase in flexion of the hind limbs. Episodes seemed to last 10 minutes to half an hour, with the longest episode lasting 3 hours, which could occur or not during periods of exercise. During the episodes, the dog did not lose consciousness and no alterations in behavior were noticed. However, it was mentioned by the owner that episodes could sometimes be avoided when he noticed some changes prior to the attack and would gently calm the animal without causing any type of excitement (Harcourt-Brown, 2008; Urkasemsin & Olby, 2014).
In regard to triggers, it seems that episodes could occur with excitement or periods of prolonged exercise or even activity that required precise control of motor.
Diagnostically, there were no abnormalities and so a trial therapy with phenobarbital was initiated. The trial showed a decrease in frequency of the episodes, even without a therapeutic serum concentration. Phenobarbital was discontinued for some time due to side effects and an increase in frequency of episodes was noticed the same as previously. Due to increase in frequency of episodes and side effects noticed with phenobarbital, potassium bromide was used to control the episodes instead and resulted well as well (Harcourt-Brown, 2008).
Study objectives
3 Study objectives
The goal of this dissertation is to:
review the literature published to date which refers to MDs;
analyse clinical cases presented to the Veterinary Teaching Hospital of the University of Trás-os-Montes e Alto Douro;
Case Presentation
4 Case Presentation
4.1 Material and Methods
The cases described in this dissertation presented all to the Veterinary Medical Teaching Hospital of the University of Trás-os-Montes e Alto Douro, in Vila Real, with a history of MDs. Three dogs of different breeds were included: a Bichon Frise, an English Bulldog and a Yorkshire Terrier, with ages between 2 and 8 years. All dogs were intact males.
The first case that will be presented is in the process of developing a case report, which has not yet been published as genetic testing is still pending. It is important to refer that this case is of extreme rarity and, to date, only one other case report has been published in another Bichon Frise. The fact that only the one report has been made in this breed, but has been published shows that this is not frequently seen, but could mean that a mode of inheritance could be present and, for that reason, further studies should be attempted.
Case Presentation
26
4.2 Clinical Cases
4.2.1 Clinical Case #1
Signalment
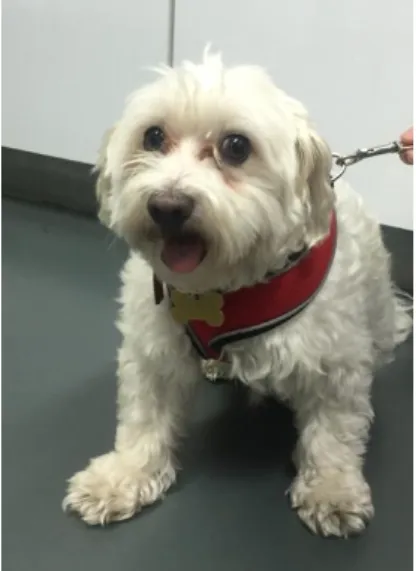
8-year-old, male intact, Bichon Frise (Figure 6).
.
Figure 6. 8-year-old, male intact, Bichon Frise of clinical case # 1.
Chief Complaint
Abnormal IMs
History
The affected animal was referred to the Veterinary Medical Teaching Hospital of the University of Trás-os-Montes e Alto Douro with a three-year history of IMs affecting its limbs.
The IMs would typically affect the limbs in the same pattern, beginning in the left thoracic limb with progression to the ipsilateral pelvic limb, while disappearing in the primarily affected one. Transition of the movements would then occur in the same manner, contralaterally (Figure 7). The thoracic limbs and pelvic limbs were dominated by a continued flexion. Muscles of the trunk would also be affected and the dog would adopt a kyphotic posture. Eventually, loss of balance would occur which led the animal to remain seated.
Case Presentation
(A) (B) (C)
Figure 7. Bichon Frise with (A) flexion of the left thoracic limb that progresses to the (B) right thoracic limb and (C) finally advances to the pelvic limbs, in this case the right side. Note that throughout the
whole episode the animal adopts a kyphotic posture.
The owner stated that, during the episodes, no loss of consciousness was noticed as the dog would respond. No hypersalivation, defecation or urination was detected and recovery from episodes was quite prompt.
Physical Exam
Unremarkable
Neurological Exam
Unremarkable
Additional Diagnostic Testing
Routine CBC and biochemistry were unremarkable as were the levels of bile acids. RT-PCR essays for infectious agents on CSF and blood, including ehrlichiosis, leishmaniasis and canine distemper virus were negative.
Case Presentation
28
(A) (B)
(C) (D)
(E) (F)
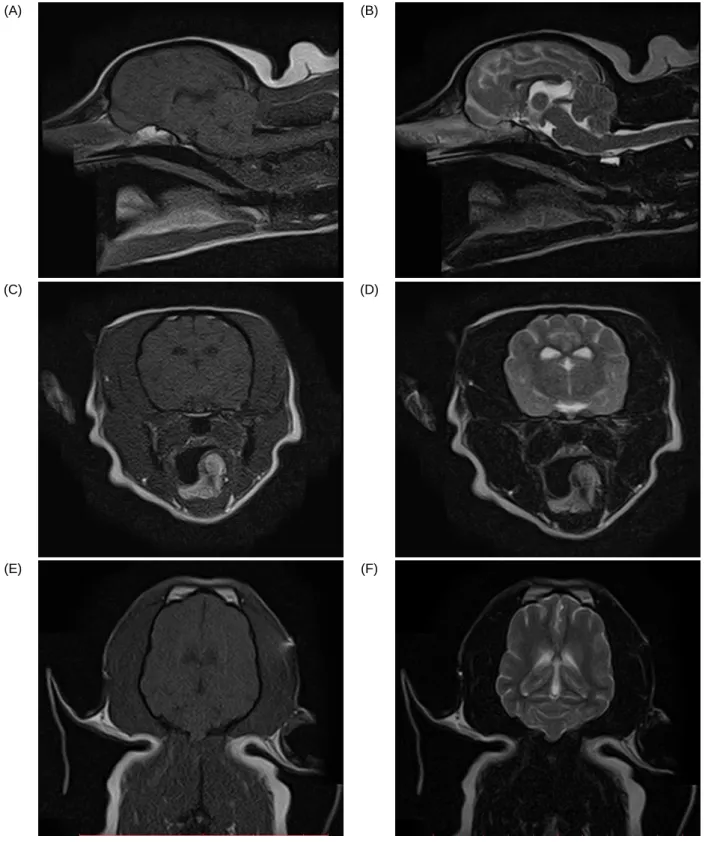
Figure 8. MRI images of different planes where no abnormalities are detected. (A) T1 sagital (B) T2 sagital (C) T1 Axial (D) T2 Axial (E) T1 Coronal (F) T2 Coronal.
Case Evolution
Case Presentation
4.2.2 Clinical Case #2
Signalment
3-year-old, male intact, English Bulldog (Figure 9).
Figure 9. 3-year-old male intact English Bulldog – case # 2.
Chief Complaint
Head bobbing
History
This dog was referred to the Veterinary Teaching Hospital of University of Trás-os-Montes e Alto Douro with a history of only two episodes of head bobbing on the day before. The tremors of the head and neck were performed in a horizontal plane and lasted around a minute.
Physical Exam
Unremarkable
Neurological Exam
Unremarkable
Additional Diagnostic Tests
A full bloodwork was performed as was biochemistry which were both unremarkable. Real-time PCR was performed in order to detect Erlichia, Anaplasma and Canine Distemper virus and results were negative.
Case Presentation
30
The animal was sent home on the same day he was presented to the hospital for observation. The day after, the dog had another episode of head bobbing, this time in a vertical plane. After this episode the owners reported that none other were observed.
Case Presentation
4.2.3 Clinical Case #3
Signalment
7-year-old, male intact, Yorkshire Terrier (Figure 10).
Figure 10. 7-year-old, male intact, Yorkshire Terrier – clinical case # 3.
Chief Complaint
Seizure-like episodes
History
This dog was referred to the Veterinary Teaching Hospital of the University of Trás-os-Montes e Alto Douro with a three-year history of possible epileptic seizures. The frequency of the episodes had been, apparently, increasing over the last week up to 2 per day.
The episodes were characterized by muscle contraction of the hind limbs and of the trunk muscles that would impair the ability of the animal to walk and stand (Figure 11).
Case Presentation
32
(A) (B) (C)
Figure 11. Yorkshire Terrier of clinical case # 3 showing signs of (A) inability to walk and stand (B) kyphotic posture with contraction and flexion of pelvic limb; (C) continued contraction of right pelvic limb.
Physical Exam
Unremarkable
Neurological Exam
Unremarkable
Additional Diagnostic Tests
Full bloodwork and chemistry were performed and were completely unremarkable. To further check for any possible abnormalities in the brain an MRI was performed which was also unremarkable (Figure 12 - Figure 14).
(A) (B)
Figure 12. MRI images in (A) T1 and (B) T2 of the brain of the Yorkshire Terrier obtained in a sagital plane that reveal no abnormalities.