Artigo
*e-mail: barreto@uel.br
DETERMINATION OF Ni(II) IN METAL ALLOYS BY SPECTROPHOTOMETRY UV-VIS USING DOPASEMIQUINONE
Wagner José Barreto*, Sonia Regina Giancoli Barreto,Ieda Spacino Scarminio, Dílson Norio Ishikawa,Miriam de Fátima Soares e Marcus Vinícius Brás de Proença
Departamento de Química, Centro de Ciências Exatas, Universidade Estadual de Londrina, 86051-990 Londrina – PR, Brasil
Recebido em 12/2/09; aceito em 10/7/09; publicado na web em 13/11/09
A spectrophotometric method was proposed for Ni(II) determination in alloys using a dopa-semiquinone (L-1) to form [Ni(II)(L1-) 3]
1-,
ε = 9.3 x 103 L mol-1 cm-1. The optimal conditions for the determination were: wavelength 590 nm, temperature 25 °C, reaction time 45 min and pH 7.5. The Beer’s law was obeyed for nickel from 3.33 x 10-5 to 1.78 x 10-4 mol L-1.The method was applied to complex samples, such as inox, nickel-titanium and cobalt-chromium alloys. A study of the potential interferents revealed that Mn was the major interferent. The limit of detection and quantiication were 2.88 x 10-5 mol L-1 and 3.06 x 10-5 mol L-1, respectively.
Keywords: UV-Vis spectrophotometry; nickel; metals.
INTRODUCTION
Ni(II) is a metal ion appearing together in a wide variety of samples of the food industry, in the manufacture of alloys, the pro-duction of heat-resistant steels and is present in small amounts in most soils, plants and animal tissues. For this reason its determination is of widespread interest. Current efforts toward the development of new methods are focused on increasing sensitivity and selectivity of the detection methods and simplifying the procedure by eliminating treatment and sampling steps.
The usual methods for determination of nickel using chemical reagents like; dimethylglyoxime, sodium diethyldithiocarbamate, (ethylenedinitrilo)tetraacetica acid disodium salt (Titriplex® III), 2,2’-Furil dioxime and dithioxamide.1 The dimethylglyoxime me-thod present ε = 14 103 L mol-1 cm-1 at 450 nm and Co, Cr(III) Cu(II),
Mn(II), Fe and Zn higher 1 μg mL-1 interfere and it is advisable to isolate the nickel by extraction with dimethylglyoxime/chloroform before the determination in aqueous solution. The method involves the addition of various chemical reagents (hydrochloric acid, bro-mine water and ammonia) and the times stated for which addition are to be strictly adhered.2 In the sodium diethyldithiocarbamate method Cu, Bi, Co and Fe are interferents and should be removed by the prior extraction with dimethylglyoxime. The method pre-sents ε = 35 x 103 L mol-1 cm-1 but in ultra-violet region (325 nm).
The method is time consuming and use extractions with carbon tetrachloride.3 The method using Titriplex® III gives a coloured complex utilized for determination of nickel by spectrophotometric observation of the band at 1000 nm.4 The 2,2’-Furil dioxime forms with nickel a yellow, water insoluble complex which is extractable with chloroform and it can be utilized for photometric determina-tion.5 The method utilizing dithiooxamide is very sensitive (limit of detection about 0.01 μg nickel) but copper and cobalt interfere seriously in the method.6
In the last ifteen years, several spectrophotometric method have been successfully employed in the individual and simultaneous determinations of nickel in mixtures.7-20
Dioxolenes are derived from 1,2-di-oxo-benzene and can assume the quinone (L), radical-anion semiquinone (L1-) and di-anion cate-cholate (L2-), structures, Figure 1.
Catecholates, semiquinones and quinones are highly reactive dioxolenes, with important participation in biological structures performing essential functions in living organisms. The dioxolene molecules show interesting magnetic and redox behaviors, forming colorful complexes with heavy metals.21,22 Studies on the preparation and spectrophotometric (UV-Vis, IR, EPR and Raman) analysis of the Co(II), Ni(II), Zn(II) and Fe(III) water soluble compounds with dioxolene derived from dopamine oxidation in a sodium thiosulfate environment, have shown that dopa-semiquinone has a high afinity for the transition metals under study (Figure 2).23 The compound resulting from the complexation reaction of the dopa-semiquinone and Ni(II) at a pH of 7 was proposed to be a soluble [Ni(II)(L1-)
3]
1-anion with the structure showed in Figure 2. The absorption spectrum shows an intense absorption band at 590 nm with ε = 3.18 x 103 L
mol-1 cm-1, attributed to an inter-ligand charge transfer. Figure 1. Oxidation states of dopamine
Figure 2. Proposed structure for the soluble [(Ni(II)(L1-)
3]
1- anion complex
The major utilization of nickel is the production of iron and non iron alloys, since its presence improves the mechanical and chemical resistance of steel. Nickel alloys also are used in odontological materials, such as Formaloy C (30% Cr, 60% Ni, 5% Mo); Ceraplus S (23% Cr, 62% Ni, 10% Mo) and Remaniun CS (Cr 26%, Ni 61%, Mo 11%). The stainless steel Inox used in orthodontics is of the austenitic type with a general composition of 18% of chromium, 8% of nickel and 0.08 to 0.15% of carbon with the remainder being iron. Iron, due to its versatility, is commonly used in orthodontics because it has a large malleability that permits the execution of curves with ease and precision. Also it is easily welded and has low attrition and low cost. The cobalt-chromium metallic alloy was developed in the early 60s, constituted of 40% cobalt, 20% chromium, 15% nickel, 15.8% iron, 7% molybde-num, 2% manganese, 0.16% carbon and 0.04% beryllium, being commercialized under the name “Elgiloy”, with properties very similar to steel, but with better malleability.24
The toxicity of Ni(II) for human beings is known. It causes damage to the central nervous system, heart and kidneys, a re-duction in the immunological capacity of the organism, eczema, allergic inlammations and carcinomas of the mucous and pul-monary membranes.25
The objective of this study was to obtain a new chromogenic reagent derived from dopamine and quantify Ni(II) in metallic alloys used in orthodontic wires, that is, Inox, nickel-cobalt and nickel-titanium alloys, by UV-Vis spectrophotometry. Response surface methodology based on a 23 central composite design was applied to optimize the conditions for spectrophotometric measurements.
EXPERIMENTAL
Reagents
The solutions were prepared using ultra pure water (Helga) and reagents of analytical grade. All the reactions were carried out in an ultra thermostatic bath (± 0.01 °C). A stock solution of NiCl2 with a concentration of 1.11 x 10-3 mol L-1 of Ni(II) was prepared and standardized with EDTA.
The buffer solutions of KH2PO4/NaOH at pH 6.0; 6.5; 7.0; 7.5; 8.0; 8.5 and of H3BO3/KCl/NaOH at pH 9.2, were prepared according to Perrin & Dempsey.26
The chromogenic reagent solution was prepared dissolving 0.3026 g (3.79 x 10-3 mol L-1) of Na
2S2O3 in 500.0 mL of water, 0.1008 g (1.05 x 10-3 mol L-1) of dopamine were added with O
2 bubbling at a constant low of 0.5 L min-1. Aliquots of 200 μL from the solution were diluted in 3 mL of water, and UV-Vis spectra (Thermo Scientiic, Genesys 2) were obtained from 200 to 900 nm in a quartz cuvette with a 1.0 cm optical pathway. The chromogenic reagent (1.05 x 10-3 mol L-1 after 48 h)has a very dark green color.
Preparation of the chromogenic reagent
To obtain the chromogenic reagent with maximum reactivity a study was carried out removing aliquots during the reaction after 24, 48, 50, 72 and 74 h to react with a ixed nickel concentration (1.11 x 10-4 mol L-1). The aliquots of 5 mL of the chromophoric reagent were mixed with 5 mL of Ni(II) solution (1.11 x 10-4 mol L-1) during 45 min, pH 7.5 (KH
2PO4/NaOH buffer) at 25 °C. Aliquots of 3 mL were transferred to a quartz cuvette to obtain the UV-Vis absorption spectra from 200 to 900 nm using water as the blank. The maximum absorbance obtained indicated the better reaction time for dopamine oxidation.
Stability of the chromogenic reagent
To establish the maximum storage time of the chromogenic reagent solution without loss of reactivity, a study was performed to investigate the stability over time. A reagent solution (500 mL) was prepared with interruption of the bubbling of O2 after 48 h of reaction and half of the volume of solution was transferred to a 250 mL lask and immediately closed. The remaining volume of the reagent solution was transferred to another 250 mL lask in which N2(g) was bubbled for 20 min to remove the O2 of the solution and then hermetically closed. The lasks were stored at 4 °C. From these solutions aliquots of 5.0 mL were transferred to lasks after 24, 48, 120, 144, 168 and 192 h and reacted with 5 mL of Ni(II) 5.55 x 10-4 mol L-1 for 40 min at pH 7.00 and 25 °C. A 3.0 mL aliquot was transferred to a quartz cuvette to obtain the UV-Vis spectra from 400 to 900 nm with a spectral resolution of 1 nm.
Complexation reaction of Ni(II) and chromogenic reagent
The Ni(II) complex in aqueous solution pH 7.5 presents an intense absorption band at 590 nm calculated from the analytical curves as ε= 9.3 x 103 L mol-1 cm-1. To optimize the conditions of
the complexation reaction of Ni(II) and the dopa-semiquinone, a 24 factorial design was carried out with a central point. The factors were; pH (6, 7, 8), reaction time (rt) (30, 45 and 60 min), reaction temperature (T) (25, 35 and 45 °C) and Ni(II) concentration (1.11 x 10-4, 9.90 x 10-5 and 8.88 x 10-5 mol L-1). The response was measured as the amount of absorption.
The 23 factorial design
A 23 central composite was carried out after the 24 factorial design because the temperature was not signiicant in affecting the response. This new factorial design was carried out at 25 °C, with pH (5.8, 6.5, 7.5, 8.5, 9.2), rt (20, 30, 45, 60 and 70 min) and Ni(II) concentration (1.80 x 10-4, 1.95 x 10-4, 2.17 x 10-4, 2.39 x 10-4, 2.54 x 10-4 mol L-1) as variables. The values in parenthesis corresponding to the levels -1.68, -1, 0, 1 and 1.68 of the variable tested in the 23 central composite designs.
Study of interference from metals
After the determination of the best conditions for the complexa-tion reaccomplexa-tion between Ni(II) and dopa-semiquinone, the possibility of interference from the transition metals Co, Cr, Cu, Fe, Mn and Zn in the determination of Ni(II) was studied. Solutions containing 6.66 x 10-5 mol L-1 of Ni(II) and the metals were prepared by the addition of different aliquots of 1000 mg L-1 standard solutions of Co(II), Cr(III), Cu(II), Fe(III), Mn(II) (Merck) and Zn(II) (Carlo Erba) with a 7.5 pH buffer. Aliquots of 5 mL of these solutions were transferred to lasks with 5.0 mL of the chromogenic reagent. The lasks were kept in an ultra thermostatic bath for 45 min at 25 °C and the absorbance was measured at 590 nm.
Digestion of the alloys
residues were iltered with quantitative ilter paper. The solutions of the digested wires and the blank were transferred to 50 mL volume-tric lasks and made up with water, then transferred to polyethylene lasks and stored at 4 ºC.
Nickel determination by atomic spectroscopy (AAS and ICP-OES)
The determination of Ni(II) in the metallic alloys by AAS (Shi-madzu AA-6601F) was carried out using a nickel hollow cathode lamp and atomic transition at 232 nm. The analytical curves from 8.50 x 10-6 to 4.25 x 10-5 mol L-1 were obtained by diluting 1.70 x 10-2 mol L-1 of Ni(II) stock solution standardized with EDTA. The ICP-OES (Perkin Elmer Optima 5200DV) analysis was performed with limits of quantiication of: Fe (0.0012 mg L-1), Ni (0.0018 mg L-1), Co (0.0015 mg L-1), Ti (0.001 mg L-1) and Cr (0.0019 mg L-1). The analytical curves were obtained using a mixture of 1000 mg L-1 standard solutions (Merck) of each metal in the range of 0.1 to 10.0 mg L-1.
Obtaining the analytical curves
The most adequate analytical curve was obtained from 3.33 x 10-5 to 1.78 x 10-4 mol L-1, for a stock solution of 1.11 x 10-3 mol L-1 NiCl2 standardized with EDTA. The volumes taken using a 10 mL burette were 0.75; 1.00; 1.25; 1.50, 1.75; 2.00; 3.00 and 4.00 mL, made up to 25.0 mL with a 7.5 pH buffer solution, in duplicate. From these solutions aliquots of 5.0 mL were transferred to 16 lasks and 5.0 mL of reagent solution added and reacted at 25 °C for 45 min. The nickel complex formed showed intense blue coloring. The blank was prepared by adding the 7.5 pH buffer solution to 5.0 mL of the chromogenic reagent in 5.0 mL lasks. The absorbances were measured at 590 nm against the blank.
Determination of the limits of detection (LOD) and quantiication (LOQ)
In seven test tubes (n=7) 5.0 mL of stock solution at 7.5 pH were mixed with 5.0 mL of the chromogenic reagent solution. After 45 min of reaction in an ultra thermostatic bath at 25 ºC, the absorbances were measured at 590 nm (n=7). The limit of detection was calculated from
the expression LOD = X_ + 3s and the limit of quantiication was
determined by the expression LOQ = X_ + 10s, where X_ is the mean
of the measured values and s is standard deviations.
Determining the Ni(II) in real mixtures
Aliquots of 2.0, 3.0 and 4.0 mL of the solutions of the digested alloys of Ni-Ti, Co-Cr and Inox, respectively, were transferred into 50 mL volumetric lasks. The volumes of the lasks were made up with a 7.5 pH buffer solution. The same procedure was carried out for the blank. An aliquot of 5.0 mL of the Ni-Ti alloy was transferred to a test tube containing 5.0 mL of the reagent solution. The same procedure was applied to the blank solution. The test tubes were kept in a bath at 25 ºC for 45 min. The absorption was measured at 590 nm against the blank.
Determination of Ni(II ) using the standard addition method
In four volumetric lasks, 2.0 mL of neutralized samples of the Inox alloy were added to 0, 200, 400 and 600 μL of the 1.11 x 10-3 mol L-1 standard solution of Ni(II) andmade up to 5 mL with a 7.5 pH buffer solution, to which 5.0 mL of the chromogenic reagent were
added. The test tubes were kept at 25.00 ± 0.01 °C for 45 min and the absorption was measured at 590 nm. The same procedure was applied to the nickel-titanium alloy.
RESULTS AND DISCUSSION
Reactivity of the chromogenic reagent
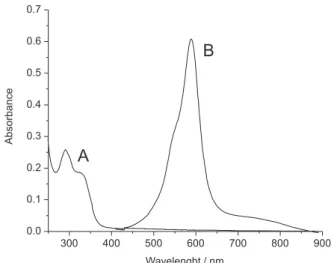
Figure 3A shows the spectra of the chromogenic reagent obtained from the oxidation reaction of the dopamine with Na2S2O3. Figure 3B shows the spectra of the Ni(II) complex in aqueous solution with an intense absorption band at 590 nm (ε= 9.3 x 103 L mol-1 cm-1)
assigned to an intra-ligand charge transference.
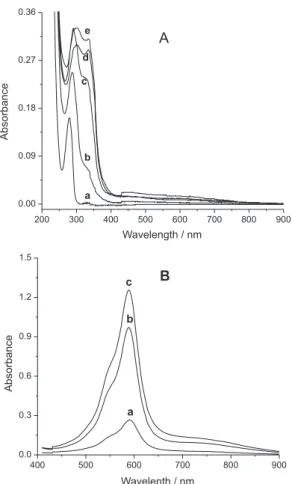
The oxidation of dopamine with O2 in the presence ofNa2S2O3 was followed spectrophotometrically (Figure 4A).At the beginning of the oxidation the spectrum has a single band at 280 nm, characte-ristic of dopamine, (Figure 4A a). After 24 h of oxidation this band disappeared and a new band at 290 nm was observed together with a shoulder at 330 nm, (Figure 4A b-d) and after 72 h two bands at 302 and 335 nm were present (Figure 4A e).
In this spectral region two electronic transitions characteristic of catecholates and semiquinonic radicals are expected due to the π → π* or n → π* electronic transitions.27 The initially colorless solution
acquired a dark green color and bands at 290 and 330 nm with 48 h of oxidation, and showed the greatest reactivity (Figure 4B). After 48 h the absorbance of the complex at 590 nm reduced indicating a progressive deactivation of the chromogenic reagent caused by a slowing polymer-ization of the dopa-semiquinone. The saturation with N2 did not improve the activity of the solution, Figure 5, indicating that polymerization processes still proceed. Although there was a progressive reduction in the reactivity, the solution remains suficiently reactive to be used to determine Ni(II), as can be observed in Figure 5, where it can be seen that after 192 h the solution absorbance was still very high (A= 0.40). The optimization parameters of the complexation reaction
The results of the experiments using the 24 factorial design sho-wed that the temperature does not have a signiicant inluence on the Figure 3. (A) UV-Vis spectrum of the chromogenic reagent, dopa-semiquinone, obtained after 48 h of dopamine oxidation by bubbling O2 in aqueous solution in the presence of Na2S2O3. (B) UV-Vis spectrum of the [Ni(II)(L
1-)
3]
1- complex
in aqueous solution obtained after 45 min of reaction, temperature 25 °C, pH 7.5, 1.11 x 10-4 mol L-1 of Ni(II) and 5.25 x 10-4 mol L-1 of the chromogenic
complexation reaction of the Ni(II) with the chromogenic reagent. The major factors of the complexation reaction which increased the response (absorbance) were the pH, reaction time and Ni(II) concentration, conirmed by conidence intervals calculated from the standard errors and Student’s critical t-distribution value for each effect. A 23 central composite design was then conducted, with
the three factors: concentration of Ni(II), pH and reaction time. The quadratic model did not show any lack of it at the 95% conidence level. Statistical signiicance of respective model equation was che-cked using F-test analysis of variance (ANOVA). The equation for the itted model eliminating the terms with non-signiicant coeficients, is represented by
y^
= 1.46 + 0.88 [Ni2+] + 0.365pH + 0.150tr - 0.79pH2 - 0.054tr2
+ 0.093[Ni2+] x pH (1)
From the regression Equation 1, the optimal conditions for the reaction between Ni(II) and chromogenic reagent were: wavelength 590 nm, temperature 25 °C, reaction time 45 min and pH 7.5. Interference of metals
It was observed previously that dopa-semiquinone react with Fe, Mn, Zn, Cu and Co, forming metal-complexes presenting bands around 600 nm, however, with lower molar absorptivity.23 The metal interference was characterized when the absorbance at 590 nm for determination of 6.66x10-5 mol L-1 of Ni(II) was changed to highest values when the interferent was present. The lowest concentrations (mol L-1) at which the most common metals used in alloys could interfere in the determination of 6.66 x 10-5 mol L-1 Ni(II) by the proposed methodology were 1.33 x 10-7 Mn(II), 4.00 x 10-7 Cu(II), 6.66 x 10-7 Fe(III), 9.33 x 10-7 Co(II), 1.06 x 10-6 Cr(III) and 1.33 x 10-6 Zn(II). The metal which showed the greatest interference in the UV-Vis method was Mn(II).
Analytical curve
The equation for the analytical curve (n = 3) of the [Ni(II)(L1-) 3]
1-complex obtained to determine Ni(II) (between 3.33 x 10-5 and 1.78 x 10-4 mol L-1) was A = -1.52 × 10-2 + 9.30 × 103[Ni]. This equation presented a high level of linearity with a correlation coeficient of 0.999. The limits of detection and quantiication determined for the methodology developed were, respectively, 2.88 x 10-5 mol L-1 and 3.06 x 10-5 mol L-1, demonstrating that the method is sensitive enough to detect nickel in the commercial cobalt-chromium and nickel-titanium metal alloys.
Applying the methodology to real samples
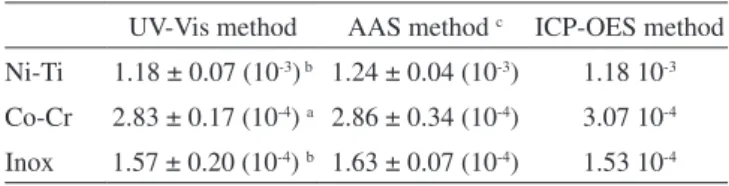
Table 1 shows the percentages of nickel recovery obtained for the metal alloys using calibration curves compared with the values obtained from the AAS and ICP-OES techniques. The UV-Vis metho-dology using analytical curves was not able to detect the Ni(II) in the samples of Inox due to the low concentration of nickel in this type of alloy, ca. 9 mg L-1, when compared with the amounts in the Ni-Ti (ca. 70 mg L-1) and Co-Cr (ca. 18 mg L-1) alloys (Table 1). The difference in the sample recoveries for the Ni-Ti alloy, i.e., 24% using the UV-Vis method and 5% with the AAS method, can be explained by the high complexity of the sample. The high concentration of titanium (30.6 mg L-1) caused a strong interference due to the formation of TiO2 during the digestion process of the sample, which precipitates adsorbing the Ni(II) ions. The results for the Co-Cr alloy show less difference between the two methods, indicating that the cobalt and chromium caused less interference than the titanium in the nickel determination. Aiming to improve the quality of the analysis, the standard addition method was used to reduce the matrix effect, for the Ni-Ti and Inox alloys. The equations for the analytical curves of the standard addition method for Inox and nickel-titanium alloys were A = 3.51×10-2 + 1.48×103 [Ni] (r =0.998) and A = 5.91×10-1 + Figure 4. (A) UV-Vis spectra of the aqueous solution of dopamine oxidized
with O2 in the presence of Na2S2O3 at reaction times of: (a) 0, (b) 24, (c) 48,
(d) 50, (e) 72 h. (B) Absorbance as a function of the time for formation of the [Ni(II)(L1-)
3]
1 anion complex in aqueous solution under optimized conditions
(pH 7.5, 25 ºC, 1.11 x 10-4 mol L-1 [Ni(II)] and 45 min of reaction) using the
chromogenic reagent prepared over: (a) 24 , (b) 48, (c) 72 h
Figure 5. UV-Vis absorbances of the [Ni(II)(L1-)
3]
1- complex in aqueous
solution at a 7.5 pH, as a function of time using the chromogenic reagent (L-1) maintained in: (a) lasks sealed after bubbling of N
2 for 15 min (•), (b)
Table 1. Concentration (mol L-1) of the Ni(II) in Ni-Ti, Co-Cr and Inox alloys using the UV-Vis methodology compared to the AAS and ICP-OES methods
UV-Vis method AAS method c ICP-OES method Ni-Ti 1.18 ± 0.07 (10-3) b 1.24 ± 0.04 (10-3) 1.18 10-3 Co-Cr 2.83 ± 0.17 (10-4) a 2.86 ± 0.34 (10-4) 3.07 10-4 Inox 1.57 ± 0.20 (10-4) b 1.63 ± 0.07 (10-4) 1.53 10-4 a Using analytical curve (N=3), b using standard addition method (N=4), c(N=2).
2.11×103[Ni] (r =0.993), respectively. The use of the standard addition method to determine Ni(II) improved the UV-Vis spectrophotometric method (Table 1), with values close to those obtained by the ICP and AAS methods. The advantages of using the UV-Vis method approach compared those by AAS and ICP-OES are, obviously, the price per unit of analysis associated with a response of good reproducibility and sensitivity.
CONCLUSIONS
The preparation of the chromophoric reagent (dopa-semiquinone) is simple, when compared to the others mentioned in the literature, since there is no need of complex organic synthesis using organic reagents. The chromogenic reagent was obtained in 48 h, through the oxidation of dopamine in the presence of sodium thiosulfate. The method does not require extraction or pre-concentration of the analyte, decreasing the manipulation time and the use of solvents. The conditions for the complexation of Ni(II) with the chromogenic reagent were optimized through the use of statistical designs, the best reaction conditions being pH 7.5, temperature 25 °C, reaction time 45 min and wavelength 590 nm. The methodology was tested for the determination of the Ni(II) analyte in very complex real samples, i.e., Inox, nickel-titanium and cobalt-chromium metal alloys, and provided good results. The study of the interference of metals Co(II), Cr(III), Cu(II), Fe(III), Mn(II) and Zn(II) revealed that Mn(II) is the major interferent in relation to the other metals. The limits of detection (2.88 x 10-5 mol L-1) and quantiication (3.06 x 10-5 mol L-1) were suficient for general usage. The use of the standard addition method can improve the quality of the results in studies of complex samples. ACKNOWLEDGMENTS
The authors would like to thank the Fundação Araucária, FAEPE/ UEL, CAPES and CNPq for the inancial support.
REFERENCES
1. Fries, J.; Getrost, H.; Organic reagents for trace analysis, E. Merck Darmstadt, 1977.
2. Specker, H.; Kartkamp, H.; Z. Anal. Chem. 1955, 145, 260.
3. Alexander, O. R.; Godar, E. M.; Linde, N. J.; Ind. Eng. Chem., Anal. Ed. 1946, 18, 206.
4. Brake, L. D.; McNabb, W. M.; Hazel, J. F.; Anal. Chim. Acta 1958, 19, 42. 5. Gabler, A. R.; Mitchel, A. M.; Mellon, M. G.; Anal. Chem. 1951, 23,
500.
6. Jacobs, W. D.; Yoe, J. H.; Anal. Chim. Acta 1959, 20, 332.
7. Rodriguez, A. M. G.; Pavon, J. M. C.; Ojeda, C. B.; de Torres, A. G.; Mikrochim. Acta 1995, 118, 229.
8. Ferreira, S. L. C.; Costa, A. C. S.; de Jesus, D. S.; Talanta 1996, 43, 1649.
9. Fan, X. Z.; Zhang, G. F.; Zhu, C. H.; Analyst 1998, 123, 109. 10. Zachariadis, G. A.; Themelis, D. G.; Kosseoglou, D. J.; Stratis, J. A.;
Talanta 1998, 47, 161.
11. Taljaard, R. E.; van Staden, J. F.; Anal. Chim. Acta 1998, 366, 177. 12. Zhao, S. L.; Xia, X. Q.; Hu, Q. F.; Anal. Chim. Acta 1999, 391, 365. 13. Teixeira, L. S. G.; Rocha, F. R. P.; Korn, M.; Reis, B. F.; Ferreira, S. L.
C.; Costa, A. C. S.; Talanta 2000, 51, 1027.
14. Ma, Q. L.; Ma, H. M.; Su, M. H.; Wang, Z. H.; Nie, L. H.; Liang, S. C.; Anal. Chim. Acta 2001, 439, 73.
15. Vicente, S.; Maniasso, N.; Queiroz, Z. F.; Zagatto, E. A. G.; Talanta 2002, 57, 475.
16. Hu, Q.; Yang, G.; Huang, Z.; Yin, J.; Anal. Sci. 2003, 19, 1449. 17. Liu, Y. W.; Chang, X. J.; Wang, S.; Guo, Y.; Din, B. J.; Meng, S. M.;
Talanta 2004, 64, 160.
18. Ghasemi, J.; Shahabadi, N.; Seraji, H. R.; Anal. Chim. Acta 2004, 510, 121. 19. Fakhari, A. R.; Khorrami, A. R.; Naeimi, H.; Talanta 2005, 66, 813. 20. Lemos, V. A.; Baliza, P. X.; Santos, J. S.; Nunes, L. S.; Jesus, A. A.;
Rocha, M. E.; Talanta 2005, 66, 174.
21. ElAyaan, U.; Herlinger, E.; Jameson, R. F.; Linert, W.; J. Chem. Soc., Dalton Trans. 1997, 2813.
22. Hider, R. C.; Howlin, B.; Miller, J. R.; Mohd-Nor, A. R.; Silver, J.; Inorg. Chim. Acta 1983, 80, 51.
23. Barreto, W. J.; Ando, R. A.; Santos, P. S.; Silva, W. P.; Spectrochim. Acta A 2007, 68, 612.
24. Burgaz, S.; Demircigil, G. C.; Yilmazer, M.; Ertas, N.; Kemaloglu, Y.; Burgaz, Y.; Mutat. Res. 2002, 521, 47.
25. Kasprzak, K. S.; Sunderman, F. W. Jr.; Salnikow, K.; Mutat. Res. 2003, 533, 67.
26. Perrin, D. D.; Dempsey, B.; Buffers for pH and metal ion control, Chapman and Hall: New York, 1974.
![Figure 2. Proposed structure for the soluble [(Ni(II)(L 1- ) 3 ] 1- anion complex where L 1- is the dopa-semiquinone radical](https://thumb-eu.123doks.com/thumbv2/123dok_br/18985244.458609/1.892.551.765.876.1088/figure-proposed-structure-soluble-anion-complex-semiquinone-radical.webp)