w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Casinga-cheirosa
organic
extract
impairment
over
Balb-c
male
mice
behavioral
phenotype
Dirce
M.
Estork
a,
Daniela
F.
Gusmão
a,
Mateus
L.B.
Paciencia
b,
Sergio
A.
Frana
b,
Ingrit
E.C.
Díaz
b,
Antonio
D.
Varella
b,
Riad
N.
Younes
b,c,
Luiz
F.L.
Reis
d,
Edna
F.S.
Montero
e,
Maria
M.
Bernardi
a,f,
Ivana
B.
Suffredini
a,b,f,∗aProgramadePós-graduac¸ãoemPatologiaExperimentaleAmbiental,Vice-ReitoriadePesquisaePós-graduac¸ão,UniversidadePaulista,SãoPaulo,SP,Brazil bNúcleodePesquisasemBiodiversidade,LaboratóriodeBotânicaeHerbárioUNIPeLaboratóriodeExtrac¸ão,UniversidadePaulista,SãoPaulo,SP,Brazil cHospitalSãoJosé,SãoPaulo,SP,Brazil
dInstitutodeEnsinoePesquisa,HospitalSírioLibanês,SãoPaulo,SP,Brazil eFaculdadedeMedicina,UniversidadedeSãoPaulo,SãoPaulo,SP,Brazil
fProgramadePós-graduac¸ãoemOdontologia,Vice-ReitoriadePesquisaePós-graduac¸ão,UniversidadePaulista,SãoPaulo,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory: Received14April2015 Accepted13October2015 Availableonline8December2015
Keywords: Laetiasuaveolens Salicaceae Openfield Elevated-plusmaze Locomotion Emotionality
a
b
s
t
r
a
c
t
Laetiasuaveolens(Poepp.)Benth.,Salicaceae,popularlyknownas“casinga-cheirosa”,“caferana”,or “laranjinha”,isnativetoBrazilbutnotendemictothiscountry.Acrudeorganicextractwasobtained fromtheleavesandstemandintraperitoneallyadministeredinmaleBalb-cmice.Itsbehavioraleffects wereevaluatedintheopenfieldandelevatedplusmazeinatwo-stageexperimentthatassessedten differentparametersrelatedtobehavioraslocomotion,emotionality,andanxiety.Inthefirststageof theexperiment,intraperitonealthecrudeorganicextractadministrationdose-dependentlyimpaired locomotionandemotionality30–120minafteradministration.Asignificantdecreaseindefecationwas observed,whichwasrelatedtoemotionality.Noalterationsintheelevatedplusmazewerefound;thus, thisapparatuswasnotusedinthenextstageoftheexperiment.Inthesecondstage,thepreviously deter-minednon-lethaldoseof0.1563g/kgwasintraperitoneallyadministered,whichimpairedlocomotion andrearingfrequencyandincreasedimmobilitytime.Necropsyrevealedsmoothintestinehemorrhage. Rutin,leucoside,nicotiflorin,guaijaverin,andastragalinwereisolatedfromthecrudeorganicextract. ThisisthefirsttimethatthesecompoundshavebeenidentifiedinL.suaveolens.Inconclusion,thecrude organicextractimpairedlocomotionandemotionalityandcausedhemorrhageinmaleBalb-cmice, indi-catingthatitsconsumptioncanbeharmfultohumansandanimals.Thepresentresultsprovideabasis forfurtherstudiesonthepharmacology,toxicology,andnaturalproductchemistryofthecrudeorganic extract.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Laetiasuaveolens(Poepp.)Benth.belongstotheSalicaceae fam-ily. It is popularly known as “casinga-cheirosa”, “caferana”, or “laranjinha”inBrazil(Gamaetal.,2007).AlthoughnativetoBrazil, itisnotendemictothiscountry(BoletimMuseuParaenseEmilio Goeldi,2013).ItiswidelydistributedinSouthAmerica,especially Brazil,Bolivia,Colombia, Venezuela, Guyana, and Peru. Accord-ing to theMissouri Botanical Garden,its basyonim is Samydia suaveolensPoepp., but it mayalso beknown asCasinga suave-olensGriseb.exBenth.,GuidoniacalophyllaKuntze,G.suaveolens
∗ Correspondingauthor.
E-mail:extractlab@unip.br(I.B.Suffredini).
Kuntze,L.calophyllaEichler,andS.petiolarisSpruceexEichler.In thepresentstudy,weadoptedthenomenclatureproposedbythe MissouriBotanicalGarden(2014).L.suaveolensisalsopopularly knownasyutubanco-del-bajoinSpanishAmazonia(Revilla,2002) andis commonlyusedinconstruction.The firstreportsonthis speciesindicatedthatanorganicextractthatwasobtainedfrom theleavesandstemofL.suaveolens(designatedEB719)showed cytotoxicactivity(30.05%lethalityinrelationtocellgrowth con-trol)againstprostatecancercelllines(Suffredinietal.,2006)and squamouscellcarcinoma(Ozietal.,2011).TheeffectsofEB719on generalactivity,includingananalysisof27parametersrelatedto thecentralnervoussystem,autonomicnervoussystem,and senso-rialandpsychomotorsystems,werepreviouslyreported,andseven moleculescontainedinthisspeciesweredescribed(Estorketal., 2014).
http://dx.doi.org/10.1016/j.bjp.2015.10.004
Inthepresentstudy,wefurtherevaluatedthebehavioraleffects ofEB719inmaleBalb-cmice,asithasneverbeendonebeforeand nofurtherinformationcouldberetainedfrompopularuses.We alsoidentifiedfivemoleculesinL.suaveolensthatwerenotreported previously.
Materialsandmethods
Plantmaterial
TheleavesandstemofLaetiasuaveolens(Poepp.)Benth., Sali-caceae,werecollectedintheBrazilianAmazonrainforestunder Braziliangovernmentlicenses(no.CGen/MMA#12A/2008andno. MMA/ICMBio/SISBIO#14895).Thecollectionwasmadeinthe sur-roundingareaofManauscity(Lat2◦58′,Long60◦26′),Amazonas,
inaseasonallyfloodedforestfromRioNegroBasin(Igapóforest). AvoucherspecimenwasdepositedattheUNIPHerbarium[A.A. Oliveira,3383(UNIP)],collectedinApril/1999,includingvoucher specimensforthefirstandsecondcollections[M.B.P.,3093(UNIP)], collectedinMarch/2009,and[M.B.P., 3203(UNIP)],collectedin March/2010,respectively.EB719wasobtainedbymacerationas previouslydescribed(Suffredinietal.,2007).
Preparationofextractanddiazepamforadministration
ThetechniqueforpreparingEB719foradministrationin ani-malswasdescribedelsewhere(Gusmãoetal.,2013b;Estorketal., 2014).Briefly,plantmaterialwassubjectedto24hmacerationwith dichloromethane:methanol(1:1).Thesolventswereremovedby rotaryevaporation,andtheextractwaskeptinafreezeruntiluse. Theextractsweredissolvedinalmondoil,whichwasalsousedas thevehiclecontrol.Diazepam(Hipolabor;lotno.AO011/11; valid-ity: 10/13; concentration, 5mg/ml; injectable medication) was administeredatadoseof1mg/kg.
Animals
Adult male Balb-c mice (Mus musculus), weighing 19–23g, were obtainedfrom theAnimal Facilities of São Paulo Univer-sity.Afterarrivalin thelaboratory,theanimalswere randomly selected, individually marked, and housedin groups of five in isolatedpolypropylenecages(38cm×32cm×16cm)under con-trolledtemperature(21±2◦C)and humidity(55–60%).Artificial lighting was provided (12h/12h light/dark cycle, lights on at 8am), withfree access toNuvilab rodent chow (Nuvital Com-pany,SãoPaulo,Brazil)andanunlimitedsupplyoffilteredwater. Theexperiments beganone weekafterthemicearrived inthe laboratory, allowing for adaptation to the new environmental conditions.Theanimalswerefastedfor1hbeforereceivingthe treatments. The animals were observed for toxic responses. If lethalityoccurredduringtheperiodofobservation,thennecropsy wasperformed.Iftheanimalssurvived untiltheend of the14 day observation period, then they were humanely euthanized inaCO2 gaschamberaccordingtoEthicsCommitteedirectives.
Theeuthanizedanimalsalsounderwentnecropsy,andindividual recordsofthenecropsywerekept.Alloftheexperimentsreceived Ethics Committee approval (CEP/ICS/UNIP 025/08, February 12, 2009).
Openfield
Anopenfield(OF)wasusedtoassesstheeffectsoftheextracton emotionalityandmotilityandconstructedaccordingtoBroadhurst (1960).Theapparatuswasadaptedtothesizeofmice. Emotional-itycanbedefinedastheobservablebehavioralandphysiological componentsofmiceemotion,yettobeusedinbehavioralstudies
forbothhumansandanimals.Whenstudiesaremadeinanimals, OFisoneoftherecommendedapparatustoevaluate emotional-ity.TheOFapparatuswasacircularwoodenbox(40cmdiameter, 50cmheight)withanopentopandfloordividedinto19squares. Illumination(400lx)wasprovidedbya100Wlampatthefloorof theapparatus.Eachanimalwasindividuallyplacedinthecenter of theOF arena, and theparameters weremeasured infive 5-minperiods:10–15min,30–35min,60–65min,120–125min,and 180–185min afterextract administration.Hand-operated coun-terswereusedtocountlocomotionfrequency(i.e.,thenumber of squares crossed with all four paws), rearing frequency (i.e., one unitof rearing correspondedtoa standingpositiononthe hindlimbs,withthetrunkperpendiculartothefloor,headtilted up,andforelimbsintheair,eithertouchingornottouchingthe walls ofthearena),and thenumber offecal boliattheend of the session. A chronometerwas used tomeasure the duration of immobility (i.e., the total time in seconds without sponta-neous movements,whenthehead,trunk,and limbswerestill) andduration ofgrooming(i.e.,thetotal timeofwashing move-mentsover thehead, licking thepawsand fur,and tail/genital cleaning).Tominimizethepossibleinfluenceofcircadianchanges onbehavior in the OF, controland experimentalanimals were alternated.Thedevicewascleanedwitha5%alcohol/water solu-tion beforeplacingthe animalsin it to eliminate possiblebias causedbyodorsleftbypreviousanimals.Theexperimentalsessions beganat1:00pmandendedbefore5:00pmtopreventcircadian influences.
Elevatedplusmaze
Theelevatedplusmaze(EPM)wasfirstconceived byBritish psychologistSheilaHandley’sgroupasamodeltoevaluate anx-iety,and itis oneof themostusedapparatus forthat purpose (Lapiz-Bluhmetal.,2008).TheEPMwasmadeofwoodandhad twoopenarms(23.5cm×8cm)andtwoclosedarmsofthesame size with 20cm high walls. The apparatuswas elevated 80cm abovethefloor.Itwaslocatedinasound-proofroomwitha100W lampatthefloorofapparatusthatprovided400lxillumination. Twobehaviors are readilynoticeable in theEPM: avoidanceof theopenarmswhilestayingintheclosedarmandescapefrom anopenarmdirectly toa closed arm(Pinheiro etal., 2007).In thepresentstudy,theapparatuswasusedtoassessanxiety-like behaviorin thefirststageof theexperiment. Theanimalswere testedintheEPMafterbeingtestedintheOF.Exploratory behav-ior wasdefined asthe number of entriesinto theclosed arms andnumberofcrosses inthecentralareaoftheapparatus.The animalswerefirstplacedin thecenter ofthemaze,which was previouslycleanedwith5%alcohol.Thenumberofopen-armand closed-armentries,timespentineacharm,andnumbercentral areacrossingsweremeasured.Observationsweremadeinfive 5-minsessions:15–20min,35–40min,65–70min,125–130min,and 185–190minafterextractadministration.Becausereexposureto theEPMmayrepresentspatialmemoryofananxiolytic/anxiogenic condition, the mice were observed in the remained sessions (Onoderaetal.,1998;Miyazakietal.,1995;SharmaandKulkarni, 1992).
Experimentaldesign
O OH
OH O
OH
HO2C
HO OH 6 OH OH O OH
HO2C
O OH 7 O R OH O OH HO O O
HO OHOH
O OOH OHOH 1 R=H 3 R=OH O OH O OH HO O O
HO OHOH
O 2 O OH OH HO O OH O OH HO O OOH OH OH 4 OH O OH O OH HO O O HO OH OH 5 OH O OH O OH HO
O O OH
8 OH
HO OH
CH2OH
O OH O OH HO O 9 OH O OH HO HO OH OH Crude extract Laetia suaveolens
RCHCH3 RBuOH RH2O
FR50%ACN FR15%ACN FRMeOH XI X IX VIII VII VI V IV III II I Liq-Liq partition
C.C. Sil C18 RP
2
7
1, 2,
3
9
8
4, 5
Scheme1.FractionationofcrudeorganicextractEB719,obtainedfromtheleavesandstemofLaetiasuaveolens.
group).AssessmentsintheOFandEPMwereperformed imme-diatelyafterassessinggeneralbehavioralchanges(Gusmãoetal., 2013a).Stage1oftheexperimentbegan withadministrationof 0.625g/kg EB719 in a group of three animals. The mice were observedforgeneralbehavioral activityintheOF andEPMand death.Becausedeathoccurredinthegroupofmicethatreceived thehigherdoseofEB719,a half-folddilutionwassubsequently administeredinasecondgroupofanimals,andtheobservations weremadeagain.Thisprocedurewasrepeateduntilanon-lethal dose(NLD)wasdetermined,definedasthedosethatcausedno deathinmiceafteri.p.administration.Inthesecondstageofthe experiment,theNLDwasadministeredi.p.intenmalemicethat weredividedintofourgroups:naive,vehicle(almondoil)control, diazepam,andNLD.Theanimalswereagainsubjectedtobehavioral observations.Toavoidcircadianinfluencesontheexperimental results,theassaysbeganat1pmandendedbefore5pm.Allofthe miceunderwentnecropsyaftertheydied.
Statisticalanalysis
Theresultswereanalyzedusingtwo-wayrepeated-measures analysisofvariance(ANOVA;Zar,1999)followedbytheBonferroni
posthoctest.ThestatisticalanalysiswasperformedusingGraphPad Prism5.0software.Valuesofp<0.05wereconsideredstatistically significant.
Extractfractionationandcompoundidentification
respectively)analysesandhigh-performanceliquid chromatogra-phy/electronsprayionization-massspectrometry(HPLC/ESI-MSn) asdescribedelsewhere(Estorketal.,2014).FractionsV(18.6mg) andVIII(27.7mg)werealsoanalyzedby1HNMR,13CNMR,and
HPLC/ESI-MSn,andtheresultsarepresentedherein.
The1HNMRand13CNMRspectraoffractionVshowedchemical
shiftsthatcorrespondedtoamixtureofthefollowingflavonoids: quercetin-3-O-rhamnopyranosyl-(1→6)-glucopyranoside (1) (Yangetal.,2011),alsocalledrutin orquercetin3-O-rutinoside, kaempferol-3-O-xylopyranosyl-(1→6)-glucopyranoside (2) (Hubner et al., 1999; Chung and Lee, 2012), also called leucoside or kaempferol 3-O-sambubioside, and kaempferol-3-O-rhamnopyranosyl-(1→6)-glucopyranoside (3) (Olennikov etal.,2012;Leeetal.,2010),alsocallednicotiflorinorkaempferol 3-O-rutinoside. The ESI-mass spectra of fraction V in negative ion modeshowed three peaksat 7.6minthat correspondedto molecularions[M−H]− at m/z609.2,579.2,and 369and three
peaksat7.7minthatcorrespondedtomolecularions[M−H]−at m/z593.2,567.3,and 413.2.Furtherinvestigationof theion at m/z609.2(1)intheLC–MS2experimentyieldedseveralfragment
ionsatm/z591.1,463,342.9,300.9,270.9,254.9,and179.1.The daughterionatm/z591.1directlyoriginatedfromtheparention atm/z609.2bythelossofH2O[M−H-H2O]−.Theionatm/z463
wasproducedfromtheneutrallossofarhamnose[M−H-Rham]−,
whichwasinagreementwithpreviousdata(CuyckensandClaeys, 2004).Theionatm/z342.9wasproducedbycross-ringcleavages of the glucose residue [M−H-Rham-120], which was also in agreementwiththe literature (Cuyckens and Claeys, 2004;Shi etal.,2007).Theadditionallossof162(glucose)ordirectlossof therutinoseresidueresultedintheaglyconequercetinionatm/z 300.9[M−H-Rham-Glc]−.Thesedatawerealsoinagreementwith
theliterature(CuyckensandClaeys,2004).Theionatm/z270.9 wasproducedbythelossofCH2Ofromaglycone[M− H-Rham-Glc-CH2O]−,andtheionatm/z254.9wasproducedbythelossof
theH2OandCOfromaglycone[M−H-Rham-Glc-H2O-CO]−.These
findings were in full agreementwith the resultsof a previous study(CuyckensandClaeys,2004).Theionatm/z179differedby amassof122Da(neutralmolecule)fromitscorrespondingionof aglycone(m/z300.9),whichisalsoconsistentwiththeliterature (Chenet al.,2002).TheLC–MS2 investigationsoftheionatm/z
579.2(2)yieldedseveralfragmentionsatm/z447,356.9,326.9, 284.9,255.0,200.1,and169.5.Thedaughterionatm/z447was produceddirectlyfromitsparentionatm/z579.2bytheneutral lossof xylose (132Da) [M−H-Xyl]−,which was alsoconsistent
withpreviousdata(Abu-Reidahetal.,2012).Theionsatm/z356.9 and326.9wereproducedbycross-ring cleavagesoftheglucose residue by the loss of [M−H-Xyl-90]− and [M−H-Xyl-120]−,
respectively,which wasinagreementwiththeliterature(Chen etal.,2002).Theadditionallossof162(glucose)ordirectlossof the sambubioside residue resulted in the aglycone kaempferol ionatm/z284.9[M−H-Xyl-Glc]−,whichwasconsistentwiththe
literature(Abu-Reidahetal.,2012).Theionatm/z255differedby amassof30Da(neutralmolecule)fromitsrelatedionofaglycone (m/z284.9), whichwasconsistentwiththeliterature(Yeetal., 2005).FurtherLC–MS2 investigationoftheionatm/z593.2(3)
showedseveralionfragmentsatm/z447,428.9,326.9,284.9,and 254.9.Theionatm/z447wasproduceddirectlyfromtheparent ionatm/z593.2bytheneutrallossofrhamnose[M−H-Rham]−. Theionatm/z428.9wasgeneratedbythelossofneutralmolecules ofrhamnose andwater [M−H-Rham-H2O]−,and theionatm/z
326.9wasproducedbycross-ringcleavagesoftheglucoseresidue, whichwasinagreementwiththeliterature(CuyckensandClaeys, 2004;Shietal.,2007).Theadditionallossof162(glucose)ordirect lossoftherutinoseresidueresultedintheaglyconekaempferol ionatm/z284.9[M−H-Rham-Glc]−,whichwasconsistentwith theliterature(Cuyckensetal.,2001).Theionatm/z254.9differed
byamassof30Da(neutralmolecule)fromtheionofaglycone(m/z 284.9),whichwasconsistentwiththeliterature(Yeetal.,2005).
The 1H NMR and 13C NMR spectra of fractionVIII showed
chemicalshiftsoftheflavonoidmixture:quercetin3-O-arabinose or guaijaverin(4) (Yoshidaet al.,1992; Prabuet al.,2006)and kaempferol3-O-glucoside(5)orastragalin(Demirezeretal.,2006; Yangetal.,2005).TheESI-massspectraoffractionVIIIin nega-tiveionmodeshowedthreepeaksat8.8minofthemolecularions [M−H]−atm/z867.2,447.1,and433.FurtherLC–MS2investigation
oftheionatm/z867.2yieldedtwofragmentionsatm/z432.9and 300.9,indicatingthatitcorrespondedtothemolecularionofthe dimer[2M−H]−fromthecompoundwithamolecularionatm/z
433[M−H]−.TheLC–MS2analysisoftheionatm/z433yielded sev-eralfragmentionsatm/z342.9,300.9,and179.Theionatm/z342.9 wasproducedbycross-ringcleavagesoftheglucoseresidue[M− H-Rham-90],whichwasinagreementwiththeliterature(Cuyckens andClaeys,2004).Anadditionalneutrallossof132(arabinose, neu-tralmolecule)resultedintheaglyconequercetinionatm/z300.9 [M−H-Ara]−,whichwasalsoconsistentwiththeliterature(Prabu etal.,2006).TheLC–MS2analysisoftheionatm/z447.1revealed
fragmentionsatm/z431.9,401.9,357,326.9,283.9,and255.The ionsatm/z431.9and401.9wereproducedbythelossof15and 45Da,respectively.Theionsatm/z357and326.9wereproducedby cross-ringcleavagesoftheglucoseresidue(lossof90and120Da, respectively),whichwasconsistentwiththeliterature(Cuyckens andClaeys,2004).Themassspectrashowedabundantionsatm/z 284.9and283.9asthebasepeakthroughfissionoftheglycosidic bondtoformanaglyconeion(m/z284.9)[M−H-Glc]−and
radi-calaglyconeion(m/z283.9)[M−H-Glc-H]−byhemolyticcleavage, respectively,whichwerealsoinagreementwiththeliterature(Kite andVeitch,2009).Theionatm/z283.9wastheprecursoroftheion atm/z255[M−H-Glc-H-CO-H]−(Ablajanetal.,2006).
Thepresenceof4-O-caffeoylquinicacid(6),5-O-feruoylquinic acid(7),andisoquercitrin(8)wasalsofound,althoughtheseresults werereportedpreviously(Estorketal.,2014).
Resultsanddiscussion
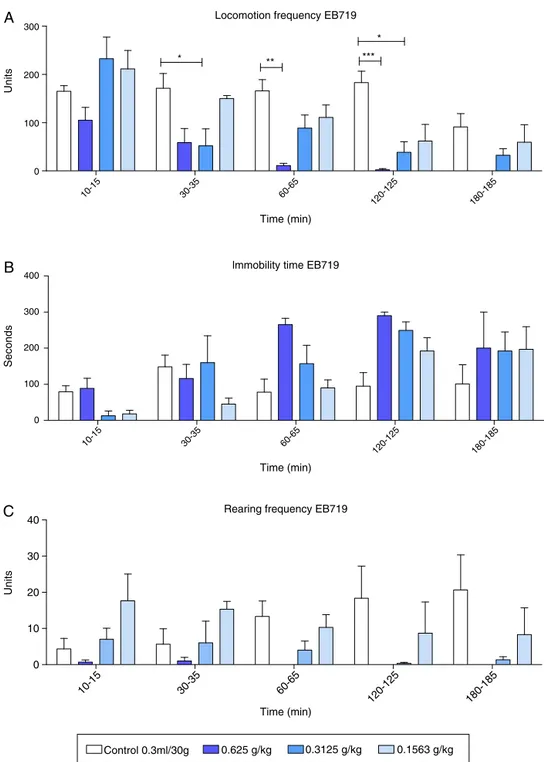
In the first stage of the behavioral phenotyping, we evalu-atedvarious parametersintheOF andEPM.TheOF resultsare showninFig.1(n=3,ntotal=12).Locomotionfrequency(Fig.1A)
wasinfluencedbythetreatment,whichaccountedfor31.12%of thetotal variance(F3,8=15.06,p<0.01),andtime accountedfor
32.37% of thetotal variance (F4,32=15.52,p<0.001). The
inter-actionbetweentime andtreatmentaccountedfor14.30%ofthe total variance(F12,32=2.29,p<0.05).ThirtyminutesafterEB719
administration,asignificantdose-andtime-dependentdecrease in locomotion was observed at all three concentrations tested. Asignificantreductionoflocomotion wasobserved30–120min afteri.p. administrationof0.625g/kgEB719(p<0.05 at30min; p<0.01at60min;p<0.001at120min)comparedwiththecontrol group.Theimmobilitytimeresults(Fig.1B)showedasignificant effectoftime, whichaccountedfor29.13% ofthetotal variance (F4,32=9.66,p<0.001),butnoeffectoftreatmentwasfound,with
Locomotion frequency EB719
10-1 5
30-35 60-65
12 0-125
180-1 85 0
100 200 300
Control 0.3ml/30g 0.625 g/kg 0.3125 g/kg 0.1563 g/kg Time (min)
Un
its
** ***
*
*
Rearing frequency EB719
10-15
30-35
60-65
120-125 180-185 0
10 20 30 40
Time (min)
Units
lmmobility time EB719
10-15 30-35 60-65
120-125 180-185 0
100 200 300 400
Time (min)
Seconds
A
B
C
Fig.1. EffectsofintraperitonealadministrationofdifferentdosesofEB719,theorganicextractobtainedfromLaetiasuaveolens,inmalemiceintheopenfieldinstage1of theexperiment.(A)Locomotionfrequency(mean±standarderror).(B)Immobilitytime(mean±standarderror).(C)Rearingfrequency(mean±standarderror).*p<0.05, **p<0.01,***p<0.001,vehiclecontrolgroupcomparedwiththethreetreatmentgroups(two-wayrepeated-measuresANOVAfollowedbyBonferroniposthoctest).
Timedidnothaveasignificanteffect(p>0.05).Groomingdecreased inmicethatreceived0.625g/kgEB71960minafter administra-tion(p<0.01).Inmicethatreceived0.625and0.3125g/kgEB719, grooming decreased 120min after administration (p<0.05). In micethatreceived0.625g/kgEB719,groomingdecreased180min afteradministration(p<0.05).Treatmentaccountedfor31.98%of thetotal variance in defecation (F3,8=14.04,p<0.01),and time
accountedfor14.98%of thetotal variance(F4,32=4.55,p<0.01).
Theinteractionbetweentreatmentandtimeaccountedfor20.65% of the total variance (F12,32=2.09, p<0.05; Fig. 2B). A
signifi-cantdecreasein defecationwasobservedinmicethatreceived 0.625g/kg(p<0.001)and0.3125g/kg(p<0.001)EB71960minafter i.p.administration.
No significantdifferences were observed in theEPM in the firstsession that evaluated anxiolytic/anxiogenic-likeeffects or
remainingsessionsthat evaluatedlearning/memory inan anxi-olytic/anxiogeniccontext(SupplementalFig.S1)inthefirststage oftheexperiment.
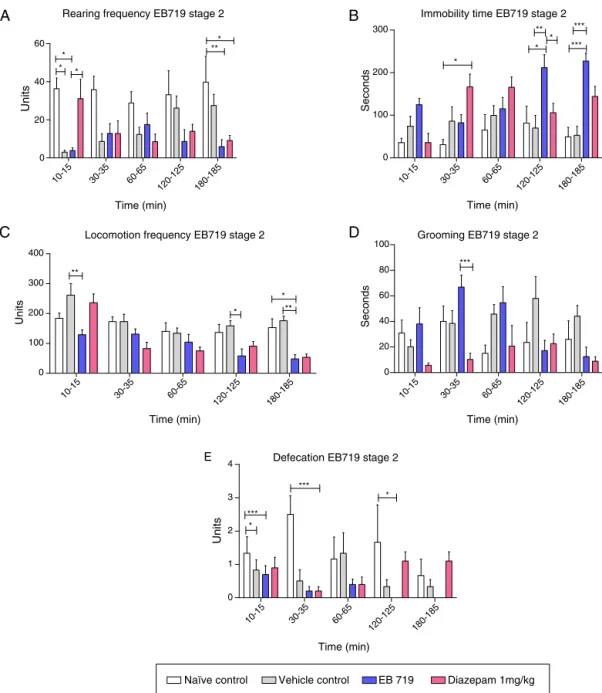
Inthesecondstageoftheexperiment,theNLDof0.1563g/kg wasadministeredi.p.inmalemice,andtheanimalswere eval-uated in the OF. Treatment accounted for 19.24% of the total varianceintheanalysisofrearingfrequency(F3,28=4.29,p<0.05; Fig.3A). Timedid not significantlyinfluence the total variance (0.55%).Theinteractionbetweentreatmentand timeaccounted for11.99%ofthetotalvariance(F12,112=4.20,p<0.001).Thefirst
Grooming EB 719
10-15 30-35 60-6
5
12 0-125
180 -185
0 5 10 15 20 25
Time (min)
Units
**
*
* *
Defecation EB719
10-15 30-35 60-65
120 -125
18 0-185
0 1 2 3 4
Time (min)
Units
****
***
A
B
Control 0.3ml/30g 0.625 g/kg 0.3125 g/kg 0.1563 g/kg
Fig.2.EffectsofintraperitonealadministrationofdifferentdosesofEB719,theorganicextractobtainedfromLaetiasuaveolens,inmalemiceintheopenfieldinstage1 oftheexperiment.(A)Grooming(mean±standarderror).(B)Defecation(mean±standarderror).*p<0.05,**p<0.01,***p<0.001,vehiclecontrolgroupcomparedwiththe threetreatmentgroups(two-wayrepeated-measuresANOVAfollowedbyBonferroniposthoctest).
administrationdecreased rearingfrequency compared withthe naivegroup10min(p<0.05)and180min(p<0.01)after admin-istration compared with the diazepam group (p<0.05). The immobilitytimedatashowedthattreatmentaccountedfor18.52% of the total variance (F3,28=5.89, p<0.01), and time accounted
for4.55% ofthetotal variance (F4,112=4.54,p<0.01).The
inter-actionbetweentreatmentandtimeaccountedfor17.24% ofthe total variance (F12,112=5.74, p<0.001). EB719 clearly increased
immobilitytime(Fig.3B)comparedwiththenaivecontrolgroup 120min(p<0.05)and180min(p<0.001)afteri.p.administration andcompared withthevehiclecontrol groupat thesametime points(p<0.01andp<0.001,respectively).Treatmentaccounted for17.87%ofthetotalvariance(F3,28=6.62,p<0.01)in
locomo-tion frequency (Fig. 3C), and time accounted for 18.26% of the total variance(F4,112=20.96,p<0.001).Theinteraction between
treatmentand time accountedfor 10.72% of thetotal variance (F12,112=4.10, p<0.001). EB719decreasedlocomotion compared
withthevehiclecontrolgroup10min(p<0.01),120min(p<0.05), and 180min(p<0.01)after administration and compared with thenaive control group180minafter administration (p<0.05). Forgrooming, treatmentaccountedfor12.07% ofthetotal vari-ance(F3,28=5.10,p<0.01),andtheinteractionbetweentreatment
andtimeaccountedfor13.49%ofthetotalvariance(F12,112=2.65, p<0.01;Fig.3D). TheanimalsthatreceivedEB719exhibitedan increase ingrooming compared withtheanimals that received diazepam30minafter i.p.administration. Fordefecation, treat-ment accounted for 14.67% of the total variance (F3,28=8.83, p<0.001), and the interaction between treatment and time accountedfor12.65%ofthetotalvariance(F12,112=2.11,p<0.05; Fig.3E).Naivecontrolanimalsaccountedformostofthedifferences inthis parameter,particularlythedecreasein fecalboli30min
(p<0.001) and120min(p<0.05)afteri.p.EB719administration and30minafterdiazepamadministration(p<0.001).
TheEPMtest wasnot performedinthe secondstageof the experimentbecausenosignificantdifferenceswereobservedinthe firststage.
Rearing frequency EB719 stage 2
10-15 30-35 60-65 120
-125 180
-185 0
20 40 60
Time (min)
Units
*
** *
*
*
Locomotion frequency EB719 stage 2
10-15 30-35 60-65 120
-125 180
-185 0
100 200 300 400
Naïve control Vehicle control EB 719 Time (min)
Units
Diazepam 1mg/kg
* **
* **
Immobility time EB719 stage 2
10-15 30-35 60-6 5
120-125 180 -185 0
100 200 300
Time (min)
Second
s
* *** *
** *** *
Grooming EB719 stage 2
10-15 30-35 60-65 120
-125 180-185 0
20 40 60 80 100
Time (min)
Second
s
***
Defecation EB719 stage 2
10-15 30-35 60-65 120
-125 180
-185 0
1 2 3 4
Time (min)
Units
*
*
*** ***
A
B
C
D
E
Fig.3. Effectsofintraperitonealadministrationofthenon-lethaldoseofEB719,theorganicextractobtainedfromLaetiasuaveolens,inmalemiceintheopenfieldinstage 2oftheexperiment.(A)Rearingfrequency(mean±standarderror).(B)Immobilitytime(mean±standarderror).(C)Locomotionfrequency(mean±standarderror).(D)
Grooming(mean±standarderror).(E)Defecation(mean±standarderror).*p<0.05,**p<0.01,***p<0.001,vehiclecontrolgroup,diazepamcontrolgroup,andnaivecontrol groupcomparedwithnon-lethaldosegroup(two-wayrepeated-measuresANOVAfollowedbyBonferroniposthoctest).
thattheycaused hemorrhage(Koch,2005),inadditiontoother plantspecies (Moro et al.,2001).Considering theimpairments inlocomotionandemotionalityandthefactthatintraperitoneal administrationofanorganicextractofcasinga-cheirosacancause hemorrhage,itsuseasapopularmedicinemaybeharmfuland shouldbeavoided.
Five flavonoids were isolated from EB719: rutin, leucoside, nicotiflorin,guaijaverin,andastragalin.Nostudieshavereported the influence of these flavonoids onhemorrhage. Nonetheless, some of these compounds, such as rutin, are well known as vascularprotectiveagentsthatactbyreducing capillary perme-ability,revertingitsfragility,maybebyananti-thromboticactivity (Gryglewskietal.,1987).Suchactivitywasnotobservedinanimals thatdied,whichmaybeattributabletootheras-yet-unidentified compoundsinEB719thatmayhaveinterferedwithhemorrhage. Rutinwasshowntointerferewiththeactivityofracemic war-farinwhen theywereconcomitantlyadministered,reducing its
coagulant effect by decreasing the elimination half-life of one specificenantiomer(Chanetal.,2009).Studiesofplantsand phe-noliccompoundswithantithrombolyticandantiplateletactivity haverecentlybeenperformed,particularlytoevaluatetheiruse asnutritionalsupplementsin menopausalwomenand in some cardiovascularconditionsbytargetingplasmacoagulationfactors (Bijaketal.,2014a,b)orfibrinogen(Chenetal.,2013;Riosetal., 2008).Althoughindicative,nooneofthefiveflavonoidsthatwas isolatedfromL.suaveolenswereindicatedasantithrombolyticor antiplateletactive,inanyofthearticles.
Conclusions
IntraperitonealEB719administrationinfluencedthebehavioral phenotypeofmaleBalb-cmicebyimpairinglocomotionand emo-tionality.EB719alsoexertedastrongeffectonthesmoothintestine, causinghemorrhage.Rutin,leucoside,nicotiflorin,guaijaverin,and astragalinwereisolatedfromEB719,whichwerenotpreviously identifiedinL.suaveolens.Ethicaldisclosures
Protectionofhumanandanimalsubjects. Theauthorsdeclare thattheproceduresfollowedwereinaccordancewiththe regula-tionsoftherelevantclinicalresearchethicscommitteeandwith thoseoftheCodeofEthicsoftheWorldMedicalAssociation (Dec-larationofHelsinki).
Confidentialityofdata. Theauthorsdeclarethatnopatientdata
appearinthisarticle.
Righttoprivacyandinformedconsent. Theauthorsdeclarethat
nopatientdataappearinthisarticle.
Authors’contributions
DME(M.Sc.student)andDFG(M.Sc.student)ranthelaboratory work.MLBPcontributedtoplantidentificationandherbarium con-fection.SAFcontributedtoplantcollection,herbariumconfection, and the laboratory work. IECD contributed to the chromato-graphicanalysisandmoleculeidentification.LFLRcriticallyread themanuscriptandcontributedtotheHPLCanalysis.EFSM con-tributedtomaintainingtheanimalfacilities.MMBandIBSdesigned thestudy,supervisedthelaboratorywork,wrotethemanuscript, performedthelaboratorywork,andcriticallyreadthemanuscript. Alloftheauthorsreadthefinalmanuscriptandapprovedthe sub-mission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgments
TheauthorsthankFundac¸ãodeAmparoàPesquisadoEstado deSãoPauloforfinancialsupport(grantno.2008/58706-8)and MicheleSanchezfortechnicalassistance.
AppendixA. Supplementarydata
Supplementarydataassociatedwiththisarticlecanbefound,in theonlineversion,atdoi:10.1016/j.bjp.2015.10.004.
References
Ablajan,K.,Abliz,Z.,Shang,X.-Y.,He,J.-M.,Zhang,R.-P.,Shi,J.-G.,2006.Structural characterizationofflavonol3,7-di-O-glycosidesanddeterminationofthe gly-cosylationpositionbyusingnegativeionelectrosprayionizationtandemmass spectrometry.J.MassSpectrom.41,352–360.
Abu-Reidah, I.M., Arráez-Román, D., Quirantes-Piné, R., Fernández-Arroyo, S., Segura-Carretero,A.,Fernández-Gutiérrez,A.,2012.HPLC–ESI-Q-TOF-MSfora comprehensivecharacterizationofbioactivephenoliccompoundsincucumber wholefruitextract.FoodRes.Int.46,108–117.
Bijak,M.,Ponczek,M.B.,Nowak,P.,2014a.Polyphenolcompoundsbelongingto flavonoidsinhibitactivityofcoagulationfactorX.Int.J.Biol.Macromol.65, 129–135.
Bijak,M.,Ziewiecki,R.,Saluk,J.,Ponczek,M.,Pawlaczyk,I.,Krotkiewski,H., Wachow-icz,B.,Nowak,P.,2014b.Thrombininhibitoryactivityofsomepolyphenolic compounds.Med.Chem.Res.23,2324–2337.
Boletim Museu Paraense Emilio Goeldi, 2013. http://scielo.iec.pa.gov.br/img/ revistas/bmpegscn/v1n1/pdf/1a07a1.pdf(accessed05.02.15).
Broadhurst,P.L.,1960.Experimentsinpsychogenetics,applicationofbiometrical geneticstotheinheritanceofbehavior.In:Hysenck,H.J.(Ed.),Experimentsin Personality,vol.1.Routledge&KeganPaul,London,pp.1–256.
Chan,E.,Hedge,A.,Chen,X.,2009.Effectofrutinonwarfarinanticoagulationand pharmacokineticsofwarfarinenantiomersinrats.J.Pharm.Pharmacol.61, 451–458.
Chen,M.,Song,F.,Guo,M.,Lui,Z.,Liu,S.,2002.Analysisofflavonoidconstituents fromleavesofAcanthopanaxsenticosusHarmsbyelectrospraytandemmass spectrometry.RapidCommun.MassSpectrom.16,264–271.
Chen,X.Q.,Wang,X.B.,Guan,R.F.,Tu,J.,Gong,Z.H.,Zheng,N.,Yang,J.H.,Zhang,Y.Y., Ying,M.M.,2013.Bloodanticoagulationandantiplateletactivityofgreentea (−)-epigallocatechin(EGC)inmice.FoodFunct.4,1521–1525.
Chung,D.-W.,Lee,S.B.,2012.Novelsynthesisofleucosidebyenzymatichydrolysis ofteaseedextract.J.Sci.FoodAgric.93,362–367.
Cuyckens,F.,Claeys,M.,2004.Massspectrometryinthestructuralanalysisof flavonoids.J.MassSpectrom.39,1–15.
Cuyckens, F., Rozenberg, R., de Hoffmann, E., Claeys, M., 2001. Structure characterizationofflavonoidO-diglycosidesbypositiveandnegative nano-electrosprayionizationiontrapmass spectrometry.J. MassSpectrom. 36, 1203–1210.
Demirezer, L.O., Gürbüz,F.,Güvenalp, Z., Ströch,K.,Zeeck, A.,2006. Iridoids, flavonoidsandmonoterpeneglycosidesfromGaliumverumsubsp.verum. Turk-ishJ.Chem.30,525–534.
Estork,D.M.,Gusmão,D.F.,Paciencia,M.L.B.,Díaz,I.E.C.,Varella,A.D.,Younes,R.N., Reis,L.F.L.,Montero,E.F.S.,Bernardi,M.M.,Suffredini,I.B.,2014.Firstchemical andtoxicologicalevaluationofCasinga-cheirosainBalb-cmalemice.Molecules 19,3973–3987.
Gama,J.R.V.,deSouza,A.L.,Calegário,N.,Lana,G.C.R.,2007.Fitossociologiadeduas fitocenosesdeflorestaombrófilaabertanomunicípiodeCodó,estadodo Maran-hão.RevistaÁrvore31,465–477.
Gryglewski,R.J.,Korbut,R.,Robak,J.,Swies,J.,1987.Onthemechanismof antithrom-boticactionofflavonoids.Biochem.Pharmacol.36,317–322.
Gusmão,D.F.,Estork,D.M.,Paciencia,M.L.B.,Diaz,I.E.C.,Frana,S.A.,Rodrigues,P.A., Suffredini,I.B.,Varella,A.D.,Younes,R.N.,Reis,L.F.L.,Montero,E.F.S.,Bernardi, M.M.,2013a.PreliminaryevaluationoftheacutetoxicityrelatedtoAbarema auriculatatomiceandinvestigationofcytotoxicityofisolatedflavonones. Phar-macologyonline(Salerno)1,113–127.
Gusmão,D.F.,Estork,D.M.,Paciencia,M.L.B.,Díaz,I.E.C.,Suffredini,I.B.,Varella,A.D., Younes,R.N.,Reis,L.F.L.,Montero,E.F.S.,Bernardi,M.M.,2013b.Influenceofthe intraperitonealadministrationofantitumorAbaremaauriculataextractonmice behavior.Rev.Bras.Farmacogn.23,903–912.
Hubner,G.,Wray,V.,Nahrstedt,A.,1999.Flavonololigosaccharidesfromtheseeds ofAesculushippocastanum.PlantaMed.65,635–642.
Kite,G.C.,Veitch,N.C.,2009.Assigningglucoseorgalactoseastheprimary glyco-sidicsugarin3-O-mono-di-andtriglycosideofkaempferolusingnegativeion electrosprayandserialmassspectrometry.RapidCommun.MassSpectrom.23, 3125–3132.
Koch,E.,2005.Inhibitionofplateletactivatingfactor(PAF)-inducedaggregation ofhumanthrombocytesbygingkolides,considerationsonpossiblebleeding complicationsafteroralintakeofGingkobilobaextracts.Phytomedicine12, 10–16.
Lapiz-Bluhm,M.D.,Bondi,C.O.,Doyen,J.,Rodriguez,G.A.,Bédard-Arana,T.,Morilak, D.S.,2008.Behavioralassaystomodelcognitiveandaffectivedimensionsof depressionandanxietyinrats.J.Neuroendocrinol.20,1115–1137.
Lee,H.-B.,Kim,E.-K.,Park,S.-J.,Bang,S.-G.,Kim,T.G.,Chung,D.-W.,2010.Isolation andcharacterizationofnicotiflorinobtainedbyenzymatichydrolysisoftwo precursorsinteaseedextract.J.Agric.FoodChem.58,4808–4813.
MissouriBotanicalGarden(www.tropicos.org,accessed10.12.14).
Miyazaki,S.,Imaizumi,M.,Machida,H.,1995.Theeffectsofanxiolyticsand anxio-genicsonevaluationoflearningandmemoryinanelevatedplus-mazetestin mice.MethodsFind.Exp.Clin.Pharmacol.17,121–127.
Moro,P.A.,Flacco,V.,Cassetti,F.,Clementi,V.,Colombo,M.L.,Chiesa,G.M., Menniti-Ippolito,F.,Raschetti,R.,Santuccio,C.,2001.Hypovolemicshockduetosevere gastrointestinalbleedinginachildtakingnaherbalsyrup.Ann.Inst.SuperSanita 47,278–283.
Olennikov, D.N., Tankhaeva,L.M.,Partilkhaev, V.V.,Rokhin, A.V.,2012. Chem-ical constituents of Caragana bungei shoots. Braz. J. Pharmacogn. 22, 490–496.
Onodera,K.,Miyazaki,S.,Imaizumi,M.,Stark,H.,Schunack,W.,1998.Improvement byFUB181,anovelhistamineH3-receptorantagonist,oflearningandmemory intheelevatedplus-mazetestinmice.NaunynSchmiedebergsArch.Pharmacol. 357,508–513.
Ozi,J.M.,Suffredini,I.B.,Paciencia,M.L.B.,Frana,S.F.,Dib,L.L.,2011.Invitrocytotoxic effectsofBrazilianplantextractsonsquamouscellcarcinomaoftheoralcavity. Braz.OralRes.25,519–525.
Pinheiro,S.H.,ZangrossiJr.,H.,Del-Bem,C.M.,Graeff,F.G.,2007.Elevatedmazesas animalmodelsofanxiety,effectsofserotonergicagents.An.Acad.Bras.Cienc. 79,71–85.
Prabu,G.R.,Gnanamani,A.,Sadulla,S.,2006.Guaijaverin–aplantflavonoidas potentialantiplaqueagentagainstStreptococcusmutans.J.Appl.Microbiol.101, 487–495.
Revilla,R.,2002.PlantasúteisdaBaciaAmazônica.Vol.1.Manaus.InstitutoNacional dePesquisasdaAmazôniaeSebrae,pp.371.
Sharma,A.C.,Kulkarni,S.K.,1992.Evaluationoflearningandmemorymechanisms employingelevatedplus-mazeinratsandmice.Prog.Neuropsychopharmacol. Biol.Psychiatry16,117–125.
Shi,P.,He,Q.,Song,Y.,Qu,H.,Cheng,Y.,2007.Characterizationandidentification ofisomericflavonoidO-diglycosidesfromgenusCitrusinnegativeelectrospray ionizationbyiontrapmassspectrometryandtime-of-flightmassspectrometry. Anal.Chim.Acta598,110–118.
Suffredini,I.B.,Paciencia,M.L.B.,Frana,S.A.,Varella,A.D.,Younes,R.N.,2007.Invitro breastcancercelllethalityofBrazilianplantextracts.Pharmazie62,798–800. Suffredini,I.B.,Paciencia,M.L.B.,Varella,A.D.,Younes,R.N.,2006.Invitroprostatecell
cancercellgrowthinhibitionbyBrazilianplantextract.Pharmazie61,722–724. Yang,H.-J.,SongM.-Ch.,Bang,M.-H.,Lee,J.-H.,Chung,I.-S.,Lee,Y.-H.,Jeong,T.-S., Kwon,B.-M.,Kim,S.-H.,Kim,D.-K.,Park,M.-H.,Baek,N.-I.,2005.Deveolopment
ofbiologicallyactivecompoundsfromedibleplantsources-XII.Flavonol glyco-sidesfromTrigonotispenduncularisBenthanditshACAT1inhibitoryactivity.J. KoreanSoc.Appl.Biol.Chem.48,98–102.
Yang,S.,Park,S.,Ahn,D.,Yang,J.H.,Kim,D.K.,2011.Antioxidativeconstituentsof theaerialpartsofGaliumspurium.Biomol.Therap.19,336–341.
Ye,M.,Yan,Y.,Gou,D.-A.,2005.Characterizationofphenoliccompoundsinthe ChineseherbaldrugTu–Si–Zibyliquidchromatographycoupledtoelectrospray ionizationmassspectrometry.RapidCommun.MassSpectrom.19,1469–1484. Yoshida,T.,Maruyama,T.,Nitta, A.,Okuda,T., 1992.EucalbaninsA,B andC, monomericanddimerichydrolysabletanninsfromEucalyptusalbaReinmw. Chem.Pharm.Bull.40,1750–1754.