w ww.e l s e v i e r . c o m / l o c a t e / b j p
Original
Article
Antioxidant,
DNA
damage
protective,
neuroprotective,
and
␣
-glucosidase
inhibitory
activities
of
a
flavonoid
glycoside
from
leaves
of
Garcinia
gracilis
Chonlakan
Supasuteekul
a,b,
Wanroong
Nonthitipong
b,
Sarin
Tadtong
c,
Kittisak
Likhitwitayawuid
b,
Parkpoom
Tengamnuay
a,
Boonchoo
Sritularak
b,∗aDepartmentofPharmaceuticsandIndustrialPharmacy,FacultyofPharmaceuticalSciences,ChulalongkornUniversity,Bangkok,Thailand bDepartmentofPharmacognosyandPharmaceuticalBotany,FacultyofPharmaceuticalSciences,ChulalongkornUniversity,Bangkok,Thailand cDepartmentofPharmacognosy,FacultyofPharmacy,SrinakharinwirotUniversity,Nakhon-nayok,Thailand
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received2November2015 Accepted24January2016 Availableonline5March2016
Keywords:
Antioxidant DNAprotective Flavonoidglycoside
Garciniagracilis
␣-Glucosidase Neuroprotective
a
b
s
t
r
a
c
t
TheleavesofGarciniagracilisPierre,Clusiaceae,havebeenusedasflavouringmaterialsinfood,with
nopreviousreportsoftheirbiologicalactivitiesandchemicalconstituents.Inthisstudy,themethanolic
extractofG.gracilisaffordedthreecompoundsnamelyapigenin-8-C-␣-l-rhamnopyranosyl-(1→2)--d
-glucopyranoside(1),5-hydroxymethyl-2-furaldehyde,andvanillicacid.Alloftheisolateswereinitially
evaluatedforsuperoxideanionradicalscavengingactivityand␣-glucosidaseinhibitoryeffects.
Com-pound1,whichwasthemajorcomponent,showedthemostpotentactivitiesamongthesethreeisolates.
Furtherbiologicalevaluationsrevealedthatcompound1couldpreventthepBR322plasmidDNAdamage
inducedbythephotochemicalreactionofriboflavinandprotectP19-derivedneuronsfromtheoxidative
stressconditioninducedbyserumdeprivation.Itwasconcludedthatthepotentbiologicalactivitiesof
G.graciliscouldbeattributedtothesynergisticeffectofcompound1withotherconstituentsfoundin
theplant.
©2016SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Ageingisanunavoidablephenomenoninlivingorganismsthat resultsinmorphological,biochemical,functionaland psychologi-calchangesintheorganism(Moreiraetal.,2014).Accordingtoa UnitedNationsreport,theaveragelifespanoftheworld popula-tionhasbeenincreasing,anditisestimatedthatthepercentage ofelderlypeople(thoseaged60yearsorover)willcontinueto growand willreach21.1% by2050due tolow birth rates and longevity(Rahman,2007;UnitedNations,2013).Thisincreasewill resultinanincreaseinage-relatedchronicdiseases,including car-diovasculardisease,cancer,diabetes,andneurologicaldisorders, suchasAlzheimer’sdiseaseandParkinson’sdisease.Therefore,itis importanttosearchforanti-ageingmedicines,foods,anddietary supplementsthataresafeandeffectivetoreducemorbidityand provideagoodqualityoflifeforelderlyindividuals(Rahman,2007). Theexcessiveproductionofreactiveoxygenspecies(ROS)and freeradicalsis consideredtobea significantcause ofoxidative
∗ Correspondingauthor.
E-mail:boonchoo.sr@chula.ac.th(B.Sritularak).
damageinbiomolecules,suchasproteins,lipids,andDNA, eventu-allyleadingtonumerousdegenerative diseases.However,these unfavourableeffectscouldbepreventedby theconsumption of antioxidantstoprotectthecellsfromROSandmaintainROS con-centrationsat a lowlevel (Gulet al.,2011; Meng etal., 2012). Variousmedicinalandfoodplantsarerich sourcesoffree radi-calscavengingmolecules,whichhavestrongantioxidantactivities (Kuateetal.,2011).Inlightofthesehealthbenefits,thesearchfor antioxidantcompoundsfromnaturalproductshasattracted inter-est.
Moreover,ROSandfreeradicalsarealsogeneratedby hypergly-caemia,andmaybeassociatedwiththemetabolicabnormalities thatoccurinpatientswithdiabetesmellitus(Tiwarietal.,2013). Onecurrentapproachtothetreatmentofdiabetesandobesityis tocontrolbloodglucoselevels.␣-Glucosidaseisakeyintestinal enzymeincarbohydratedigestion.Theinhibitionofthisenzyme coulddelaythecarbohydratehydrolysis process,leading tothe preventionof excessglucoseabsorption inthegut.Acarbose is awell-known␣-glucosidaseinhibitorthatisusedtotreat type-II diabetes mellitus, but this inhibitors appears to have major side effects, including gastrointestinal disturbance and weight gain (Hollander, 2007). Therefore, it is important to find new
http://dx.doi.org/10.1016/j.bjp.2016.01.007
C.Supasuteekuletal./RevistaBrasileiradeFarmacognosia26(2016)312–320 ␣-glucosidaseinhibitorswithfewersideeffects,andhigherpatient
approval(Kimetal.,2000;Yinetal.,2014).
ThegenusGarciniabelongstothefamilyClusiaceaeandincludes 390speciesthatarewidelydistributedintropicalAsia,Australia, Polynesia, and southern Africa. Twenty-ninespecies have been reportedinThailand.Thesespeciesareevergreentreesthatrange fromsmalltomediuminsize,andsomespeciescangrowupto 30minheight(Ritthiwigromet al.,2013;Semwal etal.,2015). PreviousstudiesreportedthatGarciniaplantscontainmany sec-ondarymetabolitesandpossessvariouspharmacologicaleffects, includingantitumour, antioxidant,anti-inflammatory, and anti-immunosuppressiveeffects(Serujietal.,2013).
Garciniagracilis Pierre,whichis alsoknownasCha-mangor Mak-paeminThai,isoneoftheGarciniaspeciesthatwere discov-eredinThailand.TheripefruitsandleavesofG.gracilisareedible. Theleavesofthisplanthavetraditionallybeenusedasflavouring materialsinfoods(Suksrietal.,2005).Therootsarealsousedas antipyreticsfolkmedicine(Chuakul,2009).However,nostudies have investigated thechemical constituentsand pharmacologi-calactivitiesofthisplanttodate.Inthis study,ourpreliminary screeningof a methanolextractprepared fromtheleavesofG. gracilisshowedavarietyofpotentbiologicalactivities,including superoxidescavengingeffects (70.65%inhibitionata concentra-tionof100g/ml),protectionagainstDNAdamage(76.46%ata concentrationof100g/ml),andneuroprotectiveeffects(100%cell viabilityataconcentrationof100ng/ml).Thisextractalso exhib-ited␣-glucosidaseinhibitoryactivity(99.49%ataconcentrationof 2mg/ml).Theseresultspromptedustoinvestigatetheextractto identifythecompoundsresponsiblefortheseactivities.
Inthepresentstudy,wedescribetheisolationofcompounds1–3 fromtheleavesofG.gracilis,aswellastheevaluationof apigenin-8-C-␣-l-rhamnopyranosyl-(1→2)--d-glucopyranoside (1), the majorisolatedcompound,forantioxidant,DNAprotective, neu-roprotective,and␣-glucosidaseinhibitoryactivities.Compound1 wasinitiallyidentifiedfromtheleavesofBambusatextilis(Wang etal.,2012).However,thispaperisthefirstreporttodescribethe presenceofaflavonoidglycosideinG.gracilis.
Materialsandmethods
Plantmaterial
Leaves of Garcinia gracilis Pierre, Clusiaceae, were collected fromPrincessMahaChakriSirindhornHerbalGardeninMueang RayongDistrictinRayong,ThailandinFebruary2011. Authenti-cationwasperformedbycomparisonwithherbariumspecimens in the National Park, Wildlife and Plant Conservation Depart-ment oftheMinistry ofNatural Resources and Environment.A voucherspecimen(GG-022554)wasdepositedintheDepartment ofPharmacognosy andPharmaceuticalBotany intheFaculty of PharmaceuticalSciencesatChulalongkornUniversityinThailand.
Chemicals
The P19 cell line (ATCC CRL-1857) was obtained from ATCC®, USA. Foetal bovine serum (FBS), new-born calf serum (NCS), alpha minimal essential medium (␣-MEM), and an antibiotic-antimycotic solution were purchased from Gibco®, USA. All trans-retinoic acid (RA), cytosine-1--d-arabinoside, 1:250 porcine trypsin, poly-l-lysine (MW>300,000), 2,3-bis(2-methoxy-4-nitro-5-sulphonyl)-2H-tetrazolium-5-carboxanilide sodium (XTT), ␣-glucosidase from Saccharomyces cerevisiae, p -nitrophenyl-␣-d-glucopyranoside(pNPG)phenazinemethosulfate (PMS),1,1-diphenyl-2-picrylhydrazyl(DPPH), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), quercetin, and
nitrobluetetrazolium(NBT)wereobtainedfromSigma–Aldrich®, USA. Riboflavin and acarbose were purchased from Fluka Analytical®,Switzerland.Ethylenediaminetetraaceticacid(EDTA) wasprocuredfromMay&Baker®,England.pBR322plasmidDNA was obtainedfromVivantis TechnologiesSdn. Bhd.®, Malaysia. Allsolvents wereanalyticalgrade and purchasedfromMerck®, Germany.
Extractionandisolation
DriedandpowderedleavesofG.gracilis(1kg)weremacerated withMeOHtoyield268.16gofmethanolextractaftersolvent evap-oration.ThiscrudeextractwassuspendedinH2Oandpartitioned withEtOAcandBuOH togenerateanEtOAcextract(60.23g),a BuOHextract(119.76g),andanaqueousextract(42.08g).
TheBuOHextractwasthenfractionatedbycolumn chromatog-raphy(CC)(MCIgel,MeOH-H2Ogradient)togeneratefivefractions (I–V).FractionII(13.53g)wasseparatedbyCC(silicagel, EtOAc-MeOHgradient)togenerateninefractions(II-AtoII-I).FractionII-E (3.41g)wasfurtherpurifiedonSephadexLH-20(MeOH)to gen-erate1(781.2mg).TheEtOAcextractwasseparatedbyvacuum liquidchromatographyVLC(CH2Cl2-Acetonegradient)togenerate sevenfractions(I–VII).FractionIV(7.58g)waschromatographed onasilicagelcolumn(CH2Cl2-EtOAcgradient)toyieldseven frac-tions(IV-AtoIV-G).FractionIV-C(0.94g)wasfurthersubjected toSephadex LH-20 (acetone)to generate 2(20mg). FractionV (5.13g)wasthenseparatedbysilicagel(CH2Cl2-EtOAcgradient) togeneratethreefractions(V-AtoV-C).FractionV-B(0.57g)was subsequently purifiedby SephadexLH-20(acetone)toobtain3 (20.2mg).Allorganicsolvents usedforextractionand isolation werecommercialgradeandredistilledpriortouse.Theisolates (1–3)showedmorethan98%purityintheNMRspectrum.
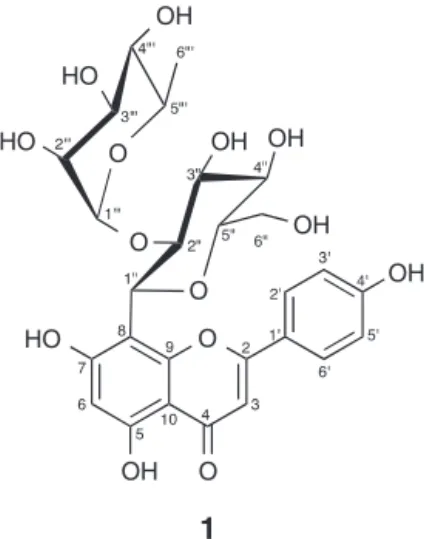
Apigenin-8-C-˛-l-rhamnopyranosyl-(1→2)-ˇ-d -glucopyranoside(1)
Yellowamorphoussolid;C27H30O14;HR-ESI-MSm/z601.1536 [M+Na]+;IRmax:3367,2935,1654,1360,837cm−1;UVmax:215 and333nm;1HNMR(DMSO-d6,300MHz)and13CNMR(DMSO-d6, 75MHz),asshowninTable1.
5-Hydroxymethyl-2-furaldehyde(2)
Brownishoil;C6H6O3;HR-ESI-MSm/z149.0216[M+Na]+; IR
max:3390,1673,1521cm−1;1HNMR(acetone-d6,300MHz):ı 9.59(1H,s,H-1),7.38(1H,d,J=3.3Hz,H-3),6.58(1H,d,J=3.3Hz, H-4),4.65(2H,s,H-6);13CNMR(acetone-d
6,75MHz):ı177.2(C-1), 162.0(C-5),152.5(C-2),122.9(C-3),109.3(C-4),56.6(C-6).
Vanillicacid(3)
Whitepowder;C8H8O4;HR-ESI-MSm/z191.0321[M+Na]+;IR
max: 3467,2917, 2847, 1671, 1433,1110, 763cm−1; 1H NMR (acetone-d6,300MHz):ı7.62(1H,dd,J=8.4,1.5Hz,H-6),7.57(1H, s,H-2),6.92(1H,d,J=8.4Hz,H-5),3.91(3H,s,3-OCH3);13CNMR (acetone-d6,75MHz): ı166.7(COOH),151.2(C-4),147.2 (C-3), 124.0(C-6),122.0(C-1),114.6(C-5),112.6(C-2),55.4(3-OCH3).
Determinationofantioxidantactivity
AssayofDPPHradicalscavengingactivity
C.Supasuteekuletal./RevistaBrasileiradeFarmacognosia26(2016)312–320
Table1
1H(300MHz)and13CNMR(75MHz)chemicalshiftsofcompound1(DMSO-d 6). Position 1H(JinHz) 13C HMBC(correlationwith1H)
2 – 164.2 2′and6′
3 6.78 s 102.6 –
4 – 182.3 –
5 – 160.8 5-OH and 6
6 6.27s 98.5 5-OH
7 – 162.5 6and1′′
8 – 104.3 6and1′′
9 – 156.0 1′′
10 – 104.6 3 and 6
1′ – 121.7 3′, 5′and 3 2′ 8.03d(8.7) 129.1 6′ 3′ 6.91d(8.7) 116.1 5′
4′ – 161.4 2′,3′,5′and6′ 5′ 6.91d(8.7) 116.1 3′
6′ 8.03 d (8.7) 129.1 2′
5-OH 13.12 s – –
Glucose
1′′ 4.75d(9.9) 71.8 2′′ 2′′ 4.06 dd (8.7, 9.1) 75.2 1′′and 1′′′ 3′′ 3.51 m 80.0 2′′and 4′′ 4′′ 3.43m 70.8 3′′and6′′
5′′ 3.24m 81.9 1′′
6′′ 3.75brd(12.0) 61.3 – 3.53m
Rhamnose
1′′′ 4.97s 100.5 2′′ 2′′′ 3.60m 70.6 4′′′ 3′′′ 3.08dd(9.0,2.3) 70.4 1′′′and4′′′ 4′′′ 2.90 t (9.0) 71.6 2′′′, 3′′′and 6′′′ 5′′′ 2.10 m 68.4 1′′′and 6′′′ 6′′′ 0.46d(6.0) 17.9 4′′′
performedforIC50determination.Thereactionmixture(200l)
ineachwellcontained20lofthesamplesolutionand180lof 50MDPPHina96-wellmicrotiterplate.Thereactionmixture wasthenincubatedfor30min,andtheabsorbanceat510nmwas measuredwithamicroplatereader.ThepercentageofDPPHradical scavengingactivitywasthencalculatedasfollows:
%DPPHradicalscavengingactivity=Ac−As Ac ×100,
whereAcistheabsorbanceofthecontrolandAsistheabsorbanceof
thesamples.Theexperimentwasperformedintriplicate(n=3),and eachexperimentconsistedofthreerepetitions.MeOHwasusedas anegativecontrol.Troloxwasusedasapositivecontrolandtreated underthesameconditionsasthesamples.
Assayofsuperoxideanion(O2•−)scavengingactivity
Thisassaymeasurestheabilityof thetestsample toinhibit thereductionofNBTtoblueformazanbyO2−(Chatsumpunetal.,
2010).Thesamplesolutionswerepreparedbydissolvingthetest sampleinasolutionof30%MeOHinpotassiumphosphatebuffer. Thereaction(200l)wasperformedbyadding40lofsample solutionand20lof750MNBTtoamixtureof20lof50mM potassiumphosphatebuffer,100lof266Mriboflavin,and20l of1mMEDTAina96-wellmicrotiterplate.Thereactionmixture wasthenilluminatedwithafluorescentlampfor10minatroom temperature.Theformationofblueformazanwasthenmonitored basedontheincreaseintheabsorbanceat570nm.Asimilar reac-tionmixturewaskeptinthedarkandservedastheblank.The percentageofO2−radicalscavengingactivitywasthencalculated asfollows:
%O−
2radicalscavengingactivity=
Ac−As
Ac ×100,
whereAcistheabsorbanceofthecontrolandAsistheabsorbanceof thesamples.Theexperimentwasperformedintriplicate(n=3),and
eachexperimentconsistedofthreerepetitions.Asolutionof30% MeOHwasusedasanegativecontrol.Troloxwasusedasapositive controlandtreatedunderthesameconditionsasthesamples.
AssayofDNAprotectiveactivity
Theinhibitoryeffectof thetestsamplesonsupercoiledDNA breakage was assessed using the agarose gel electrophoresis method(Chatsumpun etal.,2010).Briefly,thetest sample was initiallyevaluatedataconcentrationof100g/ml,andtwo-fold serialdilutionwasperformedforIC50 determination.Each reac-tionmixture(10l)contained2lofthesamplesolution,1lof 50mMpotassiumphosphatebuffer,5lof266Mriboflavin,1l of1mMEDTA,and1lof100ng/lpBR322plasmidDNA.The mix-turewasthenilluminatedwithafluorescentlampfor30min.The sameexperimentwaskeptinthedarkasablank.Themixturewas subsequentlytreatedwith2lofloadingdye(0.25% bromophe-nolblue,0.25%xylenecyanol,and40%sucroseinwater),andload onto0.7%agarosegel.Electrophoresiswasconductedat100Vina Tris–aceticacid–EDTAbuffer.Then,thegelwasstainedwith ethid-iumbromide(0.5g/mlindeionizedwater)andvisualizedunder ultravioletlight.ImageswereobtainedusingaMiniBISGel Doc-umentationsystemand analysedwithGelQuantAnalysis(DNR BioImagingSystems,Jerusalem,Israel).Theexperimentwas per-formedintriplicate(n=3),andeachexperimentconsistedofthree repetitions.Asolutionof30%MeOHwasusedasanegative con-trol.Troloxandquercetinwereusedaspositivecontrolsandtreated underthesameconditionsasthesamples.
AssayofneuroprotectiveeffectsonculturedP19-derivedneurons
Cellculture
P19cellswereculturedinP19GM(␣-MEMsupplementedwith 7.5%NCS,2.5%FBS,and1%antibiotics-antimycoticsolution)ina humidified5%CO2atmosphereat37◦C.Cellsinmonolayercultures weremaintainedintheexponentialgrowthphasebysubculturing every2days(Jones-Villeneuveetal.,1982).
DifferentiationofP19cellsintoP19-derivedneurons
Exponentiallygrownculturesweretrypsinisedandseparated intoindividualcells.ThedifferentiationofP19cellswasperformed byseeding2×106cells/mlontoa100-mmbacteriologicalculture dishcontaining10mlofP19IM(␣-MEMsupplementedwith5%FBS and1%antibiotics-antimycoticsolution)and0.5MRA.Abulky aggregateofcellswasformedinsuspension.Thecellclusterswere thendissociatedafter4daysofRAtreatmentusinga5-mlglass measuringpipetteand resuspendedonpoly-l-lysine-pre-coated multi-well plates (platescoated with 50g/ml of poly-l-lysine dissolvedinaphosphate-bufferedsaline(PBS)solutionovernight and sterilized underUV light for 30min) ata concentrationof 7×104cells/ml (150l/well) in P19SM (␣-MEM supplemented with10%FBS,and1%antibiotic-antimycoticsolution). Thecells werethen incubatedfor anadditional day.Theproliferation of non-neuronalcellswasinhibitedby theaddition of cytosine-1--d-arabinosideorAra-C (10M) onthefirstdayafterplating, andthemediumwasrenewedeveryfewdays.Thedifferentiated P19-derivedneuronswereusedafterday14ofthedifferentiation process(Jones-Villeneuveetal.,1982;Jones-Villeneuveetal.,1983; MacPhersonandMcBurney,1995;Tadtongetal.,2012).
Neuronalviabilityassay
C.Supasuteekuletal./RevistaBrasileiradeFarmacognosia26(2016)312–320 1000,and10,000ng/ml.DMSOwasaddedtotheculturesata
con-centrationof0.5%(v/v)asasolventcontrol.P19SMsupplemented with10MAra-Cwasaddedtothecontrolwells.Thecellswere keptat37◦Cfor18h.Then,50lofanXTTsolution(1mg/mlXTT in60◦C␣-MEMwith25MPMS)wasadded,and150lofthe mediumwastakenout.After4hofincubation,100lofPBS,pH 7.4wasaddedtoeachwell.Theopticaldensity(OD)wasmeasured at450nmusingmicroplatereader.Theexperimentwasperformed intriplicate(n=3),andeachexperimentconsistedofthree repe-titions,withmediumat100%cellviabilityasacontrol(Tadtong etal.,2012;Tadtongetal.,2007).Theconcentrationthatpromoted bettersurvivaloftheculturedneuronsthanthecontrolwasfurther evaluatedforneuroprotectiveactivity.
Neuritogenicityassay
TheassaywasconductedwithP19-derivedneuronscultured ina96-wellplateusingtheserumdeprivationmethod(Iacovitti etal.,1997;López-Maderueloetal.,2001;Tadtongetal.,2013). TheP19SMsupplementedwith10MAra-Cwasremovedafter 14 days of the differentiation process, and the sample solu-tionsinDMSOdiluted withP19SMcontaining10MAra-C,the ␣-MEMsupplementedwith10MAra-C,andthe1% antibiotic-antimycoticsolutionwithoutFBSwereaddedtogenerateafinal sampleconcentrationthatenhancedthesurvivalofcultured neu-rons more than the control. DMSO wasadded to the cultures at a concentration of 0.5% as a solvent control, followed by P19SMwith10MAra-C in thecontrol wells.␣-MEM supple-mentedwith10MAra-Cand1%antibiotic-antimycoticsolution withoutFBS wereused togenerate the oxidative stress condi-tion. The cells were kept at 37◦C for 18h. Cell viability was assayed usingthe XTT reduction method.The experiment was performed in triplicate (n=3), and each experiment consisted of 3 repetitions, with medium at 100% cell viability as a con-trol.
Assayof˛-glucosidaseinhibitoryactivity
The␣-glucosidaseinhibitoryeffectwasevaluatedasdescribed previously,withslightmodifications(Sunetal.,2014).Thisassay measurestheenzyme activitybyinvestigating therelease ofp -nitrophenolfromthepNPGsubstrate.Eachsamplewasinitially evaluatedataconcentrationof2mg/ml,andtwo-foldserial dilu-tionwasperformedforIC50determination.Thereactionmixturein a96-wellmicrotiterplateinitiallycontained10loftestsample and40lof0.1U/ml␣-glucosidaseandwaspre-incubatedat37◦C for10min.Then,50lof2mMpNPGwereaddedtothemixture andfurtherincubatedat37◦Cfor20min.Thereactionwasthen terminatedbytheadditionof100lofa1MNa2CO3solution.The amountofp-nitrophenolreleasedwasmeasuredusingamicroplate readertodeterminetheabsorbanceat405nm.Thepercentageof ␣-glucosidaseinhibitoryactivitywasthencalculatedasfollows:
%˛-glucosidaseinhibitoryactivity= Ac−As
Ac ×
100,
whereAcistheabsorbanceofthecontrolandAsistheabsorbance ofthesamples.Theexperimentwasperformedintriplicateand eachexperimentconsistedofthreerepetitions.FivepercentDMSO wasusedasanegativecontrol.Acarbosewasusedasapositive control and treated under the same conditions as the sam-ples.
Statisticalanalysis
Allanalyseswerecarriedoutintriplicate(n=3).Thedatawere presentedasthemean±standarddeviation(SD).One-way analy-sisofvariance(ANOVA)withtheleastsignificantdifference(LSD) testwascarriedouttoidentifysignificantdifferencesbetweenthe
control andexperimentalgroups using SPSSversion18.0 (SPSS Inc., Chicago, IL).Differenceswere considered significantwhen p<0.05.
Resultsanddiscussion
Isolationandidentificationofisolatedcompounds
ThephytochemicalinvestigationoftheMeOHextractfromG. gracilisleavesyielded aglycosidicflavonecompound (1)as the majorconstituent,alongwith5-hydroxymethyl-2-furaldehyde(2) andvanillicacid(3).The1Hand13CNMRassignmentsofcompound 1thatwerereportinapreviousstudywerebasedon1DNMR exper-iments(Wangetal.,2012).ThepreviouslyreportedNMRdataare differentfromourresults,especiallywithrespecttothe13CNMR dataforthesugarmoieties.In thisstudy,thestructure of com-pound1wasidentifiedbasedon1DNMR(1H,13CNMR),2DNMR (1H–1HCOSY,HSQC,andHMBC),HR-ESI-MS,FTIR,andUV spec-troscopy.Compound1wasobtainedasayellowamorphoussolid. TheHR-ESImassspectrumofthatcompoundshowedapeakforthe [M+Na]+ionatm/z601.1536(calculatedfor601.1533),indicating amolecularformulaofC27H30O14.TheIRspectrumofcompound1 exhibitedthebroadabsorptionofahydroxylgroupat3367cm−1 andacarbonylgroupat1654cm−1.Theseresults,alongwithUV absorptionmaximaat215and333,suggestedaflavoneglycoside structureforcompound1.
Accordingtothe1Hand13CNMRspectraof1thatarelisted inTable1,the13CNMRdataforcompound1revealedthe pres-enceoffifteenaromaticcarbonresonancesfortheaglyconepart ofthecompoundandtwelvesugarsignals,whichweredetected asonehexoseandonedeoxyhexoseunit.Basedonthe1HNMR data,theapigeninskeletonwasidentifiedfromthepresenceofone protonsingletatıH6.78(H-3)inthearomaticregion,aswellas twoprotonsignalsatıH 6.91andıH 8.03(each2H,d,J=8.7Hz) whichindicatedaparasubstituentatC-4′ofringB.FromtheHMBC spectra,thesignalatıH8.03wasassigned totwoorthoprotons ofringB(H-2′ andH-6′)basedonthecorrelationofthese pro-tonswithC-2ofringC.Ahydrogen-bondedhydroxylproton(s, 5-OH)inringAwasassignedtothesharpsingletpeakatıH13.12 inthe1H NMRspectrum.Based ontheHMBCspectra,the pro-tonat5-OHexhibitedcorrelationswithC-6atıC98.5,C-5atıC 160.8andC-10atıC104.6.Consequently,theıH6.27(1H,s)of H-6wasindicatedbyitscorrelationwiththecarbonsignalofC-6in HSQC.
C.Supasuteekuletal./RevistaBrasileiradeFarmacognosia26(2016)312–320 (ıH 4.75, d,J=9.9Hz)and H-6(ıH 6.27,s)in theHMBC
experi-ment.
Accordingtotheabovespectroscopicdata,compound1was identified as apigenin-8-C-␣-l-rhamnopyranosyl-(1→2)--d -glucopyranoside.
OH
OH OH
OH
OH
OH O
1
O O O O HOHO HO
Theother knowncompounds 2 and 3 wereidentified as 5-hydroxymethyl-2-furaldehyde(2)andvanillicacid(3)basedona comparisonoftheirNMRspectraldatawithvaluesavailableinthe literature(Espinozaetal.,2008;Changetal.,2009).
HO
OH
2
3
CHO
OCH3 CO2H
O
Preliminaryscreeningofisolatedcompounds
Based ona primary screen for superoxideanion scavenging activityat100g/ml,compound1and3exhibitedpotent scav-enger activity, with values of 96.45% and 82.61%, respectively, whilstcompound2displayednoactivity.TheIC50ofcompound1 was23.91±5.37Mandthatofcompound3was19.88±1.34M. Afterscreening for␣-glucosidase inhibitoryactivityat2mg/ml, onlycompound1showedactivity,with96.90%inhibitionandan IC50of0.56±0.01mM.Compounds2and3wereinactive. Prelim-inarystudiesofthebiologicalactivitiesoftheisolatedcompounds demonstratedtheantioxidantpotentialandrelatedeffectsof com-pound1.Moreover,thisflavonoidwithan8-Csubstitutioninthe A-ringhasnotbeenreportedpreviouslyinG.gracilis.Therefore,the presenceofan8-Cflavonoidglycosidedeservedfurther chemotax-onomicattentionandadditionalinvestigationofbiologicalactivity inthisstudy.
Antioxidantactivity
DPPHradicalscavengingactivity
DPPHisacolouredandstablenitrogenfreeradical.Thisassay determinesthereducingcapacityofanantioxidantbymeasuring thechangeofcolourfromviolettoyellowbasedonitsabsorbance. Antioxidantscaneliminatethisfreeradicalviatheprocessof hydro-genatomtransferorelectrondonation(Mohammedetal.,2015).
Based on the results, the highest scavenging activity was observedfor Trolox(IC50=7.7±1.74M). Compound1 showed a dose-dependent but weaker scavenging effect in this model
(IC50=117.47±14.14M)(Table2,Fig.1).Theweakactivityof theapigeninglycosidewasconsistentwithapreviousreportbyLu andFoo(2001)andindicatedtheimportanceofthe3′,4′-dihydroxy groupoftheB-ring,whichisakeyfactorforscavengingDPPH(Lu andFoo,2001;Lietal.,2008;Mohammedetal.,2015).In addi-tion,thesubstitutionofahydrogenatomattheC-8positioninthe flavoneAringbythetwosugarmoietiesalsodecreasedthe antioxi-dantactivityduetosterichindrance,asthosebulkygroupsreduced accessthecentreoftheDPPHradical(Prioretal.,2005;Zengetal., 2013).
Superoxideradicalscavengingactivity
Thesuperoxideradicalisasignificantcellularfreeradicaland isassociatedwithanincreaseinoxidativedamageinbiomolecules duetotheproductionofmorepowerfulreactivespecies.In our model,O2−wasgeneratednon-enzymaticallybythephotoreaction ofriboflavinandassayedbasedonthereductionofNBTtogenerate blueformazan.However,thisprocesscanbeinhibitedwhenO2− scavengersarepresent(Chatsumpunetal.,2010).
The O2− scavenging activity of compound 1 is shown in Table2 and Fig.1.Compound 1was foundtoexhibit stronger scavengingactivitythanTrolox,withIC50values of23.91±5.37 and 95.66±9.83M,respectively. Many studies reported vari-ableresultsfortheO2−scavengingactivityofflavonoidglycosides dependingonthepositionofglycosylation,theattachedhydroxyl group, and the type and number of sugars in the structures (Yokozawaetal.,1997;Zengetal.,2013;Xiaoetal.,2014;Materska, 2015).BasedonthestudiesofLuandFoo(2001),thecatecholand pyrogallolintheBringareresponsibleforstrongantioxidant activ-ity,butthescavengingactivitiesoftheflavoneglycosideswereall higherthanthatofTrolox.Thoseresultswereconsistentwiththe findingofYokozawaet al.(1997)thatapigenincaninhibitROS species,evenintheabsenceof6-or3′-OH.Thoseauthorsalso indi-catedthatthelinkedrhamnosesugaryieldedbetterpropertiesthan glucoseintheiraglycone.Therefore,thehighpotencyofcompound 1inthescavengingofsuperoxideradicalsmightalsoberelatedto thelinkedsugar.
DNAprotectiveactivity
C.Supasuteekuletal./RevistaBrasileiradeFarmacognosia26(2016)312–320
Table2
IC50values(M)ofcompound1isolatedfromGarciniagracilisforDPPHradicalscavenging,superoxideanionradicalscavenging,DNAprotective,and␣-glucosidaseinhibitory activities.
Sample DPPHa(M) Superoxideaniona(M) DNAprotectivea(M) ␣-Glucosidasea(mM)
Compound1 117.47±14.14a 23.91±0.23a 23.40±3.37a 0.56±0.01a
Trolox 7.7±1.47b 95.66±9.83b 125.75±29.91b –
Quercetin – – 21.01±1.24a –
Acarbose – – – 0.90±0.06b
Dissimilarlettersinthesamecolumnindicatesignificantlydifferentvaluesforeachparameteratp<0.05usingone-wayanalysisofvariance(ANOVA)withtheleastsignificant difference(LSD)test.
aThedatavaluesareexpressedasthemean±SDoftriplicateexperiments(n=3),andeachexperimentconsistsof3repetitions.
80
120
100
80
60
40
20
0
A
B
C
DPPH r
adical sca
ve
nging
activity
, %
Concentration (µg/ml) Concentration (µg/ml)
Concentration (µg/ml)
Supero
xide anion r
adical
sca
ve
nging activity
, %
α
-glucosidase inhibitor
y
activity
, %
100
80
60
40
20
0 60
40
20
0
1 10 100 1000 1 10 100
50 500 5000
Fig.1. Concentration-dependentinhibitoryeffectsofcompound1on:(A)theDPPHradical,(B)thesuperoxideanion(O2−),and(C)the␣-glucosidaseenzyme.Thedataare
expressedasthemean±SDoftriplicateexperiments(n=3),andeachexperimentconsistsofthreerepetitions.
Compound 1
Trolox OC
1 2 3 4 5 6 7
120
Inhibition, %
Concentration (µg/ml) 100
80
60
40
20
0
1 10 100
1 2 3 4 5 6 7
1 2 3 4 5 6 7
SC
OC
SC
OC
SC
Quercetin
D
A
B
C
C.Supasuteekuletal./RevistaBrasileiradeFarmacognosia26(2016)312–320
Neurite
Neurite
Neuron cell
Neuron cell
Neurite
Neuron cell
A
C
B
Fig.3.Phase-contrastmicrographsoftheneuritogenicityofP-19-derivedneuronsafter18hofincubationin:(A)P19SM(␣-MEM+10%,v/vFBS+10MAra-C)without treatment(control),(B)serumdeprivationconditions(␣-MEM+10MAra-C)withouttreatment(toxicconditions),and(C)serumdeprivationconditions(␣-MEM+10M Ara-C)treatedwithcompound1at100ng/ml;scalebar=10m.
flavonoidstoprotectagainstDNAdamagemaybeassociatedwith freeradicalscavengingactivity(Boyleetal.,2000).
Neuroprotectiveactivity
OxidativedamagecausedbyROSspeciesalsooccursinthebrain dueitslargeoxygenconsumption,itslargequantityoffattyacids, anditslowlevel ofantioxidantenzymes.Furthermore,neurons cannotpromptlyrecoverviamitosisand celldivision afterthey aredamagedduetotheirpostmitoticstatus(Tangsaengvitetal., 2013).P-19cells,whichareawell-knowninvitromodelderived frommurineembryonalcarcinoma,weredifferentiatedinto neu-ronsusingretinoicacid.TheP-19-derivedneuronswerefoundto beirreversiblypostmitoticandtocontainparticular neurotrans-mitters,suchas␥-aminobutyricacidandacetylcholine,whichare similartothosefoundinmatureCNSneurons(Tadtongetal.,2012). The viability of P-19-derived neurons in the presence of compound 1 was investigated using theXTT assay. Compound 1 showed 100% neuron viability at a nontoxic concentration (100ng/ml).Accordingly,theneuroprotectiveabilityofcompound 1at100ng/mlwasthenevaluatedinaserumdeprivationmodel. Serum is a mixture that contains a large amount of proteins and somevital growth factorsthat are required for the prolif-erationof cells inculture.The lackof seruminduced oxidative stressconditionsforthecells,eventuallyresultingincell apopto-sis(Tangsaengvitet al., 2013). Interestingly,at a concentration of 100ng/ml, compound 1 significantly protected the cultured neuronsagainstROStoxicity duringserumdeprivation-induced oxidativestress, asshown inFig. 3.Treatment withcompound 1 increased the neuriteoutgrowth of the cultured neurons by approximatelyfour-fold in comparison to theuntreated condi-tion(Table3).Manyreportsrevealedthatatalowconcentration, flavonoidsactasneuroprotectivesubstancesviatheactivationof themitogen-activatedprotein kinase(MAP kinase)pathway. In
Table3
Neuroprotectiveactivityduringserumdeprivation.
Sample Neuronal viabilitya(%)
Compound1(100ng/ml) 65.74±9.41b
␣-MEM+0.5%DMSO 16.34±7.73
␣-MEM 16.59±8.13
P19SMc+0.5%DMSO 100.30±0.52
P19SMc 100.00±0.00
aThedataareexpressedasthemean±SDoftriplicateexperiments(n=3),and
eachexperimentconsistsof3repetitions.
bp<0.05whencomparedwiththetoxiccondition(␣-MEM)andthesolvent
con-trolofthetoxiccondition(␣-MEM+0.5%DMSO)usingone-wayanalysisofvariance (ANOVA)withtheleastsignificantdifference(LSD)test.
c P19SMiscomposedof␣-MEM+10%(v/v)FBS.
contrast,athighconcentrations,flavonoidsactivatethecaspase pathway,leadingtoapoptosis(MandelandYoudim,2004;Tadtong etal.,2013;Williamsetal.,2004).
˛-Glucosidaseinhibitoryactivity
C.Supasuteekuletal./RevistaBrasileiradeFarmacognosia26(2016)312–320
120
∗
Neuronal viability
, %
100
80
60
40
20
0
Compound 1
a-MEM+0.5%DMSO P19SM+0.5%DMSO
a-MEM P19SM
Fig.4. Theeffectofcompound1ontheneuronalviabilityofP-19-derivedneurons intheserumdeprivationmodel.Thehistogramshowsthepercentageofcellviability relativetovehicle-treatedcontrolcultures.Eachbarexpressesthemean±SDof
trip-licateexperiments(n=3),andeachexperimentconsistsof3repetitions.Significant differenceswerefoundforthecomparisonsofcompound1withthetoxiccondition (␣-MEM)andthesolventcontrolofthetoxiccondition(␣-MEM+0.5%DMSO)using one-wayanalysisofvariance(ANOVA)withtheleastsignificantdifference(LSD) test.*p<0.05.
ThisstudyisthefirstreportthatflavoneglycosidesfromG. gra-cilisexhibit␣-glucosidaseinhibitoryactivity,suggestingthatthis plantcouldbeapotentialsourceof␣-glucosidaseinhibitorsforthe treatmentofdiabetes(Fig.4).
Inconclusion, chromatographicseparationof themethanolic extractfromtheleavesofG.gracilisledtotheisolationand identi-ficationofthreecompounds,apigenin-8-C-␣-l -rhamnopyranosyl-(1→2)--d-glucopyranoside(1),5-hydroxymethyl-2-furaldehyde (2),andvanillicacid(3).Amongtheseisolates,compound1was obtainedinthelargestquantityandexhibitedpotential superox-ideanionradicalscavengingactivity,aprotective effectagainst pBR322 plasmid DNA damage, a protective effect against P19-derivedserumdeprivation,and␣-glucosidaseinhibitoryactivity.
Authors’contributions
CS(PhDstudent)contributedtotheisolationandpurification ofthecompounds,therunningofthelaboratorywork,the anal-ysisofthedata,andthedraftingofthepaper.WNcontributedto isolationandpurificationofthecompounds.STcontributedtothe cell-basedassayofneuroprotectiveactivity.PTandKLcontributed tothecriticalreadingofthemanuscript.BScontributedtotheplant collection,thesupervisionofthelaboratoryworkandcritical read-ingofthemanuscript.Allauthorshavereadthefinalmanuscript andapprovedthesubmission.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Acknowledgements
This study was supported by the 100th Anniversary Chu-lalongkorn University Fund for Doctoral Scholarship of Chula-longkornUniversity. The authors would also like tothank The ResearchInstrumentCenteroftheFacultyofPharmaceutical Sci-encesatChulalongkornUniversityforprovidingresearchfacilities.
References
Ayepola,O.R.,Cerf,M.E.,Brooks,N.L.,Oguntibeju,O.O.,2014.Kolaviron,abiflavonoid complexofGarciniakolaseedsmodulatesapoptosisbysuppressingoxidative stressandinflammationindiabetes-inducednephrotoxicrats.Phytomedicine 21,1785–1793.
Baliga,M.S.,Bhat,H.P.,Pai,R.J.,Boloor,R.,Palatty,P.L.,2011.Thechemistryand medicinalusesoftheunderutilizedIndianfruittreeGarciniaindicaChoisy (kokum):areview.FoodRes.Int.44,1790–1799.
Boyle,S.P.,Dobson,V.L.,Duthie,S.J.,Kyle,J.A.M.,Collins,A.R.,2000.Absorptionand DNAprotectiveeffectsofflavonoidglycosidesfromanonionmeal.Eur.J.Nutr. 39,213–223.
Chang,S.W.,Kim,K.H.,Lee,I.K.,Choi,S.U.,Ryu,S.Y.,Lee,K.R.,2009.Phytochemical constituentsofBistortamanshuriensis.Nat.Prod.Sci.15,234–240.
Chatsumpun,M.,Chuanasa,T.,Sritularak,B.,Likhitwitayawuid,K.,2010. Oxyresver-atrolprotectsagainstDNAdamageinducedbyphotosensitizedriboflavin.Nat. Prod.Commun.6,41–44.
Chuakul,W.,2009.Indigenousmedicinalplantsusedasantipyretics.ThaiPharm. HealthSci.J.4,435–449.
Espinoza,C.,Viniegra-González,G.,Loera,O.,Heredia,G.,Trigos,Á.,2008. Antibac-terialactivityagainstplantpathogensbycrudedextractsandcompoundsfrom
Idriellasp.Rev.Mex.Micol.26,9–15.
George,V.C.,Kumar,D.R.N.,Suresh,P.K.,Kumar,R.A.,2015.Antioxidant,DNA pro-tectiveefficacyandHPLCanalysisofAnnonamuricata(soursop)extracts.J.Food Sci.Technol.52,2328–2335.
Gul,M.Z.,Bhakshu,L.M.,Ahmad,F.,Kondapi,A.K.,Qureshi,I.A.,Ghazi,I.A.,2011.
EvaluationofAbelmoschusmoschatusextractsforantioxidant,freeradical scav-enging,antimicrobialandantiproliferativeactivitiesusinginvitroassays.BMC Complement.Altern.Med.11,1–12.
Hollander,P.,2007.Anti-diabetesandanti-obesitymedications:effectsonweight inpeoplewithdiabetes.DiabetesSpectrum20,159–165.
Iacovitti,L.,Stull,N.D.,Johnston,K.,1997.Melatoninrescuedopamineneuronsfrom celldeathintissueculturemodelsofoxidativestress.BrainRes.768,317–326.
Jones-Villeneuve,E.M.,McBurney,M.W.,Rogers,K.A.,Kalnins,V.I.,1982.Retinoic acidinducesembryonalcarcinomacellstodifferentiateintoneuronsandglial cells.J.CellBiol.94,253–262.
Jones-Villeneuve,E.M.,Rudnicki,M.A.,Harris,J.F.,McBurney,M.W.,1983.Retionic acid-inducedneuronaldifferentiationofembryonalcarcinomacells.Mol.Cell Biol.3,2271–2279.
Kim,J.S.,Kwon,C.S.,Son,K.H.,2000.Inhibitionofalpha-glucosidaseandamylaseby luteolin,aflavonoid.Biosci.Biotechnol.Biochem.65,2458–2461.
Kuate,D.,Etoundi,B.C.O.,Soukontoua,Y.B.,Ngondi,J.L.,Oben,J.E.,2011. Compar-ativestudyoftheantioxidant,freeradicalscavengingactivityandhumanLDL oxidationinhibitionofthreeextractsfromseedsofaCameroonianSpice,Xylopia parviflora(A.Rich.)Benth.(Annonaceae).Int.J.Biomed.Pharm.Sci.5,18–30.
Li,N.,Liu,J.H.,Zhang,J.,Yu,B.Y.,2008.Comparativeevaluationofcytotoxicityand antioxidativeactivityof20flavonoids.J.Agric.FoodChem.56,3876–3883.
Likhitwitayawuid,K.,Klongsiriwet,C.,Jongbunprasert,V.,Sritularak,B., Wongserip-ipatana, S., 2006. Flavones with free radical scavenging activity from
Goniothalamustenuifolius.Arch.Pharm.Res.29,199–202.
López-Maderuelo,M.D.,Fernández-Renart,M.,Moratilla,C.,Renart,J.,2001. Oppo-siteeffectsoftheHsp90inhibitorGeldanamycin:inductionofapoptosisinPC12, anddifferentiationinN2Acells.FEBSLett.490,23–27.
Lu,Y.,Foo,L.Y.,2001.Antioxidantactivitiesofpolyphenolsfromsage(Salvia offici-nalis).FoodChem.75,197–202.
MacPherson,P.A.,McBurney,M.W.,1995.P19embryonalcarcinomacells:asource ofculturedneuronsamenabletogeneticmanipulation.Methods7,238–252.
Mandel,S.,Youdim,M.B.H.,2004.Catechinpolyphenols:neurodegenerationand neuroprotection in neurodegenerative disease. Free Radic. Biol. Med. 37, 304–317.
Materska,M.,2015.FlavoneC-glycosidesfromCapsicumannuumL.:relationships betweenantioxidantactivityandlipophilicity.Eur.FoodRes.Technol.240, 549–557.
Meng,F.,Feng,H.J.,Chen,Y.,Wang,D.B.,Yang,G.Z.,2012.Antioxidantactivityof
Garciniaxanthochymusleaf,rootandfruitextractsinvitro.Chin.J.Nat.Med.10, 129–134.
Mohammed,R.S.,Souda,S.S.E.,Taie,H.A.A.,Moharam,M.E.,Shaker,K.H.,2015.
Antioxidant,antimicrobialactivitiesofflavonoidsglycosidefromLeucaena leu-cocephalaleaves.J.Appl.Pharm.Sci.5,138–147.
Moreira,P.L.,Boas,P.J.F.V.,Ferreira,A.L.A.,2014.Associationbetweenoxidative stressandnutritionalstatusintheelderly.Rev.Assoc.Med.Bras.60,75–83.
Prior,R.L.,Wu,X.,Schaich,K.,2005.Standardizedmethodsforthedeterminationof antioxidantcapacityandphenolicsinfoodsanddietarysupplements.J.Agric. FoodChem.53,4290–4302.
Rahman,K.,2007.Studiesonfreeradicals,antioxidants,andco-factors.Clin.Interv. Aging2,219–236.
Ritthiwigrom,T.,Laphookhieo,S.,Pyne,S.G.,2013.Chemicalconstituentsand bio-logicalactivitiesofGarciniacowaRoxb.MaejoInt.J.Sci.Technol.7,212–231.
Ryu,H.W.,Cho,J.K.,Curtis-Long,M.J.,Yuk,H.J.,Kim,Y.S.,Jung,S.,Kim,Y.S.,Lee, B.W.,Park,K.H.,2011.␣-Glucosidaseinhibitionandantihyperglycemic activ-ityofprenylatedxanthonesfromGarciniamangostana.Phytochemistry 72, 2148–2154.
Semwal,R.B.,Semwal,D.K.,Vermaak,I.,Viljoen,A.,2015.Acomprehensivescientific overviewofGarciniacambogia.Fitoterapia102,134–148.
C.Supasuteekuletal./RevistaBrasileiradeFarmacognosia26(2016)312–320
Suksri,S.,Premcharoen,S.,Thawatphan,C.,Sangthongprow,S.,2005.Ethnobotany inBungKhongLongnon-huntingarea,northeastThailand.KasetsartJ.(Nat.Sci.) 39,519–533.
Sun,J.,Zhang,F.,Yang,M.,Zhang,J.,Chen,L.,Zhan,R.,Li,L.,Chen,Y.,2014.Isolation ofa-glucosidaseinhibitorsincludinganewflavonolglycosidefromDendrobium devonianum.Nat.Prod.Res.28,1900–1905.
Tadtong,S.,Athikomkulchai,S.,Sareedenchai,V.,2012.NeuritogenicactivityofThai plantextracts.J.HealthRes.26,293–296.
Tadtong,S.,Kanlayavattanakul,M.,Lourith,N.,2013.Neuritogenicand neuropro-tectiveactivitiesoffruitresidues.Nat.Prod.Commun.8,1583–1586.
Tadtong,S.,Meksuriyen,D.,Tanasupawat,S.,Isobe,M.,Suwanborirux,K.,2007.
GeldanamycinderivativesandneuroprotectiveeffectonculturedP19-derived neurons.Bioorg.Med.Chem.Lett.17,2939–2943.
Tangsaengvit,N., Kitphati,W., Tadtong,S., Bunyapraphatsara,N., Nukoolkarn, V.,2013.Neuriteoutgrowthandneuroprotectiveeffectsofquercetinfrom
CaesalpiniamimosoidesLamk.onculturedP19-derivedneurons.Evid.Based Complement.Alternat.Med.
Tiwari,B.K.,Pandey,K.B.,Abidi,A.B.,Rizvi,S.I.,2013.Markersofoxidativestress duringdiabetesmellitus.J.Biomark.,1–8.
UnitedNations,DepartmentofEconomicandSocialAffairs,PopulationDivision, 2013.Worldpopulationageing2013.ST/SEA/SER.A/348.
Wang,J.,Yue,Y.D.,Tang,F.,Sun,J.,2012.Screeningandanalysisofthepotential bioactivecomponentsinrabbitplasmaafteroraladministrationofhot-water extractsfromleavesofBambusatextilisMcClure.Molecules17,8872–8885.
Williams, R.J.,Spencer,J.P.E.,Rice-Evans,C.,2004. Flavonoids:antioxidantsor signallingmolecules.FreeRadic.Biol.Med.36,838–849.
Xiao,J.,Chen,T.,Cao,H.,2014.Flavonoidglycosylationandbiologicalbenefits.Adv. Biotechnol.
Xiao,J.,Kai,G.,Yamamoto,K.,Chen,x.,2013.Advanceindietarypolyphenolsas␣ -glucosidasesinhibitors:areviewonstructure-activityrelationshipaspect.Crit. Rev.FoodSci.Nutr.53,818–836.
Yin,Z.,Zhang,W.,Feng,F.,Zhang,Y.,Kang,W.,2014.␣-Glucosidaseinhibitors iso-latedfrommedicinalplants.FoodSci.Hum.Wellness3,136–174.
Yokozawa,T.,Dong,E.,Liu,Z.W.,Shimizu,M.,1997.Antioxidativeactivityofflavones andflavonolsinvitro.Phytother.Res.11,446–449.