w w w. s b f g n o s i a . o r g . b r / r e v i s t a
Original
Article
Hypoglycemic
and
hypolipidemic
effects
of
Solidago
chilensis
in
rats
Mariane
Schneider
a,
Adrieli
Sachett
a,
Amanda
P.
Schönell
a,
Eduarda
Ibagy
a,
Emily
Fantin
a,
Fernanda
Bevilaqua
a,
Giana
Piccinin
a,
Glaucia
D.
Santo
a,
Marta
Giachini
a,
Rafael
Chitolina
a,
Silvana
M.
Wildner
a,
Ricieri
Mocelin
b,
Leila
Zanatta
b,
Walter
A.
Roman
Junior
c,∗aNúcleodeFitoterápicos,UniversidadeComunitáriadaRegiãodeChapecó,Chapecó,SC,Brazil
bProgramadePós-graduac¸ãoemCiênciasAmbientais,UniversidadeComunitáriadaRegiãodeChapecó,Chapecó,SC,Brazil
cProgramadePós-graduac¸ãoemCiênciasdaSaúde,UniversidadeComunitáriadaRegiãodeChapecó,Chapecó,SC,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received18December2014 Accepted16May2015 Availableonline10June2015
Keywords:
Antihyperlipidemicactivity Arnica-do-brasil Asteraceae Hypoglycemic
a
b
s
t
r
a
c
t
SolidagochilensisMeyen,Asteraceae,istraditionallyusedtotreatinflammation.However,phytochemical
andpharmacologyinvestigationsarelacking.Thisstudyevaluatedthehypoglycemicandhypolipidemic
effectsofhydroalcoholicextractfromS.chilensisaerialpartsinrats.Inoralglucosetoleranceteststhe
ratsreceivedsaline(0.5ml/100g)incontrolgroup(C),hydroalcoholicextract(125,250or500mg/kg
p.o.;n=6)orglibenclamide(10mg/kgp.o.;n=6).After30min,glucose(4g/kg)wasadministered.Rats
treatedwithhydroalcoholicextract500demonstrateddecreasedglucoselevelsat180min(−22.1%),
whencomparedwithgroupC,similartoglibenclamide.Moreover,treatmentwithhydroalcoholicextract
500significantlyincreasedtheglycogencontentintheliverandsoleusmuscle,andhydroalcoholic
extract250specificallyinhibitedtheenzymemaltasewhencomparedwithgroupC.Furthermore,all
hyperglycemicratstreatedwithhydroalcoholicextract(125,250and500)exhibitedanaccentuated
decreaseintotalcholesterollevels(−36.8%,−36.7%and−41.3%,respectively).Ourresultssuggestthat
hypoglycemicandhypolipidemiceffectsofhydroalcoholicextractcouldbeassociatedwithincreased
productionandreleaseofinsulinaswellaswithinsulinotropicandantioxidanteffects.
©2015SociedadeBrasileiradeFarmacognosia.PublishedbyElsevierEditoraLtda.Allrightsreserved.
Introduction
Diabetesmellitus(DM)comprisesagroupofdisorders involv-ingdistinct pathogenicmechanismswithhyperglycemia asthe commondenominator(Teixeiraetal.,2000).Hyperglycemiain dia-betesmayberelatedtonumerousphysiologicalevents, suchas decreasedglucoseincells,reducedglucoseutilizationbyvarious tissues,and increasedhepaticproduction of glucose (gluconeo-genesis)(Prabhakar et al.,2013).Complicationsexperienced by patientswithdiabetesareoftenrelatedtochronichyperglycemia, includingretinopathy, peripheralvascular disease,renal failure, neuropathy,andcardiovasculardiseasesthatcausebothmorbidity andprematuremortality(Hiranyetal.,2000;Piaulinoetal.,2013). Itiswellestablishedthatpatientswithtype2DMfrequently have abnormal serum lipid profiles comprising elevated low densitylipoproteins(LDL)andtriglycerideslevelsalongwith mod-eratelydecreasedhighdensitylipoproteins(HDL)level(Zimmet, 2000), all of which are associated with an increased risk of
∗ Correspondingauthor.
E-mail:romanwa@unochapeco.edu.br(W.A.RomanJunior).
cardiovasculardiseases(Daietal.,2013).Manystudieshaveshown thatelevatedserumcholesterolconcentrationscancausecoronary atherosclerosis(ParkandVelasquez,2012)thatisassociatedwith heartdisease,stroke,anddeathinbothdevelopedanddeveloping countries(Raidaetal.,2008).
Medicinalplantshavebeenusedformany yearsbydifferent culturesworldwidetotreatDM(Modaketal.,2007).Investigating herbalmedicineshasbecomeprogressivelyimportantinthesearch foranew,effective,andsafetherapeuticagenttocombatDM.More than200purebioactiveprinciplesisolatedfromplantshavebeen showntolowerserumglucoselevels(Groveretal.,2002;Warjeet, 2011),includingphenolicsandflavonoids(Negri,2005).
Solidago chilensis Meyen, Asteraceae, is a species native to the southern region of South America. It is widely distributed in south and southeast Brazil, where it is popularly known as arnica-do-brasil and is used to relieve inflammation (Lorenzi
and Matos, 2002). Its main chemical constituents are
ace-tophenone,carotenes,diterpenoidswithlabdanicandclerodanic skeletons(Soares-Valverdeetal.,2009),flavonoids,glycosides, 3-methoxybenzaldehyde,essential oils, and saponins(Silvaet al., 2010),withquercetrinbeingthemajorconstituent(Torresetal.,
1987).
http://dx.doi.org/10.1016/j.bjp.2015.05.001
Ethnopharmacologicalinvestigationshave foundthis species tohaveantispasmodic,antihemorrhagic(Alonso,1998), wound-healing(Facury-Netoetal.,2004),andanti-inflammatoryeffects
(Tamura et al., 2009). Recently, there has been considerable
progressintheinvestigationofS.chilensisandgastricprotection
(Bucciarellietal.,2010)aswellasabetterunderstandingofthe
effectofS.chilensisoninsulinresistanceinobesemice(Meloetal., 2011).However,thehypoglycemicandhypolipidemiceffectsofS.
chilensisontheglucosetolerancecurvehavenotyetbeenstudied. Therefore, theobjective ofthis study wastoinvestigatethe hypoglycemicandhypolipidemiceffectsofhydroalcoholicextract (HE) from S. chilensis in rats. This study evaluatedthe glucose tolerance curve along with, liver and soleus muscle glycogen levels,disaccharidaseactivity,totalcholesterol(TC),andalanine aminotransferase(ALT)levels.Moreover,theinvitrofreeradical scavengingpropertiesofS.chilensiswereevaluated.
Materialsandmethods
Plantmaterials
AerialpartsofSolidagochilensisMeyen,Asteraceae,were col-lectedinChapecó,SC,Brazil(S27◦06′38.83′′/W52◦34′26.52′′).The
voucherspecimenwasidentifiedbyOsmardosSantosRibasand isdepositedintheherbariumoftheBotanicalMuseumofCuritiba (MBMnumber356792).
Preparationofhydroalcoholicextract
Dried aerial parts of S. chilensis (50g) of the same particle size(300m;48Tyler/Mesch)weremaceratedin80%methanol (1000ml)forsixdays.Hydroalcoholicextract(HE)fromS.chilensis
wasconcentratedtodrynessunderreducedpressureat40◦Cand
thenfreeze-driedandstoredat−20◦C.
High-performanceliquidchromatographyanalysis
ChromatographyanalysiswasperformedusingaVarian®
Pro-Star HPLC system consisting of an automatic injector, ternary gradientdetectors,pumps,andaUV/VisKromasil®C18
reversed-phase ODS column (5m; 25mm×4.5mm).The mobile phase consistedoftwosolvents:H2O:aceticacid(40:1,v/v;solventA) andCH3CN(solventB)thatwerefilteredthrough0.45mMillipore polytetrafluoroethylenemembranes.Separationswereperformed with a linear gradient: 86% solvent A and 14% solvent B for 15min,35%solventBfor20minand100%solventBfor2min.UV absorbanceat360nmwasmeasured,andtheresultswere com-paredwiththeretentiontimesofanauthenticexternalstandard followedbyaUVspectrumanalysis.Theflowrateofthemobile phasewas1ml/min−1,andtheinjectionvolumewas20l.The chromatographicrunswereperformedat22◦C.UVabsorbanceat
360nm wasmeasured(Apátiet al.,2006).Quercetrin (12.5,25, 50,100and200g/ml;Sigma–Aldrich®)wasanalyzedin
tripli-cate,andacalibrationcurvewasgenerated.HEwasdissolvedin MeOH(1mg/ml)andfilteredthroughamicroporefilter(0.45m) beforethechromatographicprofilewasgenerated.Theresultsare expressedastheconcentrationofquercetrin(%)inthedriedplant material.
Invitro2,2-diphenyl-1-picrylhydrazylfreeradicalscavenging assay
ThefreeradicalscavengingactivityofHEwasmeasuredusing themethoddescribedbyBrand-Williamsetal.(1995)withsome modifications.HE(1ml;5–200g/ml)wasaddedto2mlofa solu-tionof2,2-diphenyl-1-picrylhydrazyl(DPPH)radicals inethanol
(0.004%).Themixturewasvigorouslyshakenandallowedtostand for30minatroomtemperature(RT).Theabsorbance(Abssample) oftheresultingsolutionwasmeasuredat517nm,andthe antiox-idantactivity(AA)percentagewascalculatedusingthefollowing formula:
AA%=100−(Abssample−Absblank)×100
Abscontrol
Asolutionofethanol(2ml)andHE(1ml)wasusedastheblank (Absblank).AsolutionofDPPH(2ml)andethanol(1ml)wasusedas thecontrol(Abscontrol).Ascorbicandgallicacidswereusedas stan-dards.Freeradicalscavengingactivitywasexpressedintermsof theamountofantioxidantsnecessarytodecreasetheinitialDPPH absorbanceby50%(IC50).TheIC50valuewasdeterminedby inter-polationfromthenonlinearregressionoftheplotofpercentageof inhibitionagainsttheconcentrationofHE,whichisdefinedasthe amountofHEneededtoscavenge50%ofDPPHradicals.
Animals
TheexperimentalprotocolwasapprovedbytheEthics Com-mitteeonAnimalUseoftheCommunityUniversityintheRegion ofChapecó,Brazil(CEUANo.020/2013).Male Rattusnorvegicus, Wistar(n=30)weighing250–275gwereusedinthestudy.The animalswerehousedinwire-bottomed17cm×33.5cm×40.5cm cagesinacontrolledenvironmentat22±2◦Cwitha12hlight–dark
cycleandminimalnoise.Theratshadadlibitumaccesstowaterand commerciallypreparedrodentchowpellets(Nuvilab®
CR-1).
Oralglucosetolerancecurve
Animalswerefastedovernightanddividedintogroups con-taining six rats each. The control group (C), received saline (0.5ml/100g);theHEgroupreceivedHE(125,250or500mg/kg)
(Patiletal.,2011);andtheglibenclamidegroupreceived
gliben-clamide(10mg/kg)(Zhaoetal.,2011).Alldrugsweredilutedwith saline(0.9%)inestablisheddosesandadministeredorallyby gav-ageinavolumeof0.5ml/100gbodyweight(Trovatoetal.,1996;
Diehletal.,2001).Glucoselevelsweremeasuredbeforetherats
receivedthetreatment(zerotime)and30minafterglucosewas administrated(4g/kg)(Alametal.,2011;Pereiraetal.,2012).Blood sampleswerecollectedfromthetailveinjustpriortoand30,60 and180minafterglucoseloading,andtheglucoselevel(mg/dl) wasassayedbyaglucometer(Accu-Chek® Performa).Attheend
oftheexperimentalperiod,theanimalswereanesthetizedwith amixtureoflidocaineandsodiumthiopental(10and150mg/kg, respectively).Bloodaliquotswerecollectedforbiochemical analy-sesviacardiacpuncture,andtheanimalsweretheneuthanizedby exsanguination(Concea,2012).Theliverandsoleuswerecollected forlateranalysis,aswasasegmentofthesmallintestine.
Glycogenmeasurements
Theharvestedliverandsoleuswereassessedforglycogen con-tent3haftertreatment.Glycogenwasisolatedfromthesetissues asdescribedbyKrisman(1962).Thetissuewasweighed, homoge-nizedin33%KOH,andboiledat100◦Cfor30min,withoccasional
Disaccharidaseextractionandassays
Theextractedsmallintestinesegmentwaswashedin0.9%NaCl solution,dried onfilterpaper,weighed,trimmed, and homoge-nized(300×g)with0.9%NaCl(400mgofduodenumper1.0mlof 0.9%NaCl)for1minat4◦C.Theresultingextractwascentrifuged
at(1300×g)for8min.Thesupernatantwasassessedtomeasure
invivo maltase,sucrase,and lactase activityas wellas protein determination.The activityofmaltase(EC 3.2.1.20),lactase (EC 3.2.1.23),andsucrase(EC3.2.1.48)wasdeterminedusingaglucose diagnosiskitbasedonthereagentglucoseoxidase.Todetermined isaccharidaseactivity,duodenumhomogenates(10l)were incu-batedat37◦Cfor60minwith10lofthesubstrate(equivalentto
0.056Mofmaltase,sucrase,orlactase)(Dahlqvist,1984;Pereira etal.,2011).Oneenzymeunit(U)wasdefinedastheamountof enzymethatcatalyzedthereleaseof1molofglucoseperminute undertheassayconditions.Thespecificactivitywasdefinedas enzymeactivity(U)permilligramofprotein.Proteinconcentration wasdeterminedbythemethoddescribedbyLowryetal.(1951), usingbovineserumalbuminasthestandard.Theassayswere per-formedinduplicatealongwithappropriatecontrols.
Biochemicalanalysisofserumsamples
Uponcollection,serumsampleswereimmediatelycentrifuged (3000×g)for15min.SerumTCandALTlevelsweredetermined by enzymatic colorimetric methods (UV/vis) using commercial Labtest®kitsaccordingtothemanufacturer’sinstructions.A
semi-automatedanalyzer(BioSystems®,modelBTS310)wasusedforall
analysis(Lietal.,2012).
Statistics
Allresultsshownarepresentedasmeanvalues±SEM.Thedata wereevaluatedbyone-wayANOVAfollowedbyTukey’stestand correlationanalysesusingSPSS20.0.Ap-valueof<0.05was con-sideredstatisticallysignificant.
Results
ChemicalconstituentsofS.chilensis
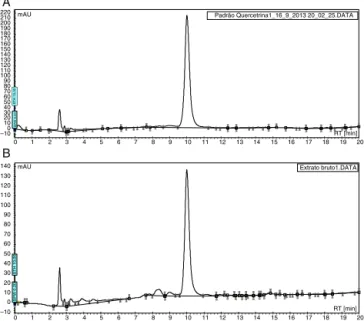
TheamountofquercetrininHEwasquantifiedbyHPLCusing ananalyticalcurve(r=0.999;y=0.735x=2.6971)witharetention timeof10.02min.TheHPLCanalysisrevealedthequercetrin con-centrationtobe2.4%intheaerialpartsofS.chilensis(Fig.1).
DeterminationofDPPHradicalscavengingactivity
TheDPPHassayshowedthatHEexhibitsantioxidantproperties
invitro(Fig.2).ThehighestscavengingeffectwasobservedforHE, withanIC50of59.12±3.14g/ml,althoughitshowedlower scav-engingabilitiesthanascorbicandgallicacids,whichwereusedas standards(16.32±2.94and2.14±1.58g/ml,respectively).
EffectofHEontheoralglucosetolerancecurve
Table1showsthatHE500hadasignificantantihyperglycemic
effectwhencomparedtotheCgroup(F(4,21)=12.0;p<0.05).Lower serumglucose (approx. 22%lower) wasdetected180minafter treatment;glibenclamideshowedsimilarresults.
EffectofHEonhepaticandsoleusglycogencontent
Fig.3showsthatHEandglibenclamidedidnotaffecthepaticand soleusglycogencontentcomparedwithothertreatmentgroups.
20 19 18 17 16 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0 140 130 120 110 100 90 80 70 60 50 40 30 20 10 0
–10 RT [min]
Extrato bruto1.DATA mAU 20 19 18 17 16 15 14 13 12 11 10 9 8 7 6 5 4 3 2 1 0 220 210 200 190 180 170 160 150 140 130 120 110 100 90 80 70 60 50 40 30 20 10 0
–10 RT [min]
Padrão Quercetrina1_16_9_2013 20_02_25.DATA mAU
A
B
Fig.1. Analysisbyhighperformanceliquidchromatography(HPLC):A.quercetrin (200g/ml);B.hydroalcoholicextractfromaerialpartsofSolidagochilensis(1mg/ml inMeOH)(RT:10.02min).HPLCVarian®,Kromasil®ODScolumn(5M)reversed
phaseC-18(25mm×4.5mm)at24±2◦C.Twosolventsystemsusedforanalysis;
H2O:aceticacid(40:1,v/v)(solventA)andCH3CN(solventB).Theflowwas1ml/min, andthegradientusedhad86%ofAfor15min,65%ofAfor20min,and100%ofB for2min.ThedetectionbyUVwasrealizedat360nm.
0 50 100 150 200 250
0 50 100 150 HE Ascorbic acid
Gallic acid
Concentration (g/ml)
% i nh ib it io n o f D P P H
Fig.2.2,2-Diphenyl-1-picrylhydrazyl(DPPH)radicalscavengeractivityof hydroal-coholicextractfromSolidagochilensis(HE)comparedwithstandardsascorbicand gallicacids.Resultsareexpressedasmeans±SEM(n=3).
However, HE 500 significantly increased hepatic (F(4,25)=6.6;
p<0.05)andsoleus(F(4,23)=3.9;p<0.05)glycogencontent com-paredwithgroupC.
EffectofHEondisaccharidaseactivity
DisaccharidaseactivitywassignificantlyaffectedbyHEonlyat adoseof250mg/kg,asitinhibitedmaltaseactivity(F(4,24)=3.4;
p<0.05)comparedwithgroupC(Fig.4).
Table1
EffectsofhydroalcoholicextractfromSolidagochilensisonaglucosetolerancecurve (mean±SEM)(n=6).
Groups Glucoselevel(mg/dl)
Initial(timezero) 30min 60min 180min
C 93.6±2.0 128.5±4.6 136.0±1.1 126.0±6.3 HE125 95.5±2.6 144.6±4.9 150.1±12.7 114.2±5.8 HE250 100.8±3.2 151.6±20.2 150.1±6.1 114.2±2.6 HE500 91.0±4.0 148.2±10.1 142.2±1.6 98.2±2.3*
GLIB 93.3±1.1 149.8±5.5 149.3±3.5 78.5±8.9*
C,control;HE,hydroalcoholicextractfromS.chilensis(125,250or500mg/kg);GLIB, glibenclamide(10mg/kg).
C HE 125 HE 250 HE 500 GLIB 0.00
0.05 0.10 0.15 0.20
*
m
g
of
gl
yc
og
e
n
/g
o
f s
o
le
u
s
m
u
s
c
le
C HE 125 HE 250 HE 500 GLIB
0.0 0.5 1.0 1.5
*
mg
o
f g
ly
c
o
g
e
n
/g
o
f h
e
p
a
tic
ti
ss
ue
Fig.3.EffectofhydroalcoholicextractfromSolidagochilensis(HE;125,250and 500mg/kg)andglibenclamide(GLIB;10mg/kg)onhepaticandsoleusglycogen con-tentinhyperglycemicrats.Valuesareexpressedasmean±SEM(n=6).*p<0.05 one-wayANOVAcomparedtothecontrolgroup(C).
EffectsofHEonTCandALT
Followingtreatment, groupC ratshad higherserumTCthan rats in the other groups. All hyperglycemic rats treated with HE (125, 250or 500) exhibited an accentuated decrease in TC (−36.8%,−36.7%and−41.3%,respectively),comparedwithgroupC
C HE 125 HE 250 HE 500 GLIB 0
50 100 150
*
Ma
lt
a
s
e
a
ct
iv
it
y
un
it
s
/g
of
pr
ot
e
in
Fig.4.EffectofhydroalcoholicextractfromSolidagochilensis(HE;125,250and 500mg/kg)andglibenclamide(GLIB;10mg/kg)onthespecificactivityofmaltase, inasegmentofthesmallintestine.Valuesareexpressedasmean±SEM(n=6). *p<0.05one-wayANOVAcomparedtocontrolgroupsaline(C).
C HE 125 HE 250 HE 500 Glib
0 20 40 60 80
*
* *
TC
(
m
g/
dl
)
Fig.5.Theeffectsoftreatmentsontotalcholesterol(TC)values(mean±SEM;n=6). Hyperglycemicratsweregivensaline(controlgroup;C)orthefollowingtreatments: hydroalcoholicextractfromSolidagochilensis(HE;125,250or500mg/kg); gliben-clamide(GLIB;10mg/kg).*p<0.05one-wayANOVAcomparedtocontrolgroup saline(C).
(F(4,23)=5.7;p<0.05;Fig.5).TherewasnodifferenceinserumALT activitybetweenthegroups(datanotshown).
Discussion
Diabetesmellitusisachronicmetabolicdisordercharacterized by hyperglycemia. It is associated with alterations in carbohy-drate,protein,andlipidmetabolism(Pereiraetal.,2011).Plants exertantihyperglycemicandhypoglycemicactivityprimarilyvia their ability torestore pancreatictissue function by increasing insulinoutput,inhibitintestinalabsorptionofglucose,orfacilitate metabolitesininsulin-dependentprocesses(Pateletal.,2012).
Thepresentstudyshowedthataglucosedoseof4g/kgcan con-siderablyincreaseratsserumglucoselevels,whichweremitigated bya singleoral doseofHE at 500mg/kg for180minfollowing glucoseadministration.Recently,itwasdemonstratedthatrutin reducesserumglucoselevelsandpotentiatesinvivoinsulin
secre-tion(Kappeletal.,2013);rutinmechanismofactioncanalsobe
explainedbymammalssynthesizingglycogentomaintain appro-priate glucose levels. Glycogen is how mammalsstore glucose for futureuse, mainlyin skeletal musclesand theliver(Jensen etal.,2011).Insulinandglucagonregulateglycogenmetabolism byactivatingandinhibitingseveralenzymesandproteins(Ferrer etal.,2003);thehealthyorganismremovesserumglucoserapidly whenglucoseisinexcess,butinsulin-stimulatedglucosedisposalis reducedinorganismswithinsulinresistanceandtype2DM(Jensen etal.,2011).Inthepresentstudy,ratsadministeredHE500had significantly increasedglycogen contentin theliver and soleus comparedwithratsingroupC,whichhelpedleadtotheHE500rats lowerserumglucoselevels.InagreementwithTorresetal.(1987), thephytochemicalanalysisbyHPLCconfirmedthatthequercetrin flavonoidisthemajorbioactivesubstanceofS.chilensis.Flavonoids mayexert beneficial effects in DMby enhancinginsulin secre-tion;reducingapoptosisandpromotingproliferationofpancreatic
-cells;improvinghyperglycemiathroughregulatinghepatocyte glucose metabolism; reducing insulin resistance, inflammation, and oxidative stressin muscleand fat;and increasingglucose uptakein skeletalmuscleand whiteadiposetissue(Babuetal., 2013).ThisfindingisinagreementwithPrasathandSubramanian
(2011),whoreportedtheantidiabeticeffectoftheflavonoidfisetin
glucosehomeostasisbymodulatingenzymesthatregulate carbo-hydratemetabolism.Itwasalsodemonstratedthatkaempferitrin, themajorflavonoidfoundinBauhiniaforficataLink.,leaves,isable todiminishserumglucoselevelsandincreaseglucoseuptakein theratsoleusasefficientlyasinsulin(Jorgeetal.,2004).Thiseffect couldberelatedtotheincreasedmuscleglycogencontentseenin thepresentstudywiththeHE500treatment.
Inthepresentstudy,we demonstratedthatHE 250reduced maltase activity. Several plants exert antihyperglycemic activ-ity viainhibiting enzymes that hydrolyze carbohydratesin the smallintestine,andtheeffectappearstoinvolveinteractionswith polyphenoliccompounds(MaiandChuyen,2007).AlthoughHE500 moresignificantlyloweredratsserumglucoselevels,itdidnot sig-nificantlyinhibitmaltaseactivity.Thisfindingisinagreementwith
Pereiraetal.(2011,2012),whofoundthathigherdosesofextracts
andsubstances,didnotaffectdisacharidaseactivity.These find-ingsreinforcetheideathatHEaffectsserumglucosebyincreasing glucosestorage(asglycogen)intheliverandmuscle.
RatstreatedwithHE(125,250or500mg/kg)showeddecreased serum TC. A previous study using other plants suggested that theseeffectscanbeattributedtotherestorationoftriacylglyceride catabolism by stimulating lipolytic pathways involving plasma lipoproteinlipase(Xieetal.,2007).Inthepresentstudy,HEcould have stimulated similar effects. Intracellular glucose and lipid metabolicdisordersarethebasisofavarietyofmetabolicdiseases. Glucoseandlipid metabolicdisordersareclosely relatedtothe occurrenceandprogressionofDM,obesity,hepaticsteatosis,and cardiovasculardisease(Mengetal.,2013).
WecannotdiscountthepossibilitythatHEalsointerfereswith cholesterol’smetabolic cycle at otherpoints, suchas intestinal uptake,endogenousmetabolism,andtransportbylipoproteins(Bei
etal.,2012;Roman-Junioretal.,2015),whichwerenotassessedin
thisstudy.
FreeradicalscavengingpropertiesofS.chilensiswereobserved in the DPPH assay. Thus, we propose that theplants hydroal-coholicextractmayhavecontributedtothehyperglycemicrats improvedlipidmetabolismandoxidativestress.Thisis character-isticofpolyphenols(Liuetal.,2014);however,furtherstudiesare requiredtoconfirmtheinvivoantioxidanteffects ofS.chilensis
anditsbenefitsin hypoglycemicandhypercholesterolemic ani-malmodels.LevelsofALTdidnotdifferbetweentreatmentgroups, indicatingtheabsenceofHEtoxicityatthedosestested.
Insummary,ourresultsshowedthatHEexertedmarked hypo-glycemiceffectsviaincreasingtheproductionandreleaseofinsulin as well as via increasing insulinotropic activity. The hypolipi-demic effect of HE in rats possibly involved reduced levels of lipoproteinsaswellasantioxidantactivity.Furthermore,therewas strongevidencethatquercetrin,themajorconstituentofS.chilensis
extracts,islargelyresponsiblefortheobservedbiologicalactivities. However,theunderlyingmechanismsoftheseeffectsneedtobe elucidatedbyfurtherstudies.
Conclusions
HydroalcoholicextractofS.chilensismaybeeffectivein main-tainingglucosehomeostasisbyreducingserumglucoselevelsand TC.
Conflictsofinterest
Theauthorsdeclarenoconflictsofinterest.
Authorscontribution
MS,APSandMGcontributedinallstepsofthisstudy.EI,FB,GDS, AS,RCandRMcontributedtobiologicalstudies.EF,GPandSMW contributedtobiochemicalanalyses.LZandWARJhaveguidedthe
laboratoryworkand contributedtodesign ofthestudy.Allthe authorshavereadthefinalmanuscriptandapprovedthe submis-sion.
Acknowledgements
ThisworkwassupportedbytheUnochapecó[modalityArt.171 –FUMDES],CNPq-PIBIC(editalN◦228/Reitoria/2014),PIBIC-FAPE
(editalN◦121/Reitoria/2013)andFAPESC.
References
Alam,M.A.,Subham,N.,Chowdhury,S.A.,Awal,M.A.,Mostofa,M.,Rashid,M.A., Hasan,C.M.,Nahar,L.,Sarker,S.D.,2011.Anthocephaluscadamba(Roxb.)Miq., Rubiaceae,extractshowshypoglycemiceffectandeasesoxidativestressin alloxan-induceddiabeticrats.Rev.Bras.Farmacogn.21,155–164.
Alonso,J.R.,1998.TratadodeFitomedicina:BasesClínicaseFarmacológicas.Buenos Aires,ISISEdiciones.
Apáti,P.,Houghton,P.J.,Kite,G.,Steventon,G.B.,Kery,A.J.,2006.In-vitroeffect offlavonoidsfromSolidagocanadensisextractonglutathioneS-transferase.J. Pharm.Pharmacol.58,251–256.
Babu,P.V.A.,Liu,D.,Gilbert,E.R.,2013.Recentadvancesinunderstandingthe anti-diabeticactionsofdietaryflavonoids.J.Nutr.Biochem.24,1–28.
Bei,W.J.,Guo,J.,Wu,H.Y.,Cao,Y.,2012.Lipid-regulatingeffectoftraditionalChinese medicine:mechanismsofactions.Evid.BasedComplement.Alternat.Med.1, 1–19.
Brand-Williams,W.,Cuvelier,M.E.,Berset,C.,1995.Useofafreeradicalmethodto evaluateantioxidantactivity.Lebenson.Wiss.Technol.28,25–30.
Bucciarelli,A.,Minetti,A.,Milczakovskig,C.,Skliar,M.,2010.Evaluationof gastro-protectiveactivityandacutetoxicityofSolidagochilensisMeyen(Asteraceae). Pharmaceut.Biol.48,1025–1030.
Concea,2012.GuiaBrasileirodeBoasPráticasemEutanásiaemAnimais:Conceitos eProcedimentosRecomendados.Brasília,62p.
Dahlqvist,A.,1984.Assayofintestinaldisaccharidases.Scand.J.Clin.Lab.Invest.44, 169–172.
Dai,F.J.,Hsu,W.H.,Huang,J.J.,Wu,S.C.,2013.Effectofpigeonpea(CajamuscajanL.) onhigh-fatdiet-inducedhypercholesterolemiainhamsters.FoodChem.Toxicol. 53,384–393.
Diehl,K.H.,Hull,R.,Morton,D.,Pfister,R.,Rabemampianina,Y.,Smith,D.,Vidal, J.M.,Vorstenbosch,C.V.,2001.Agoodpracticeguidetotheadministrationof substancesandremovalofblood,includingroutesandvolumes.J.Appl.Toxicol. 21,15–23.
Facury-Neto,M.A.,Fagundes,D.J.,Beletti,M.E.,Novo,N.F.,Penha,S.Y.J.N.,2004. Sys-temicuseofSolidagomicroglossaDCinthecicatrizationofopencutaneous woundsinrats.Braz.J.Morphol.Sci.21,207–210.
Ferrer,J.C.,Favre,C.,Gomis,R.R.,Fernández-Novell,J.M.,Garcia-Rocha,M., Dela-Iglesia,N.,Cid,E.,Guinovart,J.J.,2003.Controlofglycogendeposition.FEBSLett. 546,127–132.
Grover,J.K.,Yadav,S.,Vats,V.,2002.MedicinalplantsofIndiawithantidiabetic potential.J.Ethnopharmacol.81,81–100.
Hirany,S.,O’Byrne,D.,Devaraj,S.,Jialal,I.,2000.Remnant-likeparticle-cholesterol concentrationsinpatientswithtype2diabetesmellitusandend-stagerenal disease.Clin.Chem.46,667–672.
Jensen,J.,Rustad,P.I.,Kolnes,A.J.,Lai,Y.C.,2011.Theroleofskeletalmuscleglycogen breakdownforregulationofinsulinsensitivitybyexercise.Front.Physiol.2, 1–11.
Jorge,A.P.,Horst,H.,Sousa,E.,Pizzolatti,M.G.,Silva,F.R.,2004.Insulinomimetic effectsofkaempferitrinonglycaemiaandon14C-glucoseuptakeinratsoleus
muscle.Chem.Biol.Interact.149,89–96.
Kappel,V.D.,Frederico,M.J.S.,Postal,B.G.,Mendes,C.P.,Cazarolli,L.H.,Silva,F.R.M.B., 2013.Theroleofcalciuminintracellularpathwaysofrutininratpancreatic islets:potentialinsulinsecretagogueeffect.Eur.J.Pharmacol.702,264–268. Krisman,C.R.,1962.Amethodforthecolorimetricestimationofglycogenwith
iodine.Anal.Biochem.4,14–23.
Li,W.,Zhang,M.,Gu,J.,Meng,Z.,Zhao,L.C.,Zheng,Y.,Chen,L.,Yang,G.L.,2012. Hypoglycemiceffectofprotopanaxadiol-typeginsenosidesandcompoundKon type2diabetesmiceinducedbyhigh-fatdietcombiningwithstreptozotocin viasuppressionofhepaticgluconeogenesis.Fitoterapia83,192–198. Liu,W.,Zheng,Y.,Zhang,Z.,Yao,W.,Gao,X.,2014.Hypoglycemic,hypolipidemicand
antioxidanteffectsofSarcandraglabrapolysaccharideintype2diabeticmice. FoodFunct.22,2850–2860.
Lorenzi,H.,Matos,F.J.A.,2002.PlantasmedicinaisdoBrasil:Nativaseexóticas. InstitutoPlantarumdeEstudosdaFlora,SãoPaulo.
Lowry,O.H.,Rosebrough,N.J.,Farr,A.L.,Randall,R.J.,1951.Proteinmeasurement withthefolinphenolreagent.J.Biol.Chem.193,265–275.
Mai,T.T.,Chuyen,N.V.,2007.Anti-hyperglycemicactivityofaqueousextractfrom flowerbudsofCleistocalyxoperculatus(Roxb.)MerrandPerry.Biosci.Biotechnol. Biochem.71,69–76.
Meng,S.,Cao,J.,Feng,Q.,Peng,J.,Hu,Y.,2013.Rolesofchlorogenicacidonregulating glucoseandlipidsmetabolism:areview.Evid.BasedComplement.Alternat. Med.1,1–11.
Modak,M.,Dixit,P.,Londhe,J.,Ghaskadbi,S.,Paul,A.,2007.Indianherbsandherbal drugsforthetreatmentofdiabetes.J.Clin.Biochem.Nutr.40,163–173. Negri,G.,2005.Diabetesmelito:plantaseprincípiosativosnaturais
hipoglicemi-antes.Rev.Bras.Cienc.Farm41,121–142.
Park,J.B.,Velasquez,M.T.,2012.Potentialeffectsoflignan-enrichedflaxseedpowder onbodyweight,visceralfat,lipidprofileandbloodpressureinrats.Fitoterapia 83,941–946.
Patel,D.K.,Kumar,R.,Laloo,D.,Hemalatha,S.,2012.Diabetesmellitus:anoverview onitspharmacologicalaspectsandreportedmedicinalplantshaving antidia-beticactivity.AsianPac.J.Trop.Biomed.2,411–420.
Patil,R.N.,Patil,R.Y.,Ahirwar,A.,Ahirwar,D.,2011.Evaluationofantidiabeticand relatedactionsofsomeIndianmedicinalplantsindiabeticrats.AsianPac.J. Trop.Dis.4,20–23.
Pereira,D.F.,Cazarolli,L.H.,Lavado,C.,Mengatto,V.,Figueiredo,M.S.R.B.,Guedes,A., Pizzolatti,M.G.,Silva,F.R.M.B.,2011.Effectsofflavonoidsonalpha-glucosidase activity:potentialtargetsforglucosehomeostasis.Nutrition27,1161–1167. Pereira,D.F.,Kappel,V.D.,Cazarolli,L.H.,Boligon,A.A.,Athayde,M.L.,Guesser,S.M.,
DaSilva,E.L.,Silva,F.R.M.B.,2012.InfluenceofthetraditionalBraziliandrinkIlex paraguariensisteaonglucosehomeostasis.Phytomecine19,868–877. Piaulino,C.A.,Carvalho,F.C.,Almeida,B.C.,Chaves,M.H.,Almeida,F.R.,Brito,S.M.,
2013.ThestembarkextractsofCenostigmamacrophyllumattenuatestactile allodyniainstreptozotocin-induceddiabeticrats.Pharm.Biol.,1243–1248. Prabhakar,P.K.,Prasadb,R.,Ali,S.,Doble,M.,2013.Synergisticinteractionofferulic
acidwithcommercialhypoglycemicdrugsinstreptozotocininduceddiabetic rats.Phytomedicine20,488–494.
Prasath,G.S.,Subramanian,S.P.,2011.Modulatoryeffectsoffisetin,abioflavonoid, onhyperglycemiabyattenuatingthekeyenzymesofcarbohydratemetabolism inhepaticandrenal tissuesinstreptozotocin-induceddiabeticrats.Eur.J. Pharmacol.668,492–496.
Raida,K.,Nizar,A.,Barakat,S.,2008.TheeffectdeCrataegusaronicaaqueousextract inrabbitsfedwithhighcholesteroldiet.Eur.J.Sci.Res.22,352–360.
Roman-Junior, W.A.,Piato, A.L.,Conterato,G.M.M.,Wildner,S.M.,Marcon,M., Mocelin,R.,Emanuelli,M.P.,Emanuelli,T.,Nepel,A.,Barison,A.,Santos,C.A.M., 2015.HypolipidemiceffectsofSolidagochilensishydroalcoholicextractand itsmajorisolatedconstituentquercetrinincholesterol-fedrats.Pharm.Biol., http://dx.doi.org/10.3109/13880209.2014.989622.
Silva,A.G.,De-Sousa,C.P.G.,Koehler,J.,Fontana,J.,Christo,A.G.,Guedes-Bruni,R.R., 2010.EvaluationofanextractofBrazilianarnica(SolidagochilensisMeyen, Aster-aceae)intreatinglumbago.Phytother.Res.24,283–287.
Soares-Valverde,S.S.,Azevedo,S.R.C.,Tomassini,T.C.B.,2009.Utilizac¸ãodeCLAE, comoparadigmanaobtenc¸ãoecontroledoditerpenosolidagenonaapartirde inflorescênciasdeSolidagochilensisMeyen(arnicabrasileira).Rev.Bras.Farm. 9,196–199.
Tamura,E.K.,Jimenes,J.K.,Waismam,K.,Gobbo,N.L., Peporine,L.N., Malpezzi, M.E.A.L.,Marinho,E.A.V.,Farsky,F.H.P.,2009.InhibitoryeffectsofSolidago chilen-sisMeyenhydroalcoholicextractonacuteinflammation.J.Ethnopharmacol. 122,478–485.
Teixeira,C.C.,Rava,C.A.,Da-Silva,P.M.,Melchior,R.,Argenta,R.,Anselmi,F.,Almeida, C.R.,Fuchs,F.D.,2000.Absenceofantihyperglycemiceffectofjambolanin exper-imentalandclinicalmodels.J.Ethnopharmacol.71,343–347.
Torres,L.M.B.,Akisue,M.K.,Roque,N.F.,1987.QuercetrinaemSolidagomicroglossa DC,aarnicadoBrasil.Rev.Farm.Bioquím.Univ.S.Paulo23,33–40.
Trovato,A.,Monforte,M.T.,Barbera,R.,Rossito,A.,Galati,E.M.,Forestieri,A.M.,1996. EffectsoffruitjuicesofCitrussinensisL.andCitruslimonL.onexperimental hypercholesterolemiaintherat.Phytomedicine2,221–227.
Warjeet,S.L.,2011.TraditionalmedicinalplantsofManipurasantidiabetics.J.Med. PlantsRes.5,677–687.
Xie,W.,Wang,W.,Su,H.,Xing,D.,Cai,G.,Du,L.,2007.Hypolipidemicmechanisms ofAnanascomosusL.leavesinmice:differentfromfibratesbutsimilarofstatins. J.Pharmacol.Sci.103,267–274.
Zhao,J.,Zhang,W.,Zhu,X.,Zhao,D.,Wang,K.,Wang,R.,Qu,W.,2011.Theaqueous extractofAsparagusofficinalisL.by-productexertshypoglycaemicactivityin streptozotocin-induceddiabeticrats.J.Sci.FoodAgric.91,2095–2099. Zimmet,P.,2000.Globalization,coca-colonizationandthechronicdiseaseepidemic: