DEPARTAMENTO DE BIOLOGIA VEGETAL
Nitrification Control. How do the Natural
ecosystem do it?
Catarina Andreia Ricacho Gouveia
Tese de Mestrado
Dissertação
Mestrado em Microbiologia Aplicada
2012/2013
DEPARTAMENTO DE BIOLOGIA VEGETAL
Nitrification Control. How do the Natural
ecosystem do it?
Dissertação orientada pela Profª Dr.ª Maria Manuela Carolino
(FCUL)
Catarina Andreia Ricacho Gouveia
Mestrado em Microbiologia Aplicada
2012/2013
Nitrification Control. How do the Natural
ecosystem do it?
Catarina Andreia Ricacho Gouveia
2012/2013
This thesis was fully performed at the Department of Plant Biology in the
University of Lisbon under the direct supervision of Prof. Dr. Maria Manuela
Carolino in the scope of the Master in Applied Microbiology of the Faculty of
Science University of Lisbon.
Master in Applied Microbiology 2012-2013 Master thesis Catarina Gouveia Personal Acknowledgments………...…….……… I Summary/Resumo (Portuguese)……….. II Abstract………. 1 Introduction………..…….2
Materials and methods…………..……….………8
Study site ………8
Experimental design ……….8
Soil and Root sampling ………9
AOA and AOB enrichments cultures ………...10
Ammonia and pH susceptibility ………...10
Effect of organic compounds in AOB nitrite production……….10
AOM Indirect Growth Measurement ………10
DNA extraction ………11
PCR conditions ………...12
PCR-RFLP ………...12
Statistical analysis ………..13
Results and Dicussion ………13
Where does the ammonia oxidizing community prosper? ………...13
Nitrite production by bacteria ………13
Nitrite production by archaea ………18
How would the AOB population respond to an increase in N input…….. ……….21
Ammonia oxidizing activity by AOB from soils with and without N addition …..21
AOB community structure ……….27
Ammonia oxidizing activity by AOA from soils with and without N addition …. 29 How does the AOB population responds to abiotic factors? ………...32
Ammonia susceptibility in AOB cultures from soils with and without N- addition………..32
pH susceptibility in AOB cultures from soils with and without N- addition ………...33
Effects of organic compounds in AOB cultures from soils without N addition………..35
Conclusion ……….37
Acknowledgements ……….38
References ……….38
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
Personal Acknowledgments
I would like to thank the Department of Plant Biology (DBV) of the Faculty of Sciences, University of Lisbon for hosting and supporting the research project for the development of my Master thesis.
My greatest appreciation to Prof. Dr. Maria Manuela Carolino, who directly supervised the project, for the mentorship and help through my question filled work, for the friendship and for the interesting and fun talks that allow me to work easier in longer days. I also thank prof. Dr. Cristina Cruz and Dr. Sandra Chaves for their patience and support in supervising my Master thesis laboratory work, for teaching me how to write, not only a good scientific report, but also a story, and for the inquisitive question that have allowed me to improve my work and myself. My thanks, also, to Dr. Teresa Dias, whose ideas and work in Arrábida were the bases for my thesis and helped create my scientific questions.
I would also like to thank Prof. Dr. Rógerio Tenreiro and Dr. Luís Carvalho for the given help in a troublesome statistical analysis. To prof. Dr. Ana Reis for the stimulating discussions that raised some surprizing questions, ideas and conclusions in my work. To prof. Dr. Lélia Chambel for the help choosing the best DNA fingerprinting method when I could not make my mind. To prof. Dr. Margarida Barata and Dr. Patricia Correia for support and motivating talks. To prof. Dr. Francisco Dionisio for helping me in data analysis. My thanks to Manuela Lucas, Herculana, Teresa Granja, Célia Lima, Rute Miguel, for the indispensable help around the lab and for their teachings. To my lab collegues, Maria Calado, Raquel Costa, Marta Delgado, Inês Meleiro, Juliana Melo, Frederico Eutrópio and Florian Uhlm, whose good humour make the lab into a warm and fun environment to work.
I thank my family and friends, whose curiosity and misunderstanding to subject in the academic field and about my research made me think in a simpler and easier way to explain science. To my aunt whose strength made keep going forward. To my sister for enduring my anxiety and stress in a way only she could do. And to my parents, for their endless love and dedication that helped me follow my dreams, for the debates and discussion that pushed me to be grow as a person and for their understanding of who I am as a person and a scientist.
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
Summary/Resumo
O ciclo do N, composto por diversas etapas, é mediado principalmente por microrganismos. Os microrganismos diazotróficos fixam Azoto (N2), introduzindo-o na biosfera. Os compostos orgânicos azotados são depois mineralizados, em ambientes aeróbios, a amónia. Esta é rapidamente oxidada a nitrito (NO2-) e nitrato (NO3-) pelos microrganismos oxidantes de amónia (AOM) e oxidantes de nitrito (NOM), processo denominado de nitrificação. Em ambientes anaeróbios, pode ocorrer a desnitrificação com a redução do nitrato a óxido nítrico (NO), óxido nitroso (N2O) e azoto molecular (N2) (Bock and Wagner, 2006).
Um equilíbrio da forma e quantidade de N nos ecossistemas é vital, pois o ecossistema pode perder fertilidade com reduzida quantidade de N, mas pode sofrer eutrofização com elevadas concentrações de N. O ciclo do Azoto pode não ocorrer linearmente, sendo a resposta da comunidade microbiana responsável pelos processos de oxi-redução do azoto dependente das condições do ecossistema, tal como, a taxa de conversão de cada processo dependente do controlo da atividade microbiana, afeta a disponibilidade e a forma de azoto no ecossistema. Com excesso de N na forma orgânica, deverá haver um aumento na atividade de mineralização, seguido de nitrificação e desnitrificação, resultando na libertação de N para a atmosfera. Esta situação ocorre, se existirem condições ótimas para todos os processos, no entanto, o aumento da concentração de amónia, nitrito, nitrato, ou outros compostos resultantes dos processos oxi-redutivos do ciclo do azoto, no solo, pode ocorrer se as condições necessárias para um dos processos não se verificarem.
A nitrificação é o processo de oxidação da amónia a nitrito e nitrito a nitrato, catalisado por 2 grupos de organismos distintos (Norton and Stark, 2011; Fienck et al. 2005; Sinha and Annachhatre, 2007), sendo a oxidação da amónia considerado o passo limitante da nitrificação. A oxidação da amónia é catalisada por diversos grupos, sendo as bactérias e árqueas quimiolitotróficas oxidantes de amónia (AOB e AOA respetivamente) os principais intervenientes em ambientes terrestres aeróbios (Norton and Stark, 2011; Sinha and Annachhatre, 2007).
As bactérias oxidantes de amónia (AOB) quimiolitotróficas são membros das classes β ou γ de Proteobacteria. Nas β-proteobacteria existem 4 géneros diferentes, Nitrosomonas, Nitrosospira, Nitrosolobus e Nitrosovibrio. O género Nitrosococcus pertence às γ-Proteobacteria (Purkhold et al. 2000). As AOB estão distribuídas por diversos ambientes costeiros, marinhos e ambientes polares (maioritariamente 2 clusters, Nitrosomonas e Nitrosospira), ambientes salinos ou hipersalinos (Nitrosomonas), condições com temperaturas elevadas (Nitrosospira) e ambientes acídicos (Nitrosomonas) (Junier et al. 2010; Prosser and Nicol, 2008).
AOB são bactérias litoautotróficas, tendo como substrato principal a amónia (NH3), e como dador de eletrões a hidroxilamina. A oxidação da amónia é catalisada pela enzima amónia monoxigenase (AMO), produzindo hidroxilamina, sendo esta oxidada a nitrito pela enzima hidroxilamina oxiredutase (HAO) (Bock and Wagner, 2006; Hatzenpichler, 2012).
As árqueas oxidantes de amónia (AOA), pertencentes ao novo filo Thaumarchaeota (Norton and Stark, 2011; Hatzenpichler, 2012) aparentam estar presentes em diversos ambientes mesofilicos (marinhos, água-doce e terrestres) assim como em condições extremas (ambientes
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
acidófilos, termófilos, águas profundas e fontes termais) (Junier et al. 2010; Nicol and Schleper, 2006). A via metabólica das AOA é pouco conhecida, não tendo sido ainda identificado nenhum homologo da enzima HAO (Hatzenpichler, 2012; He et al. 2012). No entanto, parecem ter a capacidade de utilizar fontes orgânicas de energia como aminoácidos (He et al. 2012; Tourna et al. 2011; Hatzenpichler, 2012). A enzima AMO das AOA, comparativamente à AMO das AOB, tem uma maior afinidade para a amónia, podendo ter as AOA uma maior contribuição para a atividade nitrificante em ambientes oligotróficos (He et al. 2012; Martens-Habbena et al. 2009).
A importância da nitrificação baseia-se no facto de ser o único processo biológico oxidativo que liga fontes de azoto inorgânico reduzido (amónia) e oxidado (nitrito e nitrato) (Martens-Habbena et al. 2009; Norton and Stark, 2011; Fienck et al. 2005; Sinha and Annachhatre, 2007). Nitrito, o produto da oxidação da amónia, é normalmente encontrado em baixas concentrações nos solos devido à sua toxicidade, sendo essencial manter uma baixa concentração (Bock and Wagner, 2006; Norton and Stark, 2011; Cleemput and Samater, 1996). Para além do nitrito, o óxido nítrico e óxido nitroso também podem ser produzidos através da redução do nitrito pela nitrito redutase (NIR) presente em algumas bactérias oxidantes de amónia (Bremner, 1997; Burns et al. 1996). Além de promoverem a acidificação do solo com a formação de ácidos, o óxido nitroso age como um gás com efeito de estufa, 310x mais potente que o dióxido de carbono (Yamanaka, 2008; Bock and Wagner, 2006; Hatzenpichler, 2012). O nitrato pode ser utilizado como fonte de azoto por diversos organismos, e em excesso pode causar eutrofização desse ecossistema. Sendo facilmente lixiviado pode causar a contaminação de águas subterrâneas (Fienck et al, 2005).
Tendo a nitrificação uma forte influência no fluxo de azoto nos sistemas terrestres e marinhos, e os seus produtos importantes na determinação da qualidade de solos e águas, o estudo da influência dos fatores bióticos e abióticos no controlo da atividade nitrificante e crescimento dos organismos nitrificantes é essencial.
Existem diversos fatores estudados que influenciam o crescimento e atividade das comunidades oxidantes de amónia. Um dos principais fatores é a concentração de substrato, neste caso, amónia. Como foi referido, o aumento da concentração de N no ecossistema pode levar a um aumento da atividade nitrificante, dependendo da suscetibilidade das estirpes de AOB ao substrato e a sua adaptação ao aumento da amónia. Este aumento de atividade é problemática se houver acumulação de nitrito, nitrato ou outros produtos resultantes dessa atividade. A acumulação de nitrito ocorre em condições favoráveis à oxidação da amónia desfavoráveis à oxidação de nitrito e desnitrificação, como, ambientes bem oxigenados e condições alcalinas ou com pH neutro (Fienck et al. 2005), sendo as AOB muito suscetíveis a alterações no pH. Logo, a oxigenação dos solos e pH são outros fatores a ter em conta. Em ambientes pouco oxigenados (condições de microaerofilia) a formação de óxidos nitroso e nítrico é estimulada (Fienck et al. 2005; Bock and Wagner, 2006). Em solos carbonatados a produção de óxidos é reduzida pela interação química com os carbonatos, formando nitrito e nitrato.
(Bock
and Wagner, 2006).
A produção de nitritos pode ser inibida pela presença de compostosMaster in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
mixotroficamente (amónia e C orgânico como fonte de energia, podendo haver produção de nitritos) com compostos como piruvato ou acetato, ou organotroficamente (C orgânico como fonte de energia, sem produção de nitritos) com açúcares. Contudo a nitrificação pode ser importante e benéfica para algumas plantas que utilizem preferencialmente o nitrato (Bock and Wagner, 2006), logo em ambientes pobres em nitrato a nitrificação pode ser estimulada pelas plantas. Assim, fatores a considerar para o estudo de populações de microrganismos oxidantes de amónia são nicho (solo rizosférico ou superfície das raízes), a adaptação à adição de N no solo, a concentração de substrato, pH das culturas e presença de matéria orgânica.
Tendo em vista o estudo da comunidade oxidante de amónia (Bactérias e Árqueas) num ecossistema mediterrânico os principais objetivos deste trabalho foram:
1. Estudar a localização preferencial da comunidade oxidante de amónia (solo rizosférico ou superfície das raízes de Cistus ladanifer)
2. Estudar o efeito da adição de N no solo, na atividade e estrutura das comunidades oxidantes de amónia
3. Estudar o efeito da concentração de substrato (amónia), pH e presença de compostos orgânicos azotados/ glucose / acetato em culturas de AOB provenientes de solos com e sem adição de N
Num ecossistema mediterrâneo, pobre em nutrientes, os nichos preferenciais para o crescimento de microrganismos oxidantes de amónia deverão estar localizados perto da raiz das plantas, ou na superfície das raizes, proporcionando uma maior troca de nutrientes entre planta-microrganismos (Ochua-Hueso et al, 2011), além de facultar condições de oxigenação, pH e conteúdo em água favoráveis para o crescimento e atividade de oxidantes de amónia.
Sendo os solos pobres em azoto e matéria orgânica (Dias et al. 2012), espera-se encontrar populações de AOB mais adaptadas a ambientes oligotróficos. No entanto, em solos com frequente adição de N ao longo de 6 (desde 2007), o aumento da disponibilidade de N na forma de amónia, deverá determinar adaptação das populações de AOB, aumentando a atividade oxidante de amónia, e alterando a comunidade ao nível estrutural.
Em relação ao efeito da concentração de amónia nas culturas de AOB, populações de AOB adaptadas a baixas concentrações de amónia no solo, deverão ser mais suscetíveis a elevadas concentrações de amónia, comparativamente com populações de AOB adaptadas a maiores concentrações de amónia, ou seja provenientes de solos com frequente adição de azoto. Relativamente ao efeito do pH, espera-se que populações com maior atividade nitritante sejam mais tolerantes à acidificação, uma vez que o aumento da atividade leva a uma diminuição do pH, podendo resultar ou não na acidificação do solo, dependendo da composição deste. No efeito de compostos orgânicos nas culturas de AOB, espera-se que haja menor atividade com fontes energéticas orgânicas (para crescimento mixotrófico ou organotrófico), uma vez que AOB adaptadas a ambientes oligotróficos prefiram fontes energéticas e de carbono inorgânicas, como amónia e CO2.
Para o estudo de microrganismos oxidantes de amónia, amostras de solo foram recolhidas de um campo experimental, localizado no Parque Natural da Serra da Arrábida.
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
Plantas de Cistus ladanifer de solos sem adição de azoto foram recolhidas juntamente com solo rizosférico a 5-6 cm de profundidade, em julho para estudar o nicho preferencial das AOM. Solo rizosférico e raízes lavadas com água destilada foram utilizadas como inoculo para crescimento de AOB. Para o estudo do efeito da adição de N no solo, amostras de solo foram retiradas a 1 cm da raiz primária de Cistus ladanifer a 5-6 cm de profundidade dentro dos talhões dos 4 tratamentos: controlo (solo sem fertilização) e três diferentes adições de azoto: 40 e 80 kg de N /ha /y na forma de NH4NO3 (40AN e 80AN) e 40 kg de N/ ha /y na forma de NH4+ (40A). O solo foi utilizado como inoculo no crescimento de AOB e AOA. As populações de AOM foram seletivamente cultivadas por 30 (AOB) /60 (AOA) dias com 3 enriquecimentos sucessivos em meio SFC (Synthetic Freshwater Crenoarchaeota) (Konneke et al. 2005; De La Torre et al. 2008), de modo a assegurar a diluição de microrganismos heterotróficos e de matéria orgânica no inoculo de solo inicial. O meio foi suplementando de acordo com a especificidade para bactérias e/ou árqueas oxidantes de amónia (3 antibióticos diferentes). No 3º enriquecimento os meios e condições de incubação foram suplementados/alterados de acordo com o objectivo (fonte de azoto, carbono, concentração de amónia, pH). A atividade das comunidades oxidantes de amónia foi avaliado regularmente pela quantificação do produto final de nitrito, permitindo distinguir a cinética da produção de nitrito de cada cultura. A atividade de cada cultura foi estudada no 3º enriquecimento de modo a ter-se culturas de AOB sem contaminantes. A presença de árqueas e bactérias oxidantes de amónia foi confirmada pela amplificação do gene 16S rRNA e amoA, especifico para cada grupo. A estrutura das comunidades bacterianas oxidantes de amónia foi avaliado por PCR-RFLP fingerprinting, com as enzimas de restrição HinfI e HaeIII. A análise estatística dos dados foi efetuada em SPSS, versão 20 para Windows.
Comparando o número de culturas ativas, taxa de produção de nitrito e concentração máxima de nitrito, uma localização preferencial de atividade das AOB foi observado no solo rizosférico de Cistus ladanifer. No entanto, não foi detetada a presença de AOA nas culturas.
Relativamente ao efeito da adição de azoto no solo, verificou-se uma tendência para o aumento da atividade oxidante de amónia das populações de AOB. No entanto, a resposta da população ao aumento de N no solo depende da forma e quantidade de N aplicado. O controlo da atividade dependerá também da disponibilidade da amónia nos solos, havendo uma competição pelo substrato entre plantas e AOB (Verhagen et al. 1994), principalmente nos tratamentos com maior cobertura vegetal como o 40AN (Dias et al, 2011). Embora a presença de AOA não seja confirmada, as culturas de AOA provenientes de solos sem adição de azoto apresentaram uma maior atividade oxidante de amónia, indicando uma resposta das populações de AOA diferente das populações de AOB. Por PCR-RFLP verificou-se que a estrutura das populações de AOB é alterada com a adição de azoto no solo, tendo os resultados também demonstrado a heterogeneidade dos solos com o mesmo tratamento. Comparando os perfis de PCR-RFLP realizados in-silico para as espécies conhecidas do género Nitrosomonas e Nitrosospira e os perfis obtidos a partir das populações de AOB, Nitrosospira tenuis foi identificada nas populações de AOB de solos com adição de azoto.
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
Nas populações de AOB foram observadas diferentes suscetibilidades a concentrações crescentes de amónia, tendo as comunidades AOB provenientes de solo sem adição de N uma maior suscetibilidade à amónia, comparativamente a populações de AOB de solos com adição de N. A atividade oxidante de amónia, com produção cumulativa de nitrito, das culturas de populações de AOB presentes em solos sem adição de N foi inibida pela presença de compostos orgânicos como peptona, glucose e acetato. No entanto, as populações de AOB parecem ter a capacidade de utilizar ureia como fonte energética em substituição da amónia.
Considerando a influência dos fatores estudados: nicho preferencial, adição de N no ecossistema, concentração de substrato, pH e presença de compostos orgânicos na atividade das populações de AOB, estas demostraram ser suscetíveis a alterações, não são resilientes e não aparentam ter redundância funcional (não têm o mesmo nível de atividade). Mas a influência que os fatores exercem sobre a população nitrificante é necessária para controlar essa atividade, de modo a que não haja perda de N no sistema. A adição de N no solo não implica necessariamente um aumento da atividade nitritante, se este N não estiver em excesso. No entanto, N em excesso aumentará a atividade nitrificante, com consequente diminuição da quantidade de N inorgânico no solo. Este aumento de atividade levará a uma diminuição no pH, particularmente em solos sem um forte efeito tampão, o que leva a uma inibição da nitrificação. O aumento de nitrito leva também ao aumento de nitrato (oxidação do nitrito a nitrato), e ao consequente aumento da biomassa e compostos orgânicos no solo, inibindo a atividade de AOB sensíveis a matéria orgânica. Diminuindo a oxidação da amónia haverá novamente o aumento de N inorgânico no solo, de modo a não haver perda de N e consequente perda de fertilidade do solo. Esta regulação é mantida devido à sensibilidade apresentada pelas populações de AOB a estes fatores, sendo essencial para o equilíbrio de N no ecossistema.
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
Nitrification control. How do the Natural ecosystem do it?
Catarina A.R. Gouveia
Department of Plant Biology, University of Lisbon, Lisbon, Portugal
Master in Applied Microbiology, Faculty of Sciences, University of Lisbon, Lisbon, Portugal Nitrification is a fundamental and central step in the nitrogen cycle, linking the reduced and oxidized nitrogen pools, which processes are mostly accomplished by microorganisms. The first step is mainly done by ammonia oxidizing bacteria (AOB) and by ammonia oxidizing archaea (AOA). Regulation of nitrification activity is essential in ecosystem to maintain N balance, since N excess can cause eutrophication and production of toxic compounds, and N limitation can reduce soil fertility. Mediterranean ecosystem soils are poor in terms of N therefore microbial communities can be more susceptible to change when confronted with N input, pH change or presence of organic matter. This study aims to understand the niche preference, the impact of N addition in soils, pH, substrate (ammonia) concentration and presence of organic compounds in ammonia oxidizing populations from Mediterranean soils. Rhizospheric soil and root samples from Cistus Ladanifer were collected from an experimental field in which has been added 3 different N - treatments since 2007, in Serra da Arrábida Natural Park. By using serial culture enrichments and nitrite quantification through incubation time it was possible to quantify and study nitrifying activity. The presence of AOB and AOA in each enrichment was confirmed by PCR. AOB structural community’s change by N-addition in soils was assessed by PCR- RLFP. AOB susceptibility to ammonia, pH and organic compounds was studied by testing several ammonia concentrations in the medium, altering pH and supplementing the medium with organic compounds. As a preferential niche for AOB activity, AOB populations appear to be more abundant in rhizospheric soil of Cistus ladanifer. Soils with N-addition had altered AOB population with higher ammonia oxidizing activity and a different community structure. By comparing in silico PCR-RFLP profiles and the AOB profiles it was possible to identify Nitrosospira tenuis in the AOB population. Though the presence of AOA was not confirmed in any of the treatments, activity of possible AOA from soils without N –addition was higher, than of AOA from soils with N-addition. AOB activity appeared to be influenced by the substrate concentration, with AOB from soils with N-addition being less susceptible to change in substrate concentration. AOB cultures appear to be susceptible to pH, having an activity inhibition with pH above 7 and under 6. The populations are also sensible to the presence of organic matter, however, being capable of using urea as a substrate for ammonia in litotrophic growth and nitrite production activity. It was observed that ammonia oxidation activity can be stimulated by N-addition, altering not just the activity but also the population structure to have a higher tolerance to the substrate. While ammonia oxidising activity is inhibited by pH changes and presence of organic matter.
Master in Applied Microbiology 2012-2013 Master thesis Catarina Gouveia
Introduction
Nitrogen CycleNitrogen is one of the important elements for life, as it is needed to synthesize major molecules in cell components, as aminoacids and nucleotides being two of many examples. The biogeochemical cycle of nitrogen comprises the biotic and abiotic conversion of the nitrogen compounds between their oxidised and reduced forms. These conversions are done in the atmosphere, in the biosphere and the interaction between both (Bock and Wagner, 2006). In the biosphere, most of the reactions are catalysed by microorganisms. Dinitrogen (N2) can be fixed and introduced in the biosphere by diazotrophs, in the form of ammonia (NH3) or organic nitrogen (amine group). Ammonia, the most frequently found form of nitrogen in the biosphere can be released from organic compounds by mineralization. In aerobic environments ammonia is quickly oxidised to nitrite (NO2-) and nitrate (NO3-) by ammonia-oxidizing microorganisms (AOM), and by nitrite-oxidizing microorganisms (NOM) in a process called nitrification. In anoxic conditions denitrification can occur. The nitrate is used by denitrifying microorganisms, producing dinitrogen (N2), nitrous oxid (N2O) and nitric oxid (NO) (fig.1). (Bock and Wagner, 2006; Fiencke et al. 2005) The cycle is not entirely linear. Depending on the ecosystem conditions the microbial community responses change. So, the key processes stated above can directly affect the availability and form of N within the ecosystems. The increase of nitrogen can augment the concentration of toxic products, such as ammonia, nitrite, nitrous acid and nitric acid. It can also cause eutrophication of that ecosystem, and the contamination of ground water supplies (Fiencke et al. 2005). The decrease nitrogen can affect soil fertility and plant production (Bock and Wagner, 2006).
Strategies of the Natural ecosystem to balance the nitrogen availability
The natural ecosystems have developed mechanisms that involve multiple paths to nitrogen conservation (Dias et al. 2012). Paths to balance the inorganic and organic nitrogen, and to compensate the nitrogen increase or decrease in soil are essentials to the ecosystems. If there is an excess of nitrogen in soils, it can be reduced by mineralization (if the nitrogen appears as an organic form) followed by nitrification (directly if the nitrogen is in the form of ammonia or urea) and denitrification (directly with nitrate), resulting in the formation of atmospheric nitrogen. If the
Fig. 1 Nitrogen Cycle Scheme 1) Nitrogen fixation; 2) Ammonia assimilation; 3) Ammonia mineralization; 4) Ammonia oxidation (1st step in Nitrification); 5) Nitrite
oxidation (2nd step in Nitrification); 6)Nitrate reduction 7)
Nitrite reduction; 8) Nitrate leaching; 9)Nitrate assimilation; 10) Denitrification
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
system has limited nitrogen there is a need to increase nitrogen fixation and diminished nitrification and denitrification.
Nitrification and ammonia oxidation
Nitrification is the biological conversion of reduced nitrogen, ammonia, ammonium (NH4+) or organic N to oxidized N, nitrite and to nitrate successively. The importance of this process lies on the fact that it is the only oxidative biological process linking reduced and oxidized pools of inorganic nitrogen in nature (Martens-Habbena et al. 2009; Norton and Stark, 2011; Fiencke et al. 2005; Sinha and Annachhatre, 2007), and therefore has a strong influence in the fate of N in terrestrial systems.
The oxidation of ammonia by microorganisms can be done by several groups. In the presence of oxygen by the chemolithotrophic bacteria and heterotrophic bacteria. In the absence of oxygen by Planctomycetes. In aerobic soil environments the key participants in nitrification are chemolithotrophic bacteria and archaea. Heterotrophic nitrification can be catalysed by fungi, actinomycetes, and other bacteria, and seems to be significant in some forest and acidic pasture soils. The anaerobic oxidation of ammonium is known as the anammox process and it is widespread in marine and freshwater environments, but rarely found in soil. (Norton and Stark, 2011; Sinha and Annachhatre, 2007).
Ammonia-oxidizing bacteria (AOB)
Since the first chemolithotroph ammonia oxidizing bacteria isolation at the end of the nineteenth century by Winogradsky (1890), 16 more species of AOB have been described, accounting for a total of 25 culturable species (Sinha and Annachhatre, 2007). However, many not described species can be found, in culture collections at least 15 additional genospecies (Bock and Wagner, 2006) and more in natural environments.
These 25 species of chemeolitotrophic ammonia oxidizing bacteria (AOB) are comprise in the Proteobacteria phylum. Comparative 16S rRNA sequence analysis has shown that all recognized AOB are members of the β- or γ-subclass of Proteobacteria. In the β-proteobacteria there is 4 genera Nitrosomonas, Nitrosospira, Nitrosolobus and Nitrosovibrio. The Nitrosococcus genus belongs to the γ-Proteobacteria (Purkhold et al. 2000).
AOB are widespread in coastal, open ocean, polar environments (mainly 2 clusters, Nitrosomonas and Nitrosospira), hypersaline enviroments (Nitrosomonas), high temperatures (Nitrosospira) and acid environments (Nitrosomonas) (Junier et al. 2010; Prosser and Nicol, 2008). Most soil bacterial ammonia oxidizers belong to Nitrosospira genera (Purkhold et al. 2000). Ammonia oxidizing Bacteria rarely grow in soils with a pH lower tan 7, however there are several niches for AOB were the pH is optimal for AOB (Junier et al. 2010).
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
For lithotrophic AOB ammonia (NH3) but not ammonium (NH4+) is essential as the primary substrate and the intermediate hydroxylamine (NH2OH) as the real energy source. Organic substances like urea or glutamine can be used as alternatives energy sources, when ammonia is not available, by some strains of Nitrosomonas (Nitrosomonas ureae) (Bock and Wagner, 2006). An ammonia oxidation schematic model of electron transport is shown in figure. 2. Ammonia oxidation begins with the oxidation of ammonia to hydroxylamine by AMO. Oxygen (O2) is needed to form the intermediate compound and water. Hydroxylamine is oxidize to nitrite by HAO (hydroxylamine oxidoreductase). Two of the four electrons derived are required for AMO activity. The other two are used for energy generation (Bock and Wagner, 2006; Hatzenpichler, 2012). As most metabolic studies have been done in Nitrosomonas europaea, the metabolic pathways may not be the same for the other genera.
AOB are autotrophic bacteria able to fix carbon through the Calvin-Benson-Bassham (Calvin) cycle (Hatzenpichler, 2012; Sinha and Annachhatre, 2007). Some Nitrosomonas strains have the capacity to grown mixotrophically with organic compounds as carbon source (Clark and Schmidt 1967).
In presence of certain organic carbon compounds, such as pyruvate, the production of nitric oxide and nitrous
oxide is enhance.
Nitrosomonas eutropha and Nitrosomonas europaea denitrify under low oxygen concentration conditions (Yamanaka, 2008; Bock and Wagner, 2006; Hatzenpichler, 2012).
Ammonia oxidizing
archaea (AOA)
The discovery of ammonia-oxidizing archaea (AOA) (Könneke et al. 2005) fundamentally changed our view of nitrogen cycling in the environment. It was also the start of a new trend of studies related to the importance of archaea in moderate ecosystems and in nitrification processes. Since the first study by Kӧnneke (2005) several culture- independent studies with the amoA gene have shown the presence of AOA in several diverse environments. They are present in mesophyll environments (marine, freshwater, and terrestrial environments) as well as in extreme conditions (acidophilic condtions, thermophile environments, deep-sea water and hot springs) (Junier et al. 2010; Nicol and Schleper, 2006). In some ecosystems they can have a higher contribution to nitrification, out-growing the AOB that are not adapted to the environmental conditions (Junier et al. 2010; Nicol and Schleper, 2006). Anoxic conditions in river sediments
Fig. 2 Model of electron transport chain of Nitrosomonas europaea. Scheme from Fienck et al. (2005). AMO= ammonia monooxygnase; C= cytoplasmic side of membrane; Cyt= cytochrome; HAO=hydroxylamine oxireductase; NIR= nitrite reductase; P=periplasmic side of membrane; UQ= ubiquinone
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
(Liu et al. 2013), acidic soils or poor nutrient ecosystems (He et al. 2012) are examples, among other.
While in an aquatic environment the majority of AOA belong to “mesophilic crenarchaeaota” 1.1a lineage, representing approximately 20% of all prokaryotes found in these ecosystems (Karner et al. 2001; Prosser and Nicol, 2008), most soils are dominated by sequences of ammonia oxidizing archaea belonging to 1.1b lineage.
The attention and studies done in this group of archaea culminated in a proposal of a third archaeal phylum named Thaumarchaeota (Brochier-Armanet et al. 2008; Spang et al. 2010), with a set of genomic characteristics that make it and distinct phylum (Hatzenpichler, 2012; Spang et al. 2010). Currently the only common physiological trait is the chemolithoautotrophic growth by ammonia oxidation. (Norton and Stark, 2011; Hatzenpichler, 2012; He et al. 2012).
The metabolic pathways for ammonia oxidation in AOA are still unclear. No HAO homologue has been found in any AOA genome, so AMO may not catalyse the same reaction as in AOB (Hatzenpichler, 2012; He et al. 2012). In contrast to AOB, thaumarchaeotal populations seem to have the genetic potential and metabolic ability to use amino acid as energy source (Hatzenpichler, 2012). This metabolic ability can also be seen by the preference for organic sources of nitrogen, e.g. aminoacids (He et al. 2012; Tourna et al. 2011). AOA also have a higher affinity for ammonia (Martens-Habbena et al. 2009), being more adaptable to low nutrients conditions, AOA can out-number the AOB in oligotrophic environments.
Some marine species of AOA, e.g. “Candidatus Nitrosopumilus maritimus” have shown a growth inhibition in presence of organic carbon. However the terrestrial Nitrososphaera strains growth can be enhanced by small amounts of pyruvate. So in some soil environments the AOA diversity can be enhance by a rich organic carbon content (Liu et al. 2013), contrasting with most AOB species that are sensible to the presence of organic carbon. So, the preferential nutritional and environmental conditions of AOB and AOA can be distinct, allowing a separation of both communities in distinct niches.
Importance of nitrification and regulation:
Through the nitrification process, several compounds can be produced. The intermediary product of nitrification, nitrite is usually is found in trace amounts comparing with other forms of N that can appear in relatively high concentrations (Bock and Wagner, 2006; Norton and Stark, 2011, Dias et al., 2012). The maintenance of a low nitrite concentration in aerobic habitats is essential since nitrite is toxic to microorganisms, root plants and seeds (Bock and Wagner, 2006; Norton and Stark, 2011; Cleemput and Samater, 1996). So, a sequential and almost immediate nitrite oxidation to nitrate by nitrite oxidizing bacteria (NOB) is fundamental in maintaining a low nitrite concentration. The sequential nitrite consumption and oxidation is possible since ammonia oxidation to nitrite is done at low rates, being considered the rate-limiting step of nitrification (Bock and Wagner, 2006; Norton and Stark, 2011; Fiencke et al. 2005).
Nitrite accumulation can be found at low oxygen partial pressure, e.g. soil with a water content, under alkaline conditions where ammonia oxidation is enhanced, in environments with
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
high ammonia concentrations and in conditions with decreased or inhibited nitrite oxidation activity. Nitrite oxidising organisms (NOB) can be inhibited by high ammonia concentrations (Norton and Stark, 2011) or by nitrite, since high concentrations are toxic. Without nitrite oxidation by NOB, it becomes difficult to regenerate the low nitrite state of the ecosystem.
In anoxic conditions, nitrite accumulation can lead to nitric oxide and nitrous oxide production by heterotrophic bacteria denitrifcation, or by AOB denitrification.. It was though that these oxides were produced as intermediaries in ammonia oxidation (Cleemput and Samater, 1996; Bremner, 1997). However it is now known that AOB are capable producing these oxides by a denitrification process, with the reduction of nitrite by nitrite reductase (NIR) in anoxic conditions (Bremner, 1997; Burns et al. 1996), as seen in some species of AOB e.g. Nitrosomas eutropha and Nitrosomonas europaea (Yamanaka, 2008; Bock and Wagner, 2006; Hatzenpichler, 2012). The production of these oxides is problematic since both oxides form acids (nitrous acid and nitric acid) when interacting with water and may contribute to soil acidification and root damage (Fiencke et al., 2005). Nitrous oxide also acts like a greenhouse gas with a potential 310 times higher than carbon dioxide (Bock and Wagner, 2006; Fiencke et al., 2005).
Therefore regulation of nitrifying activity is essential to maintain the level of ammonia, nitrite and nitrate so that there is not a loss of fertility with low N quantities, nor a system eutrophication due to high N quantities.
As it is mentioned above the main factors that can regulate ammonia oxidation activity and AOM growth are substrate concentration (ammonia concentration), pH, presence of organic matter, oxygenation, water content, temperature and biotic regulation through plant-AOM interaction which can be by substrate competition with plants, or effect of plant exudates.
Mediterranean type ecosystem
This study focus on AOB and AOA communities found in Mediterranean-type ecosystem. Mediterranean ecosystem can be characterized by a highly seasonal climate with hot dry summers and mild wet winters. This asynchronous ecosystem with marked seasonal changes greatly influences the N fluxes. In summer when soils become water-limited the microbial activity, like decomposition and ammonia mineralization, tends to decrease, as should also occur to nitrification. The microbial activity should be being higher in spring or/and autumn due to the increase in water content (Ochua-Hueso et al. 2011; Dias et al. 2012). Concerning vegetation, the study site, in Serra da Arrábida was characterized as being a dense maquis with Cistus ladanifer as the dominant summer semi-deciduous plant (Dias et al. 2008). As for inorganic N content, it is known to vary greatly through the year, being lower in August and higher in November (Ochua-Hueso et al. 2011). Soil can be considered poor in N content, with the non-fertilized soils (without any N addition) having a very low quantity of inorganic N even in spring when soils have a greater nutrients turnover. It is an acidic soil with a pH of 5.8 ±0.2. (Dias et al. 2012). Microbial communities from these types of ecosystem should be adapted to low water content, climate changes, and low nutrient content. For microorganism adapted to low nutrient concentrations, as
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
the microbial communities found in Mediterranean soils, a nutrient input can cause abrupt and drastic alterations in community structure and activity.
Aims of this thesis
The work presented in this thesis aims to understand the ammonia oxidizing community in soils from a Mediterranean type ecosystem. The first objective was to know in which niche AOM community’s had a higher ammonia oxidation activity. Two niches were studied the root surface and rhizospheric soil of the most abundant plant species, Cistus ladanifer (Dias et al. 2008). After assessing the niche with the higher ammonia oxidation activity, the second objective was to compare the ammonia-oxidising communities from soils under nitrogen addition. The 3rd objective was to assess the effect of ammonia concentration, pH and presence of organic matter in AOB cultures.
Hypothesis:
In a poor nutrient ecosystem as the Mediterranean ecosystem, the preferential niche for the AOM growth and activity should be near the plant root, to have a higher nutrient exchange between the plant and AOM (Ochua-Hueso et al. 2011), besides giving conditions, as pH and oxygenation, favourable to AOM. Microsite in the surface of the root may have the environmental conditions to ammonia mineralization and production to occur, increasing the quantity of substrate for AOM in that area (Cleemput and Samater, 1996), while rhizospheric soil around the root may not have the same quantity of nutrients.
Bacterial or archaeal communities that are adapted to low nutrient conditions tend to suffer great changes with nutrient addition. Although some microbial groups have shown a higher degree of metabolic flexibility and physiological tolerance when changing the growth conditions this case is not seen for AOB communities, as rapid growth rates and high abundances are needed for the community’s strains to have that flexibility and adaptability (Allison et al. 2008).
Regarding the effect of N-addition in soils, the increase in ammonia-oxidation activity when the ecosystem has an increase in N quantity is excepted since it can be a strategies to counterbalance the nitrogen excess in the naturally low nutrient ecosystem. However, as AOM populations in poor nutrient soils may be adapted to oligotrophic environments, a change in the population structure may occur as adaptation to the higher N input.
As population adapted to low N content, the AOB population from soil without N addition are expected to be more susceptible to higher ammonia concentrations. Concerning pH it is described that AOB prefer pH basic to neutral (Bock and Wagner, 2006; Norton and Stark, 2011; Hatzenpichler, 2012), however AOB population can have ammonia oxidation activity at a slightly acid pH (>6, <7) (Hatzenpichler, 2012), so it is expected to have ammonia oxidation activity between pH 6.5 and pH 7.8. As the soils are poor in organic matter (Dias et al. 2012), AOM populations may prefer inorganic energy and carbon sources, though AOM can have a mixotrophic or even organotrophic growth.
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
Materials and Methods:
Study siteThe present study was conducted at Serra da Arrábida in the Arrábida Natural Park, a Natura 2000 site in south of Lisbon, Portugal (PTCON0010 Arrábida/Espichel). The study site (38º 29’ N - 9º 01’ W) is located within a region belonging to the sub-humid thermo-mediterranean bioclimatic domain (Clemente 2002). According to the climatic normal (2012) in July, when the first soil collect was done, the mean maximum temperature was 28ºC, mean minimum temperature was 15ºC and mean precipitation was 1 mm. In October, the second soil collect was done, the mean maximum temperature was 22ºC, mean minimum temperature was 14ºC and mean precipitation was 50 mm. Reported data refer to the nearest climatic station (Setúbal, 15 km distance –Instituto Português do Mar e Atmosfera). The dominant plant species is Cistus ladanifer (Dias, 2008).
Experimental design
The experimental design was defined on the scope of FCT project “Spheres of ecosystem response to nitrogen: a case Study in a Mediterranean-type ecosystem in the southern of Portugal (SERN) PTDC/BIA BEC/099323/2008. It consists of 12 plots, of 400 m2 each. N availability was modified by the addition of 40 and 80 kg N ha-1 yr-1 in the form of NH4NO3 (40AN and 80AN, respectively) and 40 kg N ha-1 yr-1 as a 1:1 mixture of NH4Cl-N and (NH4)2SO4-N (40A) (Fig.2). Each treatment had three replicates (3 plots). To prevent N ‘contamination’ through runoff from fertilized plots, the experimental plots were distributed in three rows along the slope, with the controls (without fertilization) being located in the top row. The fertilization began in January 2007 and it is still being applied. Three equal applications have been applied throughout the year: middle autumn/winter, spring and summer.
Soil and Root sampling
The soil samples used for the study of ammonia oxidizing archaea (AOA) and ammonia oxidizing bacteria (AOB) preferential distribution were
collected in July 2012. Three soil locations were randomly chosen in each control plot (C, F and E) (Fig. 3). None of the sample was taken from the modified plots. For each sample rhizospheric soil and roots were taken from seed germinated Cistus ladanifer plants at a depth of 5 - 6 cm. Samples were stored in clean plastic cups and refrigerated. In the laboratory, individual samples were immediately weighted and prepared for the microbial enrichment cultures.
For the activity and community structure comparison of ammonia-oxidizing communities in the non-fertilized and fertilized (altered) plots the sampling was performed in October 2012. Three rhizospheric soil samples were taken from different seed germinated Cistus ladanifer at a depth
Fig.3 Experimental design scheme and the relative location of the experimental plots (Green - Control; Orange - 40A; Blue - 40AN; and Grey - 80AN)
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
of 5 - 6 cm in each plot (Fig.3). Samples were maintained cold in clean plastic cups. A composite sample was obtained by homogenization of three samples from each of the three field replicate plots. 1 gram of soil from each composite sample was used as inoculum for the sequential enrichment cultures (AOB and AOA). A soil extract was prepared for each composite sample with 1 gram diluted in 10 ml of ultra-pure water for nitrite and ammonia quantification.
Ammonia oxidizing microorganism’s enrichments cultures
To compare the nitrifiying activity in Cistus ladanifer rhizospheric soil and roots three sequential enrichments cultures were prepared to allow a selection and subsequent increase of the ammonia oxidizing microorganism’s (AOM) population.
In the first enrichment 36 enrichment cultures were prepared: 18 cultures for AOB (9 for roots, 9 for rhizospheric soil) and 18 for Archaea (9 for roots, 9 for rhizospheric soil). Ammonia oxidizer Bacteria enrichment cultures were achieved in 25 mL McCartney flasks containing 20 mL of Fresh Synthetic Crenoarchaeota (SFC) with 1 mM of NH4Cl. 1 g of soil or roots were used to inoculate 20 ml of medium. SFC medium (Konneke et al. 2005; De La Torre et al. 2008) consistes of NaCl (1 g L-1), MgCl2·6H2O (0.4 g L-1), CaCl2·2H2O (0.1 g L-1), KCl (0.5 g L-1), MgSO4.7H2O (5 g L-1) and KBr (0.1 g L-1). After sterilization in autoclave (121ºC; 10min) the medium was supplemented with: per litter of medium, 1 mL of non-chelated trace element mixture, 1 mL of vitamin solution, 10 ml of KH2PO4 solution (4 g L-1), 1 ml of tungstate-selenite solution, 1 ml of sodium bicarbonate solution (1 M), and 1 ml of NH4Cl (1M). These solutions were prepared with distilled water and sterilized in autoclave (121º for 10 min) or by filtration (0.45 µm) for heat-sensitive compounds. The final pH is between 6.5 and 7. Ammonia oxidizer archaea (AOA) first enrichment cultures were prepared with streptomycin in a concentration of 2.5 µg ml-1. Cultures were incubated at 28ºC without shaking during 30 and 60 days for AOB and AOA respectively. After the first enrichment stage, AOM cultures producing more than 1 mg/L NO2- (positive cultures) were sub-cultured into a second enrichment stage transferring 5% (v/v) inoculum to 20 mL SFC fresh medium supplemented with sodium chlorate (5 mg L-1). For the second enrichment for AOA streptomycin was replaced by ampicillin at 100 µg ml-1. AOM cultures that didn’t produce nitrites (negative cultures) or those producing less than 1 mg /L of nitrites were discarded. The third enrichment for AOB was obtained by transferring 5% (V/V) of inoculum into 20 ml of fresh SFC medium. For AOA cultures ampicillin was replaced by 0.05% chloramphenicol.
For the study of ammonia oxidizing communities in the non-fertilized and in fertilized plots, rhizospheric soil was used to inoculate 24 initial enrichment cultures: 12 initial cultures for AOB and 12 for AOA. 1 g of soil was used to inoculate 20 ml of SFC medium with 1 mM of NH4Cl, supplemented with Calcium Carbonate (7.5 gl-1), Pimaricine (0.04 gl-1), and sodium chlorate (5 mg l-1). The first enrichment for Archaea had ampicillin in a concentration of 100 µg/ml. For AOB the medium was not altered in the sequential enrichments. For AOA cultures streptomycin (100 µg ml-1) was added to the 2nd and Chloranphenicol 0.05% to the 3rd enrichments.
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
In every enrichment 5 % (v/v) of inoculum was transferred from both positive and negative cultures. Cultures were incubated at 28ºC without shaking during 30 and 60 days for AOB and AOA respectively.
Ammonia susceptibility:
SFC medium was supplemented with 5mM of sodium chlorate, pimaricine (0.04 gL-1), calcium carbonate (7.5 gL-1) and different ammonia concentrations: 1 mM, 10 mM, 50 mM, 100 mM and 500 mM. The medium was inoculated at 5 % with 2nd enrichment AOB community cultures. Cultures were incubated at 28°C during 15 days without shaking. Growth was indirectly estimated by assessing cultures activity by nitrite concentrations determinations at several time-points by the Griess method.
pH susceptibility:
SFC medium was supplemented with 5mM of sodium chlorate, pimaricine (0.04 gL-1), calcium carbonate (7.5 gL-1) and 1Mof ammonia concentrations. pH was altered with 1M and 10M HCl and 1M NaOH to achieve pH 5.6, 6.7, 7.1 and 7.9. The medium was inoculated at 5 % V/V with 2nd enrichment AOB community cultures. Cultures were incubated at 28°C during 30 days without shaking. Growth was indirectly estimated by assessing cultures activity by nitrite concentrations determinations at several time-points by the Griess method.
Effect of organic compounds in AOB nitrite production
SFC medium was supplemented with 5mM of sodium chlorate, pimaricine (0.04 gL-1), calcium carbonate (7.5 gL-1) and different organic compounds: urea (46.8 mg/L), peptone (101 mg/L), sodium acetate (0.5 %) and glucose (0.5 %).Concentrations for urea and peptone were calculated to have the same amount of N as the control media (1M of NH4C, corresponding to 0.78 mM of N). Media with a combination of ammonia and urea or peptone was prepared with 0.5 M of ammonia chlorate and urea (mg/L) and peptone (mg/L). Media with sodium acetate or glucose had 1M of ammonia, but not sodium bicarbonate.The medium was inoculated at 5 % with 2nd enrichment AOB community cultures. Cultures were incubated at 28°C during 30 days without shaking. Growth was indirectly estimated by assessing cultures activity by nitrite concentrations determinations at several time-points by the Griess method.
AOM Indirect Growth Measurement
AOM indirect growth was estimated by measuring nitrite concentration at several time-points by the Griess method (Standard Methods, 1999). 200 µl were removed from each culture and the ammonia-oxidizing reaction was stopped with a 4 M KCl solution in a 1:2 proportion. 50 µl of the sample were mixed with 125 µl of a sulphanilamide solution (10 g L-1) diluted with 10% concentrated HCl and 125 µl of N-(1-naphthyl)-ethylenediamine solution (100 mg L-1). The assays were conducted in a 96-well microplate and the absorbance was read at 540 nm in a spectrophotometric microplate reader (Tecan Spectra Rainbow Micrplate Reader 400-700nm,
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
Switzerland). Nitrite standard curve was prepared using sodium nitrite solutions with concentrations ranging between 1–100 μM. Nitrite concentration of experimental samples were obtained from the resulting standard curve of nitrite concentration vs. absorbance.
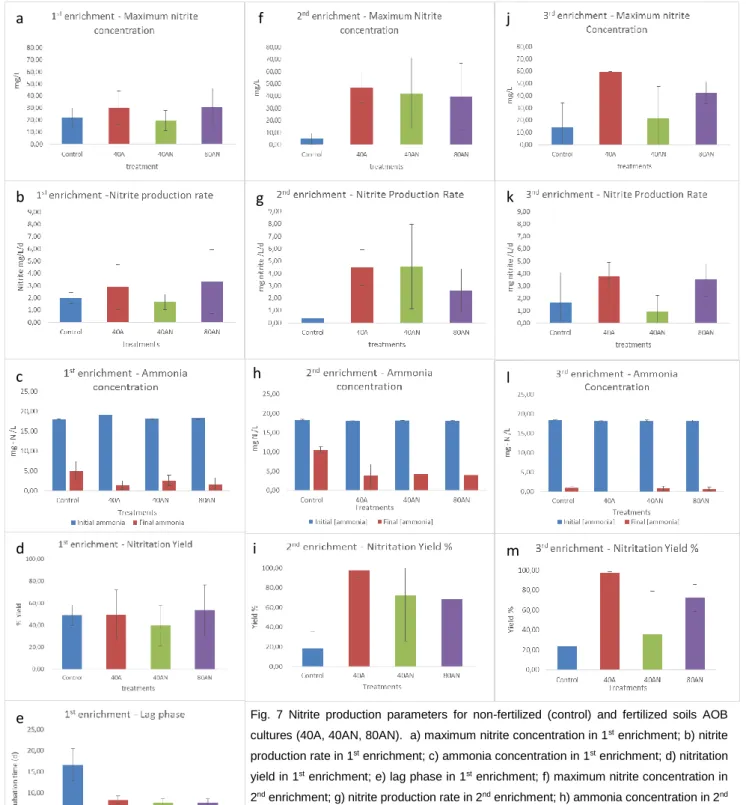
The nitrite concentration through time was used to establish a nitrite production profile and typical parameters were determined. For the 1st enrichment, length of Lag phase, nitrite production rate, maximum nitrite concentration and area under the curve were used to compare the different cultures. For the 2nd and 3rd stages of enrichment, nitrite production rate and maximum nitrite concentration were used to compare ammonia oxidizing community’s cultures. Lag phase was defined as the time segment between the beginning of incubation time and beginning of exponential nitrite production, expressed by days. Nitrite production rate was define at exponential phase with linear regression and was expressed as mg of nitrite produced per liter per incubation day (mg/L/d). Maximum nitrite concentration was determined as the highest nitrite concentration value after the 1st day of incubation, expressed as mg of nitrite per liter.
Additionally, ammonia concentration was determined in the end of each culture enrichment stage by the Berthelot reaction. 50 µl of each culture was first mixed with 50 µl of 5 % sodium citrate solution (pH 7). After 1 min of incubation at room temperature, 50 µl of 2-phenylphenol nitroprusside (PPS-nitroprusside) solution was added. Then 25 µl of hypoclorite buffer (pH 13) was added followed by 100 µl of deionized water. The microplate was incubated in the dark at room temperature for 45 min before reading. The assays were conducted in a 96-well microplate and the absorbance was read at 660 nm in a spectrophotometric microplate reader (Tecan Spectra Rainbow Micrplate Reader 400-700nm, Switzerland). The PPS-nitroprusside solution was prepared with 3.22 g of 2-hydroxybiphenyl sodium salt tetrahydrate and 0.015 g of sodium nitroprusside dehydrate dissolved in 100 ml of deionized water. The buffer was prepared by dissolving 1 g of tri-sodium phosphate dodecahydrate in deionized water and 10 ml of sodium hypochlorite.
Subsequentially ammonia consumption (%) (equation 1) and nitrite production yield (%) (equation 2) were determined. The yield is calculated given in account the N weight, not the total molecule weight. ( [𝑁𝐻4+]𝑓𝑖𝑛𝑎𝑙− [𝑁𝐻4+]𝑖𝑛𝑖𝑡𝑖𝑎𝑙) × 100 (equation 1) (([𝑁𝑂2−]𝑚𝑎𝑥𝑖𝑚𝑢𝑚× 0.31)/([𝑁𝐻4+]𝑐𝑜𝑛𝑠𝑢𝑚𝑒𝑑× 0.782)) × 100 (equation 2) DNA extraction:
DNA was extracted from 10 - 15 ml of the enrichment cultures using a direct boiling method (Sambrook et al. 2001). The samples were centrifuged at 4000 rpm for 10 minutes to remove the medium precipitated CaCO2. The supernatant was centrifuged at 4000 rpm for 10 min. The pellet was ressuspended in TE buffer (pH 8) and centrifuged at 13 000 rpm for 5 min to wash the cells.
1 30% of NO2- molecular weight is nitrogen 2 78% of NH4+ molecular weight is nitrogen
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
The supernatant was discarded and the pellet was ressuspend with TE supplemented with 0.1% tween. The samples were incubated at 100ºC for 10 min.
PCR amplification
Each 50 μl PCR reaction contained: 0.2 µl (1 unit) BioTaq DNA Polymerase (Bioline, 500 units), 5 µl (1x) NH4+ Buffer (Bioline, 10X), 2 µl (2 mM) MgCl2 (Bioline, 50 mM), 1µl (0.2 mM each) dNTPs (10 mM each), 1 µl (50 ρmol/µl) of each primer, and 2.5 µl of BSA (0.1 %). 1 μl of extracted DNA was used as template.
Thermal conditions for the bacterial 16S RNA PCR were as follows: 3 min initial denaturing step at 94 °C, followed by 35 cycles of 1 min denaturing at 94 °C, 2 min annealing at 50 °C and 1 min extension at 72 °C, with a final extension step of 3 min at 72 °C.
All PCR products were verified on 1% agarose gels stained with ethidium bromide under UV light. Primers used for amplification, targets and positive controls are listed in table 1.
Primer Sequence target Positive control Tested
dilutions reference
104F 5’-GGCGVAYGGGTGAGTAA-3’ Bacterial 16S
rRNA Escherichia coli
1:1; 1:10; 1:25
Sandra Chaves
907R 5’-CCGTCAATTCMTTTRAGTTT-3’ Muyzer et al. 1995
ArchF 5’-GAATTGGCGGGGGRGCA -3’ Archaeal 16S rRNA Halobacterium salinarium DSMZ 1:1 Sandra Chaves, personal communication ArchR 5’-TGTGTGCAAGGRGCAGGG – 3’
amoA-1F 5′-GGGGTTTCTACTGGTGGT-3′ Β-proteobacteria
amoA Nitrosomonas europaea 1:1; 1:10 Rotthauwe et al. 1997 amoA-2R 5′-CCCCTCKGSAAAGCCTTCTTC-3’ Arch-amoA-rer 5´- TTCTTCTTTGTWGCCCARTA – 3’ Archaeal amoA - 1:1 Sandra Chaves, personal communication Arch-amoA-for 5’ – CTGAYTGGGCYTGGACWTC – 3’ PCR- RFLP fingerprinting:
Selection of the restriction endonuclease was based on the in silico analysis of several selected amoA sequences from the β-ammonia oxidizing bacteria group of AOB available on GenBank, access numbers given by Purkhold et al. (2000), using the NEBCutter V2.0 (http://tools.neb.com/NEBcutter2/) from new England Biolabs. The enzyme HinfI and HaeIII was selected given its ability to discriminate between the different strains, with 2 or 3 hydrolysis region, and did not produced fragments with less than 20 bp length.
Amplification of amoA genes for RFLP analysis was performed as described above. The restriction was done separately for each enzyme. Reactions contained 10 µl of PCR product from each sample, 5 U of HinfI and 1x NEBuffer 2 (New England Biolabs GmbH, Frankfurt, Germany)
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
or 5 U of HaeIII and M buffer (TAKARA bio INC. Kyoto, Japan). Restriction reactions were performed at 37 ºC for 24 h. Full reaction volumes were loaded and visualized on a 1.5 % agarose gel 1:3 high resolution ultra-pure agarose - 1000, 2:3 ultra-pure agarose (Invitrogen Carlsbad, Estados Unidos) under UV light, after staining with ethidium bromide (EtBr) for 15 min.
Statistical analysis
The statistical analysis was performed using SPSS 20 software for Windows. All data was checked for normality with the Levine’s test. The coefficient variation (equation 3) was calculated as a normalized measure of dispersion for all the data. Linear data was checked for multiple comparisons of means with ANOVA at p<0,05. Kruskal-Wallis test was performed for non-linear data at p<0,05. Rank transformation followed by a two-way ANOVA was performed for the AOB community’s ammonia sensibility data, as described by Iman and Conover (1983).
𝐶𝑉 = 𝜎 (𝑠𝑡𝑎𝑛𝑑𝑎𝑟𝑑 𝑑𝑒𝑣𝑖𝑎𝑛𝑡𝑖𝑜𝑛)
µ (𝑚𝑒𝑎𝑛) (equation 3)
Results and Discussion
Where does the Ammonia-oxidizing community prosper?
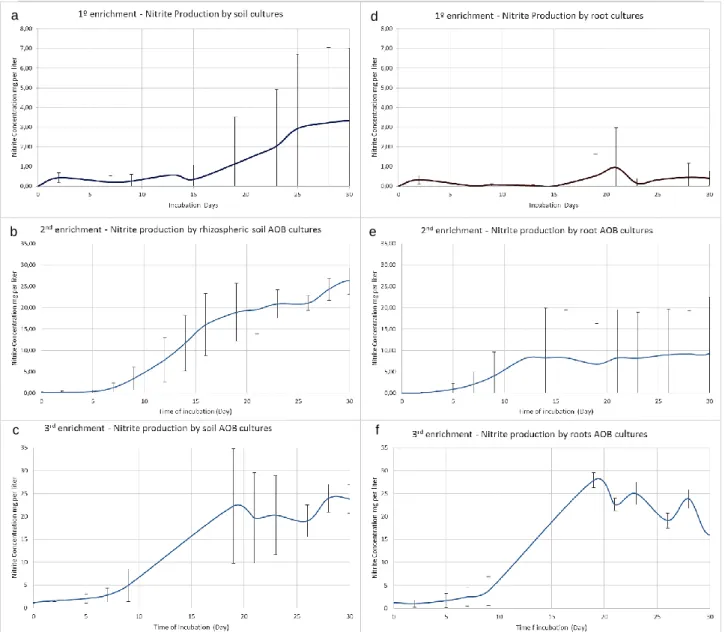
Cistus ladanifer as the most abundant plant species in the experimental study, was expected to have the highest influence on soil microbial community. So, the activity of ammonia oxidizing communities in Cistus ladanifer root surface and rhizospheric soil was assessed in order to understand in which of these niches presents a higher ammonia oxidation activity. As described in methods, 3 sequential enrichments were done. Active AOB and AOA cultures were defined as cultures with nitrite accumulation through time, where the maximum nitrite concentration is higher than the nitrite concentration found in lag phase.
Nitrite production by Ammonia-Oxidizing Bacteria
Ammonia-oxidizing bacteria growth is difficult to measure, so most of the studies are based on the activity, or nitrite production. With the nitrite measurement through the incubation time it is possible to have nitrite production curves to characterize that community.
In the rizospheric soil community 1st enrichment, nitrite accumulation can be seen during the beginning of incubation, 2 days for soil and root AOB cultures (fig.4 a and d), but decreases after that period of time. For soil AOB cultures nitrite starts to accumulate 7 days after the first decrease. Nitrite production recovery for root AOB cultures starts after 15 days of incubation. Both soil and roots AOB cultures had a 2nd decrease in nitrite concentration, more evident for root AOB cultures at 22 days of incubation (fig. 4 d) than soil AOB cultures at 13 days of incubation (fig.4 a). If in the cultures AOB where the only functional community active then the nitrite production from ammonia should be constant. However the community’s nitrite production dynamics includes diauxic growth and the variations in nitrite concentration can be considered as a result of the culture dynamics, since the coefficient variation (CV) for Griess method is 0.1, lower than these variations.
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
The 1st enrichments are prepared from soil and roots, adding nutrients and organic compounds to the medium. Since the microbial inoculum corresponds to the community present in that soil and root surface, both AOM and other heterotrophic bacteria, capable of producing nitrite, are present in the culture. AOM have a slow growth and most AOB species need several days to weeks for growth, depending on the conditions such as substrate concentration, oxygen availability, the temperature and pH values (Bock and Wagner, 2006). Since heterotrophic bacteria tend to out-grow the AOM in selective media, using the soil inoculum nutrients, the observed diauxic growth can be explained by the change in nutrient source from the ones in the medium to the nutrients in soil and roots.
Concerning the studied nitrite production parameters: lag phase, time period, in days (d), between the beginning of incubation and beginning of exponential phase; nitrite production rate, mg of nitrite produced per day per liter of medium (mg/L/d), calculated in the exponential phase; and maximum nitrite concentrations (mg/L), the enrichment community cultures from both soil and roots AOB cultures presented a high heterogeneity (table. 2 in annex) for maximum nitrite concentration (1.04 and 1.46, respectively) and nitrite production rate (1.59 and 2.3). Lag phase for soil and root AOB culture present the lowest variation (0.26 and 0.32). Root AOB cultures had higher CV for the studied parameters compared to soil AOB cultures.
The 3 studied plots (plot C, E and F) from soils without N addition, are considered field replicates in the experimental design, however considering the natural heterogeneity found in soils (Ochua-Hueso et al. 2011), it is expected to have a higher heterogeneity between communities from the same plot and between communities between the 3 plots.
Comparing the maximum nitrite concentration produced by the AOB cultures in both niches (Table 2), soil AOB cultures had higher maximum nitrite concentrations (3.59 mg/L) than root surface AOB cultures (1.35 mg/L), though significant differences were not found (ANOVA p=0.95, table 3 in annex). Particularly, communities from plot C had the highest values (9.36 mg/L, 3.06 mg/L and 11.27 mg/L) in soil AOB cultures. In root surface AOB cultures, plot C also presents the highest values for maximum nitrite concentration (2.26 mg/L, 0.75 mg/L and 6.62 mg/L) (table 2). Soil communities also present higher nitrite production rates than root surface communities (0.46 mg/L/d and 0.15 mg/L/d, respectively) (table.2), though significant differences were not observed (ANOVA, p=0.95) (table. 3 in annex). The highest nitrite production rate was also observed for plot C in soil AOB cultures and root AOB cultures (table.2).Concerning lag phase, root AOB cultures had higher lag phases than soil AOB cultures (23.33 d and 16.67 d, respectively) (table 2), lag phase was significant different between the soil and root AOB cultures (sig of 0.049, ANOVA with p= 0.95) (table. 3 in annex).
Since root cultures had cultures without constant nitrite production, nitrite production rate could not be calculated and where given a value of 0 mg/L/d. Lag phase for these cultures is 30 days, corresponding to the complete incubation time.
The soil used to prepare the 1st enrichment can have more nutrients and organic compounds available than roots with complex organic compounds that need to be degraded
Table. 2. 1st enrichment nitrite production parameters for the Rhizospheric Soil AOB community cultures and root
surface AOB community culture of Cistus ladanifer from non-fertilized soils without N addition: Plot C, E and F. Parameters include lag phase, expressed by day, nitrite production Rate expressed by mg nitrite/L/d and maximum nitrite concentrationexpressed as mg/L. Plant 1, 2, 3 are field replicates from which the samples were collected.
Master in Applied Microbiology 2012-2013
Master thesis Catarina Gouveia
before being used as energy source for heterotrophic bacteria. This could explain the higher lag phase in root community cultures, particularly in plot C communities.
To correctly compare the AOB community activity, cultures without growth of other microorganisms are needed. Therefore a 2nd enrichment was performed.
From 9 inital rhizospheric soil community cultures, 7 were considered active and used as inoculum for a 2nd serial enrichment. From root community cultures 3 were considered active and used to prepare the 2nd enrichment (table. 1 in annex).
In the 2º enrichment’s community’s cultures (fig. 4 b and e) constant nitrite production can be observed. No nitrite decrease is observed for the root or soil AOB cultures (fig. 4 b and e). The nitrite concentration decrease observed in the 1st enrichment indicated a growth of other microorganisms than AOB. With a serial transfer, nutrients and organic compounds found in the 1st enrichment were diluted, decreasing the growth of heterotrophic bacteria.
As nitrite-oxidizing bacteria could be one of the possible groups of bacteria existing in the inoculum, in the 2nd enrichment a nitratation (nitrite oxidation to nitrate) inhibitor, sodium chlorate, was added to the medium. Chlorate appears to affect specifically the nitrite-oxidizing bacteria, and has little to none inhibitory effect on the growth and activity of ammonium oxidizers (Belser and Mays, 1980). With a nitratation inhibitor the rate at which nitrite accumulates will be the rate of ammonium oxidation (Norton and Stark, 2011), allowing a more correct determination of ammonia oxidation parameters: maximum nitrite concentration and nitrite production rate.
Soil AOB cultures had a higher nitrite production rate and maximum nitrite concentrations (2.15 mg/L/d and 27.26 mg/L) than root AOB cultures (1.19 mg/L/d and 14.91 mg/L) in average (table 3). Observing each culture from soil and root AOB cultures, in soil AOB cultures Plot E presents the highest values for maximum nitrite concentration (30.22 mg/L and 29.49 mg/L) while plot C has the lowest value (23.01 mg/L), while the the lowest and highest nitrite production rates rates can be seen for in plot E (1.48 mg/L/d and 2.89 mg/L/d). For root AOB cultures the highest
Rhizospheric Soil Cultures Roots Cultures
Plot Plant Lag phase (d) Nitrite
production rate (mg/L/d) Maximum [nitrite] (mg/L) Lag phase (d) Nitrite production rate (mg/L/d) Maximum [nitrite] (mg/L) Plot C 1 7 0.65 9.36 15 0.18 2.26 2 16 0.15 3.06 15 0.05 0.75 3 21 2.48 11.27 15 1.08 6.62 Pot E 1 15 0.13 2.06 30 0 0.4 2 15 0.21 2.82 30 0 0.2 3 15 0.05 0.84 30 0 0.17 Plot F 1 21 0.07 0.46 30 0 0.43 2 21 0.45 1.52 30 0 0.26 3 19 0 0.89 15 0.07 1.06 mean 16,67 0,46 3,59 23,33 0,15 1,35 Standard deviation 4,27 0,74 3,72 7,45 0,35 1,96