SOCIEDADE BRASILEIRA DE ORTOPEDIA E TRAUMATOLOGIA
w w w . r b o . o r g . b r
Original
article
Characterization,
survival
analysis,
and
expression
of
IGFR
in
tumor
samples
from
patients
diagnosed
with
Ewing
family
tumors
treated
at
the
Barretos
Cancer
Hospital
夽
Adriano
Jander
Ferreira
a,∗,
Erica
Boldrini
b,
Rossana
Verónica
Mendoza
López
c,
Cristovam
Scapulatempo
Neto
c,
Julie
Francine
Cerutti
Santos
c,
Luiz
Fernando
Lopes
baUniversidadeFederaldoTriânguloMineiro,Uberaba,MG,Brazil
bHospitaldeCâncerInfanto-JuvenildeBarretos,OncologiaPediátrica,Barretos,SP,Brazil
cHospitaldeCâncerdeBarretos,Barretos,SP,Brazil
a
r
t
i
c
l
e
i
n
f
o
Articlehistory:
Received27May2016 Accepted4October2016
Availableonline31December2016
Keywords:
Sarcoma Ewing
Neuroectodermaltumors Primitive
Peripheral
Insulin-likegrowthfactorI Survivalanalysis
Boneneoplasms Oncology
a
b
s
t
r
a
c
t
Objectives: StudytheclinicalcharacteristicsofpatientsdiagnosedwithEwingfamilytumors (EFTs)andsurvivalanalysisbasedonriskcriteriaandexpressionofthesurfaceprotein knownasinsulin-likegrowthfactor(IGFR).
Methods:Thiswasaretrospectivecohortstudybasedonclinicaldatafrom77patients diag-nosedwithEFTstreatedbytheDepartmentofPediatricOncologyattheBarretosCancer Hospitalinaperiodbetween2003and2012.Biologicalsamplesofpatientswereexamined forthepresenceofthesurfacereceptorIGFR.
Results:Theoverallsurvivalrate(OSR)ofpatientsincludedinthestudywas45%atfiveyears, andEFSwas30%atfiveyears.Metastasisatdiagnosiswaspresentin44.2%ofthesample; 88.2%ofthesamplewasmale(p<0.001).TheevaluationoftheexpressionofIGFRin biolog-icalsamplesofpatientswasassociatedwiththevariablemetastasisatdiagnosis(p<0.001). Worseprognosiswasobservedinpatientswithextrapulmonarymetastasis(p=0.009).The localtreatmentofneoplasiapresentedbetterprognosisinpatientsundergoinglocalsurgical treatment(p<0.001).
Conclusions: Theseresultsshowedahigherincidenceofmetastasisatdiagnosisinpatients withEFTstreatedattheBarretosCancerHospital(BCH).Extrapulmonarymetastaseswere anegativeprognosticfactorinthisstudy.Surgicaltreatmentoftheprimarytumorwasa factorforbetterprognosis.StrongexpressionofIGFRwasmorefrequentinpatientswith metastasesatdiagnosis,butdidnotrepresentaprognosticfactorforEFTs.
©2016SociedadeBrasileiradeOrtopediaeTraumatologia.PublishedbyElsevierEditora Ltda.ThisisanopenaccessarticleundertheCCBY-NC-NDlicense(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
夽
StudyconductedatHospitaldeCâncerdeBarretos,Barretos,SP,Brazil.
∗ Correspondingauthor.
E-mails:adrianojander@hotmail.com,dr.adrianojander@hotmail.com(A.J.Ferreira).
http://dx.doi.org/10.1016/j.rboe.2016.10.015
Caracterizac¸ão,
análise
de
sobrevida
e
expressão
de
IGFR
nas
amostras
tumorais
dos
pacientes
com
diagnóstico
dos
tumores
da
família
Ewing
tratados
no
Hospital
de
Câncer
de
Barretos
Palavras-chave:
Sarcoma Ewing
Tumoresneuroectodérmicos Primitivos
Periféricos
Fatordecrescimentosemelhante àinsulinatipo1
Análisedesobrevivência Neoplasiasósseas Oncologia
r
e
s
u
m
o
Objetivo: Estudarascaracterísticas clínicasdos pacientescomdiagnósticode tumores dafamíliaEwing(TFEs)eanalisarasobrevidabaseadaemcritériosderiscoeexpressãoda proteínadesuperfícieconhecidacomofatordecrescimentosemelhanteàinsulina(IGFR).
Métodos: Estudodecoorteretrospectivo,combaseemdadosclínicosde77pacientescom diagnósticodeTFEstratadospeloDepartamentodeOncologiaPediátricadoHospitalde CâncerdeBarretosnoperíodoentre2003e2012.Amostrasbiológicasdepacientesforam examinadasquantoàpresenc¸adoreceptordesuperfícieIGFR.
Resultados: Emcinco anos, a taxadesobrevida global(SG)dos pacientesincluídos no estudo foi de45% ea taxa desobrevida livrede eventos(SLE) foi de30%. Metástases nomomentododiagnósticoforamobservadasem44,2%daamostra;sendoquedesses, 88,2%eramdosexomasculino(p<0,001).Aavaliac¸ãodaexpressãodeIGFRnasamostras biológicas dos pacientes apresentouassociac¸ão com a variávelmetástase ao diagnós-tico (p<0,001).Pacientescommetástase extrapulmonarapresentarampiorprognóstico (p=0,009).Amodalidadedetratamentolocaldaneoplasiaapresentoumelhorprognóstico empacientessubmetidosaotratamentocirúrgicolocal(p<0,001).
Conclusão: Osresultadosevidenciaramumamaiorincidênciademetástaseao diagnós-ticonospacientescomdiagnósticodeTFEstratadosnoHospitaldeCâncerdeBarretos. Ametástasedelocalizac¸ãoextrapulmonarfoifatordepiorprognósticonoestudo.O trata-mentocirúrgicodotumorprimáriofoifatordemelhorprognóstico.AexpressãofortedeIGFR estevemaispresentenospacientescommetástaseaodiagnóstico,porémnãosemostrou comofatorprognósticonosTFEs.
©2016SociedadeBrasileiradeOrtopediaeTraumatologia.PublicadoporElsevier EditoraLtda.Este ´eumartigoOpenAccesssobumalicenc¸aCCBY-NC-ND(http:// creativecommons.org/licenses/by-nc-nd/4.0/).
Introduction
Ewing’sBoneSarcoma(EBS)isthesecondbonemalignancy frequentinthisagegroup,itsincidencebeingsecondonlyto osteosarcoma.1 Histologically,Ewingsarcoma(ES)isdefined
astumorcomposedofsmallcells,round,immatureand usu-allyrichinglycogen.Thetumorfeaturesgeneticalterations in approximately 80% of the cases definedas the recipro-caltranslocationbetweenchromosomes11and22–t(11;22) (q24;q12),resultingintheexpressionoftheprotein EWS/FLI-1.2Thereciprocaltranslocationbetweenchromosomes11and
22isalsoassociatedwiththeoverexpressionofcellsurface glycoprotein CD99.3,4 Immunohistochemical markers,
cyto-genetics,moleculargenetics,tissuecultureandthedetection ofthe same chromosomaltranslocation t(11;22) inthe so-calledprimitiveneuroectodermaltumor(PNET)orperipheral neuroepitheliomainEwing’ssarcoma ofsoft tissues(ESST) and Askin tumorindicates that all thesetumors originate fromthesameprimordialstemcell.Thus,allthesetumors aregroupedinwhatwecallEwingFamilyofTumors(EFTs).3In
theUnitedStates,theannualincidenceofEFTsis2.9casesper millionindividualsupto20yearsofage.1IntheEuropean
con-tinent,around900newcasesarediagnosedannually.5EFTs
israre inblackindividuals, slightlypredominant inmales, occurringmostcommonlyintheseconddecadeoflife.6With
the introduction of chemotherapy in the treatment of ES
associatedwithsurgeryand/orradiotherapy,thesurvivalof patients withlocalized disease increasedfrom 10% to 70% infiveyears.Inspiteofthisgain,survivalforpatientswith metastaticdiseaseatdiagnosisis20%andforindividualswho arerefractorytotreatmentorrelapsingdisease,thesurvival ratedoesnotreach10%.7
ThereareseveralprognosticfactorsinvolvedinEFTs; lit-erature correlatesthe prognosis totheprimary tumorsite, tumor volume, age at diagnosis, gender, lactate dehydro-genase (LDH) levels and the presenceof metastasis.Some EFTsstudiesmakeinferencetoabetterprognosison extrem-ity lesions compared tolesions that compromise the axial skeleton.8,9Unresectabletumorslocatedintheaxialskeleton
are associatedwithaworseprognosis.10 However,arecent
multicenterstudywith114patientsfoundnosignificant dif-ferenceinprognosticvaluewithrespecttoaxialorextremity locationofthetumor.11ESSTsareassociatedwithworse
prog-nosisinrelationtoEBS.12Tumorsizeisapredictivefactorof
prognosisinEFTs,tumorswithsizeequaltoorgreaterthan 8.0cmareassociatedwithaworseprognosis.13Neoadjuvant
chemotherapy-inducedtumornecrosisisaprognosticfactor, andpatientswithnecrosisrateabove90%intheresected spec-imen shows increasedsurvival.13 Patientsyounger than 15
yearshaveabetterprognosiscomparedtoadolescentpatients withthesameageorolderthan15yearsandadults.7,10,14,15
diagnosis.16However,reviewoftwoGermanclinicaltrialsfor
thetreatmentofESinvolvingpatientsolderthan40yearsat diagnosishad asurvivalcomparabletoadolescentstreated inthesame trial.17 WomendiagnosedwithEFTs have
bet-ter prognosisthan men.14,18 Highserum LDH levels before
treatmentare associatedwith aworse prognosis, and this increasecanalsobecorrelatedwithlargeprimarytumorsand metastasis.14
Theparadigmformoleculartargetedtherapieshasbeen discussedtakingIGFRintoconsideration.Insulin-likegrowth factor1(IGF-1)isahormonethatfunctionsasthemajor medi-atorofgrowthhormone(GH)-stimulatedsomaticgrowth,as well as a mediatorof GH-independent anabolic responses in many cells and tissues. The IGFR-mediated molecular pathwayshave recentlyemerged as importanteffectors of neoplastictransformationinvarioustypesofcancer.Forthe oncogenictransformationinES thepresenceofthe IGF-1R receptorisneeded.19 Theinvolvementoftheexpressionof
the IGFR receptor has alsobeen studiedin squamous cell carcinomaof the larynx and may beused as an indepen-dentprognosticfactorforrecurrenceandsurvivalinpatients undergoingsurgicalresection.19
Materials
and
methods
Acohortstudywasconductedwithretrospectivedata collec-tionwhichanalyzedmedicalrecordsof101patientsageof 30yearsanddiagnosisofEFTsthatwereseenandtreatedby theDepartmentofPediatricOncologyatBCHinthe period between2003and2012,withfollowupuntil12/31/2012.The study excluded patients with a diagnosis of EFTs initially treatedatanother institution and patientstreated atBCH, butnottreatedbytheDepartmentofPediatricOncology;24 patientsmettheexclusioncriteria.
Sixty eight paraffin blocks containing biological sam-pleof patientsdiagnosed with EFTs treated atBCH inthe period 2003–2012 were separated; the blocks were stored in the Anatomical Pathology Department at BCH. Slides corresponding to the blocks were evaluated for diagnostic confirmation.
Themostrepresentativeblockofeachcasewasseparated so that the immunohistochemical staining using standard markedslidescouldbecarriedout.Slideswereincubatedwith thefollowingready-to-useprimaryantibodies:Anti-IGF1 (Rab-bitpolyclonaltoIGF1ReceptorAbcam®).Positivecontrolsare theonesaccompanyingreactionsandimmunohistochemical stainingpatternwithcytoplasmicpositivityinnormal pan-creatictissue.Standardizedevaluationformarkerswasbased onthe“Quickscore”Q=P×I20–22whereP,whichcorresponds tothepercentageofpositiveepithelialcellsthatarediffuse anduniform,wasassessedasfollows:(0:negative;1:<25% ofpositivecellsinthecytoplasm;2:26–50%;3:>50%)and I
whichassessesthestainingintensity(1:mild;2:moderate;3: intense);wegetthescorebymultiplyingthevaluesofP×I.
Theexpressionwasconsideredweakpositiveincaseswith scorefrom0to5(1),andstrongpositiveonscore6orhigher (2).23
Forthecharacterizationoftheclinicalcharacteristicsand survivalanalysisofthesample,patientdatawereenteredinto
adatabaseusingthestatisticalprogramSPSS(Version19.0, Inc.,Chicago,IL).
A descriptive analysis of the clinical characteristics of patientswasconductedbymeansofmeasuresofcentral ten-dency(meanandmedian),dispersion(standarddeviation)for quantitativevariables.Frequenciesandpercentageswere cal-culatedforcategoricalclinicalvariables.
Pearson’schi-squaretest(2)wasusedtoevaluatethe
asso-ciationbetweentwocategoricalindependentvariables. Theanalysisofoverallsurvivalbasedonriskcriteria(ageat diagnosis,genderdistribution,primarytumorlocation, local-ized or metastatic disease atdiagnosis, site ofmetastasis, treatmentprotocol,localtreatmentmodality,serumLDH lev-els and expressionofIGFR) was performed byconsidering deathbyanycauseasaneventofinterest,and,endof follow-up (live patient) or loss offollow up as reasons forstudy termination.Ontheotherhand,ontheanalysisofevent-free survival,weadopted,asaneventofinterest,thedisease pro-gression (progressionand/or localor distantrecurrence) or deathby any cause,and asreasons forstudy termination, patientsthatwerealivewithoutprogressionofthedisease, withoutlocalordistantrecurrence,orlossoffollowup. Sur-vivalcurveswerecalculatedbytheKaplan–Meiermethodand forthecomparisonofsurvivalcurvesweusedtheLog-rank test.Prognosticfactorswereevaluatedaccordingtothemodel oftheunivariateCoxregressionforOSandEFS.Forpurposesof survivalanalysis,onlypatientstreatedintheperiodbetween 2003and2010wereincluded.
AnalyseswereperformedwiththestatisticalprogramSPSS (version19.0;Inc.,IL).Weadoptedasignificancelevelof5%for allcases.
Results
Theaverageageofpatientswas15years(standarddeviation [SD]=5.42years)rangingfrom2.34to28.57yearsandthere wasapredominanceofmalesin63.6%ofcases.Upon eval-uating the ethnic group, most of the patients were white, comprising83.1%ofourstudyandonly2.6%ofsubjectswere black(Table1).
Withregardtolocation,theboneinvolvementprevailed, affecting58.4% ofallcases.On patientswithbonelesions, 28.8%affectedtheaxialskeletonandintheinjuriesthat com-promised soft tissues (ST)the axial regionwas affected in 75.5%ofpatients(Table1).
Uponevaluatingthepresenceofmetastases,weobserved that 44.2% of patients had metastatic lesions atdiagnosis (Table1).Amongpatientsmetastaticatdiagnosis88.2%were male(p<0.001).
Themainsiteofmetastasiswasthelungmakingup52.9% ofthesample,followedbybonemetastasiswith8.8%(Table1). TheanalysisofserumLDHlevelsonpatientsincludedin thisstudyshowedthat38.6%ofpatientshadvaluesabove1.5 timestheupperreferencelimit(400mg/dl)(Table1).
Immunohistochemistrywasperformedon68tumor sam-ples from patients included in the study. The strong expressionofIGFRwasobservedin51.5%ofthesamplewhile 48.5%hadweakpositiveexpression(Table1).
Table1–Clinicalcharacteristicsandprognostic variablesofinterestof77patientsdiagnosedwithEFTs treatedatBCH,period2003–2012.
Variable n (%)
Age
≤15years 35 45.5
>15years 42 54.5
Gender
Male 49 (63.6)
Female 28 (36.4)
Race
White 64 (83.1)
Black 2 (2.6)
Brown 10 (13.0)
Yellow 1 (1.3)
Locationoftheprimarytumor
Skeletal 45 (58.4)
Axial 32 (71.1)
Extremity 13 (28.8)
Softtissue 32 (41.6)
Axial 8 (25.0)
Extremity 24 (75.5)
Diseaseatdiagnosis
Localized 43 (55.8)
Metastatic 34 (44.2)
Metastasissite
Lung 18 (52.9)
Bone 3 (8.8)
Lung+bone 8 (23.6)
Centralnervoussystem 3 (8.8)
Otherlocations 2 (5.8)
Treatmentprotocol
EuroEwing 10 (13)
Brazilian 46 (59.7)
SouthAmerican 21 (27.3)
Localcontrol
Surgery 18 (22.6%)
Radiotherapy 30 (39.4%)
Surgery+radiotherapy 15 (19.7%)
Withoutlocalcontrol 14 (18.3%)
SerumLDHlevela
<600mg/dl 43 (61.4)
≥600mg/dl 27 (38.6)
ExpressionofIGFRb
Weakpositive 33 (48.5)
Strongpositive 35 (51.5)
a 7patientshadnoserumLDHlevelsatdiagnosis.
b 9patientshadnobiologicalsamplesinthefilesofthePathology
Dept.
segments. For tumors located in soft tissues, the rib cage (Askintumors)wasthemainsiteaffected totaling43.8%of patients.
Allpatientsreceivedchemotherapypriortothelocal treat-ment of the neoplasia at BCH. The local treatment ofthe lesionwithradiotherapyand/orsurgerywasasfollows:22.6% ofpatientsweresubmittedonlytosurgery,39.4%underwent exclusiveradiotherapy,19.7%underwentsurgeryand radio-therapyand18.3%patientsdiedbeforetheendofneoadjuvant chemotherapy(Table1).
1.0
0.8
0.6
0.4
0.2
0.0
0 12 24 36
n=53
48 60
Time (months)
Cum
ulativ
e sur
viv
al
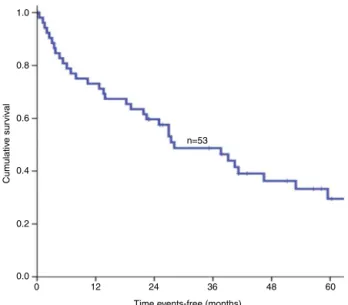
Fig.1–OScurveofpatientswithEFTstreatedatBarretos CancerHospital,2003–2010.
The treatment modality applied to the primary site of neoplasiaassociatedwiththelocationofthetumorshowed that in lesions with axial involvement, surgical treatment was performed in 29.7% oflesions withaxial involvement and55.9%inextremitylesions(p=0.023).Exclusive radiothe-rapy was indicated in 64.9% of patients withaxial lesions and 52.9% ofpatients with tumors locatedin the extrem-ities (p=0.218). A combination of modalities such as local treatment with surgery and radiotherapy was present in 18.9%ofpatientswithaxiallesions(p=0.069).Amongpatients metastatic at diagnosis 71.9% underwent local treatment with radiotherapy (p=0.048). The IGFR expression showed an association with the variable metastasis at diagnosis (p<0.001).
WefoundOSof45%at5years(Fig.1)andEFSof30%at5 years(Fig.2).
1.0
0.8
0.6
0.4
0.2
0.0
0 12 24 36
n=53
48 60
Time events-free (months)
Cum
ulativ
e sur
viv
al
Table2–Overallsurvivalprobabilityandcumulativeevent-freesurvivalaccordingtoclinicalcharacteristicsand prognosticvariablesofinterestforpatientsdiagnosedwithEFTstreatedatBCH,2003–2010.
Variable Overallsurvival Cumulativeevent-freesurvival
2-years 5-years pValue 2-years 5-years pValue
Overallsurvival 61% 45% 59% 30%
Agegroup 0.202 0.417
≤15years 72% 51% 69% 30%
>15years 47% 40% 48% 32%
Gender 0.555 0.587
Male 62% 37% 67% 30%
Female 58% 58% 50% 32%
Location 0.457 0.243
Skeletal 58% 35% 59% 18%
Softtissue 64% 53% 60% 40%
Location 0.256 0.502
Axial 59% 32% 59% 18%
Extremity 63% 58% 60% 40%
Metastasissite 0.293 0.009
Withoutmetastasis 69% 53% 63% 48%
Lung 88% 45% 75% 33%
Extrapulmonary 47% 40% 41% 10%
Localtreatment <0.001 0.008
Notreatment 20% 20% 20% 20%
Radiotherapy 63% 53% 58% 44%
Surgery 75% 64% 58% 48%
Surgery+radiotherapy 67% 33% 85% 0%
Treatmentprotocol 0.840 0.647
EuroEwing 78% 44% 67% 33%
Brazilian 57% 47% 58% 30%
SerumLDHlevel 0.710 0.747
<600mg/dl 66% 46% 64% 15%
≥600mg/dl 55% 46% 61% 34%
ExpressionofIGFR 0.502 0.628
Weakpositive 70% 43% 60% 15%
Strongpositive 65% 42% 61% 34%
Wedidnotfindstatisticallysignificantdifferenceswhen evaluatingOSand EFSforthe variables:agegroup,gender, location,treatment protocol, LDH levels and expression of IGFR(Table2).
We found a worse prognosis in EFS for patients with extrapulmonary metastases, showing a 5-year survival of 10% (p=0.009) (Table 2). Analyses of the results by the simple regression model showed statistical significance for EFS ofpatients with extrapulmonary metastases com-paredtopatientswithlungmetastasis(HR=3.13[1.33–7.37],
p=0.009).
There wasstatisticallysignificant differenceforbothOS and EFSforthe localtreatment oftumorand OS ratesfor patientsundergoingsurgeryof64%at5years(p<0.001)and 5-year EFS of 48% (p=0.008) (Table 2). The simple regres-sion model for local control with surgery was statistically significant (HR=0.11 [0.03–0.41], p=0.001)and OS (HR=0.19 [0.06–0.61],p=0.005).
Categorizingmetastaticpatientsintotwosubgroups,lung metastases and extrapulmonary metastasis, EFS was 10% at 5 years for patients with extrapulmonary metastasis (p=0.015) (Table 3). The univariate Cox regression showed
Table3–Overallsurvivalprobabilityandcumulativeevent-freesurvivalaccordingtothevariablelocationofmetastases inpatientsdiagnosedwithmetastaticEFTstreatedatBCH,intheperiod2003–2010.
Variable Overallsurvival Event-freesurvival
2-years 5-years pValue 2-years 5-years pValue
Metastasis 0.164 0.015
Lung 88% 45% 75% 33%
better prognosis for patients with lung metastases com-paredtopatientswithextrapulmonarymetastasis(HR=2.82 [1.18–6.76],p=0.009).
Discussion
The average age of patients enrolled in our study was 15 years (standard deviation [SD]=5.42 years). The literature emphasizesahigherincidenceintheseconddecadeoflife, with the majority ofpatients affected being young people under20years.6,24 Oursampleconsistedof63.6% ofmales
and 36.4% females, and literature describes the incidence ofEFTsslightly higherinmales.Retrospective studybased onthedatabasebelongingtotheNationalCancerInstitute’s Surveillance,involving725casesintheperiodbetween1989 and 2007obtaineda ratioofincidencebetween malesand femalesof3:2.25Anotherstudywithasampleof300patients
intheperiodbetween1980and2005showsafrequencyof 58%inmales.26Only2.6%ofblacksubjectsparticipatedinour
study.OurstudyisconsistentwiththeliteraturesinceEFTs isdescribedasbeingrareinblackpeople.6Astudyinvolving
220patientsfound93.2%ofwhitepatientsand6.8%ofother races.27
EFTs may be present in skeletal or soft tissues. Ofthe patientsincludedinthestudy,58.4%haddiseasewith skele-tallocationand41.6%locatedinsoftparts,whilesomestudies presentahigherincidenceinsofttissues.Amulticenterstudy involving114patientsindifferentcancertreatmentcentersin Turkeydemonstratesarateof53.5%fortumorslocatedinsoft tissues.11Incontrast,astudyconductedatSt.JudeChildren’s
ResearchHospitalfound87.9%ofpatientswithinvolvement oftheskeletalneoplasia.28Besidestheskeletalandsoft
tis-suelocation,thelocationofEFTscanbefurthersubdivided intoaxialandextremity;inourstudy,48.0%ofpatientshad neoplasiawithaxiallocationwhile52.0%wereskeletal loca-tion.Amongthoselocatedinsofttissues,75.5%wereaxial, whileinskeletal71.1%affectedtheextremity.Themainsites presentingskeletalinvolvementwerefemurandbonesofthe pelvis(22.2%foreachanatomicalregion)andintumorshaving softtissueinvolvementitwastheribcage(43.8%).The litera-tureinfersthatthemainsitesshowingEFTsskeletal involve-mentarebonesofthepelvis,femur,tibiaandhumerus.29In
EFTswithsofttissueinvolvement,themostfrequentlocation isthethoracicwall.11Incontrast,somecasesshow
involve-mentinthesofttissues,morecommonlyonextremities.8,30,31
Theevaluationoflocalizedormetastaticdiseaseatdiagnosis showedthat44.2%ofpatientshadmetastaticdisease,withthe pulmonarysiteaffectedin46.8%followedbytheskeletalsite with28.6%.Thisfindingmaysuggestandbeexplainedbythe delayeddiagnosisinsomeofthesepatientsbecauseofthe dif-ficultyofaccesstospecializedcenters,sinceaccordingto liter-atureabout25–30%ofpatientsdiagnosedwithEFTspresented metastases at diagnosis.32,33 Other studies show a rate of
metastasesatdiagnosisof13%inpatientswithprimarytumor locationinthesofttissues.34Thepreferredsiteof
metasta-sisdescribed inliteratureisconsistentwiththefindings in thisstudy,wherelungisthemainsite(50%ofcases),followed byboneinvolvement(25%)and/orbonemarrow (20%).33In
ourstudy,lungwasthemostcommonsiteofmetastasiswith
52.9%.Weobservedthat88.2%ofpatientsmetastaticat diag-nosisweremale,withanassociationbetweenthevariables genderandmetastasisatdiagnosis(p<0.001).
Among the patients included in the study, 18.3% died before local treatment. Ofpatients undergoing local treat-ment,surgerywascarriedoutin22.6%ofthecases,exclusive radiotherapy in39.4% andthecombination ofsurgerywith radiotherapyin19.7%.Thecompletesurgicalremovalis con-sideredthemodalityofchoiceforlocaltreatment,showinga lowerrateoflocalrecurrencecomparedwithisolated radio-therapy forthetreatmentofthe primarytumor.10 Thelow
rateofisolatedsurgeryand/orcombinedwithradiotherapy seen in our study may be explained once we got a rate of 44.2%for metastases atdiagnosis.Metastasis is consid-eredtheworstprognosticfactorforpatientswithEFTs.32In
metastatic patients with unresectable metastases, surgical treatment, which canoften involveloss ofbodysegments, is losing its place to local treatment with radiotherapy. Among patients metastatic at diagnosis 71.9% underwent radiotherapyastreatmentoftheprimarysiteofthetumor, displaying an associationbetween the variables metastatic disease and radiotherapy (p=0.048). Upon evaluating the local treatment modality associated tothe primary siteof the tumor, we observed that in lesions of axial involve-ment the surgical treatment was performed in 29.7% and in the lesions of extremity in 59.5%, showing an associ-ation between the variables local treatment modality and tumorlocation (p=0.023). Thisfactisexplainedbythe dif-ficulty of surgical access and the obtaining of adequate surgicalmarginsinlesionsofaxialinvolvement.The appar-entsuperiorityofthesurgerymayrepresentaselectionbias, sincemostcentralandlargerlesionsareoftentreatedwith radiotherapy.35
EFTsbiologicalsamplesusedinthisstudyshowed51.5% ofspecimenswithstronglyexpressedIGFRand 48.5%with weakpositivity.When weevaluatedthepresenceof metas-tasesatdiagnosisalongwiththeexpressionofIGFRwefound anassociationbetweenthevariables(p<0.001).Theliterature, inprinciple, doesnotcontemplatetheassociationbetween expressionofIGFRandmetastasisatdiagnosis.Perhapsthe strongpositivityfortheexpressionofIGFRmaybeapredictor ofmetastasisatdiagnosisinEFTs.
Wefound anOSof45% at5years and 30%ofEFSat5 years.TheliteratureshowsEFSaround70%withthe aware-nessofthechemotherapyadministrationconceptofinterval regimeninadditiontolocaltreatment.6Incontrast,studies
showthattheadvancedcaseshavenotpresentedencouraging resultswhensubjectedtothestandardtreatmentapproach, withregardtoimprovingsurvivalrates,wherethe5-yearrate hasbeenrecentlydescribedaslowerthan25%.36Ourstudy
involvedagreaterportionofpatientsmetastaticat diagno-sisthanthatdescribedintheliterature;sincemetastasisat diagnosisistheworstprognosticfactor,webelievethatEFS of30% at5years forthe patients includedinthe study is justified.
However,areviewoftwoGermanclinicaltrialsforthe treat-mentofESinvolvingpatientsolderthan40yearsatdiagnosis had a survival comparable to adolescents treated in the same trial.17 Our study foundthat patients agedless than
15 years,the curve shows improved survivalinthis group comparedwitholderpatients,however,therewereno statisti-callysignificantdifferencesbetweenthecurvesofOSandEFS (respectively,p=0.202andp=0.417).
OurstudysuggestsbetterOSandEFSinfemalesubjects withOSof58%at5yearsandEFSof32%,butnostatistical significancewasfound(respectively,p=0.555and p=0.587). Studies showthe variable gender asaprognostic factor in EFTs,indicatinggreatersurvivalinfemales.14
OurresultsfavorhigherOSandEFSinpatientswithtumors located in soft tissues when compared to those of skele-talsite,however,therewasnostatisticalsignificanceinthe analysis(respectively,p=0.457andp=0.243).Literaturedata makesinferencetoworseprognosisintumorslocalizedinsoft tissue.12Astudyusingcancerregistry-basedpopulationfound
nodifferenceinprognosiswithrespecttoaxialorextremity locationofthetumor.25 UponevaluatingOSandEFSinour
study,thedata suggest betterOSand EFSinpatients with extremitylesionscomparedwithlesionsofaxialinvolvement, however, there wasno statistical significance(respectively,
p=0.256andp=0.502).
EFSwasstatisticallysignificantwhenanalyzingthe vari-ablemetastasesatdiagnosis,witharateof48% at5years fornon-metastaticpatients,33%forthosewithlung metas-tasesand10%forextrapulmonarymetastases(p=0.009).Upon evaluating these data in a univariate Cox regression, we found a worse prognosis in patients with extrapulmonary metastasis(HR=3.13[1.33–7.37],p=0.009).Uponcategorizing metastaticpatientsintotwosubgroups,pulmonary metasta-sisandextrapulmonarymetastasis,wealsoobservedaworse prognosisin patientsaffected byextrapulmonary metasta-sis, with EFS of 10% at 5 years (p=0.015). The univariate Cox regression showed better prognosis for patients with lungmetastasescomparedtopatientswithextrapulmonary metastasis (HR=2.82 [1.18–6.76], p=0.009).Literature shows a worse prognosis for extrapulmonary metastases.7,10,37
The presence of bone metastasis only seems to have a better prognosis compared to patients who, in addition to extrapulmonary involvement, also displayed pulmonary involvement.38
Thelocaltreatment modality demonstrates a betterOS and EFS forpatients submitted tosurgery alone, and 64% at5yearsforOSand48%forEFS,showingastatistical sig-nificance(respectively,p<0.001andp=0.008).UnivariateCox regressionforlocalcontrolwithsurgerywasstatistically sig-nificant(HR=0.11[0.03–0.41],p=0.001).Asmentionedabove, thissuperiorityforthesurgerymayrepresentaselectionbias, sincelargerandmorecentrallesionsareoftentreatedwith radiotherapy.37Westresstheneedforrandomizedclinical
tri-alstobetterassessthesurgicalsuperiorityoverother local treatmentmodalities.
SerumLDHlevelwascategorizedinto2groups:<600mg/dl and≥600mg/dl.OS in5years was46% forthetwogroups
in question, with no noticeable statistical significance for the variable (p=0.710). Seven patients had no serum LDH level at diagnosis, which may have been responsible for
thesimilarresults,sinceincreasedserumLDHlevelsbefore treatment are associated witha worseprognosis, and this increasecanalsobecorrelatedwithlargeprimarytumorsand metastasis.14
Survival analysis consideringthe variable expressionof IGFRwas42%at5yearsforOSincaseofstrongpositiveand 42%forweakpositive(p=0.502).WhenweanalyzedEFS,we observedasurvivalof34%at5yearsforthestrongly posi-tiveand15%fortheweakpositive(p=0.628).Therewasno statistical significance in survivalanalysis considering the variableexpressionofIGFR.Thisfactmaybeexplainedbythe sizeofthesample.Ninepatientsdidnothaveanybiological samplesinthePathologyDepartment.Theuseofbiological samplesinstudies involving cytogeneticevaluationhas its limitations.Muchofthismaterialconsistsoffragmentsof tis-sueobtainedbybiopsybywayofneedles,thus,scarce.The substanceusedforfixingsuchmaterialscanalsobea deter-miningfactor,especiallyinmolecularstudies.Thecreation oftheso-calledtumorbanks,withthesnapfreezingof sam-ples,maybethewayfortheoptimizationofstudiesinvolving cytogenetics.
Severalstudiesseekingtargetedtherapiesthatarefocused on blockingthe activity ofthe IGF receptorhavebeen per-formed. A recent review demonstrates that in sometypes of tumorsthe use ofdrugs toblock the IGF1 receptorhas worsened thesurvivalofthesepatients. APhaseII clinical trialinvolvingtreatmentwithdrugsblockingtheIGF1 recep-torinbreastcanceraftermenopause,withpositivereceptor, showedworseoverallsurvival.Itwasalsomentionedthatthe resistancemechanismstotargetedtherapywiththeblocking ofIGF1-Rmaybeinvolvedincombinationwithother recep-torssuchasEGFR,andconcludedthatthesignalingpathways involvedinIGFRaremorecomplexthanoriginallythought.39
Conclusions
WeconcludethatpatientswithadiagnosisofEFTs,treatedat BarretosCancerHospital,showahigherincidenceof metas-tases at diagnosis when compared to data from current literature.Thestrong expressionofIGFRismore prevalent in patients with metastases at diagnosis. Extrapulmonary metastaseswereassociatedwithworseprognosis.The exclu-sivesurgicalmanagementoftheprimarysitewasassociated withbetterprognosiscomparedwithother localtreatment modalities.Atfirst,theexpressionofIGFRdidnotbehaveasa prognosticfactorinEFTs.
Conflicts
of
interest
Theauthorsdeclarenoconflictsofinterest.
r
e
f
e
r
e
n
c
e
s
2. DeiTosAP,DalCinP.Theroleofcytogeneticsinthe classificationofsofttissuetumours.VirchowsArch. 1997;431(2):83–94.
3. DelattreO,ZucmanJ,MelotT,GarauXS,ZuckerJM,Lenoir GM,etal.TheEwingfamilyoftumors–asubgroupof small-round-celltumorsdefinedbyspecificchimeric transcripts.NEnglJMed.1994;331(5):294–9.
4. KovarH,AryeeD,ZoubekA.TheEwingfamilyoftumorsand thesearchfortheAchilles’heel.CurrOpinOncol.
1999;11(4):275–84.
5. GattaG,vanderZwanJM,CasaliPG,SieslingS,DeiTosAP, KunklerI,etal.Rarecancersarenotsorare:therarecancer burdeninEurope.EurJCancer.2011;47(17):2493–511.
6. PizzoPA,PoplackDG.Principlesandpracticeofpediatric oncology.5thed.Philadelphia:LippincottWilliamsand Wilkins;2005.
7. Rodriguez-GalindoC,PoquetteCA,MarinaNM,HeadDR,Cain A,MeyerWH,etal.Hematologicabnormalitiesandacute myeloidleukemiainchildrenandadolescentsadministered intensifiedchemotherapyfortheEwingsarcomafamilyof tumors.JPediatrHematolOncol.2000;22(4):321–9.
8. KinsellaTJ,TricheTJ,DickmanPS,CostaJ,TepperJE, GlaubigerD.ExtraskeletalEwing’ssarcoma:resultsof combinedmodalitytreatment.JClinOncol.1983;1(8):489–95.
9. AhmadR,MayolBR,DavisM,RougraffBT.Extraskeletal Ewing’ssarcoma.Cancer.1999;85(3):725–31.
10.CotterillSJ,AhrensS,PaulussenM,JürgensHF,VoûtePA, GadnerH,etal.PrognosticfactorsinEwing’stumorofbone: analysisof975patientsfromtheEuropeanIntergroup CooperativeEwing’sSarcomaStudyGroup.JClinOncol. 2000;18(17):3108–14.
11.ArpaciE,YetisyigitT,SekerM,UncuD,UyeturkU,Oksuzoglu B,etal.Prognosticfactorsandclinicaloutcomeofpatients withEwing’ssarcomafamilyoftumorsinadults:multicentric studyoftheAnatolianSocietyofMedicalOncology.Med Oncol.2013;30(1):469.
12.AngervallL,EnzingerFM.Extraskeletalneoplasmresembling Ewing’ssarcoma.Cancer.1975;36(1):240–51.
13.TuralD,MolinasMandelN,DervisogluS,OnerDincbasF,Koca S,ColpanOksuzD,etal.ExtraskeletalEwing’ssarcomafamily oftumorsinadults:prognosticfactorsandclinicaloutcome. JpnJClinOncol.2012;42(5):420–6.
14.BacciG,LonghiA,FerrariS,MercuriM,VersariM,BertoniF. Prognosticfactorsinnon-metastaticEwing’ssarcomatumor ofbone:ananalysisof579patientstreatedatasingle institutionwithadjuvantorneoadjuvantchemotherapy between1972and1998.ActaOncol.2006;45(4):469–75.
15.vandenBergH,DirksenU,RanftA,JürgensH.Ewingtumors ininfants.PediatrBloodCancer.2008;50(4):761–4.
16.GrierHE,KrailoMD,TarbellNJ,LinkMP,FryerCJ,PritchardDJ, etal.Additionofifosfamideandetoposidetostandard chemotherapyforEwing’ssarcomaandprimitive neuroectodermaltumorofbone.NEnglJMed. 2003;348(8):694–701.
17.PieperS,RanftA,Braun-MunzingerG,JurgensH,Paulussen M,DirksenU.Ewing’stumorsovertheageof40:a
retrospectiveanalysisof47patientstreatedaccordingtothe InternationalClinicalTrialsEICESS92andEURO-E.W.I.N.G. 99.Onkologie.2008;31(12):657–63.
18.JawadMU,CheungMC,MinES,SchneiderbauerMM,Koniaris LG,ScullySP.Ewingsarcomademonstratesracialdisparities inincidence-relatedandsex-relateddifferencesinoutcome: ananalysisof1631casesfromtheSEERdatabase,1973-2005. Cancer.2009;115(15):3526–36.
19.MountziosG,KostopoulosI,KotoulaV,SfakianakiI, FountzilasE,MarkouK,etal.Insulin-likegrowthfactor1 receptor(IGF1R)expressionandsurvivalinoperable squamous-celllaryngealcancer.PLoSOne.2013;8(1):e54048.
20.HoosA,UristMJ,StojadinovicA,MastoridesS,DudasME, LeungDH,etal.Validationoftissuemicroarraysfor immunohistochemicalprofilingofcancerspecimensusing theexampleofhumanfibroblastictumors.AmJPathol. 2001;158(4):1245–51.
21.BertucciF,BorieN,GinestierC,GrouletA,Charafe-JauffretE, AdélaïdeJ,etal.IdentificationandvalidationofanERBB2 geneexpressionsignatureinbreastcancers.Oncogene. 2004;23(14):2564–75.
22.BendardafR,BuhmeidaA,RistamäkiR,SyrjänenK,Pyrhönen S.MMP-1(collagenase-1)expressioninprimarycolorectal canceranditsmetastases.ScandJGastroenterol. 2007;42(12):1473–8.
23.DongXT,YangXJ,WangHM,WangW,YuL,ZhangB,etal. Expressionanddistributioncharacteristicsofhuman orthologofmammalianenabled(hMena)inglioma.ChinJ CancerRes.2011;23(4):312–6.
24.LanzkowskyP.Manualofpediatrichematologyandoncology. 4thed.CA,USA:ElsevierInc.;2005.p.596–9.
25.LeeJ,HoangBH,ZiogasA,ZellJA.Analysisofprognostic factorsinEwingsarcomausingapopulation-basedcancer registry.Cancer.2010;116(8):1964–73.
26.PradhanA,GrimerRJ,SpoonerD,PeakeD,CarterSR,Tillman RM,etal.OncologicaloutcomesofpatientswithEwing’s sarcoma:isthereadifferencebetweenskeletaland extra-skeletalEwing’ssarcoma?JBoneJointSurgBr. 2011;93(4):531–6.
27.Rodríguez-GalindoC,LiuT,KrasinMJ,WuJ,BillupsCA,Daw NC,etal.Analysisofprognosticfactorsinewingsarcoma familyoftumors:reviewofSt.JudeChildren’sResearch Hospitalstudies.Cancer.2007;110(2):375–84.
28.KrasinMJ,DavidoffAM,Rodriguez-GalindoC,BillupsCA, FullerCE,NeelMD,etal.Definitivesurgeryandmultiagent systemictherapyforpatientswithlocalizedEwingsarcoma familyoftumors:localoutcomeandprognosticfactors. Cancer.2005;104(2):367–73.
29.JedlickaP.EwingSarcoma,anenigmaticmalignancyoflikely progenitorcellorigin,drivenbytranscriptionfactor
oncogenicfusions.IntJClinExpPathol.2010;3(4): 338–47.
30.RudNP,ReimanHM,PritchardDJ,FrassicaFJ,SmithsonWA. ExtraosseousEwing’ssarcoma.Astudyof42cases.Cancer. 1989;64(7):1548–53.
31.SiebenrockKA,NascimentoAG,RockMG.Comparisonofsoft tissueEwing’ssarcomaandperipheralneuroectodermal tumor.ClinOrthopRelatRes.1996;(329):288–99.
32.EsiashviliN,GoodmanM,MarcusRBJr.Changesinincidence andsurvivalofEwingsarcomapatientsoverthepast3 decades:surveillanceepidemiologyandendresultsdata.J PediatrHematolOncol.2008;30(6):425–30.
33.GrierHE.TheEwingfamilyoftumors.Ewing’ssarcomaand primitiveneuroectodermaltumors.PediatrClinNorthAm. 1997;44(4):991–1004.
34.OrrWS,DenboJW,BillupsCA,WuJ,NavidF,RaoBN,etal. AnalysisofprognosticfactorsinextraosseousEwingsarcoma familyoftumors:reviewofSt.JudeChildren’sResearch Hospitalexperience.AnnSurgOncol.2012;19(12): 3816–22.
35.ShambergerRC,LaquagliaMP,KrailoMD,MiserJS, PritchardDJ,GebhardtMC,etal.Ewingsarcomaoftherib: resultsofanintergroupstudywithanalysisofoutcomeby timingofresection.JThoracCardiovascSurg.
2000;119(6):1154–61.
36.RossKA,SmythNA,MurawskiCD,KennedyJG.Thebiologyof ewingsarcoma.ISRNOncol.2013;2013:759725.
ifosfamideandetoposide–aChildren’sCancerGroupand PediatricOncologyGroupstudy.JClinOncol.
2004;22(14):2873–6.
38.PaulussenM,AhrensS,BurdachS,CraftA,
Dockhorn-DworniczakB,DunstJ,etal.Primarymetastatic
(stageIV)Ewingtumor:survivalanalysisof171patientsfrom theEICESSstudies.EuropeanIntergroupCooperativeEwing SarcomaStudies.AnnOncol.1998;9(3):275–81.