DOI: http://dx.doi.org/10.1590/1516-1439.302614
Materials Research. 2014; 17(6): 1620-1627 © 2014
*e-mail: wellen.renate@gmail.com
1. Introduction
Poly(ethylene terephthalate) (PET) is a semi crystalline thermoplastic widely used by the food and health care packaging industries in the production bottles and containers for soft drink beverages, oils, creams, etc. The PET molecule is made of alternating rigid aromatic terephthalate units and
lexible aliphatic ethylene glycol units:
C O
CH
2
O CH
2 O
C O
n
Industrial production of PET bottles is performed in two steps: injection molding of a preform (Figure 1a), and
blow molding of the preform to obtain the inished product
(Figure 1d).
Crystallization may occur during cooling from the molten polymer, or during heating of a mostly amorphous
solid sample, a process deined as cold crystallization. The
degree of crystallinity and other crystallization parameters affect the final product properties. A tight control of crystallinity is a necessary condition to obtain high performance bottles. Excessive crystallinity may impair the stretching and blowing cycles, while very low crystallinity results in poor mechanical and barrier properties1-11.
Although heating and cooling rates during industrial processing are very high and the material is subjected to severe dynamical stresses, differential scanning calorimetry (DSC) static tests, carried under far milder conditions, may be used as a guide to select the processing methods. Moreover, precise determination of optimal melting
parameters is needed to avoid polymer degradation during processing and excessive energy consumption12-14.
According to packing industry cold crystallized preforms are a serious waste of money; thus, the solution to this problem would be very welcome. Much research has been, and still is, dedicated to the thermodynamics and kinetics of phase change – both from a macroscopic and microscopic point of view – in highly crystalline polymers. From previous work we know that blending a small amount of an amorphous polymer may hinder unwelcomed cold crystallization of PET and prevent excessive crystallinity under processing conditions. Thus, polystyrene, an apolar amorphous polymer appears to be a good candidate12,15-21.
The present work is concerned with an investigation of the melting and nonisothermal cold crystallization of PET and PET/PS blends with 1 to 60% of PS content. DSC and scanning electron microscopy (SEM) techniques were employed to investigate crystallization and melting parameters, and morphology. This work draws freely on previous publications of the author on the subject.
2. Experimental
2.1. Materials
Bottle grade PET, trade name Rhopet S78 was supplied
by Rhodia (Brazil), with an intrinsic viscosity [η] = 0.78 dL/g
and weight-average molar mass Mw = 48 kg/mol. Injection grade PS, trade name Styron 649D, was purchased from Dow (Brazil). Preliminary DSC scans of the polymers showed glass transitions at 70 °C and 95 °C for PET and PS, and the complete absence of crystallinity of the latter (Figure 2a). DSC scan of inished PET bottle is showed in Figure 2b; no cold crystallization peak is observed.
Effect of Polystyrene on Poly(Ethylene Terephthalate) Crystallization
Renate Maria Ramos Wellen*
Department of Materials Engineering, Federal University of Campina Grande – UFCG, CEP 58429-140, Campina Grande, PB, Brazil
Received: June 13, 2014; Revised: November 12, 2014
Blends of poly(ethylene terephthalate) (PET) and polystyrene (PS) with PS content ranging from 0% to 60% by weight, were compounded in a laboratory internal mixer, followed by quenching into iced water, resulting in substantially amorphous compounds. Blend morphology was analyzed by scanning electron microscopy (SEM). Standard differential scanning calorimetry (DSC) was used to study the cold crystallization and melting behavior of PET and PET/PS blends. SEM micrographs show a two-phase structure made up with spherical PS particles dispersed in a PET matrix. DSC scans showed that crystallization of PET is affected by the heating rate and by the addition of even
small amounts of PS. In particular, the rate of cold crystallization of PET is signiicantly reduced by
the incorporation of 1% of PS, attributed to the anti-nucleating effect of PS on PET. The amount of
PET crystallized and the melting characteristics (temperature, rate) are not signiicantly affected by
either PS content or heating rate.
2.2. Methods
Before mixing the polymers were dried in oven with forced air circulation to remove humidity and prevent PET degradation during processing. PET was dried at 120 °C for 6 hours and PS at 80 °C for 14 hours. The blends were
prepared in a Haake Rheomix 600 internal mixer itted
with high intensity (roller) rotors at 265 °C and 60 rpm for 10 min. Immediately after mixing the melt was quenched into iced water to prevent PET crystallization and to obtain substantially amorphous samples.
Nonisothermal cold crystallization and fusion were investigated using a differential scanning calorimeter Shimadzu DSC-50. Glassy samples, from 5 to 8 mg in Figure 1. PET injected preforms: (a) and (b); and blow molded bottles: (c) and (d). Unintended cold crystallization can be visualized in (b) as a whitening zone, resulting in a malformed bottle (d).
Wellen
1622 Materials Research
weight, of neat PET and PET/PS blends with 1 to 60% PS were heated from room temperature to 300 °C at 10 °C/ min. A detailed investigation was done employing heating rates ranging between 1 and 50 °C/min. All samples were tested wrapped in aluminum foil to get better heat transfer22.
Scanning electron microscopy analyses were performed in a Shimadzu SSX 550 Superscan. The samples were fractured in liquid nitrogen and covered with gold to avoid static charges.
3. Results and Discussion
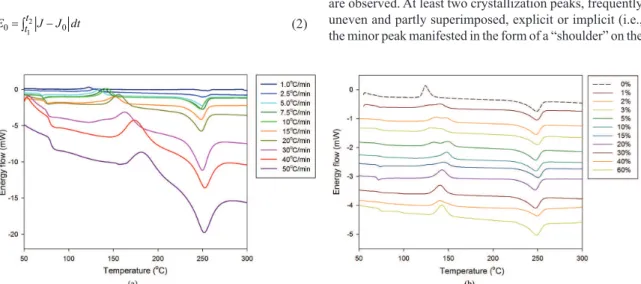
Figure 3 shows raw DSC plots of heat low versus temperature of neat PET obtained at different heating rates (a) and of PET/PS blends of different compositions, taken at 10 °C/min.
A preliminary look at the plots reveals a glass transition around 70 °C, an exothermic event at 100-200 °C, attributed to the cold crystallization of PET, and an endothermic event further down at 200-300 °C, attributed to the melting of PET. PS addition and increasing heating rates result in the displacement of the crystallization peak to higher temperatures, and contribute to a wider range of cold crystallization phenomenon which are probably linked to the segregation between PS and PET molecules and to diffusion mechanisms, however, they have little effect on the melting peak. At high heating rates (greater than 30 °C/min) a partial overlap of the cold crystallization and melting peaks was observed; it may result from the lack of time to complete the crystallization at these rates. Complex cold crystallization peaks were observed for PET/PS blends with less that 10% PS. According to literature, the shape of crystallization peaks is related to morphology of crystalline phase23-28.
Both peaks, exothermic crystallization peak and endothermic fusion peak, were integrated from starting to end points visually determined, using a straight virtual baseline between them, to obtain the mass fractions crystallized or fused, respectively:
1 0 0 1 ( ) ( ) t t
x J t J t dt
E ′ ′ ′
= ∫ − (1)
2 1
0 tt 0
E =∫ J−J dt (2)
where J is the DSC output, J0 is the virtual baseline during the event, t1 and t2 are the onset and end times of the event, and E0 is the latent heat of phase change. The normalized peak, which is also the rate of phase change (melting or crystallization):
0 0
1
dx
c J J
dt E
= = − (3)
It is convenient to measure times for the onset of the
event, τ = t – t1. Both x and c may be considered as functions
of either time (τ) or temperature (T), since temperature is a linear function of time at constant heating rate, T = T0 + ϕτ,
where ϕ is the heating rate (dT/dτ) and T0 is the temperature
at onset of the event, τ = 0.
Figure 4 shows the relative crystallinity developed during the cold crystallization of PET, and crystallization rate, as functions of temperature, at several heating rates. The crystallization process begins at very low rate, probably due to the small surface area of initial crystallites; this stage of crystal growth is sometimes associated in the literature with a nucleation process. Crystallization ends at very low rate, due to depletion of crystallizable polymer and (for high crystallinity materials) to a decrease of the interfacial area caused by interference between competing growing crystals,
among other reasons. Between initial and inal stages, the
crystallization rate reaches a maximum, at a temperature
identiied as crystallization temperature of the polymer1,28-38. The cold crystallization temperature of PET samples tested increased from 110 °C when heated at 1 °C/min to 150 °C when heated at 50 °C/min. Within the same range of heating rates, the maximum crystallization rate changed from 0.1 min–1 to 2.0 min–1. In contrast, the crystallinity, computed assuming a latent heat of fusion of the 100% crystalline PET equal to 140 kJ/kg, was found to be visually independent of the heating rate, remaining at 16 ± 1% for conditions tested39,40.
Figure 5 shows the relative crystallinity and the crystallization rate as functions of temperature for PET and PET/PS blends of different composition, measured at heating rate of 10 °C/min.
In presence of PS, complex cold crystallization peaks are observed. At least two crystallization peaks, frequently uneven and partly superimposed, explicit or implicit (i.e., the minor peak manifested in the form of a “shoulder” on the
major peak) are identiied in Figure 5b for PS contents below 30%. It should be noted that, under the tests conditions PS is a completely amorphous polymer, immiscible in PET; the solubility limit of PS in PET is believed to be less than 1%, thus it is possible that once the solubility limit between PET and PS to be reached PS is segregated at amorphous zone then producing little effect on the crystalline phase of PET. In literature database several explanations are presented for the multiple crystallization phenomenon: two kinds of amorphous regions, inter-lamellar and inter-spherulite, different crystalline geometries, co-crystallization and fractional crystallization, secondary or recrystallization effects6,9,11,12,14,28,30-31,35,41-55.
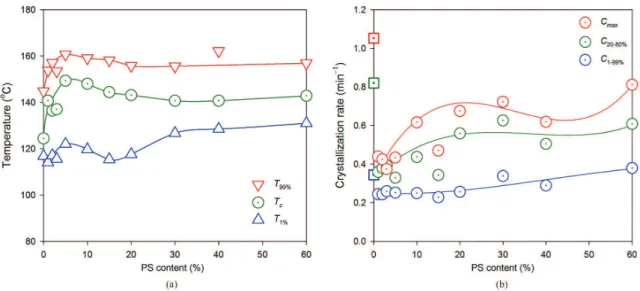
PET crystallization in PET/PS blends proceeds at a higher temperature and lower crystallization rate than in the neat resin. These features, clearly observed in Figure 5, are quantitatively illustrated in Figure 6. Figure 6a shows crystallization temperature (TC) along with initial (T1%)
and inal (T99%) values of the crystallization temperature range; Figure 6b shows the maximum crystallization rate (cMax) and the average crystallization rates in the intervals 1% to 99% and 20%-80% of relative crystallinity. An interesting characteristic of PET/PS system is the mild dependence of crystallization temperature increases
and crystallization rate dependences on the amount of PS present. The peak crystallization temperature varies between 124 °C and 150 °C depending on the PS content.
Maximum crystallization rate is signiicantly depressed by
the incorporation of small amounts of PS, from 1.1 min–1 for pure PET to 0.3-0.4 min–1 for 1-5% PS, and partially recovers with increasing PS content, up to 0.8 min–1 for 60% PS. The total amount of PET crystallized, however, is independent of PS content, remaining at 16.7 ± 1.7%. The 70% drop in the crystallization rate of PET resulting from the addition of PS in such small amounts has been attributed to the interference of amorphous PS (dissolved or segregated) on the generation of crystal PET nuclei14. Hence, we speak of the antinucleating effect of PS on the crystallization of PET. Figure 7 shows the molten fraction as a function of time measured from the onset of the event, and the rate of melting as a function of the molten fraction. In contrast with the crystallization process, melting shows a very regular, asymmetric simple peak, with peak melting temperature of 249 ± 1 °C, maximum melting rate of 0.7 ± 0.1 min−1 and crystallinity of 22 ± 2% in the interval 0-60% PS content. Comparing this value with the crystallinity recovered during cold crystallization reveals about 5% crystallinity for the “amorphous” samples.
Figure 4. Relative crystallinity (a) and crystallization rate (b) of neat PET as function of temperature.
Wellen
1624 Materials Research
Figure 6. Crystallization temperature (a) and rate of crystallization (b) as functions of PS content.
Figure 7. Molten fraction as a function of time (a) and melting rate as a function of molten fraction (b).
SEM images of fractured surface of PET/PS blends with 1% and 15% PS are shown in Figure 8. A two-phase
morphology, with 0.8 μm to 2.4 μm diameter PS spherical
particles, well dispersed in the amorphous PET matrix, is visible. The clean PS/PET interfaces suggest a poor particle/ matrix adhesion, compatible results with low solubility limit between phases.
Figure 9 presents two photographs taken during the industrial processing of PET bottles: (a) preform heating, (b) preform blowing and bottle confection. Two compositions were selected to make bottles at industrial scale, neat PET and 1% PS blend.
Apparently, 1% PS has a slightly yellowing tone, likely due to immiscible PET and PS resins, which have different refractive index; the large difference in the optimum processing temperature of PET and PS may have resulted in degraded specimens as well. In general, acceptable PET bottles are produced with both compositions.
4. Conclusions
Addition of amorphous PS to semi crystalline PET affects the nonisothermal cold crystallization process of the later, but not the total amount crystallized. The most
important effect is the signiicant reduction of the PET
cold crystallization rate by the presence of small amounts of PS. Melting of crystallized samples is not affected by the presence of PS. Neat PET and PET/PS blend preforms and bottles are successfully produced; both compositions may be applied at industrial scale.
Acknowledgements
The author wishes to thanks Rhodia (presently M&G)/ Brazil for providing the PET, CNPq/Brazil, and FACEPE/
Brazil for the inancial support, and Frederico Custodio,
manager at Sabic, by encouragement. Figure 9. Industrial production of PET bottles: (a) pre-form heating, and (b) bottle blowing.
References
1. Basset DC. Principles of polymer morphology. Cambridge: Cambridge University Press; 1981.
2. Hoffman JD and Weeks JJ. Rate of spherulitic crystallization with chain folds in polychlorotrifluoroethylene. The Journal of Chemical Physics. 1962; 37(8):1723-1741. http://dx.doi. org/10.1063/1.1733363.
3. Hoffman JD and Weeks JJ. Melting process and the equilibrium melting temperature of polyclorotrifluoroethylene. Journal of Research of the National Bureau of Standards. 1962; 66-A(1):3-28.
4. Baer E, Collier JR and Carter DR. Kinetics of spherulitic crystallization. Polymer Engineering and Science. 1965; 5(1):22-28. http://dx.doi.org/10.1002/pen.760050105.
5. Fakirov S, Seganov I and Kurdowa E. Effect of chain composition of poly(ethylene terephthalate) structure and properties. Die Makromolekulare Chemie. 1981; 182(1):185-197. http://dx.doi.org/10.1002/macp.1981.021820120.
6. Aharoni SM. Nucleation of PET crystallization by metal hydroxides. Journal of applied Polymer Science. 1984; 29(3):853-865. http://dx.doi.org/10.1002/app.1984.070290314.
7. Hu YS, Hiltner A and Baer E. Improving oxygen barrier properties of poly(ethylene terephthalate) by incorporating isophthalate. II. Effect of crystallization. Journal of applied Polymer Science. 2005; 98(4):1629-1642. http://dx.doi. org/10.1002/app.22214.
8. Alfonso GC. Crystallization in polymer blends. In: Lemstra PJ and Kleintjens LA, editors. Integration of fundamental polymer science and technology. Limburg: Springer Netherlands; 1991.
p. 143-160.
9. Keith HD and Padden FJ Jr. Spherulitic crystallization from the melt. I. Fractionation and impurity segregation and their influence on crystalline morphology. Journal of applied Physics. 1964; 35(4):1270-1285. http://dx.doi. org/10.1063/1.1713606.
10. Keith HD and Padden FJ Jr. Spherulitic crystallization from the melt. II. influence of fractionation and impurity segregation on the kinetics of crystallization. Journal of applied Physics. 1964; 35(4):1286-1296. http://dx.doi.org/10.1063/1.1713607.
Wellen
1626 Materials Research
Polymer Physics. 1987; 25(11):2371-2392. http://dx.doi. org/10.1002/polb.1987.090251113.
12. Wellen RMR and Rabello MS. Antinucleating action of polystyrene on the isothermal cold crystallization of poly(ethylene terephthalate). Journal of applied Polymer Science. 2009; 114(3):1884-1895. http://dx.doi.org/10.1002/ app.29569.
13. Wellen RMR, Canedo EL and Rabello MS. Nonisothermal
cold crystallization of poly(ethylene terephthalate). Journal of Materials Research. 2011; 26(9):1107-1115. http://dx.doi. org/10.1557/jmr.2011.44.
14. Wellen RMR. Cold crystallization of PET, PET/PS and PET/ SaN. [Thesis]. Campina Grande: Department of Materials
Engineering, Federal University of Campina Grande; 2007. 15. Wellen RMR and Rabello MS. Non-isothermal cold
crystallization kinetics and morphology of PET + SAN blends.
Journal of applied Polymer Science. 2010; 116(2):1077-1087.
16. Hu YS, Liu RYF, Rogunova M, Schiraldi DA, Nazarenko S,
Hiltner A, et al. Oxygen-barrier properties of cold-crystallized and melt-crystallized poly(ethylene terephthalate-co-4,4’-bibenzoate). Journal of applied Polymer Science, Part B: Polymer Physics. 2002; 40(22):2489-2503. http://dx.doi. org/10.1002/polb.10307.
17. Li B, Yu J, Lee S and Ree M. Poly(ethylene terephthalate
co ethylene isophthalate): relationship between physical properties and chemical structures. European Polymer Journal. 1999; 35(9):1607-1610. http://dx.doi.org/10.1016/S0014-3057(98)00252-3.
18. Liu RYF, Hu YS, Hibbs MR, Collard DM, Schiraldi DA,
Hiltner A, et al. Improving oxygen barrier properties of poly(ethylene terephthalate) by incorporating isophthalate. I. Effect of orientation. Journal of applied Polymer Science. 2005; 98(4):1615-1628. http://dx.doi.org/10.1002/app.22213.
19. Liu RYF, Hu YS, Schiraldi DA, Hiltner A and Baer E.
Crystallinity and oxygen transport properties of PET bottle walls. Journal of applied Polymer Science. 2004; 94(2):671-677. http://dx.doi.org/10.1002/app.20905.
20. Mitra D and Misra A. Study on the effect of dibenzylidene sorbitol as a nucleating agent on the crystallization and morphology of poly(ethylene terephthalate). Journal of applied Polymer Science. 1988; 36(2):387-402. http://dx.doi. org/10.1002/app.1988.070360211.
21. Wang Y, Xu Y, He D, Yao W, Liu C and Shen C. Nucleation
density reduction” effect of biodegradable cellulose acetate butyrate on the crystallization of poly(lactic acid). Materials Letters. 2014; 128:85-88. http://dx.doi.org/10.1016/j. matlet.2014.04.078.
22. Wagner M. Thermal analysis in practice. Schwerzenbach: Mettler-Toledo; 2010.
23. Schultz JM. Polymer materials science. New Jersey: Prentice-Hall; 1974.
24. Cebe P and Hong S. Crystallization behaviour of poly(ether-ether-ketone). Polymer. 1986; 27(8):1183-1192. http://dx.doi. org/10.1016/0032-3861(86)90006-6.
25. Blundell DJ. On the interpretation of multiple melting peaks in poly(ether ether ketone). Polymer. 1987; 28(13):2248-2251. http://dx.doi.org/10.1016/0032-3861(87)90382-X.
26. Mandelkern L. Crystallization of polymers. New York: McGraw-Hill Book Company; 1964.
27. Mandelkern L, Quinn FA Jr and Flory PJ. Crystallization
kinetics in high polymers. I. Bulk polymers. Journal of applied Physics. 1954; 25(7):830-839. http://dx.doi. org/10.1063/1.1721753.
28. Everaert V, Groeninckx G and Aerts L. Fractionated
crystallization in immiscible POM/(PS/PPE) blends Part 1: effect of blend phase morphology and physical state of the amorphous matrix phase. Polymer. 2000; 41(4):1409-1428. http://dx.doi.org/10.1016/S0032-3861(99)00321-3.
29. Di Lorenzo ML. Spherulite growth rates in binary polymer
blends. Progress in Polymer Science. 2003; 28(4):663-689. http://dx.doi.org/10.1016/S0079-6700(02)00035-7.
30. Koutsky JA, Walton AG and Baer E. Nucleation of polymer droplets. Journal of applied Physics. 1967; 38(4):1832-1839. http://dx.doi.org/10.1063/1.1709769.
31. Lee CH. Non-linear spherulite growth and phase change at
the spherulite growth front in isotactic polypropylene/partially hydrogenated oligo(styrene-co-indene) blend. Polymer. 1998; 39(21):5197-5201. http://dx.doi.org/10.1016/S0032-3861(97)10112-4.
32. Price FP. A phenomenological theory of spherulitic crystallization: Primary and secondary crystallization process.
Journal of Polymer Science Part a. 1965; 3:3079-3086.
33. Apiwanthanakorn N, Supaphol P and Nithitanakul M. Non-isothermal melt-crystallization kinetics of poly(trimethylene terephthalate). Polymer Testing. 2004; 23(7):817-826. http:// dx.doi.org/10.1016/j.polymertesting.2004.03.001.
34. Avramova N. Amorphous poly(ethylene terephthalate)/ poly(butylene terephthalate) blends: miscibility and properties.
Polymer. 1995; 36(4):801-808. http://dx.doi.org/10.1016/0032-3861(95)93111-X.
35. Keller A and Cheng SZD. The role of metastability in polymer phase transitions. Polymer. 1998; 39(19):4461-4487. http:// dx.doi.org/10.1016/S0032-3861(97)10320-2.
36. Kim SH, Ahn SH and Hirai T. Crystallization kinetics and nucleation activity of silica nanoparticle-filled poly(ethylene 2,6-naphthalate). Polymer. 2003; 44(19):5625-5634. http:// dx.doi.org/10.1016/S0032-3861(03)00623-2.
37. Kim WN and Burns CM. Phase behavior of blends of polycarbonate with partially miscible polymers. Journal of applied Polymer Science. 1990; 41(78):1575-1593. http:// dx.doi.org/10.1002/app.1990.070410718.
38. Kissinger HE. Variation of peak temperature with heating rate in differential thermal analysis. Journal of Research of the National Bureau of Standards. 1956; 57(4):217-221. http:// dx.doi.org/10.6028/jres.057.026.
39. Roberts RC. Poly(ethylene terephthalate) I. Heat of fusion.
Polymer. 1969; 10:113-116. http://dx.doi.org/10.1016/0032-3861(69)90014-7.
40. Starkweather HW Jr, Zoller P and Jones GA. The heat of fusion of poly(ethylene terephthalate). Polymer Physics. 1983; 21(2):295-299. http://dx.doi.org/10.1002/pol.1983.180210211.
41. Martuscelli E, Pracella M and Crispino L. Crystallization
behaviour of fractions of isotactic polypropylene with different degrees of stereoregularity. Polymer. 1983; 24(6):693-699. http://dx.doi.org/10.1016/0032-3861(83)90005-8.
42. Nadkarni VM, Shingankuli VL and Jog JP. Effect of blending on
the crystallization behavior of PET. Journal of applied Polymer Science. 1992; 46(2):339-351. http://dx.doi.org/10.1002/ app.1992.070460215.
43. Norton DR and Keller A. The spherulitic and lamellar morphology of melt-crystallized isotactic polypropylene.
Polymer. 1985; 26(5):704-716. http://dx.doi.org/10.1016/0032-3861(85)90108-9.
45. Padden FJ and Keith HD. Spherulitic crystallization in polypropylene. Journal of applied Physics. 1959; 30(10):1479-1484. http://dx.doi.org/10.1063/1.1734985.
46. Pingping Z and Dezhu M. Study on the double cold crystallization peaks of poly(ethylene terephthalate) 3. The influence of the addition of calcium carbonate (CaCO3).
European Polymer Journal. 2000; 36(11):2471-2475. http:// dx.doi.org/10.1016/S0014-3057(00)00042-2.
47. Kong Y and Hay JN. Miscibility and crystallisation behaviour of poly(ethylene terephthalate)/polycarbonate blends. Polymer. 2002; 43(6):1805-1811. http://dx.doi.org/10.1016/S0032-3861(01)00772-8.
48. Kwei K and Frisch HL. Interaction parameter in polymer
mixtures. Macromolecules. 1978; 11(6):1267-1271. http:// dx.doi.org/10.1021/ma60066a039.
49. Lee JK, Choi WS, Kwon HK and Lee KH. Liquid–
liquid phase separation and crystallization behavior of poly(ethylene terephthalate)/poly(ether imide) blend. Polymer. 2002; 43(9):2827-2833. http://dx.doi.org/10.1016/S0032-3861(02)00054-X.
50. Minakov AA, Mordvintsev DA and Schick C. Melting and reorganization of poly(ethylene terephthalate) on fast heating (1000 K/s). Polymer. 2004; 45(11):3755-3763. http://dx.doi. org/10.1016/j.polymer.2004.03.072.
51. Rabello MS. The properties and crystallization behavior of photo-degraded polypropylene. [Thesis]. United Kingdom:
Department of Mechanical, Materials and Manufacturing Engineering, University of Newcastle upon Tyne; 1996.
52. Liu T, Mo Z, Wang S and Zhang H. Isothermal melt and cold
crystallization kinetics of poly(aryl ether ether ketone ketone) (PEEKK). European Polymer Journal. 1997; 33(9):1405-1414. http://dx.doi.org/10.1016/S0014-3057(97)00016-5.
53. Rabello MS and White JR. Crystallization and melting behaviour of photodegraded polypropylene — II. Re-crystallization of degraded molecules. Polymer. 1997; 38(26):6389-6399. http:// dx.doi.org/10.1016/S0032-3861(97)00214-0.
54. Nakamura K, Watanabe T, Katayama K and Amano T. Some aspects of nonisothermal crystallization of polymers. I. Relationship between crystallization temperature, crystallinity, and cooling conditions. Journal of applied Polymer Science. 1972; 16(5):1077-1091. http://dx.doi.org/10.1002/ app.1972.070160503.
55. Lodefier P, Jonas AM and Legras R. Chemical Heterogeneity
of poly(ethylene terephthalate) as revealed by temperature rising elution fractionation and its influence on polymer thermal behavior: a comparison with poly(ethylene terephthalate- co