Estimation of the age and biometry of Salminus brasiliensis (Cuvier 1816)
captured in the Funil hydroelectric plants
Estimativa da idade e biometria de Salminus brasiliensis (Cuvier 1816)
capturada nas hidrelétricas de Funil
DOI:10.34117/bjdv6n4-441
Recebimento dos originais: 02/03/2020 Aceitação para publicação: 01/04/2020
Athalita Ester Mendonça da Silva Piva Ferreira
Mestre em Engenharia Agrícola pela Universidade Federal de Lavras Instituição: Universidade Federal de Lavras
Campus Universitário, Caixa Postal 3037, CEP: 37200-900, Lavras-MG, Brasil E-mail: athalitaester@hotmail.com
Carlos Cicinato Vieira Melo
Doutor em Zootecnia pela Universidade Federal de Lavras
Instituição: Centro Universitário Tocantinense Presidente Antônio Carlos Av. Filadélfia, 568 - St. Oeste, CEP: 77816-540, Araguaína-TO, Brasil
E-mail: carloscicinato85@gmail.com
Natália Michele Nonato Mourad
Doutora em Zootecnia pela Universidade Federal de Lavras Instituição: Universidade Federal de Lavras
Campus Universitário, Caixa Postal: 3037, CEP: 37200-900, Lavras-MG, Brasil E-mail:natminonato@gmail.com
Viviane de Oliveira Felizardo
Doutora em Zootecnia pela Universidade Federal de Lavras Instituição: Universidade Federal de Lavras
Campus Universitário, Caixa Postal 3037, CEP: 37200-900, Lavras-MG, Brasil E-mail: viviofbio@yahoo.com.br
William Franco Carneiro
Doutorando em Zootecnia pela Universidade Federal de Lavras Instituição: Universidade Federal de Lavras
Campus Universitário, Caixa Postal 3037, CEP: 37200-900, Lavras-MG, Brasil E-mail: willfc14@gmail.com
Rilke Tadeu Fonseca de Freitas
Doutor em Zootecnia pela Universidade Federal de Viçosa Instituição: Universidade Federal de Lavras
Campus Universitário, Caixa Postal: 3037, CEP: 37200-900, Lavras-MG, Brasil E-mail: rilke@ufla.com
Luis David Solis Murgas*
Doutor em Zootecnia pela Universidade Federal de Lavras Instituição: Universidade Federal de Lavras
Campus Universitário, Caixa Postal: 3037, CEP: 37200-900, Lavras-MG, Brasil *Corresponding Author: E-mail: lsmurgas@ufla.br
ABSTRACT
In order to better understand the ecology and biology of Salminus brasiliensis, and to provide basic information for the evaluation of fish stocks, this study verified the age and biometry S. brasiliensis. Sixty-six fish (aged 2-15 years) were captured at the Funil Hydroelectric Power Plant, Brazil, between September 2006 and August 2007. The biometric data recorded were weight, total and standard length, height, thickness and head length. Sex was determined by macroscopic evaluation of gonads. The gonads and liver were removed and weighed for calculation of gonadosomatic index and hepatosomatic index. The age of each fish was determined by analysis of growth rings in the scales, while the scale radius was measured from the focus to the end of the scale. Differences between seasonal biometric averages and reproductive indexes were verified using the Newman Keuls test at 5%. Results indicate that in the spring and summer seasons, smaller fish were caught compared to those caught during the winter and autumn. Females tend to have greater weight and morphometric measurements than males. The age and radius of the scale were positively correlated with the biometric variables. The biometric variables of S. brasiliensis can be used as an indicator of age in this species.
Keywords:Dourado, growth; fish; morphometry; ray of scale. RESUMO
Com o objetivo de melhor compreender a ecologia e a biologia de Salminus brasiliensis e fornecer informações básicas para a avaliação dos estoques de peixes, este estudo verificou a idade e a biometria de S. brasiliensis. Sessenta e seis peixes (de 2 a 15 anos) foram capturados na Usina Hidrelétrica de Funil, Brasil, entre setembro de 2006 e agosto de 2007. Os dados biométricos registrados foram peso, comprimento total e padrão, altura, espessura e comprimento da cabeça. O sexo foi determinado por avaliação macroscópica das gônadas. As gônadas e o fígado foram removidos e pesados para o cálculo do índice gonadossomático e do índice hepato-somático. A idade de cada peixe foi determinada pela análise dos anéis de crescimento nas escalas, enquanto o raio da escala foi medido do foco até o final da escala. As diferenças entre as médias biométricas sazonais e os índices reprodutivos foram verificadas pelo teste de Newman Keuls a 5%. Os resultados indicam que, nas estações primavera e verão, peixes menores foram capturados em comparação com os capturados durante o inverno e o outono. As mulheres tendem a ter maior peso e medidas morfométricas do que os homens. A idade e o raio da escala foram positivamente correlacionados com as variáveis biométricas. As variáveis biométricas de S. brasiliensis podem ser usadas como um indicador de idade nesta espécie.
Palavras-chave: Dourado, crescimento; peixe; morfometria; raio de escala. 1 INTRODUCTION
The estimation of the age of the fish provides parameters necessary to evaluate the fish population dynamics (recruitment, growth, and mortality) and stock structure(Koch and Quist, 2007; Maceina et al., 2007; Methot and Wetzel, 2013). The data obtained can be used in the creation of age-structured population models(Maceina et al., 2007), which can guide the management and implementationof conservation programs(Spurgeon et al., 2015). Information used to determine age
in fish populations is usually derived from calcified structures, such as gills, pectoral fin rays, scales and sagittal otoliths (Buckmeier et al., 2018).These marks are usually associated with environmental factors (Felizardo et al., 2010).
Scales are a very useful biometric structure since they are easy to collect and do not negatively impact the health of the fish after removal. The scales have certain characteristics that can be used to estimate the age via counting the number of annuli, growth, spawning activity, migrations, diseases, environmental conditions related to temperature, and lack of food, among others (Rojo, 1991). A number of studies such as Araya et al. (2005) andAgostinho et al. (1999)have been carried out on correlation between the formation of rings andwater temperature, fluctuations in water levels, photoperiod, transparency, water velocity in the habitat, and food availability.
In Brazil, one of the criteria adopted by the government to guide decisions related to species conservation is related to fish age estimates (Santana and Minte-Vera, 2017). Consequently, using scales for determination of age have been widely used in native fish in Brazil, such as
Hypophthalmusmarginatus(Cutrim and Batista, 2005), Leporinusobtusidens(Araya et al., 2005), L. acutidens(Araya et al., 2008) and Salminusbrasiliensis(Dei Tos et al., 2009).The S. brasiliensis scale
is of the elasmoid type, specifically of the cycloid type. According toHolden & Raitt (1974)this type of scale is subcircular, disk-shaped and thin and is found in most soft-rays fish in the fins, presenting on its outer surface concentric deposited sclera or striations following a regular pattern, from the center and origin of the scale, the foccus.
The estimation of the age of the fish, using the scaling methodology is widelyemployed in South America, due to its low sampling cost, ease of preparation of the samples, and sidestepping the use of expensive equipment(Isely and Grabowski, 2007; Santana and Minte-Vera, 2017).
Salminusbrasiliensiscommonly known as dourado,is a characin found in the rivers of Bolivia,
Brazil, Paraguay and Uruguay (Gagne et al., 2017) and travel long distances (up to 1000 km)to reach reproductive areas(Petrere Junior, 1985). Despite its importance, this species is considered vulnerable according to the red list of species, being threatened with extinction in some basins(Barletta et al., 2010). Agostinho et al. (2007)noted that fish usually come under threat due to the construction of dams, which leads to the destruction of habitat, interruptions of migratory routes, blocking access to spawning and changes in water quality.
In order to better understand the ecology and biology of S. brasiliensis and to provide basic information for the evaluation of fish stocks, this study aimed to determine the age, a variation of biometric and seasonal parameters for each age and the correlation between age and the scales rays with the biometric parameters and reproductive indices of S. Brasiliensis in the Funil Hydroelectric Plant, Brazil, three years after the dam closed.
2 MATERIAL AND METHODS
2.1 STUDY AREA
The study was conducted in the upper course of the Rio Grande, between the municipalities of Lavras and Perdões, in the south of Minas Gerais (21 ° 08 'S and 44 ° 55'W), downstream of the Funil Hydroelectric Power Plant UHE Funil). The construction of the Funil HPP started in 2000 and started operating in 2003. The Funil HPP has a fish transposition system (FTS) of the lift type designed to allow for the migration of different species of fish upstream of the dam(Murgas et al., 2018).
2.2 SAMPLING
A total of 32 males and 32 females of S. brasiliensis were evaluated between 2006 and 2007. Individuals of S. brasiliensis were caught between September 2006 and August 2007 in the Rio Grande, downstream of the Funil HPP, with authorization from the State Forestry Institute (IEF), through the scientific fishing license category D-N. 063-07. Fish were collected at intervals of 15 days in the morning, using a rod and windlass, using as bait earthworms and bovine heart.
The highest pluviometric indexes observed during the period of capture of the animals were between November and February, with a peak of 17.9 mm in January. The mean temperature was 20.5°C (± 3.2) using a portable digital thermometer.After thecapture, the animals were desensitized in benzocaine (80 mg L-1) and kept immersed in ice during transportfor the Animal Physiology and
Pharmacology Laboratory, Veterinary Medicine Department at the Federal University of Lavras. Afterward, the specimens were taxonomically identified and the following morphometric parameters were measured with a pachymeter and an ichthyometer: (i) total length (TL - cm), (ii) standard length (SL - cm), (iii)height (H - cm), (iv) depth (DP - cm) and (v) head length (HL - cm). The total weight (TW - g) of each animal was also obtained through a digital balance. Width measurements were obtained in the region of the 1st radius of the dorsal fin, the SL, between the head end and the lower perimeter of the stem (caudal fin insertion), the HLbetween the anterior end of the head and the caudal part of the operculum, the body height was measured from the radius of 1° of the dorsal fin (Figure 1).
Figure 1: Morphometric measurements performed on a golden specimen of S. brasiliensis.
After obtaining the morphometric data, the animals were incised longitudinally and the walls resulting from this incision were folded, andthe gonads removed, and macroscopically analyzed for sex determination and weighed to calculate the gonadosomatic index [GSI = (gw/tw) x 100], where gw is the weight of the gonads (g) and twis the total weight (g) of the fish (Vazzoler, 1996). Likewise, the liver was removed and weighed to obtain the hepatosomatic index, calculated as follows: HSI (%) = hepatopancreas weight (g) x 100 / bodyweight (g).
Prior to the extraction of the scales, the fish were washed in running water to remove any dirt residues andlost fish scales from other fish. The scales were collected with forcepsfromthe region below the dorsal fin and above the lateral line. All the scales collected for each specimen were inspected for deformities, scratches or any changes that made it difficult to count the annuli. Three scales (symmetrical) were selected from each animal, identified and stored in envelopes for later determinationof age. Due to the hydrophilic characteristics of the scales, a hydration treatment by immersion in distilled water was performed prior to readings.
The scales of each specimen were placedon microscope slides and their readings were performed witha binocular loupe (Wild ST4, Swiss) ata magnification of 20x, allowing for the complete visualization of the scales. The reading was made by visualizing the zones where the striae are more spaced (fast growing areas, which usually occurs in summer), followed by areas where the scales are less spaced (slow growth zones - which occurs in winter) that form a mark on the scale (ring or annulus). Together these two zones correspond to an annual growth zone. These growth marks should be observed throughout the surface of the scale (Holden, 1974). As the age was read, the radius (mm) of the scale was also measured from the foccus (center of the scale) to the edgeof the scale (Figure 2).
Figure 2: Measurement of the scale radius.
2.3 DATA ANALYSIS
For the study of the effects of sex and seasonal season on the biometrics variables of the fish collected, the data were submitted to analysis of variance using a statistical model with two cross-classification criteria, which included the effects of sex, season of the year and of the interaction between these factors. The means were compared using the Newman Keuls (SNK) test at a 5% probability level. A correlation of age and radius of the scales was made with the variables related to the biometric data and Pearson's correlation coefficient. Statistical analyzes were performed using R software version 3.0.2 (R Development Core Team, 2013).
3 RESULTS AND DISCUSSION
3.1 SEX RATIO
The sexual ratio found of 1:1 (Table 1) was within the expected range for this population. Not relevant to this study as the 1:1 ratio was within expectations for a normal population. Dala-Corte e Azevedo, (2010)suggest that for species with a ratio of 1:1 it implies that it does not present differences in the birth, mortality and growth rates, nor does it have sexual dimorphism.
Table 1. Specimens of Salminus brasiliensis captured downstream of the Funil HPP, grouped by season and sex.
Season Sex Total Male Female Winter 6 9 15 Spring 6 8 14 Summer 10 7 17 Autumn 10 8 18 Total 32 32 64
3.2 AGE OF INDIVIDUALS
Although the Scales of S. brasiliensiswas generally well defined, thus facilitatingidentification and counting, approximately 20% of the scales presented irregularities in the rings, making it impossible to identify the age of the fish. Irregularity of the growth rings is common due to the occurrence of losses and regeneration of the scales. Regenerated scales can be easily identified by the irregular shape of the striations and by the absence of concentric striations near the center of the scale (Holden, 1974). The 20% irregular scales are on the low side seeing that Araya et al. (2008)discarded 45% of the scales in a study of L. acutidens.Indicate possible reason(s) for your low count of irregular scales as compared to Araya et al. (2008).
The age of the individuals sampled varied from 2 to 15 years, and the age group with the highest frequency of capture were those of 6 (n=10), 7 (n=12) and 8 (n=11) years of age, decreasing in number for the lowest or highest observed age (Table 2). A similar finding(most individuals in the 9-year-old categoryand a decrease in animals collected at higher or lower ages) was observed byFelizardo et al. (2015), in a study evaluating the age of L. obtusidens. According to Dei Tos et al. (2010), freshwater fish in South America do not present high longevity, reaching a maximum of 15 years of age.
Table 2. Biometric data, reproductive indexes, radius of scale according to the ages (N) of Salminus brasiliensis captured downstream of the Funil hydroelectric plant
Age (N)* Parameters Weight(g) TL (cm) SL (cm) Width (cm) Height (cm) HL (cm) Ray (mm) GSI (%) HSI (%) 2 (3) 916.6± 381.8 44.8±4.3 40.1±1.7 555.0±0.7 9.6±1.2 9.7±1.5 3.3± 1.1 0.2±0.1 0.4±0.1 3 (3) 1250.0± 661.4 48.0±8.8 40.3±5.8 4.9±0.8 11.2±2.3 10.4±2.2 4.3± 1.5 0.3±0.3 0.5±0.0 4 (4) 1600.0± 424.2 51.2±2.0 45.0±3.5 6.1±0.5 11.9±0.6 10.9±1 5.1±1.3 0.4±0.4 0.7±0.3 5 (8) 2590.2± 1164.3 56.7±7.2 49.8±7.2 6.4±1.0 14.4±3.1 12.4±2.5 5.8±0.8 0.2±0.1 0.5±0.1 6 (10) 3284.1± 827.7 63.6±4.3 55.2±3.8 6.9±1.5 15.7±2.1 13.7±1.4 6.2±0.8 0.1±0.1 0.5±0.1 7 (12) 3505.0± 1014.1 62.6±5.3 55.2±3.5 7.0±0.5 15.9±2.4 14.3±1.9 6.6±0.4 0.2±0.1 0.5±0.1 8 (11) 3940.9± 1182.1 64.0±5.8 55.9±6.1 7.0±0.5 16.7±3.5 14.8±2.6 6.8±0.9 0.2±0.1 0.6±0.2 9 (7) 5045.7± 1106.7 66.0±8.8 60.8±5.0 8.3±0.8 19.5±2.6 16.0±1.4 8.1±0.3 0.4±0.1 0.5±0.0 11 (2) 5250.0± 1060.6 72.0±7.0 63.5±4.9 7.7±0.4 20.5±0.7 15.5±0.7 9.5±0.7 0.2±0.1 0.7±0.2 12 (2) 6400.0± 424.2 78.2±6.0 71.5±2.1 8.1±0.0 19.2±3.1 19.2±0.3 10.0±0.7 0.4±0.2 0.4±0.1
13
(1) 5600.0±0.0 75.0±0.0 66.0±0.0 7.3±0.0 18.5±0.0 14.5±0.0 10.6±0.0 0.1±0.0 0.9±0.0 15
(1) 8000.0±0.0 83.0±0.0 75.0±0.0 9.5±0.0 24.0±0.0 19.5±0.0 11±0.0 0.40±0.0 0.6±0.0
* N number of animals. TL: Total length, SL: Standard length, HL: Head Length, GSI: Gonadsomatic index, HIS: Hepatosomatic index
3.3 SIZE OF INDIVIDUALS
As there was no significant interaction (P>0.05) of the biometric data of males and females seasonally, the values obtained for each sex were grouped for analysis. In this way, it was observed that in the spring and summer season smaller fish were captured in relation to those captured during the winter and fall (Table 3).With anincrease in the water current caused by the floods, the hook that was thrown on the river was dragged to the embarkment edge, where smaller fish were found, since in deeper places smaller animals become easy prey for larger fish. On the other hand, the increase in river flow may also stimulate fish to seek margins. The increase in the current was generally caused by the release of water from the spillways, opened during the flood season to control the level of the upstream dam.
3.4 WEIGHT OF INDIVIDUALS
Females had a greater weight and morphometric measurements than males (Table 3). The mean total length of females observed was 64.8cm, whereas in males it was 58.6cm These results are similar to those obtained by Barbieri et al. (2001), evaluating S. brasiliensis in the Mogi-Guaçu river and by Araya et al. (2005), for fish collected in the Paraná River. Felizardo et al. (2010) evaluating
Megaleporinus obtusidens(piapara) swimming bladder abnormalities collected in the same place of
urstudy, verified that the females were larger than males, although they were of the same age. This difference is due to the fact that females present a higher growth rate than males and, as a consequence, reach higher lengths for the same age (Felizardo et al., 2015).
Table 3. Seasonal biometric data of S. brasiliensis caught downstream of the Funil hydroelectric plant.
* Different overwritten letters in columns indicate difference at 5% probability. TL: Total length, SL: Standard length, Width, HL: Head length. Season* Weight (g) Age (years) Ray (mm) TL (cm) SL (cm) Width (cm) Height(cm) HL (cm) Spring 4521.2±1429.9a 8.3±2.8ª 7.0±0.7a 66.8±8.0ª 60.3±6.4ª 7.4±1.5ª 17.8±2.6ª 16.0±2.1ª Summer 1997.9±1544.8b 5.1±2.3d 5.1±1.5b 53.5±10.1b 46.8±8.7b 6.2±1.4b 12.4±3.4c 11.4±2.7b Autumn 4128.6±1278.0a 7.4±2.1b 6.6±0.9a 64.9±6.3ª 56.3±6.4ª 7.2±0.8a 18.2±2.7ª 14.5±1.7ª Winter 3501.3±1250.7ª 6.9±2.3c 6.6±0.9a 62.5±7.4a 55.7±6.9a 7.0±0.7a 15.3±2.7b 14.3±2.6a Sex Male 2975.1±1668.1 6.8±2.8 5.9±1.5 58.6±10.0 51.9±8.9 6.7±1.2 14.9±3.7 12.7±2.5 Female 4027.8±1508.2 6.8±2.3 6.7±0.9 64.8±7.8 57.0±7.5 7.2±1.2 16.9±3.5 15.1±2.5
3.5 REPRODUCTION OF INDIVIDUALS
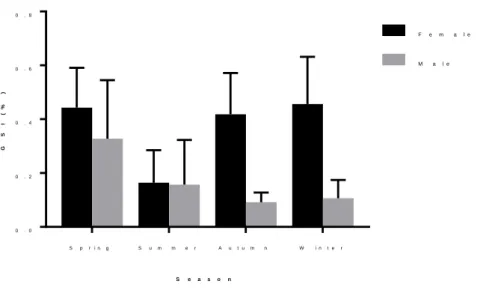
In the present study, we observed that the seasonal gonadosomatic index (GSI) of the females was higher (P<.05) than in the males, except in the summer, where males and females showed similar GSI (P>.05). The most discrete variation in the GSI of males during the seasons, when compared to females can be attributed to the differences between testes and ovaries, in terms of volume, mass and energy demand required for the production of gametes(Ribeiro et al., 2007). On the other hand, the similarity between GSI in the summer can be explained, due to the emptying of the gonads of the females after spawning, whereas the gonads of the males do not present seasonal variations. The fact that the GSI of the females has decreased significantly (P< .05) in summer in relation to spring, may indicate that the reproductive period of this species occurs preferentially in spring, where the highest GSI was observed (Figure 3).
Figure 3: Gonadossomatic index (GSI) (%) seasonal of males and females of S. brasiliensis.
3.6 LIVER WEIGHT
The hepatosomatic index (HSI) for both sexes of S. brasiliensis can be used as indicator of the reproductive period, since the highest HSI for females and males was observed in spring and summer, differing significantly (P <0.05) of the other stations (Figure 4). This observed result coincides with the reproductive period of the species. According to Querol et al. (2005), the HSI is related to the mobilization of energy reserves necessary for the (i) process of vitellogenesis, (ii) reproduction or (iii) preparation for the winter period. The importance of the HSI was also commented on byCosta et al. (2005), citing the changes caused by the liver, since this organ synthesizes and secretes the precursor of the proteins of the vitellogeninThe vitellogenin is removed from the bloodstream for the development of the oocytes, while the rapid accumulation of the yolk probably
S p r i n g S u m m e r A u t u m n W i n t e r 0 . 0 0 . 2 0 . 4 0 . 6 0 . 8 S e a s o n G S I ( % ) F e m a l e M a l e
happens by the decrease of the weight of the liver(Yoneda et al., 2001). On the other hand, during testicular and sperm maturation, the liver also participates as an energy supplier for the testicular reabsorption and reorganization process, as well as the transfer of liver substances to the metabolism involved in the production of gametes in the testes. Thus, it is expected that a variation in liver weight will occur during the seasons, reflecting the assimilation or use of energy reserves by the fish. In the present study, the lower HSI were observed in the months before the reproductive period, this fact may be related to the high energy cost for oocyte production, in addition to other costs, such as male demand and the high risk of predation during the cut and copula (Querol et al., 2005). Similar results were observed in Loricariichthysplatymetopon (Querol et al., 2005) and Cynoscion acoupa(Almeida et al., 2016), which indicated a decrease in HSI at spawning peaks.
S p r i n g S u m m e r A u t u m n W i n t e r 0 . 0 0 . 2 0 . 4 0 . 6 0 . 8 1 . 0 S e a s o n H S I ( % ) F e m a l e M a l e
Figure 4: Seasonal Hepatosomatic (%) index of males and females of S. brasiliensis.
3.7 CORRELATION BETWEEN AGE AND RADIUS OF THE SCALE
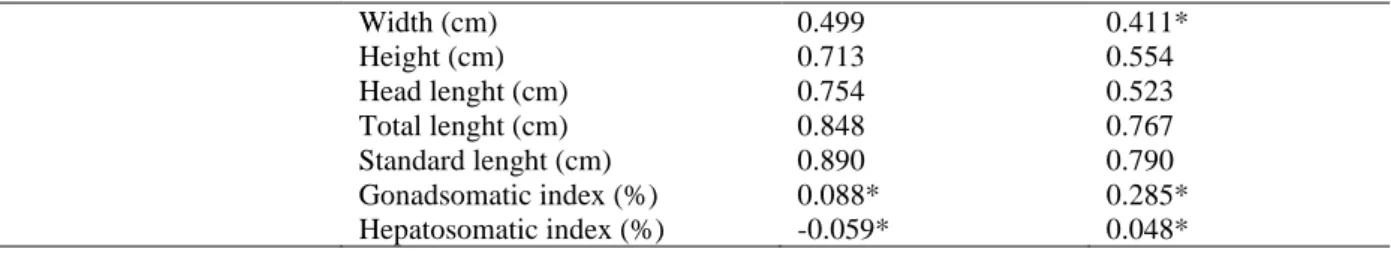
A strong correlation (r> 0.7) was present for weight, total length and pattern, with the age of the animals in both sexes (Table 4).
Table 4. Pearson's correlation coefficient between age and radius of the scale with the biometric variables of males and females of S. brasiliensis
Variable Variable Correlation (r)
Male Female Age Weight (g) 0.873 0.802 Ray (mm) 0.819 0.658 Width (cm) 0.699 0.560 Height (cm) 0.819 0.612 Head length (cm) 0.791 0.681 Total length (cm) 0.858 0.790 Standard length (cm) 0.820 0.838 Gonadsomatic index (%) -0.027* -0.129* Hepatosomatic index (%) 0.231* -0.127* Ray Weight (g) 0.775 0.681
Width (cm) 0.499 0.411* Height (cm) 0.713 0.554 Head lenght (cm) 0.754 0.523 Total lenght (cm) 0.848 0.767 Standard lenght (cm) 0.890 0.790 Gonadsomatic index (%) 0.088* 0.285* Hepatosomatic index (%) -0.059* 0.048*
* There is no significant correlation with the Pearson correlation at 5% probability.
Similarly, we observed a strong correlation between the radius and the total and standard-length measurements in S. brasiliensis, and a moderate correlation (r>0.6) between the radius scale and the total and standard length of females. The high correlation coefficient (r=0.74) was also observed between the radius and the standard length independently of the sex of the fish. In this case a regression model was applied to describe the relation between the radius and the standard length (Figure 5). Radius growth showed a positive increase in relation to fish SL. As reported byIbáñez and O’Higgins, (2011), the size of the fish has a high correlation with the size of the scales. In Leporinus
obtusidens, a correlation above 80% between the radius of the scales and the standard length were
reported by Felizardo et al. (2015), similar to that observed in the present study. As reported byHaimovici e Reis, (1984), the radius of the scales of Umbrinacanosai increase in proportion to the length, in addition, these same authors assure that the length of this fish can be used as an indicator of the age. 3 0 4 0 5 0 6 0 7 0 8 0 0 3 6 9 1 2 1 5 S t a n d a r d l e n g t h ( c m ) R a y ( m m ) Y = 0.2075*X - 4.634 r ² = 0 . 7 0 8 6
Figure 5: Radius (mm) vs Standard length (cm) of S. brasiliensis.
It was also verified that the age of S. brasiliensis does not influence (P<0.05) in the GSI and HSI, suggesting that the age does not affect the reproductive performance of this species. However, the quality of the gametes should be verified in animals with advanced age (Felizardo et al., 2015), since the reproductive potential of the animals may be influenced by bioticand
abioticfactors(Jakobsen et al., 2009). The results observed in this study demonstrate that the age identification of S. brasiliensis can be estimated using the biometric length data of the animals.
4 CONCLUSION
Based on this work, we can conclude that the dourado captured downstream of the Funil Hydroelectric Power Plant had ages between 2 and 15 years; the age and radius of S. brasiliensis scales are highly correlated with biometric variables, thus indicating the possibility of using these variables as an indication of age in this species.
ACKNOWLEDGEMENTS
This work was supported by the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), theCoordenação de Aperfeiçoamento de Nível Superior(CAPES), the Fundação de Amparo à Pesquisa do Estadode Minas Gerais (FAPEMIG) and Universidade Federalde Lavras (UFLA) and The Compahia Energética de Minas Gerais (CEMIG)
REFERENCES
Agostinho, A., Miranda, L.E., Bini, L.M., Gomes, L.C., Thomaz, S.M., Suzuki, H., 1999. Patterns of colonization in neotropical reservoirs, and prognoses on aging. Theor. Reserv. Ecol. its Appl. 227– 265.
Agostinho, A.A., Gomes, L.C., Pelicice, F.M., 2007. Ecologia e manejo de recursos pesqueiros em reservatórios do Brasil 501.
Almeida, Z.S., Santos, N.B., Sousa, H.L., Carvalho Neta, R.N.F., Andrade, T.S.O.M., 2016. Biologia Reprodutiva da Pescada Amarela (Cynoscion acoupa) Capturada na Baía de São Marcos, Maranhão, Brasil, Biota Amazônia. https://doi.org/10.18561/2179-5746/biotaamazonia.v6n1p46-54
Araya, P.R., Agostinho, A.A., Bechara, J.A., 2008. Population structure, growth and fishery yield of Leporinus acutidens (Valenciennes, 1837) (Teleostei: Anostomidae) in Yacyretá Reservoir (Argentina). Neotrop. Ichthyol. 6, 57–66. https://doi.org/10.1590/s1679-62252008000100007
Araya, P.R., Agostinho, A.A., Bechara, J.A., 2005. The influence of dam construction on a population of Leporinus obtusidens (Valenciennes, 1847) (Pisces, Anostomidae) in the Yacyretá Reservoir (Argentina). Fish. Res. 74, 198–209. https://doi.org/10.1016/j.fishres.2005.02.007
Barbieri, G., Salles, F.A., Cestarolli, M.A., 2001. Growth and first sexual maturation size of Salminus maxillosus Valenciennes, 1849 (Characiformes, Characidae), in Mogi Guaçu river, state of São Paulo, Brazil. Acta Sci. 23, 453–459.
Barletta, M., Jaureguizar, A.J., Baigun, C., Fontoura, N.F., Agostinho, A.A., Almeida-Val, V.M.F., Val, A.L., Torres, R.A., Jimenes-Segura, L.F., Giarrizzo, T., Fabré, N.N., Batista, V.S., Lasso, C., Taphorn, D.C., Costa, M.F., Chaves, P.T., Vieira, J.P., Corrêa, M.F.M., 2010. Fish and aquatic habitat conservation in South America: A continental overview with emphasis on neotropical systems. J. Fish Biol. 76, 2118–2176. https://doi.org/10.1111/j.1095-8649.2010.02684.x
Buckmeier, D.L., Snow, R., Smith, N.G., Porter, C., 2018. Are Age Estimates for Longnose Gar and Spotted Gar Accurate? An Evaluation of Sagittal Otoliths, Pectoral Fin Rays, and Branchiostegal Rays. Trans. Am. Fish. Soc. 147, 639–648. https://doi.org/10.1002/tafs.10039
Costa, A.P.R., De Andrade, D.R., Vidal, M.V., Souza, G., 2005. Indicadores quantitativos da biologia reprodutiva de fêmeas de piau-vermelho no Rio Paraíba do Sul. Pesqui. Agropecu. Bras. 40, 789– 795. https://doi.org/10.1590/s0100-204x2005000800009
Cutrim, L., Batista, V. da S., 2005. Determinação de idade e crescimento do mapará (Hypophthalmus marginatus) na Amazônia Central, Acta Amazonica. https://doi.org/10.1590/s0044-59672005000100013
Dala-Corte, R.B., Azevedo, M.A., 2010. Biologia reprodutiva de Astyanax henseli (Teleostei, Characidae) do curso superior do rio dos Sinos, RS, Brasil. Iheringia - Ser. Zool. 100, 259–266. https://doi.org/10.1590/s0073-47212010000300012
Dei Tos, C., Gomes, L.C., Agostinho, A.A., Batista, R.P., 2009. Age, growth, mortality and yield per recruit of the dourado Salminus brasiliensis, Corumbá Reservoir, Goiás State, Brazil. Neotrop. Ichthyol. 7, 223–230. https://doi.org/10.1590/s1679-62252009000200014
Felizardo, V. de O., de Andrade, E.A., Melo, C.C.V., Murgas, L.D.S., de Freitas, R.T.F., Andrade, E. de S., 2015. Determinação da idade e sua correlação com as variáveis biométricas e indíces reprodutivos sazonais de Leporinus obtusidens. Acta Sci. - Biol. Sci. 37, 265–271. https://doi.org/10.4025/actascibiolsci.v37i3.28003
Felizardo, V. de O., de Souza Andrade, E., Murgas, L.D.S., Winkaler, E.U., Wouters, F., 2010. Swimbladder abnormalities in piapara (leporinus obtusidens) captured downstream of the funil dam. Neotrop. Ichthyol. 8, 661–665. https://doi.org/10.1590/s1679-62252010000300012
Gagne, T.O., Ovitz, K.L., Griffin, L.P., Brownscombe, J.W., Cooke, S.J., Danylchuk, A.J., 2017. Evaluating the consequences of catch-and-release recreational angling on golden dorado (Salminus brasiliensis) in Salta, Argentina. Fish. Res. 186, 625–633. https://doi.org/10.1016/j.fishres.2016.07.012
Haimovici, M., Reis, E.G., 1984. Determinação de idade e crescimento da Castanha Umbrina Canosai,(Pisces, Sciaeinidae) do Sul do Brasil, Atlântica.
Holden, M.J., Raitt, D.F.S., 1974. Manuel de Sciences Halieutique, deuxième partie-Méthodes de recherches sur les ressources et leur application, in: FAO, Documents Techniques Sur Les Pêches. p. 223.
Ibáñez, A.L., O’Higgins, P., 2011. Identifying fish scales: The influence of allometry on scale shape and classification. Fish. Res. 109, 54–60. https://doi.org/10.1016/j.fishres.2011.01.016
Isely, J.J., Grabowski, T.B., 2007. Age and Growth, in: Guy, C.S., Brown, M.L. (Eds.), Analysis and Interpretation of Freshwater Fisheries Data. American Fisheries Society, p. 961.
Jakobsen, T., Fogarty, M.J., Megrey, B.A., Moksness, E., 2009. Fish Reproductive Biology: Implications for Assessment and Management, Fish Reproductive Biology: Implications for Assessment and Management. Wiley-Blackwell, Oxford, UK. https://doi.org/10.1002/9781444312133
Koch, J.D., Quist, M.C., 2007. A Technique for Preparing Fin Rays and Spines for Age and Growth Analysis. North Am. J. Fish. Manag. 27, 782–784. https://doi.org/10.1577/m06-224.1
Maceina, M.J., Boxrucker, J., Buckmeier, D.L., Gangl, R.S., Lucchesi, D.O., Isermann, D. a, Jackson, J.R., Martinez, P.J., 2007. Current Status and Review of Freshwater Fish Aging Procedures Used by State and Provincial Fisheries. Fish. Res. 32, 329–340. https://doi.org/https://doi.org/10.1577/1548-8446(2007)32[329:CSAROF]2.0.CO;2
Methot, R.D., Wetzel, C.R., 2013. Stock synthesis: A biological and statistical framework for fish stock assessment and fishery management. Fish. Res. 142, 86–99. https://doi.org/10.1016/j.fishres.2012.10.012
Murgas, L.D.S., Alves, M.F., Carneiro, W.F., Felizardo, V.O., Mello, R.A., Machado, G.J., Andrade, E.S., Pompeu, P.S., 2018. Reproductive biology of pequira Bryconamericus stramineus (Eigenmann, 1908) in Funil Reservoir-MG, Brazil. Brazilian J. Biol. 79, 639–645. https://doi.org/10.1590/1519-6984.186925
Petrere Junior, M., 1985. Migraciones de peces de agua dulce en America Latina: algunos comentarios. COPESCAL Doc. Ocas. Roma.
Querol, M.V.M., Querol, E., Gomes, N.N.A., 2005. Fator de condição gonadal, índice hepatossomático e recrutamento como indicadores do período de reprodução de Loricariichthys platymetopon (Osteichthyes, Loricariidae), bacia do rio Uruguai médio, sul do Brasil. Iheringia. Série Zool. 92, 79–84. https://doi.org/10.1590/s0073-47212002000300008
Ribeiro, V.M.A., Santos, G.B., Bazzoli, N., 2007. Reproductive biology of Steindachnerina insculpta (Fernandez-Yépez) (Teleostei, Curimatidae) in Furnas reservoir, Minas Gerais, Brazil. Rev. Bras. Zool. 24, 71–76. https://doi.org/10.1590/s0101-81752007000100009
Rojo, A.L., 1991. Dictionary of Evolutionary Fish Osteology, CRC Press. CRC Press, Boca Raton. https://doi.org/10.2307/1446697
Santana, H.S., Minte-Vera, C. V., 2017. Age and growth of Prochilodus lineatus in a spatially structured population: is there concordance between otoliths and scales? Environ. Biol. Fishes 100, 223–235. https://doi.org/10.1007/s10641-017-0574-5
Spurgeon, J.J., Hamel, M.J., Pope, K.L., Pegg, M.A., 2015. The Global Status of Freshwater Fish Age Validation Studies and a Prioritization Framework for Further Research. Rev. Fish. Sci. Aquac. 23, 329–345. https://doi.org/10.1080/23308249.2015.1068737
UEM), SBI, São Paulo 169, 169.
Yoneda, M., Tokimura, M., Fujita, H., Takeshita, N., Takeshita, K., Matsuyama, M., Matsuura, S., 2001. Reproductive cycle, fecundity, and seasonal distribution of the anglerfish Lophius litulon in the East China and Yellow Seas. Fish. Bull. 99, 356–370.