2018
UNIVERSIDADE DE LISBOA
FACULDADE DE CIÊNCIAS
DEPARTAMENTO DE BIOLOGIA ANIMAL
Mestrado em Ecologia e Gestão Ambiental
Afforestation in Portuguese drylands: is the wild rabbit a good
success indicator?
Bárbara Carlota Rua Pinto O’Neill
Dissertação orientada por:
Prof. Doutora Margarida Santos-Reis
i
Acknowledgements
I would like to gratefully thank my supervisors, Profª Margarida Santos-Reis and Profª Cristina Branquinho, for having accepted since the very beginning to supervise me in this work, for always believing in me (even when I did not believe in myself…), and for being confident that I would be able to finish the thesis despite all my difficulties and resistance to statistics in general. Thank you so much for all your kindness, tolerance and patience (which had to be considerably high given the circum-stances!), and for sharing this bittersweet journey with me.
Very special thanks go to Melanie Köbel, Sara Lobo Dias, Alice Nunes, Adriana Príncipe, Eusebiu Stamate and to all my beloved friends from the AdaptforChange Project (our dear Project!) and to the eChanges group. Thank you so much Melanie and Eusebiu for making those two months of fieldwork unforgettable, filled with such humorous moments! Melanie, Alice, Sara (that precious help with ArcGIS and endless hours doing cartography!), thank you so very much for all your patience, kindness, for the vital help with pretty much every single doubt, and statistical issue, for being there for me and always ready to help no matter what or how busy you were, and for all the countless and endless work sessions we had together. I would also like to thank my friend Carina Neto for giving me such vital help with all the QGIS related issues and numerous problems I had with the maps. Thank you all so much for believing in me in a way that I really didn’t, and for always trusting that I would finish the disserta-tion. This thesis is also for you! Also, special thanks to Henrique Borges for his precious help with the pagination and formatting of the whole thesis.
Thank you very much also, Sandra Alcobia and Tiago, for all the help given me in the beginning of my thesis, and for being so sweet and receiving me (even just for a short stay!) at the Companhia das Lezírias. Sandra, thank you so much for teaching me all the relevant information regarding bunnies and their latrines and pellets and other animal traces. It was certainly vital to help me do my own fieldwork later in the Alentejo with the rabbits in the reforestations, so I am gratefully thankful to you.
I would also like to thank Catarina Costa, Andreia Anjos, Inês Santos, Inês Mirra, Patrícia dos Santos, Paolo Carril, Catarina Gouveia, Lorenzo and everyone else who works in the Ecology Scholar-ship-holders Room and to other friends on the 5th and 4th floor of C2 where I spent endless hours working during these last months. Thank you so very much and everyone else that I might have forgotten. Thanks for having received me so well in a room where I wasn’t really supposed to be! Thank you for all your kindness, encouraging words, interest for my hard work, for all the good times, the social gatherings and long talks, and for witnessing all my moods and the weird adventure it was to finish this thesis on time. Thank you for always being there for me, for being my second family and for providing me a second home. It would not have been the same to finish this work without your support and daily com-pany and friendship!
I would also need to thank Patrícia Atalaya for appearing at exactly the right moment of my life and for helping me regain track of everything and making me believe that I would be able to finish this thesis once and for all. I am truly thankful to you; without your guidance I would not be where I am now.
Thank you to all my amazing friends who were there all along and supported me unconditionally, always believing that I would make it. Thank you, Joana, Sara, Daniela, Pedro, Rúben, Teresa, Rafael and Alice, Joaquina, Guida, Vitória, Paula and my dear Tomás, and everyone I might not remember, but somehow helped me or gave me some support along this journey.
To my family, especially to my aunt São, Michael, Bia and Bella for being so present and sup-portive in the last critical work stage, and to my mother Isabel and my father Brian, for always believing unconditionally in me, for giving me all the strength needed to face and complete this work and believing that I had all the capacities to conclude it. We surely know that finishing this thesis is a vital academic victory, but most importantly than that, a personal one and a turning point in my life! I thank you emo-tionally.
ii
Abstract
Dryland ecosystems are characterised by high aridity levels and extremely low precipitation rates, being highly vulnerable to desertification and land degradation (DLD) due to these high aridity levels and considerable human pressure. Such vulnerability is expected to increase under a climate change scenario predicting a general increase in aridity, temperatures, frequency of extreme events and an accentuated decrease of precipitation. A significant proportion of drylands can be found within Mediterranean ecosystems, which have been experiencing a marked expansion of semi-arid climate in recent years due to climate change. This is becoming particularly alarming in the Iberian Peninsula, mainly in the southern Portuguese regions of Alentejo and Algarve. Drylands’ DLD susceptibility will lead to a significant decrease in ecosystems’ productivity, calling for the implementation of adaptation strategies. Reforestation with holm-oak (Quercus ilex), cork-oak (Quercus suber) and stone-pine (Pinus pinea) native species is a restoration practice that has been widely used in southern Portuguese drylands for the last sixty years. However, there are several lacunae concerning the evaluation of reforestation’s characteristics and success, which have not yet been properly evaluated, while it is also quite unusual to have recourse to animal indicators to perform such evaluation.
The present work aims to understand whether the European wild rabbit (Oryctolagus cuniculus - Linnaeus, 1758) is a good indicator of the success of historical reforestations in southern Portuguese drylands. The wild rabbit is a multifunctional keystone species in Mediterranean ecosystems, especially in the Iberian Peninsula, due to the multiplicity of relevant roles and impacts it has in habitat’s structure and complexity as ecosystem engineers and “landscape shapers”, and as a main prey for a wide range of major predators, many of which are emblematic endangered species. Therefore, the wild rabbit exhibits several favourable conditions for being a good ecological indicator.
In this work, the wild rabbit’s relative abundance was estimated within holm-oak, cork-oak and stone-pine reforestation sites along an aridity gradient through southern regions of continental Portugal through latrine counting along foot-transects, using the number of latrines to calculate wild rabbits’ Kilometric Abundance Index (KAI) for each sampling site. The relation between wild rabbits’ relative abundance and environmental parameters representative of habitat’s structure and complexity within reforestations (vegetation, soil and climate characterisation performed by the AdaptforChange Project’s research team, which examined the success of such reforestations) was evaluated, to determine whether the wild rabbit performs relevant roles in reforestations’ ecosystems, thus having the potential to be a good indicator of the success of Portuguese dryland reforestations. Spearman correlations were performed to identify possible relations between wild rabbit and reforestation variables, as well as ANOVA analysis to test the effects between environmental parameters and rabbit variables. Furthermore, buffers and land use cartography were carried out for each sampling site to understand how the composition and structure of the landscape may influence the presence and abundance of the wild rabbit in the study area. Spearman correlations were carried out between rabbit variables and habitat/landscape variables (areas and percentages of occupation of each land-use class).
The lack of association between rabbits’ abundance, environmental parameters associated with reforestations and plantation areas determines that, unfortunately, the wild rabbit is indeed not a good indicator of the success of reforestations in southern Portuguese drylands. The wild rabbit’s current distribution and density situation in the Iberian Peninsula prevents the species from acting as a good ecological indicator, which is reinforced and confirmed by our results. Therefore, management and recovery measures should be taken so that the species can regain a fundamental role as an ecological indicator.
iii
Resumo
As drylands são ecossistemas que ocupam aproximadamente metade da superfície terrestre, sendo caracterizadas por elevados índices de aridez e taxas de precipitação extremamente reduzidas. As dry-lands incluem áreas que estão sob influência dos climas árido, semiárido e seco sub-húmido, sendo altamente susceptíveis à desertificação e degradação das terras devido aos elevados índices de aridez e considerável ocupação e pressão humana.
Uma considerável proporção de drylands pode ser encontrada nos ecossistemas mediterrânicos, particularmente na bacia do Mediterrâneo. Nos últimos anos, estes ecossistemas têm sofrido uma ex-pansão significativa do clima semiárido em resultado das alterações climáticas. Esta situação tem-se tornado particularmente relevante e preocupante na Península Ibérica, nomeadamente no sudeste de Portugal continental, nas regiões do Alentejo e Algarve, que têm sofrido uma expansão dos climas se-miárido e seco sub-húmido.
A elevada susceptibilidade destas regiões à desertificação e degradação será agravada num cená-rio atual de alterações climáticas que prevê um aumento generalizado dos índices de aridez, das tempe-raturas, da ocorrência e intensidade de fenómenos extremos e decréscimo acentuado da precipitação. Essa susceptibilidade acrescida conduzirá certamente a uma redução significativa da produtividade bi-ológica e económica dos ecossistemas, apelando à tomada de estratégias de adaptação no sentido de mitigar os efeitos das alterações climáticas, através da promoção da resiliência dos ecossistemas e mi-nimização das suas vulnerabilidades aos efeitos climáticos adversos.
A floresta contribuirá nesse sentido ao desempenhar um importante papel no sequestro de car-bono, regulação climática, promoção e conservação da biodiversidade, complexidade do habitat, solo e água, auxiliando na minimização dos efeitos climáticos adversos, na prevenção da desertificação e de-gradação e no aumento da resiliência dos ecossistemas a eventos extremos, de modo que estes possam continuar a produzir bens e serviços com importância económica, ambiental e social.
Deste modo, a reflorestação com espécies autóctones, como sejam a azinheira (Quercus ilex), o sobreiro (Quercus suber) e o pinheiro-manso (Pinus pinea), é um importante método de restauro ecoló-gico de ecossistemas degradados que tem sido praticado no sudeste semiárido português, em regiões não-produtivas ou agrícolas abandonadas, no decurso dos últimos 40 a 60 anos. As espécies autóctones são ideais neste contexto dada a sua excepcional resiliência e capacidade de adaptação a climas áridos, conseguindo atenuar efeitos climáticos adversos. Apesar disso, reflorestações com espécies nativas em regiões semiáridas estão geralmente associadas a reduzidas probabilidades de sucesso, dado o reduzido crescimento das árvores e elevada mortalidade das plântulas nos primeiros anos de vida, sob condições de secura extrema e escassez hídrica.
Em Portugal, existem diversas lacunas no que concerne a avaliação das características, do estado atual e até do sucesso das reflorestações realizadas no semiárido, não tendo ocorrido ainda uma avalia-ção integrada e sistemática das mesmas. As poucas avaliações que são realizadas restringem-se frequen-temente ao estudo da estrutura, crescimento e funcionamento das comunidades vegetais, sendo pouco usual o recurso a indicadores animais para tal avaliação.
O presente trabalho teve assim por objetivo determinar se o coelho-bravo (Oryctolagus cuniculus - Linnaeus, 1758) é um bom indicador do sucesso de reflorestações realizadas no semiárido histórico português no decurso das últimas décadas.
O coelho-bravo é uma espécie-chave nos ecossistemas mediterrânicos, especialmente na Penín-sula Ibérica, devido à multiplicidade de papéis e efeitos relevantes que desempenha a nível da estrutura, funcionamento, dinâmica e complexidade nesses habitats. Eles são importantes engenheiros de ecossis-temas e desempenham um papel preponderante enquanto modeladores da paisagem, dada a sua influ-ência e efeitos significativos a nível da composição e estrutura da vegetação e capacidade incrível de
iv produzir alterações relevantes na paisagem. Além disto, estão na base das cadeias tróficas mediterrâni-cas, constituindo a presa principal de um alargado leque de predadores de topo, sendo que alguns são espécies emblemáticas ameaçadas (lince-ibérico, águia-imperial-ibérica e abutre-preto), sendo impor-tantes a nível da dinâmica das cadeias alimentares pela influência que têm nas flutuações nas populações de predadores. Além disso, as paisagens heterogéneas mediterrânicas compostas por mosaicos de áreas florestadas de azinheira e sobreiro intercaladas com patches de arbustos e regiões abertas (áreas agríco-las, prados e pastagens) constituem os habitats ideais para o coelho, uma vez que lhe permitem optimizar o esforço da procura de alimento (em regiões abertas ricas em plantas herbáceas e cereais) e minimizar o risco de predação (nas regiões fechadas, como as florestais e com arbustos para proteção).
Deste modo, e dada a estrita relação entre a presença e abundância do coelho-bravo e a estrutura e complexidade do habitat, o coelho-bravo reúne todas as características favoráveis para ser um bom indicador ecológico.
Neste trabalho, a abundância relativa de coelho-bravo foi estimada num total de vinte e três re-florestações de Quercus suber, Quercus ilex e Pinus pinea localizadas no semiárido histórico português, nas regiões do Alentejo e Algarve, ao longo de um gradiente de aridez. A abundância relativa do coelho foi estimada através da contagem de latrinas ao longo de transetos lineares, tendo sido posteriormente usado o número total de latrinas detectadas e a distância total percorrida para calcular o Índice Quilo-métrico de Abundância (IQA) para cada ponto de amostragem.
Foi posteriormente avaliada a relação entre a abundância relativa de coelho-bravo e parâmetros ambientais representativos da estrutura e complexidade do habitat das reflorestações (dados resultantes da caracterização da vegetação, solo e clima realizados pela equipa de investigação do Projeto Nacional AdaptforChange, que avaliou o sucesso de reflorestações realizadas no semiárido histórico português). Tais relações foram avaliadas no sentido de determinar se o coelho-bravo desempenha papéis relevantes nos ecossistemas associados às reflorestações, logo se pode ser classificado como um bom indicador do sucesso de reflorestações em regiões semiáridas portuguesas.
Foram realizadas correlações de Spearman para identificar possíveis relações entre as variáveis associadas ao coelho-bravo e as variáveis ambientais associadas às reflorestações, bem como Análises de Variância (ANOVA) para testar os efeitos entre determinados parâmetros ambientais, como sejam o tipo dominante de reflorestação, a presença de Pinus pinea, a distribuição espacial dos arbustos e o tipo de solo dominante, e o conjunto de variáveis relativas ao coelho-bravo.
Para além disso, foram realizados buffers com um raio de 250 metros para cada um dos pontos de amostragem, tendo sido posteriormente elaborada uma cartografia ao nível da ocupação e usos do solo, essencialmente focada nos tipos de coberto vegetal (plantações dominadas por Quercus ou por Pinus; montados; prados e pastagens; matos), presença de água (massas de água) e indícios de presença e ocu-pação humana (habitações e pomares; infra-estruturas; ruínas e poços abandonados; estradas alcatroa-das), para os buffers previamente obtidos. A cartografia foi elaborada com o intuito de realizar uma análise da diversidade de usos do solo, e assim perceber como a composição e estrutura da paisagem influenciam a presença e abundância de coelho-bravo na área de estudo. Foram realizadas correlações de Spearman para determinar as relações entre as variáveis relativas ao coelho-bravo e as variáveis de habitat (áreas e percentagens de ocupação de cada classe de uso do solo).
Apesar da área de estudo apresentar uma paisagem heterogénea composta por mosaicos de dife-rentes habitats sendo as plantações o uso do solo dominante, e de se terem verificado algumas relações significativas entre a abundância de coelho e variáveis de habitat, a verdade é que não se verificou nenhuma relação significativa com as plantações. A ausência de associação significativa entre a abun-dância relativa de coelho-bravo, as variáveis ambientais associadas à estrutura e complexidade das re-florestações e as áreas das plantações determina que, infelizmente, o coelho-bravo não é efetivamente um bom indicador do sucesso de reflorestações nas drylands do sudeste de Portugal continental. Apesar
v do coelho-bravo reunir todos os requisitos necessários para ser um bom indicador ecológico, a verdade é que ele acaba por não o ser na Península Ibérica, especialmente em Portugal, dada a sua atual distri-buição desigual e as suas densidades extremamente variáveis e altamente fragmentadas ao longo do território de Portugal e Espanha. Esta situação é o resultado infeliz de um declínio massivo nas popula-ções desta espécie no decurso das últimas décadas, principalmente devido à perda e fragmentação do seu habitat, da emergência de doenças virais (Mixomatose e Doença Hemorrágica Viral), e mortalidade por interferência humana, particularmente devido à caça. Desta forma, o número reduzido de efetivos nas populações impede que o coelho seja um bom indicador, e os resultados deste trabalho infelizmente corroboram esta constatação.
Dada a situação delicada do coelho-bravo na Península Ibérica, o seu estatuto de espécie-chave multifuncional nos ecossistemas mediterrânicos apela à tomada urgente de medidas destinadas à gestão e recuperação das suas populações, com vista ao restabelecimento do seu papel fundamental como in-dicador ecológico num futuro próximo.
Palavras-chave: Coelho-bravo, drylands portuguesas, abundância relativa, reflorestações, indicadores
vi
Table of Contents
Acknowledgements ... i
Abstract ... ii
Resumo ... iii
List of Figures ... vii
List of Tables ... viii
1. GENERAL INTRODUCTION ... 1
1.1 - Drylands & Reforestations in Mediterranean semi-arid regions ... 1
A brief overview of drylands’ main features and vulnerabilities ... 1
Drylands amidst Mediterranean regions in a climate change perspective... 1
Reforestation with native species - restoration strategy for drylands under climate change ... 2
Forestry & reforestation policies in the Portuguese territory ... 3
Reforestations with native species within Southern Portuguese drylands ... 4
Low success rates of reforestation & natural regeneration in Portuguese drylands ... 5
Afforestations’ success evaluation - limitations, difficulties & weaknesses ... 5
Project AdaptforChange - finding ways to improve reforestation success in semi-arid lands ... 6
The potential of animal indicators for evaluating reforestation success ... 6
1.2 - The European wild rabbit amidst Mediterranean drylands... 7
A brief overview of rabbit’s taxonomy & distribution ... 7
Short description of rabbit’s behaviour, social organization & reproduction ... 7
On wild rabbit’s basic trophic ecology ... 8
Wild rabbit’s presence & abundance estimation methods - a summary ... 9
Wild rabbit’s fundamental ecological requirements & preferential habitat ... 10
Factors regulating wild rabbit’s distribution & abundance in Mediterranean regions ... 10
Main threats associated with wild rabbit populations’ decline in the Iberian Peninsula ... 11
The wild rabbit as a leading actor in Mediterranean ecosystems ... 12
Wild rabbit’s potential as an ecological indicator ... 13
Framework and relevance of this study ... 13
Objectives ... 13
2. GEOGRAPHICAL AND METHODOLOGICAL FRAMEWORK ... 14
2.1 - Study area characterisation ... 14
2.2 - Sampling sites’ selection ... 15
2.3 - Wild rabbit sampling ... 16
2.3.1 - Sampling strategy: rabbit latrines & droppings’ counts along linear transects ... 16
2.3.2 - Field sampling ... 17
2.4 - Characterization of reforestations by AdaptforChange’s research team ... 19
2.4.1 - Definition of sampling plots for vegetation and soil sampling ... 19
2.4.2 - Vegetation sampling: trees, shrubs & herbaceous features ... 20
2.4.3 - Climate characterization ... 20
2.5 - Data organization & analysis ... 20
2.5.1 - Wild rabbits’ data assembly, organization & analysis ... 20
2.5.2 - Determination of wild rabbits’ relative abundance ... 21
2.5.3 - Integration of reforestation-related variables within the wild rabbit database ... 21
2.5.4 - Spatial organization of wild rabbit data in the Geographic Information System ... 22
2.6 - Data analysis ... 22
2.6.1 - Histograms and correlations ... 22
2.6.2 - Analysis of variance ... 22
2.7 - Buffers and land use cartography of sampling sites ... 23
3. RESULTS ... 24
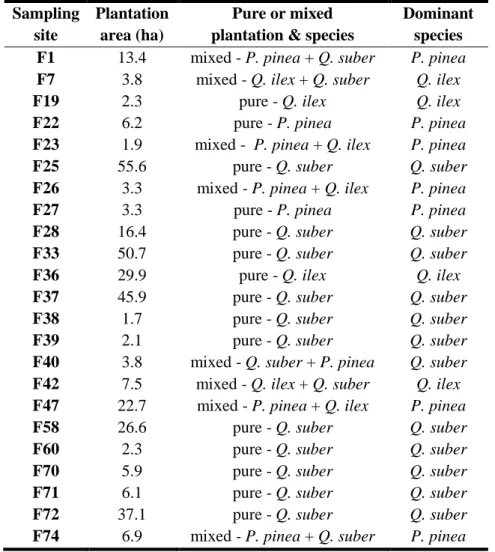
3.1 - Characterisation of sampling sites ... 24
3.2 - Wild rabbit sampled sites ... 25
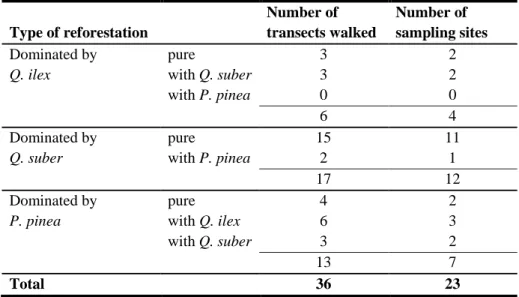
3.2.1 - Characterisation of linear transects ... 25
3.2.2 - Wild rabbit sampling strategy ... 26
3.2.3 - Correlations ... 30
3.2.4 - Relationships between wild rabbit variables and indicators of the success of reforestations ... 31
Correlations ... 31
Analyses of variance ... 33
3.2.5 - Buffers and land-use cartography ... 35
Correlations between wild rabbit variables and habitat variables ... 40
4. DISCUSSION... 42
5. FINAL CONSIDERATIONS ... 49
BIBLIOGRAPHICAL REFERENCES ... 50
vii
List of Figures
Figure 1.1 - Worldwide distribution of drylands according to their specific subtypes Figure 1.2 - Aridity expansion over past years in continental Portugal
Figure 1.3 - Reforestation restoration projects carried out in the Mediterranean Basin within dryland and
non-dryland areas
Figure 1.4 - Examples of reforestations in the southeast region of the Alentejo, continental Portugal Figure 1.5 - Illustration of the wild rabbit
Figure 1.6 - Wild rabbit's current distribution across the world Figure 1.7 - Examples of wild rabbit’s latrines
Figure 1.8 - Mosaic landscape, typical among Mediterranean habitats Figure 2.1 - Map illustrating the study area along an aridity gradient
Figure 2.2 - Map illustrating an example of rabbit latrines and pellet counts within one sampling site Figure 2.3 - Example of a sampling area for vegetation sampling including 3 plantation lines, with an
area of 1000 m2
Figure 3.1 - Representation of the total number of latrines found in each sampling site Figure 3.2 - Representation of latrines’ size index (average diameters within pre-established
average diameter classes) per sampling sites
Figure 3.3 - Representation of pellets’ quantity index (pellets’ average number within each latrine
found in pre-established pellets’ estimated number classes) per sampling sites
Figure 3.4 - Representation of the wild rabbit’s Kilometric Abundance Index for each sampling site Figure 3.5 - Map illustrating the variability of the wild rabbit’s Kilometric Abundance Index among the
set of sampling sites
Figure 3.6 - Box plots between environmental parameters and rabbit variables Figure 3.7 - Example of a final buffer prior to the land-use cartography
Figure 3.8 - Examples of the land-use classes for plantations created in the cartography Figure 3.9 - Examples of the land-use classes for montados created in the cartography
Figure 3.10 - Examples of the land-use classes for other vegetation covers and the presence of water
created in the cartography
Figure 3.11 - Examples of the land-use classes for human presence and/or occupation created in the
cartography
Figure 3.12 - Percentages of each land-use class within the set of sampling sites
viii
List of Tables
Table 2.1 - Wild rabbit sampling sites according to type of reforestation
Table 3.1 - Number of sampling sites where wild rabbit sampling was carried out, according to each
dominant type of reforestation
Table 3.2 - Characterisation of Quercus suber, Quercus ilex and Pinus pinea plantations studied, according
to each sampling site
Table 3.3 - Number of transects walked according to dominant type of reforestation, and number of
sampling sites within each reforestation type
Table 3.4 - Number of latrines detected according to each dominant type of reforestation, and to the number
of transects walked within different reforestation types
Table 3.5 - Characterisation of wild rabbit latrines and pellet data obtained through latrine counting along
foot-transects, according to each sampling site
Table 3.6 - Spearman correlations between the set of wild rabbit variables
Table 3.7 - Spearman correlations between the set of wild rabbit variables and indicators of the success of
reforestations (vegetation complexity and structure variables)
Table 3.8 - Spearman correlations between the set of wild rabbit variables and indicators of the success of
reforestations (soil, topography and climatic variables)
Table 3.9 - Land uses and occupation in the study area
Table 3.10 - Total areas of the main land-use classes created in the cartography and buffers’ total area
according to each sampling site
Table 3.11 - Spearman correlations between the set of wild rabbit variables and landscape variables (total
areas of the land-use classes)
Table 3.12 - Spearman correlations between the set of wild rabbit variables and landscape variables
(percentages of the land-use classes’ areas)
APPENDIX
Table 1 - Template field sheet used for registering the field data collected for wild rabbit sampling Table 2 - Summary of main results concerning the characterisation of the wild rabbit’s linear transects Table 3 - Representation of latrines’ average diameter classes and latrines’ size index per sampling site Table 4 - Representation of pellets’ average number classes and pellets’ quantity index per sampling site
1
1. GENERAL INTRODUCTION
1.1 - Drylands & Reforestations in Mediterranean semi-arid regions A brief overview of drylands’ main features and vulnerabilities
Drylands are characterised by high aridity levels which encompass hyper-arid (Aridity Index lower than 0.05), arid (Aridity Index between 0.05 and 0.20), semi-arid (aridity ranging from 0.20 to 0.50) and dry sub-humid (aridity gradient from 0.50 to 0.65) areas (MEA 2005a; Reynolds et al. 2007) and extremely low precipitation rates, in accordance with the Aridity Index of the United Nations Envi-ronment Programme (UNEP), and in relation to the ratio between annual mean precipitation and annual potential evapotranspiration (Middleton & Thomas 1997; Nunes 2017).
Drylands occupy almost 50% of the Earth’s land surface (6.15×106 ha), distributed throughout four continents, covering from 31% (South America) to 75% (Australia) of the continental land area, and are inhabited by more than 38% of the total global population (Figure 1.1) (Reynolds & Stafford Smith 2002; Reynolds et al. 2007; UNCCD 2012).
This type of land is extremely vulnerable to desertification and land degradation (DLD) due to high aridity levels coupled with intense human occupation, pressure, and land cover changes over past decades, such as removal of vegetation caused by land-use intensification for crop production, agricul-ture, grazing, and deforestation (Belo et al. 2009; Cortina & Maestre 2005; MEA 2005a; MEA 2005b; Middleton & Thomas 1997; Príncipe et al. 2014; UNCCD 2012). These factors unfortunately contribute to a significant decrease or even loss of ecosystem functionality, composition and biological or eco-nomic productivity over time (Costa et al. 2008; Príncipe et al. 2014; UNCCD 2012), which directly influences their provision of ecosystem services (MEA 2005a). Besides being extremely vulnerable to DLD effects, drylands are also highly vulnerable to climate change (Nunes 2017; Reynolds et al. 2007).
Drylands amidst Mediterranean regions in a climate change perspective
A considerable proportion of drylands is found within Mediterranean ecosystems, such as the Mediterranean Basin (where approximately 67% of the territory is occupied by drylands) (Nunes 2017; Nunes et al. 2016b; Príncipe et al. 2014). Their DLD susceptibility is expected to increase under a climate change scenario that estimates an accentuated increase of average temperatures, a decrease in rainfall and shifts in precipitation frequency and patterns, more frequent and severe occurrence of ex-treme events (heat waves, droughts and storms), and higher aridity levels, for the Southeastern
Figure 1.1 - Worldwide distribution of drylands according to their specific subtypes. Adapted from MEA 2005b.
2 Mediterranean, especially in Europe and in the Iberian Peninsula (Costa et al. 2008; ICNF 2013; IPCC 2014; MEA 2005a; Miranda et al. 2006).
Over the past decades, a significant expansion of semi-arid climate has been occurring in Medi-terranean ecosystems, mainly due to climate change (Köbel et al. 2017; Nunes et al. 2016a).
This situation is unfortunately gaining relevance in the Iberian Peninsula, especially in the south-east Alentejo and Algarve regions of Portugal, in which a significative expansion of both semi-arid and dry sub-humid climate over past years has been occurring (Figure 1.2) (Costa et al. 2008; Köbel et al. 2017; Nunes et al. 2016a). This expansion in the Portuguese territory is mainly due to a global increase of temperatures that has been under way since 1970, responsible for more frequent and intense drought events, storms and heavy rainfall periods, and decreased precipitation rates within irregular rainfall re-gimes (MAMAOT 2013; Miranda et al. 2006; Nunes et al. 2016a; Príncipe et al. 2014).
Given drylands’ enhanced vulnerability under this scenario, especially in southern Portuguese
regions, it is vital to adopt adaptation strategies, such as the restoration of degraded ecosystems, to mit-igate climate change effects and minimize ecosystems’ vulnerabilities under climate constraints (Cortina & Maestre 2005; MAMAOT 2013; Nunes et al. 2016a; Vizinho 2015). This can be achieved through the promotion of ecosystem species and habitats’ resilience, adaptation, and sustainability, and of eco-system services and assets’ provision (providing products related to economic welfare such as wood and cork) (MAMAOT 2013; Nunes et al. 2016a; Pereira et al. 2009; Vizinho 2015).
Reforestation with native species - restoration strategy for drylands under climate change
Among ecological restoration programs concerning plant species, reforestations with native species is a frequently used forestry management strategy, as it helps to ameliorate desertification and land degradation effects on drylands and promote their resilience and adaptation capacities to climate change (AdaptforChange 2015; Köbel et al. 2017; MAMAOT 2013; Nunes et al. 2016a; Pereira et al. 2009; Príncipe et al. 2014).
Autochthonous forests play fundamental roles in support services, such as primary productivity, and in regulation services - soil erosion’s prevention and protection through plant cover; facilitation of carbon sequestration within plant biomass and in the soil; climatic regulation; increase of biodiversity,
Figure 1.2 - Aridity expansion over past years in continental Portugal.
(left) historical aridity gradient [1961-1990]; (right) current aridity [1980-2010]. Adapted from AdaptforChange 2015.
3 organic matter, habitat complexity, water infiltration in the soil, productivity; decrease in the risk of fires and the incidence of plagues and diseases; and promotion of a wider production of ecosystem assets and services with economic, environmental and social relevance (Köbel et al. 2017; MAMAOT 2013; Nunes 2017; Pereira et al. 2009; Príncipe et al. 2014).
Reforesting wide dryland areas with different native species will contribute to a majorincrease of tree cover and a wider diversity of species, which is fundamental to mitigate aridity effects and to potentiate forestry ecosystems’ resilience in the face of extreme events. These forests promote the re-covery of the structure, composition and function of such ecosystems so they can continue producing assets and services with higher economic return (Cortina & Maestre 2005; Köbel et al. 2017; Nunes 2017; Pereira et al. 2009; Príncipe et al. 2014; Rey Benayas & Camacho-Cruz 2004). On the other hand, they will also help revert downshifts in tree growth and natural regeneration among forest settlements in highly susceptible dryland areas (Middleton & Thomas 1997; Príncipe et al. 2014; Silva 2012).
Native species have an exceptional adaptation and resistance to arid climates, making them highly suitable for reforestations undertaken in dryland ecosystems, as they can attenuate negative climatic effects imposed by high aridity levels and decreased precipitation rates (Köbel et al. 2017; Príncipe et al. 2014).
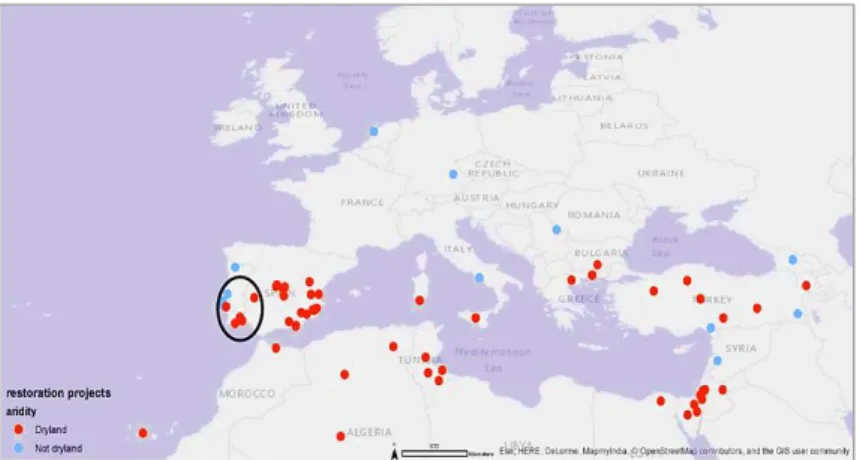
According to a global survey of dryland reforestations, a significant number of reforestation pro-jects have been carried out within these regions for the restoration of degraded ecosystems. Such propro-jects have been performed both worldwide and across the Mediterranean Basin, with emphasis in the Iberian Peninsula’s dryland areas (Figure 1.3) (Nunes et al. 2016b).
Forestry & reforestation policies in the Portuguese territory
For millennia, holm-oaks and cork-oaks were the dominant woodlands system within continental Portugal, and were used for the harvesting of economically and industrially valuable resources in re-sponse to the demands of industry and urban centres (wood, cork, fibre, among others). However, until the middle of the 20th century, rural populations converted such forested areas into subsistence agricul-tural fields and livestock grazing areas (Pereira et al. 2009; Príncipe et al. 2014). Throughout the fol-lowing years, the economic return associated with such activities ceased and so those areas became unproductive, which led to their abandonment and to an increase in rural exodus (Nunes et al. 2016a; Pereira et al. 2009; Príncipe et al. 2014).
Such non-productive agricultural and grazing lands or marginal terrains were later converted to forested areas, especially since Portugal’s entry in the European Union (Nunes et al. 2016a; Pereira et
Figure 1.3 - Reforestation restoration projects carried out in the Mediter-ranean Basin within dryland and non-dryland areas.
4 al. 2009; Príncipe et al. 2014). This was mainly due to higher private investments related to economic interests, to the promotion of afforestation initiatives and to forests’ natural regeneration (Köbel et al. 2017; MAMAOT 2013). Nowadays forests are indeed the dominant form of land use in continental Portugal, occupying 35.4% of the territory, one of the highest afforestation rates within the European Union (ICNF 2013; ICNF 2014; Köbel et al. 2017; MAMAOT 2013).
Reforestations with native species within Southern Portuguese drylands
The Portuguese government, along with the Instituto da Conservação da Natureza e Florestas (ICNF), the Agência Portuguesa do Ambiente (APA), landowners, national park managers, municipali-ties, Environmental Non-Governmental Agencies (ENGOs), and private and public companies, have been promoting and implementing in the last forty to sixty years several reforestation initiatives with cork-oak (Quercus suber), holm-oak (Quercus ilex) and stone-pine (Pinus pinea) native species (Köbel et al. 2017; MAMAOT 2013; Nunes et al. 2016a; Príncipe et al. 2014). Such reforestations, a vital component of the National Strategy for Adaptation to Climate Change (ENAAC), have been carried out in non-productive or abandoned agricultural and grazing areas with higher aridity (semiarid and dry sub-humid areas), particularly in the southeast region of the Alentejo (Figure 1.4) (Köbel et al. 2017; Nunes et al. 2016a; Príncipe et al. 2014).
According to the National Forestry Inventory, in 2010 there were 328 578 ha occupied by refor-estations of holm-oak, 730 399 ha of cork-oak and finally 173 716 ha of stone-pine trees (ICNF 2016, cited by Köbel et al. 2017). Reforestation projects that were submitted between 2013 and 2016 encom-passed a total of 53 659 ha that were reforested, and from these, 8 432 ha were occupied by cork-oak (16%), 4 887 ha by stone-pine (9%), while only 1 029 ha by holm-oak (2%). There was a total of 280 projects that were approved and validated in the Alentejo region, occupying a total area of 6 101 ha (ICNF 2016, cited by Köbel et al. 2017; Nunes et al. 2016a). Many reforestation efforts took place in the sub-region of Alentejo Interior, such as holm-oak reforestations undertaken in 1995 and 2009 in the Herdade da Contenda (Príncipe et al. 2014; Rey Benayas & Camacho-Cruz 2004; Silva 2012).
Holm-oak, cork-oak and stone-pine are the most commonly used species for the restoration of woodlands in Mediterranean ecosystems (ICNF 2014; Nunes 2017; Oliet et al. 2015; Príncipe et al. 2014; Ramos et al. 2015), due to their exceptionally high resilience to drought events, being physiolog-ically adapted to water scarcity (ICNF 2014; MAMAOT 2013), and since they are extensively
Figure 1.4 - Examples of reforestations in the southeast region of the Alentejo, continental Portugal. (top) Quercus ilex; (left) Quercus suber; (right)
5 distributed and considerably abundant within drier Mediterranean areas (Belo et al. 2009; Bravo-Oviedo & Montero 2005; MAMAOT 2013; Pereira et al. 2009; Ramos et al. 2015).
Investing in differential reforestation activities and practices and in more than one tree species will considerably reduce the risks of both forest settlements’ failure and of ecological and economic losses under a more arid scenario (AdaptforChange 2015; ICNF 2014).
Low success rates of reforestation & natural regeneration in Portuguese drylands
Unfortunately, most of the reforestation efforts carried out within southern Portuguese drylands proved to be unsuccessful in the long term or at least had a very minimal success rate, which is consistent with the extremely low success rates and prominent failure of most reforestation programs carried out for the ecological restoration of Mediterranean drylands (Oliet et al. 2015; Príncipe et al. 2014).
Mediterranean dryland reforestations’ low success rates or even failure is mainly due to climatic constraints imposed by high aridity, summer droughts, and high variability in rainfall regimes coupled with water scarcity (Príncipe et al. 2014; Rey Benayas & Camacho-Cruz 2004; Silva 2012). The latter greatly improve stressing effects in plants and are responsible for trees’ limited survival, growth and productivity during the first years after planting, and for restricted tree recruitment (ICNF 2014; MAMAOT 2013; Oliet et al. 2015; Príncipe et al. 2014), as well as for extremely low rates of natural tree regeneration related to the slow growth and high seedling mortality rates (ICNF 2014; Príncipe et al. 2014; Rey Benayas & Camacho-Cruz 2004; Silva 2012).
Afforestations’ success evaluation - limitations, difficulties & weaknesses
In Portugal, information regarding reforestation characteristics, results, and even their success rates are extremely scarce and dispersed, mainly due to the limitations and difficulties associated with the use of a very heterogeneous set of reforestation and evaluation methodologies in different macrocli-matic areas (Köbel et al. 2017; Nunes et al. 2016a). In fact, until now there has been no proper systemacrocli-matic and integrated evaluation of the success associated with different Portuguese dryland afforestations per-formed in recent years.
Ecological restoration success evaluations are essentially focused on the structure and functioning of plant communities (Mexia 2008), and as such, these evaluations are usually restricted to punctual inspections or evaluations concerning tree density, as to guarantee the maintenance of a certain mini-mum tree density that is required for the preservation and viability of afforestations (Köbel et al. 2017; Nunes et al. 2016a).
For these cases, evaluations were restricted since they only concerned the establishment and growth of planted trees, without considering the potential effects and impacts of reforestation actions upon ecosystems’ parameters - such as the natural regeneration of trees, plant diversity and productivity and soil quality, functionality and sustainability, and evaluation of the impacts of climatic constraints related to more arid conditions and lower water availability in afforestations. From this perspective, an efficient and successful evaluation of Portuguese afforestations is lacking, and it is vital not only to understand whether reforestations are fulfilling the objectives proposed for the arid areas, but also to evaluate their resilience under a climate change scenario (Köbel et al. 2017).
6
Project AdaptforChange - finding ways to improve reforestation success in semi-arid lands
The Project AdaptforChange is a National Research Project carried out in 2015 and 2016 that has aimed to assess the status and the degree of success of historical reforestations with native species: holm-oak (Quercus ilex subsp. rotundifolia); cork-holm-oak (Quercus suber), and stone-pine (Pinus pinea), imple-mented in southern Portuguese drylands along an aridity gradient, in order to understand in which ways reforestation practices and environmental conditions (such as climate) affect the ecosystem (Köbel et al. 2017; Nunes et al. 2016a).
To evaluate reforestation success, the relationship between certain ecosystem parameters (tree, shrub and herbaceous diversity and productivity; natural tree regeneration; soil quality; structural habitat diversity; ecosystem services [including biodiversity evaluations]), and climatic data (mainly aridity, since climate imposes strong constrains on plant development in the study area) was evaluated for a set of holm-oak, cork-oak and stone pine reforestations throughout the Alentejo region (Köbel et al. 2017; Nunes et al. 2016a).
The results obtained have demonstrated that both reforestation practices and environmental con-ditions play vital roles in shaping the ecosystem. Reforestation objectives should be planned and man-aged in a more flexible way and be more adapted according to the specific objectives or ecosystem services for each case, taking into consideration the specific climatic and topographic context of the target areas. Reforestations should also be implemented in such a way as to guarantee long-term sus-tainability, to increase ecosystem resilience and to diversify income sources for local populations under a climate change scenario of increasing aridity in the coming decades (Köbel et al. 2017; Nunes et al. 2016a).
The potential of animal indicators for evaluating reforestation success
In many restoration projects, especially in those concerning afforestations, using animal indica-tors for success evaluations on habitat and food webs’ structure and complexity (ecological succession) is indeed not very frequent (Mexia 2008).
An international inquiry in areas with conservation interest has shown that, from a total of fifty restoration projects undertaken in Portuguese semiarid regions that performed success evaluations, only six of them truly measured the number of species and/or animal abundance and used it as an indicator of such success (Alice Nunes & Cristina Branquinho - unpublished data).
This reinforces the need for alternative success indicators, such as animal ones, that may have the potential to help understand whether reforestation efforts were indeed successful, if they represented a good investment in the Portuguese territory, and if they are contributing somehow to increase ecosys-tems’ resilience to Climate Change through an enhanced habitat structure, complexity, and diversity.
7
1.2 - The European wild rabbit amidst Mediterranean drylands A brief overview of rabbit’s taxonomy & distribution
The European wild rabbit Oryctolagus cuniculus (Linnaeus, 1758) is a small herbivorous mammal that be-longs to the Lagomorpha Order and Leporidae Family (Fig-ure 1.5) (ICNF 2005; Smith & Boyer 2008).
The wild rabbit is a native species from the Iberian Peninsula (Alves et al. 2008; Ferreira 2003; Mira et al. 2007) and the North-western African regions of Morocco and Algeria, only occurring naturally within those regions of the Mediterranean Basin (Gálvez-Bravo 2011; ICNF 2005; Rodrigues de Barros 2016).
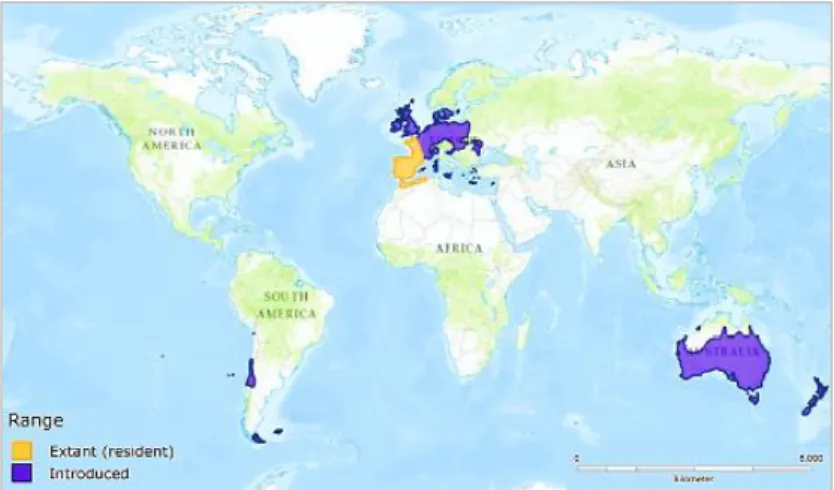
Their current worldwide distribution, from across the European continent, including Madeira and the Azores is-lands, Canarias and Baleares, to several other regions of the world, such as Australia, New Zealand and some parts of South America and North Africa (Figure 1.6), is due to
sev-eral deliberate introductions over past decades, given their relevance as a food or cinegetic resource (Alves et al. 2008; Ferreira 2003; ICNF 2005; Rodrigues de Barros 2016; Smith & Boyer 2008). Wild rabbit’s successful establishment in other countries is mainly due to their high ecological plasticity, colonizing and adaptation capacities within several types of habitat, coupled with elevated reproduction rates and favourable ecological conditions (Fernandes dos Santos 2009; Ferreira 2003; Gálvez et al. 2008; Smith & Boyer 2008; Ward 2005). However, it is considered a plague in many countries where it has been introduced, such as in Australia, New Zealand and England, due to their damaging effects to agriculture, native species and ecosystems (Alves et al. 2008; Smith & Boyer 2008; Ward 2005).
Short description of rabbit’s behaviour, social organization & reproduction
Wild rabbits are active during twilight and at night (Ferreira 2003; Gálvez-Bravo 2011), spending much of the day resting underground. Rabbits usually only come out of their burrows at dawn and dusk time to graze and feed in areas relatively close to their warrens. Such nocturnal habits constitute an
Figure 1.5 - Illustration of the wild rabbit.
Adapted from ICNF 2005.
Figure 1.6 - Wild rabbit's current distribution across the world.
8 efficient strategy to reduce the predation risk related to hunting pressure and/or diurnal predators (Fer-reira 2003; Fernandez-de-Simon et al. 2011; Gonçalves 2015). They also tend to avoid dirt tracks during the day to prevent human disturbance, although they can be spotted in broad daylight on warm, sunny days in undisturbed places (Fernandez-de-Simon et al. 2011; Zim & Hoffmeister 1955).
The wild rabbit is indeed the lagomorph species that exhibits the most complex social organization. Each social group is formed by a dominant male and several reproductive females along with juveniles and subordinated males, being its social structure determined by habitat quality as well as shelter and food availability (Gálvez-Bravo 2011; Gonçalves 2015). Digging burrows is a relevant component of wild rabbits’ social organization, which is used by different social groups (Ferreira 2003; Gálvez-Bravo 2011; Gálvez et al. 2008). Warrens are a complex group of burrows that several rabbits have been building up for a considerable amount of time. They are of vital importance to rabbit populations, as rabbits establish their vital domain around them, and are indispensable for rabbit’s reproduction and protection against potential predators (Ferreira 2003; Gálvez-Bravo 2011; Gonçalves 2015).
Rabbits are very popular for their prolific nature, as they are one of the fewest vertebrate species, and unlike other lagomorphs, in which females can be sexually receptive during the whole year (Gálvez-Bravo 2011; Smith & Boyer 2008; Solinas 1998; Zim & Hoffmeister 1955). Therefore, they exhibit a considerably accelerated reproductive rate, being able to produce more than twenty cubs annually (Gonçalves 2015; Rodrigues de Barros 2016). Their breeding period is highly dependent on plant availability and climatic conditions. Nonetheless, such a prolific nature is usually counterbalanced by numerous casualties due to the action of external factors such as predators, disease, road traffic, shooting and trapping (Ferreira 2003; Gonçalves et al. 2014; Ward 2005).
On wild rabbit’s basic trophic ecology
The wild rabbit is a generalist herbivorous species that feeds on a wide set of herbaceous and cereal species, mainly grasses and leguminous plants, though they preferably chose to feed on grasses, especially from dry pastures, as these have the highest nutritive value, protein levels and reduced fibres’ content (Fernandes dos Santos 2009; Ferreira 2003; Gonçalves 2015).
However, they can alter their dietary choices and feeding strategy according to plant availability and nutritional content in their habitats (Ferreira 2003; Gonçalves 2015; Morgado 2008; Simões 2010). This nutritional adaptation is highly relevant especially within Mediterranean climate and ecosystems, where food resources’ availability can be quite differential (Gonçalves 2015; Simões 2010). Therefore, they feed on alternative plants with lower nutritive value, such as Cistaceae, Compositae, and Ericaceae, when grasses are less abundant or not present, especially during theSummer season, and may have to feed on other poorly nutritional parts of those plants (leaves, barks and small pieces of roots) (Fernandes dos Santos 2009; Ferreira 2003; Gálvez-Bravo 2011).
Rabbits go through a special physiological mechanism called caecotrophy, in which they re-ingest their own cecotrophic pellets (soft white, bacteria and protein-rich droppings), and then go through a second digestion to later produce a second type of pellets, which are definitive harsh droppings that are permanently deposited in the soil (Gálvez-Bravo 2011; Gil-Jiménez et al. 2015). This is a very efficient strategy as it maximizes nutrient and water intake by rabbits, especially when they feed on plants with lower nutritive value (Ferreira 2003; Gálvez-Bravo 2011; Gil-Jiménez et al. 2015). These definitive pellets can be found either dispersed or deposited in latrines by rabbits that belong to the same social group (Ferreira 2003; Gálvez-Bravo 2011).
Latrines are faecal pellet clusters of at least twenty or thirty droppings typically found inside rabbits’ vital domain that play vital roles in the demarcation of their territory (Figure 1.7) (Ferreira 2003;
9 Gálvez et al. 2008; Morgado 2008). The act of depositing droppings in latrinesconstitutes a vital social interaction between rabbits, being important for olfactive communication among individuals (Gálvez-Bravo 2011; Gonçalves 2015; Sneddon 1991). Rabbits show evidence of a behavioural pattern concern-ing droppconcern-ings and latrines’ location and deposition, as they recurrently tend to choose specific locations within their territories to deposit them (Gonçalves 2015; Morgado 2008; Sneddon 1991). There is indeed a strong behavioural component of latrine abundance, which may not be necessarily linked to rabbit abundance per se, given that rabbits tendentially choose the same regions to deposit their pellets (Fer-nandez-de-Simon et al. 2011; Sneddon 1991). Latrines can be usually found in more opened regions that do not exhibit significant degrees of steepness, preferably in undisturbed arenaceous and/or granitic soils with some herbaceous cover, such as along dirt tracks, earthen, tilled land, among pasture regions and in the proximity of burrows (Margarida Santos-Reis & Sandra Alcobia - personal communication). Rabbit latrines are in this way visible elements that reveal wild rabbit’s presence in a certain region, and can be used to obtain indirect estimates of their relative abundance in those regions (Virgós et al. 2003).
Dropping accumulation within latrines presents very particular physical and chemical character-istics that provide an important nutrient source, contributing to soil fertility, the increase of plant diver-sity and biomass, altering its floristic composition, and being beneficial for coprophagous beetle com-munities (Fernandez-de-Simon et al. 2011; Gálvez-Bravo 2011; Gálvez et al. 2008).
Wild rabbit’s presence & abundance estimation methods - a summary
Until the present time, no methods capable of performing direct and exact measures of wild rabbit population size(s) are known. It is indeed only possible to estimate relative abundance, and todetect and evaluate population density fluctuations, through resource to indirect methods (Fernandez-de-Simon et al. 2011; Gonçalves & Santos-Reis 2014).
These allow us to obtain species’ abundance indices through the quantification of presence and activity traces, such as droppings, latrines, scratchings, footprints, burrows, warrens, among others, which are usually visible and easily identifiable elements (Ferreira 2003; Simões 2010). The most frequently used trace presence prospection methods include linear transects on foot recording rabbit signs (Gonçalves & Santos-Reis 2014; Sarmento et al. 2004); cleared-plot pellet counts at permanent plots (Catalán et al. 2008; Fernandes dos Santos 2009; Ferreira 2003); and finally standing crop counts (Fernandez-de-Simon et al. 2011; Moreno & Villafuerte 1995).
In general, indirect methods do not involve large economic nor human resources and are easily applicable, allowing for the obtaining of reasonably accurate presence and relative abundance estimates in a simple, time-efficient and non-expensive way (Ferreira 2003; Simões 2009; Simões 2010). Since their precision does not depend on animals’ detection or capture, presence trace prospections - such as pellet-count indices - are the most suitable and reliable ones to study less conspicuous species and that
Figure 1.7 - Examples of wild rabbit’s latrines.
10 occur in low to very low densities, such as the wild rabbit in Portugal nowadays (Fernandes dos Santos 2009; Ferreira 2003). From this perspective, LATR (Latrine index - latrines/km) has been recently adopted as a standardized method for monitoring rabbits in Portugal (Gonçalves & Santos-Reis 2014; LPN 2014; Sarmento et al. 2004).
Despite its simplicity and versatility, there are nevertheless some negative aspects that should be pointed out, such as the strict relation between differential defecation rates, dietary composition and social patterns; the relevant direct role of environmental conditions on the deterioration and detectability of traces, and the strong connection between the degree of the observer’s experience and the reliability of species traces identification (Ferreira 2003; Simões 2010).
Wild rabbit’s fundamental ecological requirements & preferential habitat
Wild rabbit’s presence, abundance and distribution are essentially dependent and related to habitat structure, complexity and quality (Ferreira 2003; Gonçalves 2015; Gonçalves & Santos-Reis 2014; Virgós et al. 2003), namely landscape features regarding the nature of soil cover. Their most suitable habitats should include non-fragmented landscapes with a combination of both open and closed areas, exhibiting heterogeneous vegetation cover that allow for nutritionally rich food choices and shelter, and soft soils with adequate substrates that facilitate the construction of burrows and shelters for refuge purposes (Delibes-Mateos et al. 2010; Ferreira 2003; Gonçalves & Santos-Reis 2014; Gonçalves et al. 2014; Gonçalves 2015; Virgós et al. 2003).
Having stated this, heterogeneous mosaic landscapes, highly abundant within Mediterranean ecosystems, are indeed wild rabbit’s preferential habitats (Gonçalves & Santos-Reis 2014; LPN 2014; Simões 2009). These landscapes are composed by complex mixed mosaics of holm and cork-oak forested areas mingled with shrub patches and scrublands, and opened regions, namely pasturelands and agricultural areas (Figure 1.8) (Gonçalves et al. 2014; Gonçalves 2015; ICNF 2005; Simões 2010; Virgós et al. 2003).
In these mixed habitats, the transition between open and closed areas and the discontinuity in the vegetation is vital, as it ensures simultaneously the presence and proximity of the most favourable areas for rabbits to find shelter and protection from predators and/or adverse (climatic) conditions (within closed areas: shrub, scrublands and forest), as well as to feed (within opened areas: pastures and agricultural areas, with high herbaceous or pasture covers that support their diet of grasses and
cereals) (Fernandes dos Santos 2009; Ferreira 2003; Gonçalves 2015; ICNF 2005; Simões 2009; Virgós et al. 2003). This spatial interspersion of mosaic areas suitable for rabbits allows them to optimize their efforts in their search for food resources and minimizes the risk of predation, which is considerably high especially within the Iberian Peninsula (Ferreira 2003; Gonçalves 2015; Gonçalves & Santos-Reis 2014).
Figure 1.8 - Mosaic landscape, typical among Mediterranean habitats. Adapted from Gonçalves 2015.
11
Factors regulating wild rabbit’s distribution & abundance in Mediterranean regions
Within the Iberian Peninsula, the wild rabbit’s presence and abundance is mostly affected by a set of environmental and climatic parameters, such as altitude, slope, type of soil, habitat availability, disturbance factors or threats (predators, pathogens and hunters), precipitation rates and temperature (Ferreira 2003; Gálvez-Bravo 2011; Gonçalves 2015; LPN 2014). Oryctolagus cuniculus tend to be rare in regions that surpass 1500 m in elevation (Smith & Boyer 2008), having been reported elsewhere that rabbits usually avoid areas with low temperatures, high precipitation rates and any kind of human dis-turbance (Gálvez-Bravo 2011; LPN 2014).
Most studies concerning rabbit population trends in the Iberian Peninsula are mainly focused on the associations between present habitat parameters and recent distribution and abundance of their pop-ulations (Delibes-Mateos et al. 2010; Fernández 2005). There is an evident lack of information concern-ing the effects of habitat loss on Iberian rabbit populations, beconcern-ing extremely difficult to understand clearly how landscape changes have affected rabbit populations, and their influence on rabbit popula-tions’ decline in the Iberian Peninsula (Delibes-Mateos et al. 2010). Despite that, there are some studies that substantiate the existence of a strong correlation between rabbit distribution and abundance and significant land-use changes over recent decades in Mediterranean regions (Delibes-Mateos et al. 2010).
Main threats associated with wild rabbit populations’ decline in the Iberian Peninsula
Wild rabbit abundance, once significantly high in the Iberian Peninsula, has been suffering an accentuated decline since the first half of the 20th century (Delibes-Mateos et al. 2010; ICNF 2005; LPN 2014; Mira et al. 2007; Ward 2005). This delicate situation is mainly due to habitat loss, fragmentation and deterioration, viral diseases, and high cinegetic pressure (Ferreira 2003; Gonçalves et al. 2014; ICNF 2005; Ward 2005). The resultant reduction in rabbit populations has caused a major impairment of trophic webs, as well as relevant ecological and economic consequences in Mediterranean ecosys-tems’ functioning (Cabezas-Díaz et al. 2009; Delibes-Mateos et al. 2008; ICNF 2005; LPN 2014).
Habitat deterioration and fragmentation in Mediterranean landscapes are a consequence of the abandonment of traditional agricultural methods, intensive agriculture, forestry intensification, con-struction of urban infrastructures due to socio-economic growth, and recurrent forest fires potentiated by increasing aridity (Fernandes dos Santos 2009; Gálvez-Bravo 2011; ICNF 2005; LPN 2014; Ward 2005). These changes in habitat structure and complexity have promoted the decrease and impoverish-ment of the suitability of rabbits’ most favourable heterogeneous mosaic habitats, thus affecting directly this species’ presence and abundance (Fernandes dos Santos 2009; Gonçalves et al. 2014; ICNF 2005; Smith & Boyer 2008; Ward 2005).
Two major viral diseases, Myxomatosis, and Rabbit haemorrhagic disease (RHD), emerged in 1953 and 1988 respectively within the Iberian Peninsula, being responsible for high mortality rates and for reductions in rabbit populations of approximately 24% in Portugal and of 73% in Spain overthe last thirty years (Gálvez-Bravo 2011; Gonçalves 2015; ICNF 2005; LPN 2014; Virgós et al. 2003). In addi-tion, the European wild rabbit is a very popular game species with a major hunting value, especially in the Iberian Peninsula, where rabbit hunting is a very important recreational, social and economic activity (Delibes-Mateos et al. 2007; Ferreira 2003; Gonçalves et al. 2014; ICNF 2005; Mira et al. 2007; Mor-gado 2008; Simões 2009).
Nowadays wild rabbit abundances are extremely variable within the Iberian Peninsula, because of population decline in recent years (Fernández 2005; Gálvez-Bravo 2011; Gonçalves et al. 2014). Although present throughout the entire Portuguese territory (ICNF 2005), currently their distribution is highly fragmented and heterogeneous, favouring the existence of many low-dense areas and fewer areas still containing rabbits at a relatively high density (Cabezas-Díaz et al. 2009; Delibes-Mateos et al. 2010;
12 Gonçalves 2015; Gonçalves & Santos-Reis 2014; ICNF 2005). For this reason, their conservation status is classified as “Near Threatened”, according to both the IUCN Red List of Threatened Species (Smith & Boyer 2008), and the Red Book of Vertebrates of Portugal (ICNF 2005).
The wild rabbit as a leading actor in Mediterranean ecosystems
Wild rabbits are a fundamental key-species in Mediterranean habitats (Delibes-Mateos et al. 2010; Gonçalves & Santos-Reis 2014; Mira et al. 2007), due to the significant influence that they have in such ecosystem structures, functions and dynamics, and to their ecological importance and the mul-tiplicity of relevant roles they play in these ecosystems (Delibes-Mateos et al. 2008; Delibes-Mateos et al. 2010; Ferreira 2003; Gonçalves et al. 2014; Mira et al. 2007). They are impressive ecosystem engi-neers and a relevant ‘landscape shaper’, given their significant influence and effects on plant commu-nities’ composition and vegetation structure, and their extraordinary capacity to produce relevant changes in landscapes (Ferreira 2003; Gil-Jiménez et al. 2015; Gonçalves & Santos-Reis 2014; Gon-çalves et al. 2014).
The rabbit’s herbivorous role promotes seed dispersion of both herbaceous and woody species, producing changes within the composition of vegetation communities, thus being very beneficial for their regeneration and theincrease of plant populations, and promote the maintenance of opened areas, contributing to a more complex and heterogeneous habitat (Delibes-Mateos et al. 2008; Delibes-Mateos et al. 2010; Ferreira 2003; Gálvez-Bravo 2011; Gonçalves 2015). On the other hand, rabbits’ extraordi-nary excavator capacity through burrow construction is responsible for producing changes in soil char-acteristics, and besides burrows provide foraging, shelter and nesting habitats for other species (Gálvez-Bravo et al. 2009; Gálvez-(Gálvez-Bravo 2011; Gonçalves 2015; Gonçalves et al. 2014).
Moreover, rabbit pellets’ deposition within latrines and rabbits’ urine are fundamental for soil fertilisation, plant growth and higher plant species’ diversity and biomass (Cabezas-Díaz et al. 2009; Delibes-Mateos et al. 2008; Fernandez-de-Simon et al. 2011; Gálvez-Bravo 2011; Gonçalves 2015). The combination of herbivory, excavating activities and pellet accumulation all contribute to an increase in the physical heterogeneity of the ecosystem due to exchanges in edaphic conditions, the creation of water, nutrients and seed clusters (Gálvez-Bravo 2011; Gonçalves et al. 2014).
Wild rabbits have a vital importance in the Mediterranean ecosystem food chains’ dynamic, in their quality as a basic food resource for a wide variety of top predators (Ferreira 2003; Gonçalves et al. 2014; Simões 2010). For being a vital food resource for such a broad number of predators, they have a very significant influence in predators’ population fluctuations (Delibes-Mateos et al. 2008; Fernández 2005; Ferreira 2003; Gonçalves et al. 2012; Simões 2010).
Within the Iberian Peninsula, rabbits are indeed the main stable prey of about 40 relevant species of predators, many of which are emblematic endemic endangered species, some of them being indeed critically endangered, such as the Iberian lynx (Lynx pardinus - Temminck, 1827), the Iberian Imperial eagle (Aquila adalberti - Brehm, 1861), the Black vulture (Aegypius monachus - Linnaeus, 1766), and the Bonelli’s eagle (Hieraaetus fasciatus - Vieillot, 1822) (Cabezas-Díaz et al. 2009; Ferreira 2003; Gonçalves et al. 2012; Loureiro et al. 2011; LPN 2014; Mira et al. 2007). Among these, the Iberian lynx and Iberian Imperial eagle are indeed specialized in rabbit’s consumption (Delibes-Mateos et al. 2008; Rodrigues de Barros 2016), being unfortunately highly dependent on rabbits to survive. In this context, the wild rabbit’s decline in recent years has posed serious consequences for Iberian lynx populations, depriving the lynx from directly interfering in their behaviour, reproduction and long-term survival (Gil-Jiménez et al. 2015; Gonçalves 2015; Loureiro et al. 2011; LPN 2014; Mira et al. 2007).
13
Wild rabbit’s potential as an ecological indicator
The wild rabbit is indeed an essential keystone species in Mediterranean ecosystems, especially in the Iberian Peninsula (Delibes-Mateos et al. 2010; Ferreira 2003; Gonçalves & Santos-Reis 2014). This is due to the multiplicity of relevant roles and impacts they have within these habitats, in the quality of relevant ecosystem engineers and as a ‘landscape shaper’ (Delibes-Mateos et al. 2008; Ferreira 2003; Gálvez-Bravo 2011; Gonçalves et al. 2014). Moreover, they are among the vital base of food chains, being one of the most significant food resources for a considerable set of top predators (Cabezas-Díaz et al. 2009; Fernández 2005; Gonçalves et al. 2014; Virgós et al. 2003).
Given the proven relevance of such species within Mediterranean ecosystems and their strict re-lation and vital roles in habitat structure, quality and complexity, the wild rabbit has all the potential to be a good ecological indicator in such ecosystems.
Framework and relevance of this study
The present work was carried out within the context of the previously mentioned AdaptforChange Project, a Portuguese Research Project carried out in 2015 and 2016 that aimed to evaluate the success of reforestations that were implemented in Portuguese historical semi-arid lands.
Reforestation with native species is a vital environmental management tool, as it is an ecological restoration method frequently used for degraded ecosystems. The study of the potential of the wild rabbit as an ecological indicator of ecosystem management, particularly in the context of reforestations with native species in Mediterranean drylands, poses a perfect conciliation between two vital Biology aspects, the Ecology (wild rabbit & habitat ecology) and the Environmental Management (reforestation with native species) ones.
Objectives
The present work intends to understand whether the wild rabbit is a good success indicator for historical reforestations in southern Portuguese drylands.
14
2. GEOGRAPHICAL AND METHODOLOGICAL FRAMEWORK
2.1 - Study area characterisation
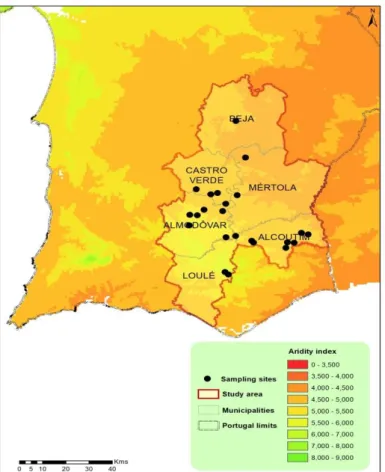
The present study was carried out in holm-oak (Quercus ilex subsp. rotundifolia), cork-oak (Quercus suber) and stone-pine (Pinus pinea) reforestation areas, located in the southern dryland regions of continental Portugal (Baixo Alentejo and Algarve - Figure 2.1), southwestern Iberian Peninsula, Europe. These reforestation actions took place under semi-arid and dry sub-humid climates, over the last forty to sixty years.
The study area has a typical Mediterranean climate with a strong temperature and rainfall seasonality, and a high inter-annual variability in precipitation. It is characterized by cold and moist winters with strong precipitation periods and very hot, dry summers with scarce precipitation and average temperatures above 22ºC (Belo et al. 2009). However, due to a considerable increase in aridity levels in past decades, nowadays these regions are dominated by semi-arid (Aridity Index between 0.20 and 0.50) and dry sub-humid climates (Aridity Index from 0.50 to 0.65). These characteristics impose limitations to plant species establishment and growth (Köbel et al. 2017; Vallejo et al. 2012), which are expected to worsen under a climate change scenario of increased aridity and a higher frequency of extreme events, such as prolonged droughts or seasonal floods (Belo et al. 2009; Costa et al. 2008; Miranda et al. 2006).
Figure 2.1 - Map illustrating the study area along an aridity gradient.
The set of sampling sites is shown here according to the municipalities where they are located, and along an aridity gradient from dry sub-humid (AI=0.58) to semi-arid (AI=0.44). Regions in orange are more arid (lower aridity index). Map scale 1:880.000. The map was projected using the coordinate system