Structural study of a series of synthetic goethites obtained in aqueous
solutions containing cadmium
„
II
…
ions
E. E. Sileoa)and P. S. Solı´s
INQUIMAE, Facultad de Ciencias Exactas y Naturales, Universidad de Buenos Aires, Ciudad Universitaria, Pab. II, Piso 3, C1428EHA, Buenos Aires, Argentina
C. O. Paiva-Santos
Instituto de Quı´mica, Universidade Estadual Paulista, UNESP, Araraquara, 14801-970 Sa˜o Paulo, Brazil
~Received 3 June 2002; accepted 8 November 2002!
The capacity of goethite for CdII substitution has been explored in a series of synthetic samples prepared from FeIIIand CdIInitrate solutions aged 21 days in alkaline media. The total metal content (@Fe#1@Cd#) was 0.071 M in all preparations. The samples have been characterized by chemical and X-ray diffraction analysis; the morphology of the solids is described. The cell parameters for all samples were obtained by the Rietveld fits to the X-ray diffraction data. Refined structures show that for samples prepared at the final molar ratio mCd<5.50 ~expressed as mCd51003@Cd#/@Cd# 1@Fe#), a~Cd, Fe!-goethite is the only crystalline product. In these samples, the unit cell parameters increased as a function of Cd concentration, indicating Cd incorporation in the structural frame. At the preparative ratio, mCd57.03, the incorporation of Cd in the goethite structure is drastically reduced and a probable Cd-substituted hematite is formed together with the Fe,Cd-goethite. © 2003 International Centre for Diffraction Data. @DOI: 10.1154/1.1538188#
Key words: X-ray powder diffraction, Rietveld analysis, substituted goethite, cadmium, substituted hematite
I. INTRODUCTION
Goethite, ~a-FeOOH!, is widespread in the ecosystem, and it occurs in oxidizing and reducing environments. It is found in Fe ore deposits and is the most important iron oxide hydroxide present in river and marine sediments. The natural phase commonly occurs as very fine particles that accumu-late heavy metals by adsorption and complexing and/or co-precipitation~Cornell and Schwertmann, 1996!.
In alkaline media, goethite forms from ferrihydrite, the initial oxyhydroxide of Fe~III!. Ferrihydrite is a metastable compound that may transform into goethite, hematite, or a mixture of the two, via two competing pathways. The inter-conversion to goethite proceeds via a dissolution-reprecipitation process ~Fisher, 1971; Blesa and Matijevic, 1989!. Formation of hematite involves a solid-state rear-rangement ~Feitknecht and Michaelis, 1962; Schwertmann and Fisher, 1966! that starts in denser zones within the fer-rihydrite aggregate. Giovanoli and Cornell ~1992! have shown that some first row transition elements stabilize ferri-hydrite toward dissolution so that hematite forms more eas-ily. Aluminum also promotes the formation of hematite, as this effect is attributed to a lower solubility of the Al-containing ferrihydrite precursor relative to pure ferrihydrite ~Schwertmann et al., 2000!. The metal-mixed ferrihydrite may form substituted goethite and/or hematite.
Insertion in synthetic goethites has been demonstrated for the following elements: Al, Cr, Cd, Ni, Ga, Sc, Ge, Si, U, Th, Co, Cu, Pb, V, Mn, and Zn ~Norrish and Taylor, 1961; Lim-Numez and Gilkes, 1985; Stiers and Schwertmann, 1985; Vandenberghe et al., 1986; Cornell and Giovanoli, 1987, 1989; Diaz et al., 1989; Ebinger and Schulze, 1989;
Schwertmann et al., 1989; Gerth, 1990; Schwertmann and Pfab, 1994; Gasseret al., 1999; Manceauet al., 2000; Sileo et al., 2001!.
Cadmium is a heavy metal that is known to bioaccumu-late~Jinet al., 1998; Lehoczkyet al., 1998!. Its solutions are highly toxic to humans and many other biota. It occurs in some mine wastes and as a waste from various industrial activities. The CdII ion is one of the species that may be adsorbed and/or coprecipitated from solution into Fe oxide hydroxides. As the ionic radius of CdIIis significantly larger than that of FeIII~0.0920 vs 0.0615 nm!the substitution may considerably alter the structural framework of pure goethite, changing its morphology and its physicochemical properties. The processes of CdII uptake onto goethite surfaces have been fully analyzed~Forbeset al., 1979; Balistrieri and Mur-ray, 1982; Venemaet al., 1996; Collinset al., 1999; Parkman et al., 1999!. However, only basic studies of cadmium incor-poration into the structural frame of goethite have been re-ported ~Gerth, 1990!.
Goethite shows an orthorhombic unit cell with four groups FeOOH. The structure is based on HCP packing of oxygen (OI) and hydroxide ions (OII) with half of the octa-hedral sites filled with FeIII ions. The structure may be de-scribed in terms of Fe octahedra (FeO3(OH)3) linked in pairs by an edge, through two OII atoms. These pairs form double chains that run parallel to @001# ~Pbnm!. Along the chain, the octahedra are joined by edges through OIand OII atoms. Also each octahedron shares two corners (OI) with two polyhedra from a neighboring double row. This arrange-ment leads to double chains separated by vacant double rows ~see Figure 1!.
Cations MII, MIII, and MIVmay substitute into the octa-hedral Fe positions in the goethite structure; for MIIand MIV substitution, an uptake or release of protons is expected, to
account for charge balance. Although FeIII and CdII have very different ionic radii, their electronic configuration is symmetric; FeIII is a d5 and CdIIis a d10 ion. Thus, in the Cd-for-Fe substitution, the symmetry of the coordination sphere for both ions is expected to be approximately undis-torted and only an enlargement of thea,b, andcparameters is expected.
In this work, we investigated the morphological and cell parameter variations for a series ofa-FeOOH samples syn-thesized from alkaline solutions of FeIII containing different concentrations of CdIIions. We determined the concentration range in which cadmium is incorporated into the goethite structure, and the maximum capacity of Cd in goethite. As the synthetic Cd-rich goethites obtained are poorly crystal-line, structural changes are quantified through the Rietveld analysis ~Rietveld, 1969!of powder X-ray diffraction data.
II. EXPERIMENTAL
A. Preparation of the solids
Pure and Cd-rich goethites were prepared by adding at 70 °C, 50 mL of a 1 M ferric nitrate or 1 M iron plus cad-mium nitrates solution, to 650 mL of sodium hydroxide so-lutions~final OH2concentration was in all cases 0.5 M!. The
suspensions obtained were aged for 21 days at 70 °C in closed polyethylene flasks. Once a day, the flasks were vig-orously shaken. The final products were centrifuged and washed three times with doubly distilled water. In order to remove any non-structurally incorporated cations or ferrihy-drite from the surfaces of the products, an acid oxalate ex-traction ~Schwertmann and Cornell, 1991! was carried out twice. After the extraction process, the solids were washed with doubly distilled water and dialyzed until the conductiv-ity of the solution was similar to that of bidistilled water. Finally, the solids were dried for three days at 40 °C. Prepa-ration molar ratio mCd for the samples, expressed as 100 3@Cd#/@Cd#1@Fe#, were 0; 0.99; 2.88; 3.63; 5.05, and 7.03.
B. Chemical and morphological analysis
The Fe and Cd content in the solids were measured using a Varian Techtrom A-A5R atomic absorption spectrometer.
Morphological observations were carried out using a Philips 515 scanning electron microscope~SEM!operated at 30 keV.
C. X-ray diffraction measurements
Samples were hand-ground in an agate mortar and pestle, and side-loaded into an Al sample holder. XRD data were collected at the measurement conditions given in Table I. The step width, 0.025°, assured a minimum of about 12 intensity points for the narrower peaks.
D. Structure refinements
The structures were refined in the 2-theta range 18.500°–131.000° to ensure that only the specimen is irradi-ated. The Rietveld method was applied using the GSAS ~General Structure Analysis System! program ~Larson and Von Dreele, 1994!. Starting unit-cell parameters and atomic coordinates for goethite ~Szytula et al., 1980!, space group Pbnm, Z54 and hematite~Blakeet al., 1966!, space group R-3c,Z56, were taken from published works. Although the space group of goethite was reoriented toPnma, in order to facilitate comparisons with previous works, we present the results of the refinements as goethite having the space group Pbnm. The measured background was fitted with a simple linear interpolation. The Thompson–Cox–Hasting pseudo-Voigt function~Thompsonet al., 1987!with the microstrain broadening description of Stephens ~Stephens, 1999! was used for fitting peak profiles. Only the scale factors and the background coefficients were adjusted during the first cycles, and then gradually in successive cycles, the following pa-rameters were varied in the least-squares refinement proce-dure: the sample displacement and the unit cell values, the size related LX and Xe and the strain relatedShklparameters, the asymmetry parameters for the peak shapes, and the atomic positional parameters. The isotropic thermal param-eters were varied for all atoms. The preferred orientation was corrected using the March function~Dollase, 1986!. The oc-cupancy factor of the metal site,f, was refined in two stages, first, the starting occupancy factor of the Cd atom was fixed to 0.0 (fFe51.0) and the occupancy factor of the site was obtained, then, and according to chemical analysis, the Cd ion was situated in the Fe position and the occupancy factors of Cd and Fe were refined; this two-step procedure is dis-cussed later. In S5, containing goethite and hematite,mCdin the goethite phase was calculated from regression data as explained later.
Figure 1. Arrangement of polyhedra in the structure of goethite, the octa-hedra share edges within the rows, and vertices between chains.
TABLE I. Chemical analyses for the samples.
Sample Preparation ratioa Cd contenta
S0 0 0
S1 0.99 0.96 –1.06
S2 2.74 4.05– 4.06
S3 3.63 4.55– 4.56
S4 5.50 5.91–5.98
S5 7.03 4.54 – 4.73
aCadmium content measured by duplicate and expressed as 100
III. RESULTS AND DISCUSSION
A. Chemical and XRD analysis of the solids
Table II shows the preparation ratio and the chemical analysis for Fe and Cd for samples S0 to S5. The metal content of each sample was determined by duplicate. Elec-tron microscopy observations of the solids were performed to rule out the presence of Cd and/or Fe amorphous materials or traces of metal oxides not detectable by XRD. As can be seen, from S1 to S4, the level of cadmium incorporation increases in accordance with the preparation ratio. In S5 the process is inverted and an increase in the Cd content of the initial solution provokes a decrease in the Cd content of the solid obtained.
In samples S2– S4, themCdin the final sample is higher than the preparation ratio; this fact is attributed to the extrac-tion process by which only Fe amorphous phases are dis-solved by the oxalic-oxalate solution.
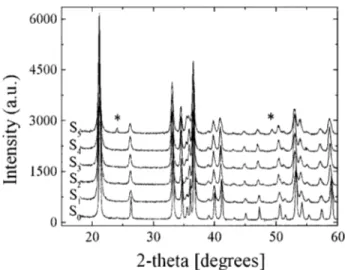
Figure 2 presents the XRD diagrams obtained for the six samples prepared. For S0to S4, the only phase detected was goethite~PDF: 29-0713!and a peak displacement to smaller 2u values was observed as the Cd content in the sample increased. For S5 new peaks are present at 2u: 24.22° and 49.32°. These additional peaks indicate the presence of he-matite ~PDF: 33-0664!.
B. Crystal morphology
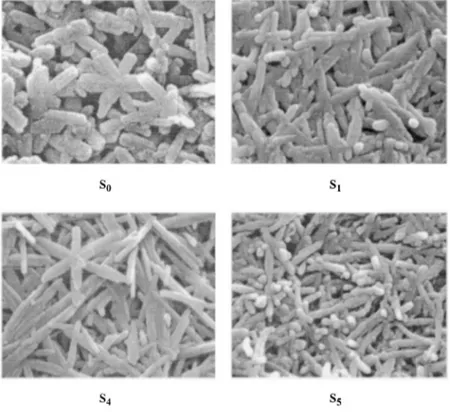
Morphological information obtained via electron micro-scopic imaging was obtained for all samples. Figure 3 shows the scanning electron micrographs~SEM!of samples S0, S1, S4, and S5. S0~pure goethite!displays a variety of acicular
habits where some lengthy needles ~average 1.330.2mm) coexist with a majority of short needles ~average 0.5 30.2mm) and star-shaped twins. From S1 to S4, stars are present together with needles where the length-to-width ratio is increased; the largest length-to-width ratio is found in S3 ~average 1.230.1mm). A change in morphology is detected in S5 where only a few stars are present with needles that show a drastic reduction in crystal lengths ~average 0.6 30.1mm and 0.330.1mm). Some imperfections in crystal morphologies observed by SEM pictures in substituted-goethites, were probably caused by the extraction processes.
TABLE II. Results of the refinements for samples S0– S5.
Sample
~Anis. axis! Rwp Rp x2
RBragg Wt~%!
Pref Or~hkl!
~ratio, fraction! Ocup a~nm! b~nm! c~nm! V ~nm3!
S0 8.84 6.57 1.28 4.53 100 021 Fe: 0.461 14~1! 0.995 89~2! 0.302 30~1! 0.138 830~6!
~1.04, 0.99! 0.875~3!
~001! 140 Cd:
~1.36, 0.01! 0.000
S1 9.25 6.64 1.49 3.33 100 021 Fe: 0.461 67~2! 0.997 04~4! 0.302 67~1! 0.139 316~12!
~1.15, 0.60! 0.879
~021! 140 Cd:
~1.10, 0.40! 0.010a
S2 9.53 7.40 1.53 5.26 100 021 Fe: 0.462 33~3! 1.000 58~4! 0.303 43~1! 0.140 366~13!
~1.49, 0.60! 0.799~1!
~021! 140 Cd:
~1.23, 0.40! 0.033~1!
S3 8.50 6.44 1.25 3.18 100 021 Fe: 0.462 40~2! 1.001 07~3! 0.303 59~1! 0.140 512~05!
~1.13, 0.55! 0.806~1!
~021! 140 Cd:
~1.01, 0.45! 0.038~1!
S4 9.02 6.89 1.42 4.99 100 021 Fe: 0.462 55~3! 1.002 78~4! 0.303 93~1! 0.140 976~13!
~1.13, 0.94! 0.861~4!
~021! 140 Cd:
~1.87, 0.06! 0.054~4!
S5 8.54 6.54 1.25 3.14 89.14 021 Fe: 0.462 45~4! 1.002 13~6! 0.303 74~2! 0.140 763~18!
~60.09! ~0.96, 0.43! 0.820~4! 0.505 43~5!b 0.505 43~5!b 1.380 57~26!b 0.305 426~54!b
~001! 10.86b 140 Cd:
~60.25! ~0.99, 0.57! 0.046~4!
aValues in parentheses are uesd for the least significant figures of the data shown, and are taken from the final cycle of the Rietveld refinements. bNot refined.
cData for the hematite-like phase.
C. Results of the X-ray diffraction refinements
The structure of sample S5 was refined considering the two phases, goethite and hematite. Several values of the pre-ferred orientation function were used during the refinement, but only the 021 and 140 planes markedly improved the quality of the refinements. All samples show both preferred orientations but the ratio and the fraction changed from one
sample to another ~cf. Table II!. The 140 plane showed the highest ratio in S0 and S4 ~1.36 and 1.88, respectively!, in contrast the corresponding fractions were small ~0.01 and 0.06!.
Anisotropic line-shape broadening was detected in the solids; from S1 to S4, the anisotropic broadening axis was 021, and in S0 and S5, was 001. The reliability factors for
Figure 4. Lattice parameters and cell volume for the goethite phase vs % to-tal Cd content in samples S0to S5. Figure 3. SEM images for selected samples~ magnifi-cation 40 0003!showing the needles and some star-like crystals and the increment of the length-to-width ratio from S0 to S4. The drastic change in morphology of
S0– S5are presented in Table II.Rwp,GoF, andRBraggvalues are in the range 8.84 –9.53, 1.3–1.5, and 2.94 –5.26, respec-tively, and they are, in general, satisfactory.
In all samples, the refined metal occupancy factor (fFe1Cd) is smaller than 1.0, the fFe1Cd values varied be-tween 0.832 and 0.915~see Table II!. This fact indicates the presence of cation-deficient compounds of the type
a-(Fe12xMex)12y/3O12y(OH)11y, where the OH/O ratio exceeds the expected 1.0 value. Similar features have been observed in Al~III!-~Wolska and Schwertmann, 1989! and Cr~III!-goethites ~Sileo et al., unpublished!. After this step, and keeping constant the refined fFe1Cdvalue, the singlefFe and fCd occupancy factors were also refined. The obtained fCd/fFe1fCd relations agree with the Cd contents obtained from the chemical analyses thus confirming a complete iso-structural Cd-for-Fe substitution.
The calculated lattice parameters and unit-cell volume for the sample S0– S4 are listed in Table II. A plot of these values versus the total cadmium content in the sample (mCd) is shown in Figure 4. As mCd in the goethite phase was not available for sample S5, it was obtained from an initial fit where the cell parameters were refined. From these cell pa-rameters and the corresponding linear regressions, an aver-age mCd was calculated ~estimated mCd value from linear regression was 5.46!. This value was used in the final refine-ment of S5.
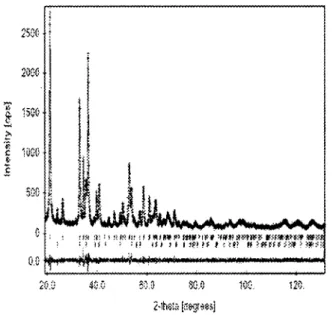
A plot of the observed and the calculated data for S5 is given in Figure 5. As can be observed, the experimental dia-gram is well described by the goethite and the hematite-like structural models that have been employed.
Figure 4 shows that the unit cell parameters increase with the increase of Cd content in the solid in agreement with Cd incorporation in the frame structure. An abrupt change is observed in S5 where a reduction in cell param-eters is detected for the goethite phase. At this highest pre-parative condition, as indicated by the chemical analyses and Rietveld refinement, the total incorporation of Cd is de-creased and the formation of hematite becomes competitive
with that of the Cd-substituted goethite. In turn, a and c parameters for the hematite-phase are larger than reported ~0.5054~1!and 1.3806~3!nm vs 0.5034 and 1.3752 nm!, in-dicating a partial Cd-for-Fe substitution in the hematite struc-ture.
IV. CONCLUSION
In the preparative rangemCd50.99– 7.03, the results ob-tained confirm the incorporation of CdII in the goethite framework. According to the Rietveld refinements, the maxi-mum Cd-for-Fe substitution in the goethite structure was
mCd55.90, and was achieved at the preparative condition
mCd55.50. At the preparation ratiomCd57.03, nucleation of hematite is started within the Cd-rich ferrihydrite aggregate hindering the formation of goethite, and the formation of hematite becomes competitive with that of the Cd-substituted goethite. The values of the cell parameters reveal that the Cd incorporation in the goethite phase diminishes in favor of Cd-substituted hematite.
This work indicates that goethite binds significant amounts of Cd through Cd-for-Fe substitution and can con-trol the Cd distribution and concentration in aqueous sys-tems. The formation of goethite in Cd containing solutions may be used to remove the metal from waste water and liq-uid waste.
ACKNOWLEDGMENTS
This work was supported by a grant from Universidad de Buenos Aires ~I015, RS 5009/2000!. The authors acknowl-edge M. Villegas for SEM measurements.
Balistrieri, I. S. and Murray, J. W.~1982!. ‘‘The Adsortion of Cu, Pb, Zn and
Cd on Goethite from Mayor Ion Seawater,’’ Geochim. Cosmochim. Acta
46, 1253–1265.
Blake, R. L., Hessevick, R. E., Zoltai, T., and Finger, L. W.~1966!.
‘‘Re-finement of the Hematite Structure,’’ Am. Mineral.74, 177–186.
Blesa, M. A. and Matiejevic, E. ~1989!. ‘‘Phase Transformations of iron oxides, oxohydroxides, and hydrous oxides in aqueous media,’’ Ad. Coll. and Int. Science29, 173–221.
Collins, C. R., Vala Ragnarsdottir, K., and Sherman, D. M.~1999!. ‘‘Effect
of Inorganic and Organic Ligands on the Mechanism of Cadmium Sor-tion to Goethite,’’ Geochim. Cosmochim. Acta63, 1989–3002. Cornell, R. M. and Giovanoli, R. ~1987!. ‘‘Effect of Manganese on the
Transformation of Ferrihydrite into Goethite and Jacobsite in Alkaline Media,’’ Clays Clay Miner.35, 11–20.
Cornell, R. M. and Giovanoli, R.~1989!. ‘‘Effect of Cobalt on the Formation
of Crystalline Iron Oxides from Ferrihydrites in Alkaline Media,’’ Clays Clay Miner.37, 65–70.
Cornell, R. M. and Schwertmann, U.~1996!. ‘‘The Iron Oxides: Structure,
Properties, Reactions, Occurrence and Uses,’’ 573, VHC, New York. Diaz, C., Furet, N. R., Nikolaev, V. L., Rusakov, V. S., and Cordeiro, M. C.
~1989!. ‘‘Mo¨ssbauer Effect Study of Co, Ni, Mn, and Al Bearing
Goet-hites,’’ Hyperfine Interact.46, 689– 693.
Dollase, W. A.~1986!. ‘‘Corrections of Intensities for Preferred Orientations
in Powder Diffractometry: Applications of the March Model,’’ J. Appl. Crystallogr.19, 267–272.
Ebinger, M. H. and Schulze, D. G.~1989!. ‘‘Mn-substituted Goethite and
Fe-substituted Groutite Synthesized at Acid pH,’’ Clays Clay Miner.37,
151–156.
Feitknecht, W. and Michaelis, W. ~1962!. ‘‘U¨ ber die Hydrolyse von Eisen~III!-perchlorat-Lo¨sungen,’’ Helv. Chim. Acta45, 212–224.
Fisher, W. R. ~1971!. ‘‘Modellversuche zur Bildung und Auf-lo¨sung von
Goethit und amorphen Eisenoxiden im Bodem,’’ Diss. T. U. Mu¨nchen.
Forbes, E. A., Posner, A. M., and Quirk, J. P.~1979!. ‘‘The Specific Adsorp-tion of Divalent Cd, Co, Cu, Pb and Zn on Goethite,’’ J. Soil Sci.27,
154 –166.
Gasser, U. G., Nuesch, R., Singer, M. J., and Jeanroy, E.~1999!.
‘‘Distribu-tion of Manganese in Synthetic Goethite,’’ Clay Miner.34, 291–299.
Gerth, J.~1990!. ‘‘Unit-cell Dimensions of Pure and Trace Metal-associated
Goethites,’’ Geochim. Cosmochim. Acta54, 363–371.
Giovanoli, R. and Cornell, R. M.~1992!. ‘‘Crystallization of Metal
Substi-tuted Ferrihydrites,’’ Z. Pflanzenernahr. Bodenk.155, 455– 460.
Jin, T. Y., Lu, J., and Nordberg, M.~1998!. ‘‘Toxicokinetics and Biochem-istry of Cadmium with Special Emphasis on the Role of Metallothion-ein,’’ Neurotoxicology19, 529–535.
Larson, A. C. and Von Dreele, R. B. ~1994!. General Structure Analysis
System~GSAS!, Los Alamos National Laboratory report LAUR 86-748. Lehoczky, E., Szabo, L., Horvath, S., Marth, P., and Szabados, I. ~1998!. ‘‘Cadmium Uptake by Lettuce in Different Soils,’’ Comm. Soil. Sci. Plant Anal.29, 1903–1912.
Lim-Numez, R. and Gilkes, R. J.~1985!. ‘‘Acid Dissolution of Synthetic
Metal-containing Goethites and Hematites,’’ Proc. Int. Clay Conf. Clay Mineral Soc. Am., pp. 197–204.
Manceau, A., Schlegel, M. L., Musso, M., Sole, V. A., Gauthier, C., Petit, P. E., and Trolard, F. ~2000!. ‘‘Crystal Chemistry of Trace Elements in
Natural and Synthetic Goethite,’’ Geochim. Cosmochim. Acta64, 3643–
3661.
Norrish, K. and Taylor, R. M.~1961!. ‘‘The Isomorphous Replacement of
Iron by Aluminium in Soil Goethites,’’ J. Soil Sci.12, 294 –306.
Parkman, R. H., Charnock, J. M., Bryan, N. D., Livens, F. R., and Vaughan, D. J.~1999!. ‘‘Reactions of Copper and Cadmium Ions in Aqueous
So-lution with Goethite, Lepidocrocite, Mackinawite, and Pyrite,’’ Am. Mineral.84, 407– 419.
Rietveld, H. M. ~1969!. ‘‘A Profile Refinement Method for Nuclear and
Magnetic Structures,’’ J. Appl. Crystallogr.2, 65–71.
Schwertmann, U., Friedl, J., Stanjek, H., and Schulze, D. G.~2000!. ‘‘The effect of Al on Fe Oxides. XIX Formation of Al-substituted Hematite from Ferrihydrite at 25° and pH 4 to 7,’’ Clays Clay Miner.48, 259–172.
Schwertmann, U., Gasser, U., and Sticher, H.~1989!. ‘‘Chromium-for-iron
Substitution in Synthetic Goethites,’’ Geochim. Cosmochim. Acta 53, 1293–1297.
Schwertmann, U. and Cornell, R. M.~1991!. ‘‘Iron Oxides in the
Labora-tory,’’ pp. 46, VHC, New York, USA.
Schwertmann, U. and Pfab, G.~1994!. ‘‘Structural Vanadium in Synthetic
Goethite,’’ Geochim. Cosmochim. Acta58, 4349– 4352.
Schwertmann, U. and Fisher, W. R.~1966!. ‘‘ZurBildung vona-FeOOH and
a-Fe2O3 aus amorphem Eisen~III!-hydroxid. III.’’ Z. Anorg. Allg.
Chem.346, 137–142.
Sileo, E. E., Ramos, A. Y., Magaz, G. E., and Blesa, M. A. ‘‘Long-range vs. Short-range Ordering in Synthetic Cr-substituted Goethites,’’ in prepara-tion.
Sileo, E. E., Alvarez, M., and Rueda, E. H.~2001!. ‘‘Structural Studies on
the Manganese for Iron Substitution in the Synthetic Goethite-Jacobsite System,’’ Int. Inorg. Mater.3, 271–279.
Stephens, P. W.~1999!. ‘‘Phenomenological model of anisotropic bradening
in powder diffraction,’’ J. Appl. Crystallogr.32, 281–289.
Stiers, W. and Schwertmann, U.~1985!. ‘‘Evidence for Manganese
Substi-tution in Synthetic Goethite.’’ Geochim. Cosmochim. Acta 49, 1909–
1911.
Szytula, A., Burewicz, A., Dimitrijevic, Z., Krasnicki, S., Rzany, H., Todor-ovic, J., Wanic, A., and Wolski, W.~1980!. ‘‘Neutron Diffraction Studies
ofa-FeOOH,’’ Phys. Stat. Solidi26, 429– 434.
Thompson, P., Cox, D. E., and Hastings, J. B.~1987!. ‘‘Rietveld Refinement
of Debye-Scherrer Synchrotron X-ray data from Al2O3,’’ J. Appl.
Crys-tallogr.20, 79– 83.
Vandenberghe, R. E., Verbeeck, A. E., De Grave, E., and Stiers, W.~1986!.
‘‘Mo¨ssbauer Effect Study of Mn-substituted Goethite and Hematite,’’ Hyperfine Interact.29, 1157–1160.
Venema, P., Hiemstra, T., and Van Riemsdijk, W. H. ~1996!. ‘‘Multisite
Adsortion of Cadmium on Goethite,’’ J. Colloid Interface Sci.183, 515–
527.
Wolska, E. and Schwertmann, U.~1989!. ‘‘Nonstoichiometric structures