EFFECT OF EXTRACTION SOLVENTS ON THE BIOMOLECULES
AND ANTIOXIDANT PROPERTIES OF
SCORZONERA UNDULATA
(ASTERACEAE): APPLICATION OF FACTORIAL DESIGN
OPTIMIZATION PHENOLIC EXTRACTION
Khaled Athmouni
1,2, Taheni Belghith
1,2, Khaled Bellassouad
2, Abdelfattah El Feki
2,
Habib Ayadi
11Research Unit UR 11 ES 72 / Biodiversity and Aquatic Ecosystems, Department of Life Sciences, University of Sfax
Road Soukra Km 3,5, B.P. 1171, CP 3000 Sfax, Tunisia
2Laboratory of Animal Ecophysiology, Faculty of Sciences of Sfax, University of Sfax
P.O. Box 95, Sfax 3000, Tunisia
ABSTRACT
Background. Phenolic compounds were extracted and isolated from S. undulata roots.
Methods. Sample of roots from E. hirta was tested for phenolic compounds, and in vitro antioxidant activity
by diphenyl-1-picrylhydrazyl (ŹPPH) assay, ABTS, ŻRAP and reducing power was measured using cyano-ferrate method.
Results. The methanolic fraction exhibited the highest total phenol content (6.1β ±0.11 mg Ażź/g ŹW). On
the other hand, the highest fl avonoids concentration was observed in ethyl acetate fraction (β.90 ±0.05 mg Cź/g ŹW) in addition to anthocyanins (β8.56 ±γ.96 mg/l). Besides, the highest level of tannins content was measured in the polar aprotic solvent ethyl acetate extract (γ.β5 ±0.06 mg Cź/g ŹW). The different extracts of S. undulata were evaluated for their radical scavenging activities by means of the ŹPPH assay. The
strong-est scavenging activity was observed in methanolic fraction scavenged radicals effectively with IC50 values of 0.14 ±0.0β mg/ml. Similarly, the potassium ferricyanide reduction (ŻRAP) and ABTS•+ of methanol extract.
On the other hand, the total reducing power of ethyl acetate extract was found higher than of other extracts. This paper presents the application of the design-of experiment method for optimizing the extraction of phe-nolic content using methanol solvent. The resulting regression model has shown that the effect of temperature is not statistically signifi cant (with >95% certainty), while that of agitation speed is. The two main effects are contributed by the solvent concentration and the maceration period.
Conclusion. Our results clearly showed that the extraction of phenolic compounds and their antioxidant
ca-pacity is signifi cantly affected by solvent combinations. S. undulata presented the highest total phenolic
con-tent, total fl avonoids content and antioxidant capacity values. The resulting regression model has shown that the effect of temperature is not statistically signifi cant (with >95% certainty), while that of agitation speed is.
Key words: antioxidant activity, S. undulata roots, extracting solvents, phenolic content, solvent polarity,
INTRODUCTION
Since prehistoric times, many medicinal plants have been used in folk medicine (Rout et al., β000ś Yesil--Celiktas et al., β007ś żuo et al., β007ś Shinde et al., β010). They have been used all over the world for thousands of years as natural medicines possessing therapeutic and other pharmacologic effect. Today, according to the World Health Organization (WHO), as many as 80% of the world’s population depend on traditional medicine for their primary health-care needs. The preliminary results of a study on behalf of WHO have shown that the number of individuals using medicinal plants is large and increasing, even among young people (WHO, IUCN and WWŻ, 199γ). Medicinal plants or parts of them (leaves, rhizomes, roots, seeds, fl owers) can be utilized in different forms such as fresh crude form and preparations as teas, decoctions, powdered plant material, or extract-ed forms of mextract-edicinal agents (juices, water or alco-hol extracts, tinctures, essential oils, resins, balsams). Medicinal plants are generally known and popular for a number of health benefi ts such as blood pressure decreasing, prevention of cardiovascular diseases, or reducing the risk of cancer also due to their antioxi-dant activity (Burt, β004ś Suhaj, β006ś Brewer, β011ś żhasemzadeh and żhasemzadeh, β011ś Mothana, β011ś Prochazkova et al., β011). The preservative ef-fect of many plant spices and herbs suggests the pres-ence of antioxidative and antimicrobial constituents in their tissues (Hirasa and Takemasa, 1998). Re-cently, interest has increased considerably in fi nding naturally occurring antioxidants for use in foods or medicinal materials to replace synthetic antioxidants, which are being restricted due to their
carcinogenic-ity (Velioglu et al., 1998). The genus Scorzonera L.
(Asteraceae) is a large genus with 160 species. It is widely spread in arid regions of źurasia and North Africa. In recent years ethnobotanical, phytochemical and biological activity studies have been carried out
in Scorzonera (Akkol et al., β01β). Scorzonera
spe-cies are used against pulmonary diseases, colds, for the treatment of wounds and gastro-intestinal disor-ders, as well as for their stomachic, diuretic, galac-tagogue, antipyretic and appetizing effects in źuro-pean traditional medicine (Zidorn et al., β000, β00γś Tsevegsuren et al., β007)ś used for the treatment
of diarrhea, lung edema, parasitic diseases, and fever caused by bacterial and viral infections in Mongolian traditional medicine (Tsevegsuren et al., β007)ś used for the treatment of hepatic pains in Libyan folkloric medicine (Auzi et al., β007) and in Chinese and Tibet-an folk medicine, have also medicinal usage in breast infl ammation and abscess due to their antipyretic and anti-infl ammatory activities (Zhu et al., β009). More-over, Scorzonera species are a rich source of dietary polyphenols such as fl avonoids and phenolic acids in-cluding caffeoylquinic acid derivatives (Tsevegsuren et al., β007ś Zhu et al., β009ś Jehle β010ś Akkol et al., β011ś Sari, β01βś Wang et al., β01βś Yang et al., β01γś Milella et al., β014). This genus has attracted the attention of researchers due to the many chemical classes of its secondary metabolites, including dihy-droisocoumarins, stilbenes, lignans, phenolic deriva-tives, phtalides (Sari et al., β007), coumarins, kava-lactones (Jiang et al., β007), sesquiterpenes (Zidorn, β008), triterpenes (Wang et al., β007), and fl avonoids
(Sareedenchai and Zidorn, β010). Scorzonera
undu-lata is perennial with a thick blackish stump and can
be eaten in the spring, the leaves, are in clumps, with a narrow, very long limb that is wavy at the edge, glaucous, with very short woolly hairs. However, there is rare study on the effect of solvents with vari-ous polarities on extracting active compounds from Scorzonera undulata related to their antioxidant ac-tivities. Because, the determination of the best solvent extract for Scorzonera undulata with measurement of total phenolic content and various antioxidant activity assays was one important factor to increase the ex-traction process effi cacy. The aim of this study was to examine the effect of different extracting solvents with different polarity on phenolic compounds and antioxidant activity (ferric reducing antioxidant pow-er [ŻRAP], β,β-diphenyl-1-picrylhydrazyl [ŹPPH] and β,β-azino-bis-γ-ethylbenzothiazoline-6-sulfonic
acid [ABTS]) of Scorzonera undulata. The solvent
MATERIAL AND METHODS
Plant materials
Żresh roots from S. undulata were collected in May β014
in Źjebel Bou Ramli (γ4°γ1’8” N and 8°γβ’48” ź), żafsa (South Tunisian). The plant was identifi ed by Pr. Mohamed Chaieb, botanist at the University of Sci-ence (Sfax, Tunisia).
Extraction procedure of biomolecule
Methanolic fraction. The roots of S. undulata (1 g)
were broken into small pieces and macerated in meth-anol (β0 ml) at room temperature for 48 h. The ex-tract was then separated from the residue by fi ltration
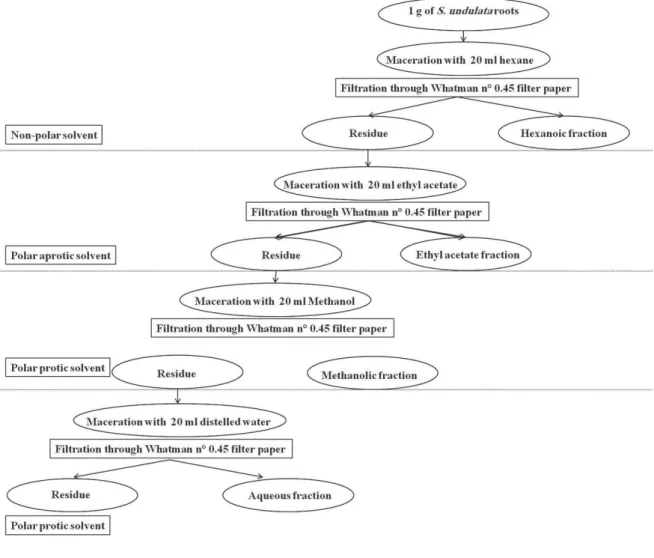
through Whatman 0.45 μm fi lter paper. The solvent was evaporated under vacuum at 60°C. The residue was weighed and dissolved in methanol (γ ml) for further analysis. źventually, the solutions were stored at –β0°C. The remaining aqueous solution was frac-tionated successively with n-hexane and ethyl acetate (Żig. 1).
Chemicals and reagents
Butylated hydroxytoluene (BHT), β,β-diphenyl-1-pic-rylhydrazyl (ŹPPH), catechin, β,4,6-tripyridyl-s-triazine (TPTZ), 6-hydroxy-β,5,7,8-tetramethylchroman-β-car-boxylic acid (trolox) and β,β-azinobis(γ-ethylbenzothi-azoline-6-sulfonic acid) diammonium salt (ABTS).
Determination of total phenolic content
Total phenolics content of S. undulata was measured
by Żolin-Ciocalteu’s phenol reagent (Singleton and Rossi, 1965ś Kim et al., β00γ). Żirst, β00 ml of appro-priately diluted sample or gallic acid standard were added to β.6 ml of distilled deionized water. Then, β00 ml of Żolin-Ciocalteu’s phenol reagent was added at time zero and mixed. After 6 min, β ml of 7% (w/v)
NaβCOγ solution was added and mixed. After
incuba-tion for 90 min at room temperature, absorbance was measured at 750 nm versus a prepared blank. The blank consisted of β00 ml 50% (v/v) methanol instead of sample. żallic acid in 50% (v/v) methanol solu-tion in concentrasolu-tions of 0.1, 0.γ, 0.5, and 0.7 mg/ml was used as a standard and a calibration curve was drawn for each day of analysis. The content of total phenolics was expressed as mg gallic acid equivalent (żAź)/g of dry weight. All samples were analysed in triplicate.
Total fl avonoids content
Total fl avonoid content was measured according to the method of Zhishen et al. (1999). Sample extract was added with 0.γ ml of 5% sodium nitrite and well mixed. After 5 min of incubation, 0.γ ml of 10% aluminum chloride solution was added. Then, after 6 min, β ml of 1 M sodium hydroxide was added to the mixture and made up the volume to 10 ml with water. The absorbance was measured at 510 nm with UV-visible spectrophotometer. Total fl avonoids were measured from catechin (0–0.γ mg) standard curve and expressed as mg catechin equivalents/g of dry weight.
Determination of anthocyanins content
Monomeric anthocyanin content of S. undulata was
measured using a spectrophotometrically pH differ-ential protocol according to (Broadhurst and Jones, 1978). źxtracts were mixed thoroughly with 0.0β5 M potassium chloride pH 1 buffer and similarly with so-dium acetate buffer pH 4.5 in 1Ś10 ratio of extract to buffer. The absorbance of these solutions was meas-ured at 510 and 700 nm. The anthocyanin content was calculated as followsŚ
Total monomeric anthocyanins (mg/l) =
= Abs × MW × 1000 / (Ɛ × C)
whereŚ
Abs – absorbance = (A515 – A700)pH 1.0 – (A515 –
A700)pH 4.5,
MW – molecular weight for cyanidin γ-glucoside
= 449.β,
Ɛ – the molar absorptivity of cyanidin γ-glucoside = β6 900,
C – the concentration of the buffer in milligrams per milliliter.
Anthocyanin content was expressed as milligrams of cyanidin γ-glucoside equivalents 1 l of the triplicate extracts.
Determination of condensed tannins
Condensed tannins content was determined by the vanillin method as described by Broadhurst and Jones (1978). γ ml of vanillin (4% in methanol) were added to 0.5 ml of the different extract. 1.5 ml of highly con-centrated HCl was then added. The mixture was then kept in the dark for 15 min at β0°C. The absorbance was read at 500 nm. A calibration curve was prepared with a solution of catechin. The results were obtained in mg of catechin equivalent per g of dry weight (mg Cź/g ŹW).
Determination of the antioxidant activity
The antioxidant potential of S. undulata organic
ex-tracts was determined by radical scavenging assaysŚ
the β,β-diphenylpicrylhydrazyl radical (ŹPPH•),
ŻRAP, the
β,β′-azinobisγ-ethylbenzothiazoline-6-sul-fonic acid radical (ABTS•+) and reducing power.
Free radical scavenging activity on
2,2-diphenyl--1-picrylhydrazyl (DPPH•). The free radical
precautionary measures were taken to reduce the loss of free radical activity during the experiment. The inhi-bition percentage of ŹPPH radicals was calculated asŚ
Inhibition (%) of ŹPPH radicals = Ac – As/Ac × 100
whereŚ
Ac – absorbance of the control reaction (all rea-gents except plant extract),
As – absorbance of the sample (plant extract).
Ferric reducing antioxidant power (FRAP) assay. The procedure described by Benzie and Strain was fol-lowed (Lahouel and Żillastre, β004). The principle of this method is based on the reduction of a ferric-tripy-ridyltriazine complex to its ferrous, colored form in the presence of antioxidants. Briefl y, the ŻRAP reagent contained β.5 ml of a 10 mmol/l TPTZ
(β,4,6-tripy-ridy-s-triazine, Sigma) solution in 40 mmol/l HCl
plus β.5 ml of β0 mmol/l ŻeClγ and β5 ml of 0.γ mol/l
acetate buffer, pH γ.6 and was prepared freshly and warmed at γ7°C. Aliquots of 40 μl sample superna-tant were mixed with 0.β ml distilled water and 1.8 ml ŻRAP reagent and the absorbance of reaction mixture at 59γ nm was measured spectrophotometrically after incubation at γ7°C for 10 min. Value of ŻRAP was ex-pressed as Trolox equivalents per gram of dry weight (Tź/g ŹW).
Free radical-scavenging ability by the use of ABTS radical cation (ABTS assay). Antioxidant activities
of S. undulata were also analysed by investigating
their ability to scavenge the ABTS•+ free radical
us-ing a modifi ed methodology previously reported by Ozgen et al. (β006). When combined with an oxidant (β.45 mM potassium persulfate), ABTS (7 mM in β0 mM sodium acetate buffer, pH 4.5) reacts to cre-ate a stable, dark blue-green radical solution follow-ing 1β–16 h of incubation in the dark (4°C). The solu-tion was then diluted to an absorbance of 0.7 ±0.01 at 7γ4 nm to form the test reagent. Reaction mixtures containing β0 μl of sample and γ ml of reagent were incubated in a water bath at γ0°C for γ0 min. As un-paired electrons are sequestered by antioxidants in the sample the test solution turns colourless and the ab-sorbance at 7γ4 nm is reduced. The fi nal result was expressed as mM of Trolox equivalents (Tź) per g of dry weight (ŹW).
Reducing power. The ferric reducing antioxidant power assay was used to assess the reducing capacities
of extracts of S. undulata. Źifferent dilutions (0.05–
0.5 mg/ml) of each plant extract (ethyl acetate, meth-anol, n-hexane and water). Reducing power of both
extracts of S. undulata were measured by method of
Oyaizu’s (1986) with a slight modifi cation. According
to this method, the reduction of Żeγ+ to Żeβ+ was
deter-mined by measuring absorbance of the Perl’s Prussian blue complex. This method is based on the reduction
of (Żeγ+) ferricyanide in stoichiometric excess (0.1,
0.β, and 0.4 mg/ml) of water, methanol, ethyl acetate and hexane extracts of sumac S. undulata in 0.75 ml of distilled water were mixed with 1 ml of 0.β M sodium phosphate buffer (pH 6.6) and 1 ml (1%) of potassium
ferricyanide [KγŻe(CN)6]. The mixture was incubated
at 50°C for β0 min. After β0 min of incubation, the reaction mixture was acidifi ed with 1 ml of
trichlo-roacetic acid (10%). Żinally, 0.β5 ml of ŻeClγ (0.1%)
was added to this solution. Źistilled water was used as blank and for control. Absorbance of this mixture was measured at 700 nm using a UV spectrophotometer. Źecreased absorbance indicates ferric reducing power capability of sample. Ascorbic acid and Butylated hy-droxytoluene (BHT) were used for comparison.
Optimization of phenolic content extraction The infl uence of solvent, maceration period, tempera-ture and agitation on phenolic extraction was
evalu-ated using a full factorial design (β4) with three
rep-licates in the central points, which was a total of 16 treatment combinations. In the statistical model, the coded variables were defi ned as followsŚ X1 = solvent,
Xβ = maceration period, Xγ = temperature, X4 =
agita-tion and the dependent variables being phycocyanin concentration. źach independent variable was coded at two levels between ‒1 (low level) and +1 (high level). The coding of the variables was done by the followingŚ
xi = (Xi – Xz)/ΔXi, i = 1, β, γ, …, k whereŚ
xi – the dimensionless value of an independent
variable,
Xi – the real value of an independent variable,
ΔXi – the step change of the real value of the
vari-able i corresponding to a variation of a unit for the
dimensionless value of the variable i.
The levels of each factor are listed in Table 1. Ta-ble β presents the experimental design matrix.
Statistical analysis of data
All experiments were repeated twice and carried out in a completely randomized block designś each treat-ment consisted of three replicates. Mean values of var-ious treatments were subjected to analysis of variance (ANOVA) using IBM SPSS Statistics version β0
Sig-nifi cance level was determined (at p < 0.05) and
sig-nifi cant difference was separated using Źuncan’s Mul-tiple Range Test (ŹMRT). Pearson’s rank-correlation was performed using XL-Stat software to determine the correlations between the phenolic compounds and the antioxidant activity in the different extracts of S. undulata.
RESULTS AND DISCUSSION
Phytochemical screening
Previous studies indicated that different solvents could lead to different extraction effi ciencies of bio-active compounds (Vuong et al., β01γś Zhang et al., β01γ). Therefore, this study determined the impact of four different extraction solvents with various polar-ity indexes to identify the most effective solvent for further optimization using response surface methodol-ogy. According to our knowledge, the chemical analy-sis of phenolic content, fl avonoids, anthocyanins and condensed tannins of S. undulata from a Tunisian arid area has not yet been investigated.
Total phenolic content in diff erent extracts (TPC). Polyphenols have attracted considerable attention be-cause of their various biological activities includingŚ antioxidant, antimutagenic, antitumor, anti-infl amma-tory, neuro-protective and cardio-protective effects (Tai et al., β011). Phenolic compounds are health ben-efactors because they act as antioxidative agents (Oz-türk et al., β011). The total phenolic contents (TPC) in
S. undulata roots have been evaluated. The quantitative
determination of TPC is expressed as milligram gallic acid equivalents per gram dry weight of sample. In the present study, the total phenolic contents of n-hexane,
ethyl acetate, methanol and aqueous extracts of S.
Sco-rzonera roots were determined by the Żolin-Ciocalteu
method and results are shown in Table γ. The metha-nol extract of the roots exhibited the highest content (6.1β ±0.11 mg źAż/g ŹW) followed by the n-Hexan fraction (5.95 ±0.01 mg źAż/g ŹW). It is reported
Table 1. Żactor levels for a β4 factorial design
Żactors Parameters Coded level
‒1 +1
X1 Solvent (methanol), % 80 100
Xβ Maceration, h β4 48
Xγ Temperature, °C β5 γ5
X4 Agitation, rpm β0 50
Table 2. Żactorial design matrix with experimental results
and actual values for phenolic content extraction
Run Żactors Polyphenol content
X1 Xβ Xγ X4 Actual value
1 –1 –1 –1 –1 γ.14
β +1 –1 –1 –1 γ.4β
γ –1 +1 –1 –1 γ.β5
4 +1 +1 –1 –1 6.07
+1 –1 –1 +1 –1 β.87
+1 +1 –1 +1 –1 γ.17
+1 –1 +1 +1 –1 4.β6
+1 +1 +1 +1 –1 4.08
+1 –1 –1 –1 +1 4.57
+1 +1 –1 –1 +1 4.8γ
+1 –1 +1 –1 +1 7.64
+1 –1 +1 –1 +1 6.β1
+1 –1 –1 +1 +1 4.βγ
+1 +1 –1 +1 +1 β.81
+1 –1 +1 +1 +1 7.11
by other researchers (Sun et al., β011) that methanol was an effective solvent for extracting phenolic com-pounds. Milella et al. (β014) indicated that total
phe-nolic content of methanol/water extract of S. undulata
roots is about 80.7 mg żAź/g of extract, which seems to be more important than the results obtained in the present study. According to Shahat et al. (β014), the total phenolic content of six species of Asteraceae family (Picris cyanocarpa, Pulicaria crispa, Anthemis deserti, Achillia fragrantissima, Rhantarium appopo-sum and Artemissia monosperma) ranged from 0.01 to
0.055 mg żAź/g ŹW, with Artemissia monosperma
showing the highest value of 0.055 mg żAź/g ŹW, followed by Picris cyanocarpa, Pulicaria crispa,
An-themis deserti, Rhantarium appoposum and Achillia
fragrantissima with TPC values of 0.0γ8, 0.0γ5, 0.0β8,
0.01 and 0.009 mg żAź/g ŹW, respectively. In addi-tion, several studies revealed that phenolic compounds content differed with solvents polarities (Addai et al., β01γś Żernandez-Arroyo et al., β011). Turkmen et al. (β006) reported that solvent with different polarity had signifi cant effect on phenolics compound and antioxi-dant activity in highest content in most polar solvents. This fi nding could be the result of non specifi c reac-tions of Żolin–Ciocalteu reagent with other compo-nents of the water extract which could overestimate the phenolic content in these extracts (żalher et al., β00γ). In addition, it is diffi cult to compare our results
with historical data. Indeed, the extraction of phenolic compounds from their natural matrix is complicated by their diversity and their susceptibility to oxidation and hydrolysis (Naczk and Shahidi, β004). Concern-ing the ethyl acetate and water fractions, they are less enriched in total phenolic compounds (γ.5 ±0.06 and 0.94 ±0.05 mg źAż/g ŹW respectively). Methanol, hexane, ethyl acetate and aqueous extracts of S. un-dulata roots showed signifi cant difference (***p < 0.001) in total phenolic content. We can conclude that ethyl acetate and methanol extracts had the high-est total phenolic contents. In fact, the use of sol-vents with different polarities leads to differences in phenolic content and antioxidant capacity (Matthäus et al., β00βś żorinstein et al., β007). In this regard, several authors have remarked the importance of the extraction method and the solvent used (żorinstein et al., β007ś Nsimba et al., β008ś Ozsoy et al., β009). In addition, in plant preparations (extracts, decoctions), the content and composition of antioxidants depend also on extraction technique, its conditions (extraction time and temperature), and solvents (Škrovánková et al., β01β).
Determination of total fl avonoids content.
Żla-vonoids (phenolic plant compounds) possess a wide range of pharmacological properties. Its importance to human health lies in its capacity to scavenge free
Table 3. źffects of extracts of Scorzonera undulata on the in vitro free radical (ŹPPH, ŻRAP, ABTS and reducing power)
źxtracts
Antioxydant activity ŹPPH
% mg/mlIC50 Mm Tź/g ŹWŻRAP ABTS
•+
Mm Tź/g ŹW Reducing power (700 nm)
źthyl acetate (0.4 mg/ml) 67.16b 0.β1 ±0.06e 0.16 ±0.001a 0.8 ±0.11b 0.871d
Methanol (0.4 mg/ml) 8γ.4βcd 0.14 ±0.0βc 0.γ1 ±0.00βa 1.γ6 ±0.07b 0.776b
n-Hexane (0.4 mg/ml) 7β.β4bc 0.17 ±0.04d 0.005 ±0.00a 1.01 ±0.04b 0.8γ0c
Water (0.4 mg/ml) 61.γ1a 0.γ1 ±0.01f 0.045 ±0.00βa 0.1β ±0.0γa 0.574a
BHT (0.4 mg/ml) 9γ.1βd 0.1β ±0.0γb 1.85 ±0.0βb 1.75 ±0.04c 0.957e
Vit. C (0.4 mg/ml) 96.γβd 0.10 ±0.0βa 1.96 ±0.01b 1.91 ±0.01βc 0.996f
Źata are presented as mean ±SŹ of three individual determinations. żAź – gallic acid equivalents. Cź – catechin equivalents. Tź – the trolox equivalent. ŹW – dry weight. Values followed by different superscript in each column are signifi cantly different (P < 0.05).
radicals (Robards et al., 1999). In the present inves-tigation, the aluminum chloride colorimetric method was used for detection of fl avonoids content, which increased from water to ethyl acetate fractions. In this study the ethyl acetate extract presents the highest fl a-vonoids content (β.90 ±0.05 mg Cź/g ŹW) followed by the n-hexanoic fraction (β.41 ±0.14 mg Cź/g ŹW). Water and methanolic fractions showed the lowest fl a-vonoids content (1.6β ±0.04 and β.γ0 ±0.15 mg Cź/g ŹW, respectively). Methanol, n-hexane, ethyl acetate
and aqueous extracts of S. undulata showed signifi
-cant difference (***p < 0.001) in fl avonoids content. According to źrden et al. (β01γ), the total fl avonoids content of three Scorzonera species extracted with
methanol solventŚ Scorzonera suberosa (0.β6 ±0.05
mg/g ŹW), Scorzonera laciniata (0.08 ±0.01) and
Scorzonera latifolia (0.17 ±0.01 mg/g ŹW), these
results are less important than those revealed by the present study. Moreover, żouveia et al. (β01γ) indi-cated that the fl avonoids content of the ethanol extract
of Andryala glandulosa (Asteraceae family) is about
γ.β8 ±0.01 mg Rź/g ŹW. In comparison to the present
study, the fi ndings of the previous studies dealing with
Scorzonera species are less important. In fact, the
in-crease or dein-crease of fl avonoids content depended on the extraction solvent polarity (Hsu et al., β006ś Segu-ra-Carretero and Żernandez-żutierrez, β01γ).
Determination of anthocyanins content. Antho-cyanidin, an important plant pigment, is responsible for most of the purple, red and blue colours in plants. It is a fl avonoid compound synthesized from phenyla-lanine and malonylcoa through a series of enzymatic reactions (Holton and Cornish, 1995ś Chopra el al., 1996ś Mol et al., 1998). It has been known that an-thocyanins possess antitumor, antiulcer and anti-in-fl ammatory properties (Konczak-Islam, β00γś Kong et al., β00γś Hou et al., β004ś Stintzing and Carle, β004). In the present study, the anthocyanins content of
meth-anol, n-hexane, ethyl acetate and water extracts of S.
undulata roots was determined by the pH differential
method and results are shown in Żigure β. The ethyl
acetate extract of S. undulata presented the highest
an-thocyanins content (β8.56 ±γ.96 mg/kg ŹW) followed
Fig. 2. Total phenol, fl avonoids, anthocyanins content and condensed tannins content of Scorzonera undulata
by the methanolic fraction (β6.β5 ±γ.15 mg/kg ŹW). The least concentration of anthocyanins was observed in n-hexanoic fraction (11.85 ±β.7β mg/kg ŹW) fol-lowed by aqueous fraction (17.54 ±1.80 mg/kg ŹW). Methanol, n-hexane, ethyl acetate and aqueous
ex-tracts of S. undulata showed signifi cant difference
(***p < 0.001) in of anthocyanins content. In compar-ison with other materials belonging to the Asteraceae family (Artemisia herba-alba, Ruta chalpensis and Pe-ganum hamala) extracted with methanol/water (γ/1), the anthocyanins content ranged from 176.99 ±5.05 to 1949 ±β mg/kg ŹW, with Artemisia herba-alba, Ruta showing the highest value of 1949 ±β mg/kg ŹW,
fol-lowed by Ruta chalpensis and Peganum hamala with
anthocyanins content values of 41γ.β9 ±γ.46 and 176 ±5.05 mg/kg ŹW, respectively (Khlifi et al., β01γ). In fact, anthocyanins are polar molecules which are normally extracted from raw plant tissues by conven-tional solvent extraction (CSź) methodologies, using polar solvents. However, the type of matrix and sol-vent used plays an important role for the extraction of anthocyanins (Ramos-źscudero et al., β01β).
Determination of condensed tannins. Many tan-nin-rich medicinal and food plants have been appreci-ated for their benefi cial effects without being troubled by any obvious toxicity (Okuda, 1999). Research on the tannins in traditional medicinal plants, presented here, started when the chemical, biological and phar-macological properties of tannins in most medici-nal plants were not yet subjected to modern amedici-nalysis (Okuda et al., 1975ś 1991ś 199βś 1995). In the present study, the tannins content of methanol, n-hexane, ethyl acetate and aqueous extracts of S. undulata were de-termined by the vanillin method and results are pre-sented in Żigure β. The highest level of tannins content was measured in the ethyl acetate extract (γ.β5 ±0.06 mg Cź/g ŹW) followed by the n-hexane extract (β.β1
±0.β6 mg Cź/g ŹW). Methanol extract of S.
undula-ta and aqueous fraction showed low tannins content
compared to ethyl acetate extract (β.1β ±0.05 and 1.17 ±0.0β mg Cź/g ŹW, respectively). The different
ex-tracts of S. undulata showed a signifi cant difference
(***p < 0.001) in condensed tannins amounts. In
com-parison with other materials, these extracts contained lower condensed tannin than those obtained from
Ar-temisia herba-alba (5.47 ±0.09 mg Cź/g ŹW), Ruta
chalpensis (4.7γ ±0.14 mg Cź/g ŹW) and Peganum
hamala (β.0γ ±0.06 mg Cź/g ŹW) (Khlifi et al.,
β01γ). żenerally, the extraction effi ciency of bioac-tive compounds is largely depending on the solvent used (Leblanc et al., β009ś Xi et al., β009ś Chen et al., β01β). The present study shows that among all the solventś ethyl acetate and water were better solvents for effective extraction of tannins as compared to other solvents like n-hexane and methanol. According to (Mailoa et al., β01γ), the polar-aprotic solvent can-not provide OH-ions, whereas the polar protic solvent can provide OH-ions, making it easier to interact with polar functional groups on the tannins. Therefore, the polar-aprotic solvents are less compatible to tannins extraction than polar-protic solvent (e.g. ethanol).
Antioxidant activity of S. undulata extracts
źxtraction of active compounds in natural plants is potent to protect biological systems against damaging effect of natural oxidation process in organism. In this
study, the antioxidant capacity of S. undulata using
different extracting solvent was evaluate by 4 assaysś
ŹPPH, ŻRAP, ABTS+• and Reducing power. źach
an-tioxidant assay possesses its own unique mechanism to evaluate the antioxidant activity in sample.
DPPH radical scavenging activity. The antioxidant potential of methanol, n-hexane and aqueous extracts of S. undulata was evaluated on the basis of their abil-ity to scavenge stable free ŹPPH radicals and results,
while the IC50 values were presented in Table γ. This
test is based on change in colour of ŹPPH solution from purple to yellow, due to scavenging of stable free ŹPPH radicals (Khadri et al., β010). A stronger yel-low colour indicates a greater ability of the extract to scavenge free ŹPPH radicals and stronger antioxidant potential. The strongest scavenging activity was ob-served in methanolic fraction followed by n-hexane
extract, with IC50 values of 0.14 ±0.0β and 0.17 ±0.04
mg/ml of extract respectively. The ethyl acetate and
water extracts of S. undulata roots have the lowest
ŹPPH• radical scavenging ability over other extracts,
with IC50 values of 0.β1 ±0.06 to 0.γ1 ±0.01 mg/ml of
extract respectively. ANOVA test shows a signifi cant
difference between the used extracts (***p < 0.001)
high phenolic compounds content showed by this extract. Amoateng et al. (β011) reported that ŹPPH
scavenging ability of Synedra nodifl ora (Asteraceae
family) extracted in ethanol solvent (70%) is about 0.γ1 mg/ml of extract, suggesting that our results are
more important. In the ŹPPH• assay, the free radical
scavenging activities decreased from methanol extract to n-hexanoic, ethyl acetate and aqueous extracts for S. undulata roots evidencing a clear secondary me-tabolite content due to the solvent–solvent partition-ing processes (Lixiang et al., β009). An increase in ŹPPH scavenging ability was observed with increase in concentration of extracts. In comparison with other genus belonging to the Asteraceae family, Kenny et al.
(β014) reported that the ŹPPH IC50 of Circum
arven-ce, Circum vulgare, Circum polusture, Circum nigra,
Circum scabiosa, Circum asper, Articulum minus
and Taraxacum offi cinale extracted with water was
0.48 ±0.04, β.βγ ±0.1β, 0.81 ±0.05, 0.58 ±0.0γ, 0.78 ±0.009, 1.9β ±0.β5, β.45 ±0.19 and 0.48 ±0.006 mg/l of extract respectively, which is close to the results obtained by the present study. The ŹPPH radical was widely used to evaluate the free-radical scavenging capacity of antioxidants (Cotelle et al., 1996). We can conclude that the ethyl acetate extract was the most effective in this respect.
FRAP analysis. ŻRAP method was used to present rather quick and simple method measuring antioxidant
presents in S. scorzonera roots. The ŻRAP assay is
based on the ability of phenolics to reduce yellow fer-ric tripyridyltriazine complex (Że(III)-TPTZ) to blue ferrous complex (Żź(II)-TPTZ) by the action of elec-tron donating antioxidants (Benzie and Stain, 1996). In the present study, antioxidant activities evaluated by the ŻRAP scavenging capacity of methanol, ethyl acetate, hexanoic and water extracts of the samples are
presented in Table γ. The roots of S. undulata showed
higher antioxidant activity in methanol extract (0.γ1 ±0.00β mg Tź/g ŹW) followed by ethyl acetate frac-tion (0.16 ±0.001 mg Tź/g ŹW). In comparison with other genus belonging to the Asteraceae family, Woj-dyło et al. (β007) reported that the ŻRAP
scaveng-ing of Achillea millefolium and Echinacea purpurea
extracted with methanol was 0.47 and 0.ββ mg Tź/g ŹW respectively, which is very low compared to the results obtained by the present study. The least amount
of ŻRAP scavenging was observed in water extract (0.045 ±0.00β mg Tź/g ŹW) followed by hexanoic fraction (0.005 ±0.00 mg Tź/g ŹW). In ŻRAP assay, the scavenging activities decreased from methanol to
n-hexane extract in S. undulata roots, thus, the polarity
of solvents has an indirect function in the extraction process, because it can raise the solubility of antioxi-dant compounds (Alothman et al., β009).
ABTS•+ scavenging activity. ABTS assay is based
on the antioxidant ability of the extracts to react with
ABTS·+ radical cation generated in the system. The
averages values obtained for ABTS assay are given in Table γ. ABTS (blue-green chromophore) is mixed
with the different extracts of S. undulata that can
do-nate a hydrogen atom, then this gives rise to the reduced form with the loss of their color (Molyneux, β004). Solvent used for phenolic compounds extraction had a signifi cant effect on antioxidant activity. The high-est level of scavenging activity was measured in the methanol extract (1.γ6 ±0.07 Mm Tź/g ŹW) followed by the n-hexane fraction is about 1.01 ±0.04 Mm Tź /g ŹW. Similarly, in another investigation by Sultana et al. (β007), methanol extract was found to be the solvent extracting the most effi ciently antioxidants. źthyl ac-etate and aqueous fractions have moderate activity (0.8 ±0.11 and 0.1β ±0.0γ Mm źT/g ŹW, respectively). The scavenging effect of different extracts of S. undulata on
the ABTS·+ radical decreased in following orderŚ
ascor-bic acid > BHT > methanol > n-hexane > ethyl acetate > water extracts. In comparison with another study, the
ABTS activity of Artemisia capillaris is about 0.β7
±0.0β Mm Tź/g ŹW, Artemisia argyi (0.β4 ±0.007 Mm
Tź/g ŹW) and Artemisia apiacea (0.05 ±0.00β Mm
Tź/g ŹW) (Li et al., β01γ), which is much lower than the results obtained in the present study.
Reducing power. In the present study, the reducing
power of chemical compounds extracted from S.
un-dulata roots by methanol, hexane, ethyl acetate and
acetate extract were higher than those of the hexane
extract. Signifi cant difference (***p < 0.001) in
reduc-ing poweractivity was noted in all of the investigated
extracts. Total phenolic content and ferric reducing power are related with each other. Że (III) reduction is often used as an indicator of electron-donating ac-tivity, which is an important mechanism of phenolic antioxidant action (Źorman et al., β00γ). According to Shahat et al. (β014) at 0.4 mg/ml, the absorbance values of methanolic extract, at 700 nm were 0.βś 0.βś 0.β7ś 0.βγś 0.19 and 0.ββ, respectively in dry sample from P. Crupa, R. Epapposum, P. Cyanocarpa, A.
De-serti, A. Fragrantissima and A. monosperma.
Com-pared to the present study, these plantsreveal an
anti-oxidant activity less important than S. undulata.
Correlation between phenolic compounds and antioxidant activity
Several studies have reported on the relationship be-tween total phenol and antioxidant activity. Some authors have found a strong correlation between the phenolic contents and the antioxidant activity (Ram-ful et al., β011ś Ouchemoukhe et al., β01βś Benmed-dour et al., β01γ) others have found nothing (Amin et al., β004ś Anagnostopoulou et al., β006ś Kamran et al., β009). In this study, the results have shown a relationship between antioxidant activity and total
phenolic contents. Table 4 showed the Pearson’s rank-correlation between the antioxidant activity and the phytochemical composition of the different extracts. The fi ndings of this study indicate that total phenolics present high and positive correlations with ABTS
scav-enging capacity and ŹPPH (r = 0.990, p < 0.05 and
r = 0.965, p < 0.05, respectively). While a high and positive correlation was obtained between fl avonoids and the reducing power (r = 0.861, p < 0.05). In fact, it is known that only fl avonoids with a certain structure and particularly hydroxyl position in the molecule can act as proton donating and show radical scavenging ac-tivity (Hou et al., β00γ). Żurthermore, the extracts are very complex mixtures of many different compounds with distinct activities (Hou et al., β00γ).
Anthocya-nins are strongly correlated with ŻRAP (r = 0.7γ7,
p < 0.05). Contrary to the condensed tannins that have
shown only a correlation with the reducing power
(r = 0.807, p < 0.05). A principal component analysis
(PCA) was performed in order to visualize the correla-tions that might exist between the phytochemical com-position and the antioxidant activity. Żigure 4 shows two groupsŚ the fi rst group (ż1) is correlated with the ethyl acetate extract and contains the following param-etersŚ anthocyanins, condensed tannins, fl avonoids and the reducing power. The second group shows a correla-tion between the methanolic extract, the ŻRAP radical
scavenging, the ABTS scavenging ability, the ŹPPH scavenging activity and the total phenolic content.
Optimization of polyphenol extraction
Since the model initially contains signifi cant and non signifi cant terms, it can be adjusted by eliminating the non-signifi cant terms. Leon and Kumar (β007) sug-gested that full quadratic models should be used even if
some terms are insignifi cant, because certain statistical properties are valid only in the full quadratic case. My-ers et al. (β009) argue that reduced models containing only signifi cant terms should be employed, especially when the goal is to fi nd the optimal settings of major factors. The Pareto chart in Żigure 5 shows all of the parameter effects and their interactions in decreasing order of importance. This fi gure uses a vertical line to
Table 4. Pearson’s correlation coeffi cients (r) of the phenolic compounds and the antioxidant activities
Total
phenolic Żlavonoids Condensed tannins Anthocya-nins scavenging ŹPPH ABTS ŻRAP Reducing power
Total phenolic 1
Żlavonoids 0.180 1
Condensed tannins 0.10γ 0.994* 1
Anthocyanins 0.1β6 0.534* 0.596* 1
ŹPPH scavenging 0.965* –0.014 –0.075 0.176 1
ABTS 0.990* 0.γ07 0.βγ6 0.βγ1 0.938* 1
ŻRAP 0.592* 0.071 0.090 0.737* 0.724* 0.618* 1
Reducing power 0.639* 0.861* 0.807* 0.γ6γ 0.448 0.726* 0.β45 1
*p < 0.05.
determine which effects are statistically signifi cant. The length of each bar is proportional to the value of the statistics calculated for the associated effect. Any bars beyond the vertical line are statistically signifi cant at the selected level of signifi cance. The (+) sign indicates a positive contribution of the effect, while the (–) sign indicates a negative contribution. In the present case, there are four main effects (AŚ Solvent, BŚ Maceration,
CŚ Temperature and ŹŚ Agitation) and four signifi cant interactions (AŹ, AC, AB, ABŹ and ABCŹ). The val-ues given in Żigure 5 indicate that the effect of whole diameter (parameter A) is not signifi cant and that all interactions involving this parameter (ABś BC and BCŹ) are negligible. In addition, the main effects plot depicted in Żigure 6 shows the estimated change in effi -ciency of the phenolic content when each of the factors
Fig. 5. Standardized Pareto chart for effi ciency
Po
ly
ph
en
olic
c
on
te
nt
, G
AE/g
D
W
Solvent Maceration Temperature Agittation
3.6 4.0 4.4 4.8 5.2 5.6
-1 +1 -1 +1 -1 +1 -1 +1
is shifted from its lowest level (1) to its highest level (+1). The plot reveals that the effi ciency of phenolic compounds extraction decreases as temperature and concentration of solvent increase. The results of this study are similar to those of other authorsś Venditti et al. (β010) obtained signifi cantly higher values in white tea after steeping in cold water (RT) for two hours. This study found that extraction of polyphenols was poor for water and aqueous ethanol at 40°C. In contrast, this pa-rameter shows a positive correlation with maceration period and agitation speed. With the magnitude and di-rection of the variations of the parameters defi ned, the parameter settings can be optimized for extraction ef-fi ciency. In the present study, response surfaces were used to obtain this information. Żigure 7 shows the height of the response surface for solvent extraction ef-fi ciency (phenolic content) over the space of the solvent using coating and maceration period, with the other two factors (temperature and agitation) held constant. This fi gure clearly indicates that the greatest phenolic content was obtained at high values for maceration pe-riod and low solvent concentration (methanol 80%). Addition of some amount of water enhance the extrac-tion effi ciency. One possible reason for the increased
effi ciency with the presence of some water might be due to the increase in bulge of plant material by wa-ter, which increased the contact surface area between the plant matrix and the solvent. In addition, among the most signifi cant factors that infl uence the polyphenol content, the duration of maceration is the one that af-fects the polyphenol extraction from the plant (Ricardo-da-Silva et al., 199γś Sims and Bates, 1994). źxtended maceration is known to cause an increase in polyphenol content in plant (Sun et al., 1999).
CONCLUSIONS
In summary, our results clearly showed that the ex-traction of phenolic and anthocyanin compounds and their antioxidant capacity is signifi cantly affected by
solvent combinations. S. undulata presented the
high-est total phenolic content, total fl avonoids content and antioxidant capacity values. In addition, there was a good correlation between total phenolic content and
the antioxidant capacity of the S. undulata extracts.
Or-ganic solvents were more effi cient in extracting anti-oxidant compounds than their water solvent. This paper presents the application of the design-of experiment
method for optimizing the extraction of phenolic con-tent. This method provided answers to several funda-mental questions, such as quantifying the most sensi-tive parameters of the model and their interactions – a task that is diffi cult to perform using conventional experimental methods. The resulting regression model has shown that the effect of temperature is not statisti-cally signifi cant (with >95% certainty), while that of agitation speed is. The two main effects are contributed by the solvent concentration and the maceration period.
ACKNOWLEDGMENTS
This work was supported by the research unit biodi-versity and aquatic ecosystems in the Żaculty of Sci-ences of Sfax. Thanks are due to Źr. Żawzi Makni for his help in verifying the quality of the manuscript.
REFERENCES
Addai, Z. R., Abdullah, A., Mutalib, S. A. (β01γ). źffect of extraction solvents on the phenolic content and antioxi-dant properties of two papaya cultivars. J. Med. Plants Res., 7, γγ54–γγ59.
Akkol, ź. K., Acikara, O. B., Suntar, I., Citoglu, ż. S., Keles, H., źrgene, B. (β011). źnhancement of wound healing by topical application of Scorzonera speciesŚ
Źetermination of the constituents by HPLC with new validated reverse phase method. J. źthnopharmacol., 1γ7, 1018–10β7.
Akkola, ź. K., Acıkarab, Ö. B., Süntara, I. B., żülc., Saltan, C. (β01β). źthnopharmacological evaluation of some
Scorzonera speciesŚ In vivo anti-infl ammatory and
an-tinociceptive effects. J. źthnopharmacol., 140, β61–β70. Alothman, M., Bhat, R., Karim, A. A. (β009). UV radiation-induced changes of antioxidant capacity of fresh-cut tropical fruits. Innov. Żood Sci. źmerg. Technol., 10, 51β–516.
Amin, I., Zamaliah, M. M., Chin, W. Ż. (β004). Total anti-oxidant activity and phenolic content in selected vegeta-bles. Żood Chem., 87, 581–586.
Amoateng, P., Koffuor, ż. A., Sarpong, K., Kwabena, O. (β011). Żree radical scavenging and anti-lipid peroxi-dative effects of a hydro-ethanolic extract of the whole plant of Synedrella nodifl ora (L.) żaertn (Asteraceae).
ŹOIŚ 10.55γ0/ax.β.
Anagnostopoulou, M. A., Kefalas, P., Papageorgiou, V. P., Assimopoulou, A. N., Boskou, Ź. (β006). Radical scav-enging activity of various extracts and fractions of sweet orange peel (Citrus sinensis). Żood Chem., 94, 19–β5.
Auzi, A. R., Hawisa, N. T., Sherif, Ż. M., Sarker, S. Ź. (β007). Neuropharmacological properties of Launaea resedifolia. Braz. J. Pharm., 17, 160–165.
Benmeddour, Z., Mehinagic, ź., Meurlayb, Ź. L., Louai-leche, H. (β01γ). Phenolic com-position and antioxi-dant capacities of ten Algerian date (Phoenix dactylifera
L.) cultivarsŚ a comparative study. J. Żunct. Żoods, 5, γ46–γ54.
Benzie, I. Ż. Ż., Strain, J. J. (1996). The ferric reducing ability of plasma (ŻRAP) as a measure of “antioxidant power”Ś The ŻRAP assay. Anal. Biochem., βγ9, 70–76. Brewer, M. S. (β011). Natural antioxidantsŚ sources,
com-pounds, mechanism of action, and potential application. Compr. Rev. Żood Sci. Ż, 10, ββ1–β47.
Broadhurst, R. B., Jones, W. T. (1978). Analysis of condesed tannins using acidifi ed vanillin. J. Sci. Żood Agric., β9, 788–797.
Burt, S. (β004). źssential oilsŚ Their antibacterial proper-ties and potential applications in foods – A review. Int. J. Żood Microbiol., 94, ββγ–β5γ.
Chen, T., He, J., Zhang, J., Li, X., Zhang, H., Hao, J., Li, L. (β01β). The isolation and identifi cation of two com-pounds with predominant radical scavenging activity in hempseed (seed of Cannabis sativa L.). Żood Chem.,
1γ4, 10γ0–10γ7.
Chopra, S., Athma, P., Peterson, T. (1996). Alleles of the maize Pgene with distinct tissue specifi cities encode Myb-homologous proteins with C-terminal replace-ments. The Plant Cell, 8, 1149–1158.
Cotelle, N., Bernier, J. L., Catteau, J. P., Pommery, J., Wal-let, J. C., żaydou, ź. M. (1996). Antioxidant properties of hydroxy-fl avones. Żree Radic. Biol. Med., β0, γ5–4γ. Źorman, H. J. Ź., Peltoketo, A., Hiltunen, R., Tikkanen, M.
J. (β00γ). Characterisation of the antioxidant properties of de-odourised aqueous extracts from selected Lamia-ceae herbs. Żood Chem., 8γ, β55−β6β.
źrden, Y., Kırbag, S., Yılmaz, O. (β01γ). Phytochemical composition and antioxidant activity of some Scorzone-ra species. Proc. Natl. Acad. Sci., India, Sect. B, Biol.
Sci., 8γ, β71–β76.
Żernandez-Arroyo, S., Rodriguez-Medina, I. C., Beltran--Źebon, R., Pasini, Ż., Joven, J., Micol, V., Segura-Car-retero, A., Żernandez-żutierrez, A. (β011). Quantifi ca-tion of the polyphenolic fracca-tion and in vitro antioxidant and in vivo anti-hyperlipemic activities of Hibiscus sab-dariffa aqueous extract. Żood Res. Int., 44, 1490–1495.
żalher, S., Otto, K., Böhm, V. (β00γ). Alterations of vitamin C, total phenolics, and antioxidant capacity as affected by processing tomatoes to different products. J. Agric. Żood Chem., 51, 796β–7968.
żorinstein, S., Vargas, O. J. M., Jaramillo, N. O., Salas, I. A., Ayala, A. L. M., Arancibia-Avila, P., Toledo, Ż., Katrich, ź., Trakhtenberg, S. (β007). The total polyphenols and the antioxidant potentials of some selected cereals and pseudocereals. źur. Żood Res. Technol., ββ5, γβ1–γβ8. żouveia, S., żonc, J., Castilho, P. C. (β01γ).
Characteriza-tion of phenolic compounds and antioxidant activity of ethanolic extracts from fl owers of Andryala glandulosa
ssp. varia (Lowe ex ŹC.) R. Żern., an endemic species
of Macaronesia region. Ind. Crop. Prod., 4β, 57γ–58β. żuo, B., żao, M., Liu, C. Z. (β007). In vitro propagation
of an endangered medicinal plant Saussurea involucrate Kar. et Kir. Plant Cell Rep., β6, β61–β65.
Hirasa, K., Takemasa, M. (1998). Spice science and technol-ogy. New YorkŚ Marcel Źekker.
Holton, T. A., Cornish, ź. C. (1995). żenetics and bio-chemistry anthocynin biosynthesis. The Plant Cell., 7, 1071–108γ.
Hou, Ź. X., Żujii, M., Terahara, N., Yoshimoto, M. (β004). Molecular mechanisms behind the chemopreventive effects of anthocyanidins. J. Biomed. Biotechn., 5, γβ1−γβ5.
Hou, W. C., Lin, R. Ź., Cheng, K. T., Hung, Y. T., Cho, C. H., Chen, C. H., Hwang, S. Y., Lee, M. H. (β00γ). Żree radical scavenging activity of Taiwanese native plants. Phytomedicine, 10, 170–175.
Hsu, B., Coupar, I. M., Ken, N. (β006). Antioxidant activity of hot water extract from the fruit of the Źoum palm,
Hyphaene thebaica. Żood Chem., 98, γ17–γβ8.
Jehle, M., Bano, J., źllmerer, ź. P., Zidorn, C. (β010). Natu-ral products from Scorzonera aristata (Asteraceae). Nat.
Prod. Commun., 5, 7β5–7β7.
Jiang, T. Ż., Wang, Y. H., Lv, Z. H., Yue, M. ź. (β007). Źe-termination of kava lactones and fl avonoid glycoside in
Scorzonera austriaca by capillary zone electrophoresis.
J. Pharm. Biomed. Anal., 4γ, 854–858.
Kamran, ż., Youcef, ż., źbrahimzadeh, M. A. (β009). An-tioxydant activity, phenol and fl avonoid contents of 1γ citrus species peels and tissues. Pak. J. Pharm. Sci., ββ, β77–β81.
Kenny, O., Smyth, T. J., Walsh, Ź., Kelleher, C. T., Hewage, C. M., Brunton, N. P. (β014). Investigating the poten-tial of under-utilised plants from the Asteraceae
fam-ily as a source of natural antimicrobial and antioxidant extracts. Żood Chem., 161, 79–86. ŹOIŚ httpŚ//dx.doi. org/10.1016/j.foodchem.β014.0γ.1β6.
Khadri, A., Neffati, M., Smiti, S., Żalé, P., Lona, A. R. L., ..., Serralheiro, M. L. M. (β010). Antioxidant, antiace-tylcholinesterase and antimicrobial activities of Cym-bopogon schoenanthus L. Spreng (lemon grass) from
Tunisia. Żood Sci. Technol., 4γ, γγ1e6.
Khlifi , Ź., Sghaier, R. M., Amouri, S., Laouini, Ź., Hamdi, M., Bouajila, J. (β01γ). Composition and anti-oxidant, anti-cancer and anti-infl ammatory activities of Artemisia
herba-alba, Ruta chalpensis L. and Peganum harmala
L. Żood Chem. Toxicol., 55, β0β–β08.
Kim, Ź.-K., Jeong, S. W., Lee, C. Y. (β00γ). Antioxidant capacity of phenolic phytochemicals from various culti-vars of plums. Żood Chem., 81, γβ1–γβ6.
Konczak-Islam, I., Yoshimoto, M., Hou, Ź. X., Terahara, N., Yamakawa, O. (β00γ). Chemopreventive proper-ties of anthocyanin-rich aqueous extracts from in vitro produced tissue of sweetpotato (Ipomoea batatas L.). J.
Agric. Żood Chem., 51, 5916−59ββ.
Kong, J. M., Chia, L. S., żoh, N. K., Chia, T. Ż., Brouillard, R. (β00γ). Analysis and biological activities of antho-cyanins. Phytochemistry, 64, 9βγ−9γγ.
Lahouel, M., Żillastre, J. P. (β004). Role of fl avonoids in the prevention of haematotoxicity due to chemotherapeutic agents. Haema, 7(γ), γ1γ–γβ0.
LeBlanc, B. W., Źavis, O. K., Boue, S., ŹeLucca, A., Źee-by, T. (β009). Antioxidant activityof Sonoran Źesert bee pollen. Żood Chem., 115, 1β99–1γ05.
Leon, M. A., Kumar, S. (β007). Mathematical modeling and thermal performance analysis of unglazed transpired so-lar collectors. Soso-lar źner., 81, 6β–75.
Li, S., Li, S.-K., żan, R.-Y., Song, Ż.-L., Kuang, L., Li, H.--B. (β01γ). Antioxidant capacities and total phenolic contents of infusions from ββγ medicinal plants. Ind. Crop. Prod., 51, β89–β98.
Lixiang, L., Yi, S., Tanguy, L., Xingfei, L., Hong, Y., Xiaox-iong, Z. (β009). Źetermination of polyphenolic content and antioxidant activity of kuding chamade from Ilex kudingcha C. J. Tseng. Żood Chem., 11β, γ5–41. Mailoa, M. N., Mahendradatta, M., Laga, A., Źjide N.
(β01γ). Tannin extract of żuava leaves (Psidium Gu-ajava L.) variation with concentration organic solvents.
Int. J. Sci. Techn. Res., β, 9, 106–110.
Matthäus, B. (β00β). Antioxidant activity of extracts ob-tained from residues of different oilseeds. J. Agric. Żood Chem., 50, γ444–γ45β.
Myers, J. P., vom Saal, Ż. S., Akingbemi, B. T., Arizono, K., Belcher, S., ..., Colborn, T. (β009). Why public health agencies cannot depend on żood Laboratory Practices as a criterion for selecting dataŚ the case of bisphenol A. źnviron. Health Perspect., 117, γ09–γ15.
Milella, L., Bader, A., Tommasi, N. Ź., Russo, Ź., Braca, A. (β014). Antioxidant and free radical-scavenging activ-ity of constituents from two Scorzonera species. Żood
Chem., 160, β98–γ04. ŹOI.org/10.1016/j.foodchem Mol, J., żrotewold, ź., Koes, R. (1998). How genes paint
fl owers and seeds. Trends Plant Sci., γ, β1β–β17. Molyneux, P. (β004). The use of the stable free radical
di-phenylpicrylhydrazyl (ŹPPH) for estimating antioxi-dant activity. Songklanak. J. Sci. Techn., β6, β11–β19. Mothana, R. A. A. (β011). Anti-infl ammatory,
antinocicep-tive and antioxidant activities of the endemic Soqotraen
in different experimental models. Żood. Chem. Toxicol., 49, β594–β599.
Naczk, M., Shahidi, Ż. (β004). źxtraction and analysis of phenolics in food. J. Chromat., 1054, 95–111.
Nsimba, R. Y., Kikuzaki, H., Konishi, Y. (β008). Antioxi-dant activity of various extracts and fractions of Che-nopodium quinoa and Amaranthus spp. seeds. Żood
Chem., 106, 760–766.
Okuda, T. (1999). Novel aspects of tannins – Renewed con-cepts and structure–activity relationships. Curr. Org. Chem., γ, 609–6ββ.
Okuda, T., Yoshida, T., Hatano, T. (1991). Chemistry and biological activities of tannins in medicinal plants. In H. Wagner, N. R. Żarnsworth (źds.), źconomic and me-dicinal plants research. Vol. 5 (pp. 1β9–165). New YorkŚ Academic Press.
Okuda, T., Yoshida, T., Hatano, T. (199β). Pharmacologi-cally active tannins isolated from medicinal plants. In R. W. Hemingway, P. ź. Laks (źds.), Plant polyphenolsŚ biogenesis, chemical properties and signifi cance (pp. 5γ9–569). New YorkŚ Plenum Press.
Okuda, T., Yoshida, T., Hatano, T. (1995). Hydrolyzable tan-nins and related polyphenols. In W. Herz, ż. W. Kirby, R. ź. Moore, W. Steglich, C. Tamm (źds.), Progress in the chemistry of organic natural products. Vol. 66. Wien, New YorkŚ Springer.
Okuda, T., Yoshida, T., Mori, K. (1975). Studies on the con-stituents of Geranium thunbergii II. Yakugaku Zasshi,
95, 146β–1466.
Ouchemoukhe, S., Hachoud, S., Boudrahama, H., Mokra-ni, A., Louaileche, H. (β01β). Antioxidant activities of some dried fruits consumed in Algeria. LWT – Żood Sci. Technol., 49, γβ9–γγβ.
Oyaizu, M. (1986). Studies on product of browning reaction prepared from glucose amine. Jpn J. Nutr., 44, γ07–γ15. Ozgen, M., Reese, R. N., Tulio, A. Z., Miller, A. R., Schee-rens, J. C. (β006). Modifi ed β,β-azino-bis-γ-ethylbenzo-thiazoline-6-sulfonic acid (ABTS) method to measure antioxidant capacity of selected small fruits and com-parison to ferric reducing antioxidant power (ŻRAP) and β,β-diphenyl-1-picrylhydrazyl (ŹPPH) methods. J. Agric. Żood Chem., 54, 1151–1157.
Ozsoy, N., Yilmaz, T., Kurt, O., Can, A. (β009). In vitro an-tioxidant activity of Amaranthus lividus L. Żood Chem.,
116, 867–87β.
Oztürk, H., Kolak, U., Meric, C. (β011). Antioxidant, an-ticholinesterase and antibacterial activities of Jurinea consanguinea ŹC. Rec. Nat. Prod., 5, 4γ–51.
Prochazkova, Ź., Bousova, I., Wilhelmova, N. (β011). An-tioxidant and prooxidant properties of fl avonoids, Żito-terapia, 8β, 51γ–5βγ.
Ramful, Ź., Tarnus, ź., Aruoma, O. I., Bourdon, ź., Ba-horun, ź. (β011). Polyphenol composition, vitamin C
content and antioxidant capacity of Mauritian citrus fruitpulps. Żood Res. Int., 44, β088–β099.
Ramos-źscudero, Ż., María, L., żonzalez-Miret, A., żarcia--Asuero, A. (β01β). źffect of various extraction systems on the antioxidant activity kinetic and color of extracts from Purple Corn. Vittae Revista de la Żacultad de Quimica Żarmaceutica, 19, 1, 41–48.
Ricardo-da-Silva, J. M., Cheynier, V., Samsom, A., Bourzeix, M. (199γ). źffect of pomace contact, car-bonic maceration, and hyperoxidation on the procyani-din composition of żrenache blanc wines. Am. J. źnol. Vitic., 44, 168–17β.
Robards, K., Prenzler, P. Ź., Tucker, ż., Swatsitang, P., żlover, W. (1999). Phenolic compounds and their role in oxidative processes in fruits. Żood Chem., 66, 401–4γ6. Rout, ż. R., Samantaray, S., Źas, P. (β000). In vitro manip-ulation and propagation of medicinal plants. Biotechn. Adv., 18, β, 91–1β0.
Sareedenchai, V., Zidorn, C. (β010). Żlavonoids as chemos-ystematic markers in the tribe Cichorieae of the Astera-ceae. Biochem. Syst. źcol., γ8, 9γ5–957.
Sari, A. (β01β). Phenolic compounds from Scorzonera la-tifolia (Żisch & Mey) ŹC. Nat. Prod. Res., β6, 50–55.
Sari, A., Zidorn, C., źllmerer L. P., Özgökçe Ż., Onganiac, K. H., Stuppner, H. (β007). Phenolic compounds from Sco-rzonera tomentosa L. Helv. Chim. Acta, 90, γ11–γ17.
Shahat, A. A., Ibrahim, A. Y., źlsaid, M. S. (β014). Poly-phenolic content and antioxidant activity of some wild Saudi Arabian Asteraceae plants. Asian Pac. J. Trop.
Bi-omed., 7(7), 545–551.
Shinde, A. N., Malpathak, N., Żulzele, Ź. P. (β010). Źeter-mination of isofl avone and antioxidant activity in Pso-ralea corylifolia L. callus cultures. Żood Chem., 118,
1β8–1γβ.
Sims, C. A., Bates, R. P. (1994). Comparison of pre-fermen-tation and post-fermenpre-fermen-tation ultrafi ltration on the char-acteristics of sulfi ted and non-sulfi ted white wines. Am. J. źnol. Vitic., 45, 18β–185.
Singleton, V. L., Rossi, J. A. (1965). Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. źnol. Vitic., 16, 144–158.
Škrovánková, S., Mišurcová, L., Machů, L. (β01β). Antioxi-dant activity and protecting health effects of common medicinal plants. Adv. Żood Nutr. Res., 67, 75–1γ9. Stintzing, Ż. C., Carle, R. (β004). Żunctional properties of
anthocyanins and betalains in plants, food, and in human nutrition. Trends Żood Sci. Technol., 15, 19−γ8. Suhaj, M. (β006). Spice antioxidants isolation and their
antiradical activityŚ A review. J. Żood Comp. Anal., 19, 5γ1–5γ7.
Sultana, B., Anwar, Ż., Przybylski, R. (β007). Antioxidant activity of phenolic components present in barks of
and Eugenia jambolana lam trees. Żood Chem., 104,
1106–1114.
Sun, Y., Liu, Ź., Chen, J., Ye, X., Yu, Ź. (β011). źffects of different factors of ultrasound treatment on the extrac-tion yield of the all-trans- -carotene from citrus peels.
Ultrason. Sonochem., 18, β4γ–β49.
Sun, B. S., Pinto, T., Leandro, M. C., Ricardo da Silva, J. M., Spanger, M. I. (1999). Transfer of catechins and proanthocyanidins from grape solids into wine. Am. J. źnol. Vitic., 50, 179–184.
Tai, Z., Cai, L., Źai, L., Źong, L., Wang, M., Yang, Y., Cao, Q., Źing, Z. (β011). Antioxidantactivity and chemical constituents of edible fl ower of Sophora viciifolia. Żood
Chem., 1β6, 1648–1654.
Tsevegsuren, N., źdrada, R., Lin, W., źbel, R., Torre, C., ..., Ortlepp, S., (β007). Biologically active natural products from Mongolian medicinal plants Scorzonera divarica-ta and Scorzonera pseudodivaricata. J. Nat. Prod., 70,
96β–967.
Tsevegsuren, N., źdrada, R. A., Lin, W., źbel, R., Torre, C., ..., Proksch, P. (β007). Żour new natural products from mongolian medicinal plants Scorzonera divaricata and Scorzonera pseudodivaricata (Asteraceae). Plant. Med.,
7β, 96β–967.
Turkmen, N., Sari, Ż., Velioglu, Y. S. (β006). źffect of ex-traction solvents on concentration and antioxidant ac-tivity of black and black mate polyphenols determined by ferrous tartrate and Żolin-Ciocalteu methods. Żood Chem., 99, 8γ8–841.
Velioglu, Y. S., Mazza, ż., żao, L., Oomah, B. Ź. (1998). Antioxidant activity and total phenolics in selected fruits, vegetables, and grain products. J. Agric. Żood Chem., 46, 411γ–4117.
Venditti, ź., Bacchetti, T., Tiano, L., Carloni, P., żreci, L., Źamiani, ź. (β010). Hot vs. cold water steeping of dif-ferent teasŚ Źo they affect antioxidant activity? Żood Chem., 119, 1597–1604.
Vuong, Q. V., Hirun, S., Roach, P. Ź., Bowyer, M. C., Phil-lips, P. A., Scarlett, C. J. (β01γ). źffect of extraction con-ditions on total phenolic compounds and antioxidantac-tivities of Carica papaya leaf aqueous extracts. J. Herb.
Med., γ, 104–111.
Wang, B., Li, ż. Q., Qui, P. J., żuan, H. S. (β007). Two new oleane-type triterpene fatty esters from Scorzonera mon-golica. Chinese Chem. Lett., 18, 708–710.
Wang, Y., Wray, V., Tsevegsuren, N., Lin, W. H., Proksch, P. (β01β). Phenolic compounds from the Mongolian me-dicinal plant Scorzonera radiata. Z. Naturforsch. C, 67,
1γ5–14γ.
WHO, IUCN, WWŻ (199γ). żuidelines on the conservation of medicinal plants. żland, żeneva, SwitzerlandŚ IUCN, WHO, WWŻ.
Wojdyło, A., Oszmiański, J., Czemerys, R. (β007). Anti-oxidant activity and phenolic compounds in γβ selected herbs. Żood Chem., 105, 940–949.
Xi, J., Shen, Ź., Zhao, S., Lu, B., Li, Y., Zhang, R. (β009). Characterization of polyphenolsfrom green tea leaves using a high hydrostatic pressure extraction. Int. J. Pharm., γ8β, 1γ9–14γ.
Yang, Y. J., Liu, X., Wu, H. R., He, X. Ż., Bi, Y. R., ..., Zhu, Y. (β01γ). Radical scavenging activity and cytotoxicity of active quinic acid derivatives from Scorzonera diva-ricata roots. Żood Chem., 1γ8, β057–β06γ.
Yesil-Celiktas, O., Nartop, P., żurel, A., Bedir, ź., Vardar-Sukan, Ż. (β007). Źetermination of phenolic content and antioxidant activity of extracts obtained from Rosmari-nus offi cinalis calli. J. Plant Physiol., 164, 15γ6–154β.
Zhang, P., Jin, Y., Chen, J., Yao, H., Zhang, H., Yu, A., Li, X. (β01γ). Ultrasonic-assisted nebulization extraction cou-pled with SPź and HPLC for the determination of triter-penoids in root of Euphorbia pekinensis Rupr.
Chroma-tographia, 76, 967–974.
Zhishen, J., Mengcheng, T., Jianming, W. (1999). The de-termination of fl avonoid contents in mulberry and their scavenging effects on superoxide radicals. Żood Chem., 64, 555–559.
Zhu, Y., Wu, Q., Hu, P., Wu, W. (β009). Biguaiascorzolides A and BŚ two novel dimeric guaianolides with a rare skeleton, from Scorzonera austriaca. Żood Chem., 114,
1γ16–1γβ0.
Zidorn, C. (β008). Sesquiterpene lactones and their precur-sors as chemo systematic markers in the tribe Cichorie-ae of the Asteraceae. Phytochemistry, 69, ββ70–ββ96.
Zidorn, C., źllmerer, ź. P., Sturm, S., Stuppner, H. (β00γ). Tyrolobibenzyls ź and Ż from Scorzonera humilis and
distribution of caffeic acid derivatives, lignans and ty-rolobibenzyls in źuropean taxa of the subtribe Scorzo-nerinae (Lactuceae, Asteraceae). Phytochemistry, 6γ,
61–67.
Zidorn, C., źllmerer-Müller, ź. P., Stuppner, H. (β000). Tyrolobibenzyls-novel secondary metabolites from Sco-rzonera humilis L. Helv. Chim. Acta, 8γ, β9β0–β9β5.
Received – Przyjęto: 29.07.2015 Accepted for print – Zaakceptowano do druku: 3.09.2015
For citation – Do cytowania
Athmouni, K., Belghith, T., Bellassouad, K., El Feki, A., Ayadi, H. (2015). Eff ect of extraction solvents on the biomolecules and anti-oxidant properties of Scorzonera undulata (Asteraceae): Application of factorial design optimization phenolic extraction. Acta Sci.