AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
197
AACL BI OFLUX
Aqu a cu lt u r e , Aqu a r iu m , Con se r va t ion & Le gisla t ion
I n t e r n a t ion a l Jou r n a l of t h e Bioflu x Socie t y
D ist r ibu t ion a n d com m u n it y st r u ct u re of fish in
Obit su - ga w a Rive r Est u a r y of in ne r Tok y o Ba y ,
ce n t ra l Ja pa n
Joeppet t e J. Herm osilla, Yasushi Tam ura, Masat o Mot eki,
and Hiroshi Kohno
Tok y o Univ ersit y of Marine Science and Technology , Minat o, Toky o 108- 8477, Japan. Corresponding aut hor: J. J. Herm osilla, j oeppet t e@y ahoo.com
Abst r a ct. The dist r ibut ion and com m unit y st r uct ur e of fish in Obit su- gawa River Est uar y of inner Toky o Bay, cent r al Japan was st udied fr om May t o Decem ber 200 5 and Mar ch t o Apr il 200 6. A t ot al of 1 9,006 individuals, r epr esent ed by 25 species and som e unident ified species under fam ily Clupeidae, Cypr inidae, Gobiidae, Hem ir am phidae, Mugilidae, Plat ycephidae, Pleur onect idae and Tr iglidae wer e collect ed. Fam ily Gobiidae had t he m ost num ber of t axa wit h 13 gener a and 10 species. Gr eat est fish abundance happened in August and secondar ily in Apr il and May. Species r ichness was evident in t he war m er m ont hs par t icular ly in May ( 17 t axa) , August ( 21 t axa) , Sep t em ber ( 15 t axa) and Oct ober ( 17 t axa) . Mar ine t eleost s significant ly cont r ibut ed t o t he species r ichness and abu ndance of fish, which cor r esponded t o 52.9% ( 10,04 6 individuals) of t he t ot al cat ch while t he est uar ine fishes wer e t he second m ost abundant gr oup wit h 33.5% ( 6,372 individuals) of t h e t ot al cat ch. Species dom inance was a coher ent feat ur e of t his com m u nit y. The pr opor t ional con t r ibut ion of m ar ine t eleost s t o t he fish com m unit y decr eased wit h incr ease dist ance upst r eam while t hat of est uar ine fishes incr eased wit h incr ease dist ance upst r eam . The developm ent al st ages of gobies r ange fr om lar vae t o adult but j uveniles const it ut e 77.06% of t he t ot al sam ple. The dist r ibut ion of developm ent al st age of est uar ine gobies was influenced t o a gr eat er ext ent by var iat ion in m ont hly wat er t em per at ur e and st at ion or t he int er act ion of bot h. Adult est uar ine gobies had t he t endency t o aggr egat e in t he m iddle est uar y r eflect ing t heir high t oler ance t o a wide r ange of wat er salinit y inher ent in t his st at ion but avoided t he lower est uar y m ost likely due t o t he pr edom inance of high salinit y wat er s.
Ke y W or ds: Tokyo Bay, est uar y, gobies.
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
198
2000, 2004a, 2004b, 2005a, 2005b, 2007; Ok azaki et al 2011) t hat generat ed a considerable am ount of k now ledge on t he lifecycle pat t erns as w ell t he developm ent al st ages of som e of t he com m on fish t ax a present in t he est uarine t idelands of inner Tok y o Bay . These dat a w ere used in t he current st udy t o furt her underst and t he dist ribut ion and com m unit y st ruct ure of fish in Obit su- gaw a Riv er Est uary and provided som e pert inent det ails regarding t he relev ance of w at er t em perat ure and sit e in predict ing t he dy nam ics in t he dist ribut ion of t he dev elopm ent al st ages of select ed est uarine species.
M a t e r ial an d M e th od. Sam pling w as perform ed from May t o Decem ber 2005 and March t o April 2006 along Obit su- gaw a Riv er sit uat ed at t he Boso Peninsula near Egaw a, Kisarazu Cit y , Chiba Prefect ure nort heast of Tok yo Bay, Japan. There w ere 3 designat ed st at ions along t he saline reaches of t he river. The lower est uary ( Longit ude: 139° 53’50” ; Lat it ude: 35° 24’33” ) w as t he st at ion sit uat ed adj acent t o t he riv er m out h along t he coast front ing Tok y o Bay w hile t he m iddle est uary ( Longit ude: 139° 54’10” ; Lat it ude: 35° 24’39” ) w as sit uat ed inner t o t he previous st at ion at a dist ance of 0.5 k m . The upper est uary on t he ot her hand w as sit uat ed furt her upst ream at a dist ance of 2 k m from t he m iddle est uary ( Figure 1) . Sam pling w as not m ade possible in January and February in t he 3 st at ions as w ell as in t he low er est uary st at ion in March and April.
LOWER
M IDDLE
UPPER
Figur e 1. Map of Tokyo Bay and t he locat ion of t he sam pling st at ions along t he Obit su- gawa River est uary [ Maps court esy of t he Nat ional Geophysical Dat a Cent er
( ht t p: / / w ww.n gdc.noaa.gov/ m gg/ coast / ) and Environm ent al Sciences Research I nst it ut e ( ht t p: / / w ww. esr i.com / ) ] .
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
199
t ak en from each st at ion. I n t he laborat ory , fish w as ident ified t o species level as possible. St andard lengt h ( SL) w as m easured t o t he nearest 0.1 m m . Dev elopm ent al st ages w ere det erm ined for gobies according t o t he descript ions m ade by Kendall Jr et al ( 1984) w hile j uv eniles w ere furt her divided int o 3 groups on t he basis of it s body pigm ent at ion ( Kanou et al 1999, 2005b) . Juv enile 1 ( J1) had pigm ent at ion sim ilar t o post flexion larv ae while j uv enile 2 ( J2) w as t he t ransit ion period bet ween J1 and J3. Juv enile 3 ( J3) had t he sam e pigm ent at ion pat t ern as adult s. Addit ionally , species w ere grouped based on lifecy cle cat egory in accordance w it h t he w ork of Kanou et al ( 2000) . Sam ples w ere t hen fix ed in 70% et hanol and k ept for furt her analy sis.
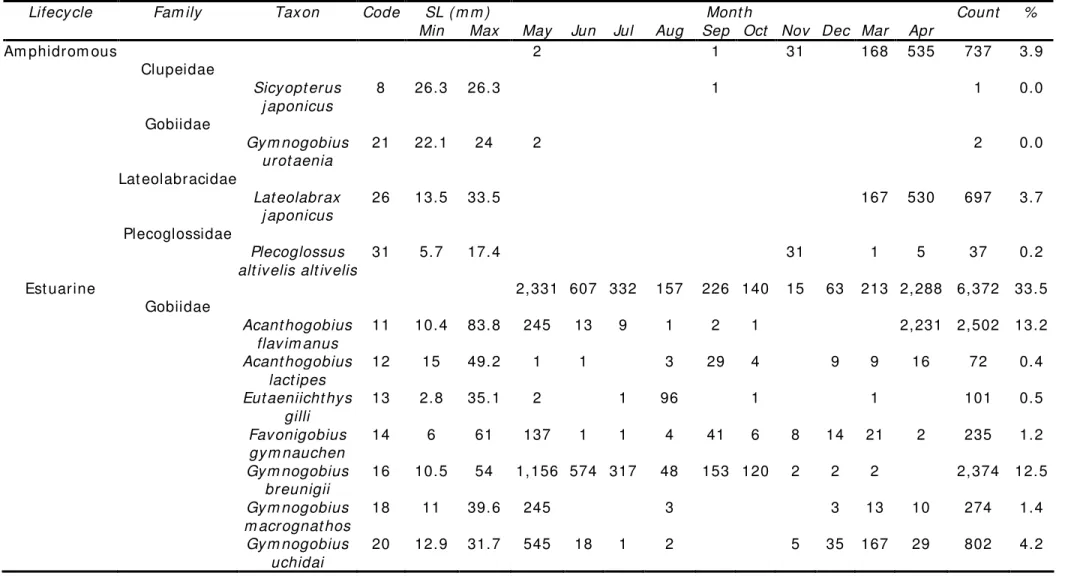
Rény i div ersit y profile w as used t o describe t he species richness and species ev enness propert ies of t he fish com m unit y in t he 3 st at ions. Rényi div ersit y offers a m ore com plet e sum m arizat ion of com m unit y div ersit y t hat uses a param et ric fam ily of div ersit y indices ( alpha "α" div ersit y ) w hose m em bers hav e v ary ing sensit ivit ies t o t he presence of rare and abundant species in a com m unit y , w hich t hen becom es increasingly dom inat ed by t he com m onest species for increasing v alues of t he param et er α ( Ricot t a 2003) . Tót hm érész ( 1995) recom m ended t he use of Rény i div ersit y since it conv ey s t he degree of dom inance in t he com m unit y such t hat a hom ogenous com m unit y has a perfect horizont al profile w hile a com m unit y wit h high degree of dom inance has a st eep profile. Div ersit y profile v alues w ere calculat ed at fix ed scales (α = 0, 0.25, 0.5, 1, 2, 4, 8, ∞). The v alues of t he Rény i div ersit y at scales 0, ≈1, 2 and ∞ (or infinit y ) reflect t he logarit hm of species richness, Shannon div ersit y index, and t he logarit hm s of reciprocal Sim pson and Berger- Park er div ersit y indices, respect iv ely ( Ricot t a 2003) . A fish com m unit y in a given st at ion w as m ore div erse if all t he v alues of t he div ersit y profile w ere higher. Rényi diversit y curv e for each st at ion w as calculat ed by random ized pooling of m ont hs t hat belong t o a part icular st at ion and a div ersit y profile was calculat ed on t hese pooled m ont hs.
Abundance for each t ax on w as fit t ed int o ordinat ion space using a nonm et ric m ult idim ent ional scaling ( NMDS) in search for pat t erns in t heir dist ribut ion in relat ion w it h m ont hs and st at ions. Moreov er, a st at ist ical t est w as perform ed using t he m axim um likelihoood approach on t he SL of select ed est uarine species w hose dist ribut ion w ere evident for a prot ract ed period and cov ering at least 2 st at ions wit hin t his period t o ex plore any v ariat ion in size in response t o w at er t em perat ure and st at ions. To do t his, t he SL for each species w as init ially fit t ed t o 2 feasible st at ist ical dist ribut ion fam ilies ( e.g., Gaussian, Gam m a) appropriat e for size dat a ( Craw ley 2007) and w as int egrat ed int o a Generalized Linear Model ( GLM) fram ew ork . The Ak aik e` s I nform at ion Crit eria or AI C ( Ak aik e 1981; Burnham & Anderson 2004) w as used t o ident ify w hich of t he 2 dist ribut ion fam ilies best ex plained t he v ariat ion in t he SL of a giv en species. Model wit h t he low est AI C score w as select ed as t he “ best ” approx im at ing m odel ( Burnham & Anderson 2004; Ellison 2004) . St at ion and w at er t em perat ure were considered as t he ex planat ory v ariables in t he m odel- fit t ing process wit h 4 cov ariat es ( e.g., w at er t em perat ure only, st at ion only, w at er t em perat ure and st at ion as w ell as w at er t em perat ure and st at ion int eract ion) . These cov ariat es w ere fit t ed t o t he SL dat a for each species wit h t he appropriat e dist ribut ion fam ily . The AI C w as used t o det erm ine w hich m odel best ex plained t he v ariat ion in size of a part icular species. The best m odel w as t hen plot t ed. A plot cont aining t he SL dist ribut ion for each species against t he w at er t em perat ure gradient s for each st at ion w as creat ed and a line w as draw n t hat corresponded t o t he predict ed response of SL t o gradient s in w at er t em perat ure observ ed for each st at ion.
St at ist ical t est s w ere carried out under t he st at ist ical com put ing language R ( v ersion 2.12.0) . NMDS w as perform ed using t he “ v egan” pack age ( Dix on 2003; Ok sanen et al 2007) w hile t he Rényi div ersit y w as perform ed using t he “ Biodiv ersit yR” pack age ( Kindt & Coe 2005) . The m odel fit t ing of SL t o t he best dist ribut ion fam ily and subsequent ly, t o t he m ost appropriat e com binat ion of ex planat ory v ariables w ere perform ed using t he “ MASS” pack age ( Venables & Ripley 2002) .
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
200
w at er salinit y in t he said est uary ( Table 1) . As for w at er t em perat ure, how ev er, m ont h ( p= 0.001) w as m ore im port ant t han st at ion ( p= 0.05) . Figure 2 show ed t he m ont hly v ariat ion in w at er t em perat ure as well as v ariat ion in w at er salinit y observ ed in t he 3 st at ions. High w at er t em perat ure w as observed from June t o August ( 26± 2.3 – 26.7± 1.2° C) , w hich decreased gradually from Sept em ber ( 19.4± 0.6° C) t o Decem ber ( 6.1± 0.6° C) . Wat er t em perat ure w as increasing from March ( 11.4± 3.5° C) t o May ( 18.2± 0.2° C) . Highest salinit y w as observ ed in t he lower est uary ( 24.1± 6.7 psu) , w hich begun t o decrease upst ream ( m iddle est uary = 13.1± 7.1 psu; upper est uary = 3.1± 3.4 psu) .
Table 1 AI C scores for different com binat ion of covariat es for wat er t em perat ure and salinit y. The covariat e or covar iat e com binat ion w it h t he lowest AI C score ( * ) was considered t he suit able approxim at ing m odel t hat explained t he variat ion in wat er t em perat ure and salinit y
Phy sical v ariable Model Cov ariat es AI C Score Dist ribut ion fam ily
Wat er t em perat ure 1 Wat er t em perat ure 185.3 Gaussian
2 St at ion 188.8
3 Mont h 101.4
4 St at ion + Mont h 97.5*
Wat er salinit y 1 Wat er salinit y 175.3 Gam m a
2 St at ion 160.1*
3 Mont h 186.2
4 St at ion + Mont h 172.4
( a) Wat er Tem perat ure ( b) Wat er Salinit y
Figur e 2. Average m ont hly variat ion in wat er t em perat u re ( a) and spat ial var iat ion in wat er salin it y ( b) in Obit su- gawa River Est uary.
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
201
Nev ert heless, secondary peak s in fish abundance w ere realized in spring part icularly in April ( 15% ; 2,848 individuals) and May ( 13.3% ; 2,534 individuals) .
Species richness w as evident in t he w arm er m ont hs part icularly in May ( 17 t ax a) , August ( 21 t ax a) , Sept em ber ( 15 t ax a) and Oct ober ( 17 t ax a) , w hich w as congruent wit h st udies on t he icht hy ofauna in t he t idelands ( Kanou et al 2000) and euhaline sy st em s ( Kohara & Kohno 1999; Kanou et al 2002) of inner Tok y o Bay . NMDS based on act ual count per t ax on in Figure 3 show ed t hat t he occurrence as w ell as incidence of peak abundance of fish w ere prev alent in m ont hs w hen t he w at er t em perat ure w as increasing w it h t he ex cept ion of t he am phidrom ous fish, Plecoglossus alt iv elis alt iv elis ( Tem m inck & Schlegel, 1846) , w hose larv ae ( 13.7± 2.9 m m SL) w ere prev alent in Nov em ber in t he low er est uary ( Table 2) . The larv ae of t his species w as k now n t o occur in t he est uarine and coast al sy st em s following hat ching period around Oct ober t o Decem ber in t he upst ream s of t he river ( Ham ada & Kinoshit a 1988; Tak ahashi et al 2000; Kensak u et al 2003; Yo et al 2006) and t he brack ish w at ers were considered an im port ant habit at for t he larv ae of t his species ( Saruw at ari 1995; Yasuhik o 2002) . Generally , t he occurrence and abundance of fish in Obit su- gaw a River Est uary v ary w it h changed in m ont hly w at er t em perat ure and a subst ant ial num ber of t ax a had abundance peak in t he w arm m ont hs. Part icularly, 7 out of t he 12 m arine species had t heir occurrence and abundance peak in sum m er ( June t o August ) and 6 of w hich w ere prev alent in August ( Table 2) suggest ing t hat t he connect ivit y bet w een t hese t w o sy st em s becam e m ore relev ant around sum m er part icularly in August w hen a considerable num ber of m arine species had m ov ed t o t he est uary . This st udy proposed t hat t he Obit su- gaw a Riv er Est uary serv ed as a t ransient habit at for som e m arine species part icularly during sum m er. Conv ersely , t he low wint er t em perat ures in com binat ion w it h low salinit ies in est uaries can cause sev ere phy siological st ress for m ost fishes including t he est uarine species (Whit field et al 1981) , w hich lik ely lim it s t he fish colonizat ion of t hese sy st em s during t he cold m ont hs ( Whit field 1999) .
Figur e 3. Nonm et ric m ult idim ensional scalin g ( NMDS) for t he num erical abundance of t he 36 t axa in relat ion t o m ont hs. The Bray–Curt is was t he dissim ilarit y index used t o generat e t he NMS. The bubble plot was proport ional t o t he average wat er t em perat ure for each m ont h while t he v ect or
( arrow) was dir ect ed t owards t he m ont hs wit h incr easin g gradient in wat er t em perat ure. Each num ber in t he plot corr esponds t o a code for a part icular t axon given in Table 2.
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
202
relev ant in t he w arm m ont hs reflect ing it s t endency t o aggregat e in t he est uary during t he w arm m ont hs. Gym nogobius hept acant hus ( Hilgendorf, 1879) w as prom inent in May w hile Hy poat herina v alenciennei ( Bleek er, 1854) in July . Alt hough Sardinella zunasi
( Bleek er, 1854) w as t he m ost dom inant species in t his group because of it s rem ark able abundance in August , ot her m arine t eleost s part icularly Konosirus punct at us ( Tem m inck & Schlegel, 1846) and Salangicht hy s ishikaw ae Wakiy a & Takahashi, 1913 w ere also prev alent in t his m ont h. Nuchequula nuchalis ( Tem m inck & Schlegel, 1845) w as t he second m ost abundant m arine species t hat w as prev alent in Sept em ber. The rem ark able num ber of S. zunasi can be at t ribut ed t o t he seasonal spaw ning period of t his species t hat peak around August ( Oda 2007) . I t w as likely t hat a subst ant ial num ber of larv ae ( 11.2± 1.4 m m SL) m igrat ed t o t he est uary following hat ching from t he adj acent euhaline w at ers. All t hese species can be considered as est uarine opport unist s since t hey w ere present in subst ant ial num ber in t his sy st em but only for a short period w hile t he rem aining species w ere m arine st ragglers because t hey w ere v ery few and assum ed t o be st enohaline ( Claridge et al 1986; Lenant on & Pot t er 1987; Loneragan et al 1989; Loneragan & Pot t er 1990; Whit field 1999; Elliot t et al 2007) . Nev ert heless, t he lat t er cont ribut ed considerably t o t he species richness in t his syst em .
Est uarine fishes w ere t he second m ost abundant group t hat corresponded t o 33.5% ( 6,372 individuals) of t he t ot al cat ch wit h 8 represent at iv e species ex clusiv ely under fam ily Gobiidae ( Table 2) . They w ere part icularly prom inent from March t o Sept em ber but m ost im port ant ly in April ( 35.9% ; 2,288 individuals) and May ( 36.6% ; 2,331 individuals) . Acant hogobius flav im anus ( Tem m inck and Schlegel, 1845) and
Gy m nogobius breunigii ( St eindachner, 1879) w ere t he m ost abundant species, w hich w ere responsible for t he subst ant ial num ber of individuals in April and May , respect ively. How ev er, t he abundance of Gy m nogobius uchidai ( Tak agi, 1957) , Gy m nogobius m acrognat hos ( Bleek er, 1860) and Fav onigobius gy m nauchen ( Bleek er, 1860) in May as w ell as Eut aeniicht hy s gilli Jordan & Sny der, 1901 in August w ere also im port ant .
Acant hogobius lact ipes ( Hilgendorf, 1879) and Pseudogobius m asago ( Tom iy am a, 1936) w ere relat iv ely few in t his sy st em . Generally, a subst ant ial num ber of gobies in April w ere J1 ( 78.1% ; 386 individuals) w hile J1 ( 31% ; 728 individuals) and J2 ( 47% ; 1,102 individuals) in May . A m ore or less sim ilar result w as observ ed in t he t em perat e Sw an Est uary of Aust ralia w herein influx of 0+ recruit s of est uarine gobies were relev ant in t he w arm m ont hs ( Gill & Pot t er 1993) . One part icular adv ant age in t he t im ing of reproduct ion around lat e spring w as t he higher grow t h rat e of t he progeny result ing from rise in w at er t em perat ure like in t he case of t he est uarine goby , Pseudogobius olorum ( Sauv age, 1880) , of Sw an Est uary ( Gill et al 1996) . Significant recruit m ent of est uarine gobies around spring can also be at t ribut ed t o t he high prim ary product ivit y of t he coast al sy st em of t he bay during t his season ( Ogaw a & Ogura 1997; Nak ane et al 2008; Boum an et al 2010) especially if t he ut ilizat ion of t he int ert idal habit at by epibent hic fishes were relat ed t o feeding ( Cat t rij sse et al 1994; Kneib & Wagner 1994; Kneib 1997; Laffaille et al 2000; Nem erson & Able 2003; Kanou et al 2004a) giv en t he fact t hat t he highly eut rophic est uaries locat ed east of inner Tok y o Bay w ere generally described as areas w it h not able phy t oplank t on grow t h and high chlorophy ll a concent rat ion in t he part iculat e organic m at t er during t he w arm m ont hs ( Ogaw a & Ogura 1997; Suzum ura et al 2004) . Moreov er, t he prev alent w arm w at ers from lat e spring t o early aut um n can prom ot e t em perat ure- m ediat ed grow t h for t he y oung fish cohort s ( Gill et al 1996; Krück et al 2009) . Thus, t he current st udy proposed t hat t he abundance of early j uv eniles around spring w as a st rat egy t hat increase t he chances of surv iv al and t he subsequent recruit m ent of est uarine gobies int o t he sy st em .
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
203
March from t heir spaw ning ground in t he wat ers off Kum am ot o and inhabit t he freshw at er t hrough spring ( Shoj i et al 2006) . The 3 rem aining species of am phidrom ous fish cont ribut ed only t o t he species richness of fish in Obit su- gaw a Riv er Est uary .
Lepom is m acrochirus Rafinesque, 1819 w as t he only freshw at er represent at ive ident ified in t his syst em , w hich corresponded t o 0.2% ( 47 individuals) of t he t ot al cat ch ( Table 2) . This species w as prev alent in August . Considering t hat freshw at er fish in general is a not capable of osm oregulat ion in high saline w at ers ( Pot t er & Hy ndes 1999) , it w as lik ely t hat t he occasional presence of t his species in t he est uary w as coherent w it h t he incidence of low w at er salinit y in t he low er reaches of t he riv er, w hich happened in August w hen t he av erage w at er salinit y w as 8.4± 11.9 psu.
Teleost s wit h unk now n lifecy cle corresponded t o 9.5% ( 1,804 individuals) of t he t ot al cat ch wit h 8 fam ilies and 8 genera ( Table 2) . Abundance of t his group w as prom inent in March and August but m ost im port ant ly in August . Kareius spp. and som e unident ified m ugilids ( Mugilidae spp.) w ere prevalent in March. On t he ot her hand, t he goby , Trident iger spp., t oget her wit h som e unident ified clupeids ( Clupeidae spp.) w ere prom inent in August . Tribolodon spp., Gy m nogobius spp., Rhinogobius spp.
Hem iram phus spp., Plat y cephalus spp., Chelidonicht hy s spp. and som e unident ified gobies ( Gobiidae spp.) w ere im port ant cont ribut ions t o t he richness of icht hy ofauna in Obit su- gaw a Riv er est uary .
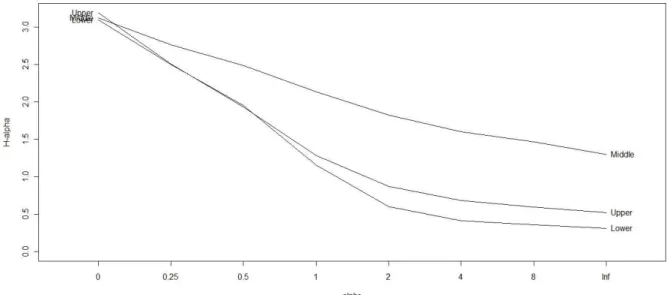
At least 6 dist ribut ional pat t erns w ere ident ified by NMDS based on act ual count of t he 36 t ax a ( Figure 4) . Prev alent in t he lower est uary w ere one am phidrom ous (P. alt iv elis alt iv elis) , 2 est uarine (E. gilli and G. m acrognat hos) , 2 m arine (G. hept acant hus
and S. zunasi) and 4 t ax a w it h unk now n lifecy cle ( Clupeidae spp., Gobiidae spp.,
Rhinogobius spp. and Trident iger spp.) . Prev alent in t he m iddle est uary w ere 2 m arine (Liza haem at ocheilus ( Tem m inck & Schlegel, 1845) , and S. ishikaw ae) and 3 genera w it h unk now n lifecy cle (Hem iram phus spp., Kareius spp. and Chelidonicht hy s spp.). A conspicuous com ponent of t he upper est uary w ere 2 am phidrom ous (Gy m nogobius urot aenia ( Hilgendorf, 1879) and Sicy opt erus j aponicus ( Tanak a, 1909) , 3 est uarine (A. flav im anus, A. lact ipes and P. m asago) , 4 m arine (Caranx sex fasciat us Quoy & Gaim ard, 1825, H. v alenciennei, Takifugu niphobles ( Jordan & Sny der, 1901) and Terapon j arbua
( Forssk ål, 1775) ) and 4 t ax a w it h unk now n lifecycle (Gy m nogobius spp., Mugilidae spp.,
Plat y cephalus spp. and Tribolodon spp.) . Prev alent in bot h low er and m iddle est uaries w ere one est uarine (G. uchidai) and 3 m arine (K. punct at us, Om obranchus elegans
( St eindachner, 1876) and N. nuchalis) species. Prev alent in bot h m iddle and upper est uaries w ere one am phidrom ous (L. j aponicus) and one freshw at er (L. m acr ochirus) . Prev alent in bot h low er and upper est uaries but wit h considerable represent at ive individuals in t he m iddle est uary w ere 2 gobiid (G. breunigii and F. gy m nauchen) and one m arine species (Engraulis j aponicus Tem m inck & Schlegel, 1846) . Generally , a paucit y in peak abundance of est uarine species w as observed in t he m iddle est uary t hat w as lik ely at t ribut ed t o a wider range of w at er salinit y ( 3- 25.8 psu) inherent in t his st at ion as com pared t o t he low er est uary ( 14- 30 psu) and upper est uary ( 0.2- 10.5 psu) . Nev ert heless, about 63.4% ( 350 individuals) of t he adult est uarine gobies w ere collect ed from t he m iddle est uary suggest ing t he high t olerance of t his part icular dev elopm ent al st age t o a wide range of w at er salinit y in t his st at ion.
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
204
Table 2 Act ual count of fish across t he sam pling m ont hs grouped according t o lifecy cle cat egory . Minim um (Min) and m axim um ( Max ) st andard lengt h ( SL) as w ell as proportional abundance ( % ) of fish w ere also present ed for each t ax on ( each tax on w as assigned a specific num eric
code t hat w as used as a reference in Figures 3, 4 and 6)
SL ( m m ) Mont h Lifecy cle Fam ily Tax on Code
Min Max May Jun Jul Aug Sep Oct Nov Dec Mar Apr
Count %
Am phidrom ous 2 1 31 168 535 737 3.9
Clupeidae
Sicy opt erus j aponicus
8 26.3 26.3 1 1 0.0
Gobiidae
Gy m nogobius urot aenia
21 22.1 24 2 2 0.0
Lat eolabracidae
Lat eolabrax j aponicus
26 13.5 33.5 167 530 697 3.7
Plecoglossidae
Plecoglossus alt iv elis alt iv elis
31 5.7 17.4 31 1 5 37 0.2
Est uarine 2,331 607 332 157 226 140 15 63 213 2,288 6,372 33.5
Gobiidae
Acant hogobius flav im anus
11 10.4 83.8 245 13 9 1 2 1 2,231 2,502 13.2
Acant hogobius lact ipes
12 15 49.2 1 1 3 29 4 9 9 16 72 0.4
Eut aeniicht hy s gilli
13 2.8 35.1 2 1 96 1 1 101 0.5
Fav onigobius gy m nauchen
14 6 61 137 1 1 4 41 6 8 14 21 2 235 1.2
Gy m nogobius breunigii
16 10.5 54 1,156 574 317 48 153 120 2 2 2 2,374 12.5
Gy m nogobius m acrognat hos
18 11 39.6 245 3 3 13 10 274 1.4
Gy m nogobius uchidai
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
205
Pseudogobius m asago
22 5.6 21.3 3 1 8 12 0.1
Freshw at er 3 44 47 0.2
Cent rarchidae
Lepom is m acrochirus
4 14.2 35.5 3 44 47 0.2
Marine 189 10 89 9,559 158 4 17 1 3 16 10,046 52.9
At herinidae
Hy poat herina v alenciennei
1 6.4 104.7 0 0 78 16 94 0.5
Blenniidae
Om obranchus elegans
2 9.3 10.8 2 2 0.0
Carangidae
Carnx sex fasciat us
3 52.5 52.5 1 1 0.0
Clupeidae
Konosirus punct at us
6 7.5 44.2 12 6 6 116 140 0.7
Sardinella zunasi
7 8.2 16 85 2 9,210 1 9,298 48.9
Engraulidae
Engraulis j aponicus
10 15.6 39.8 8 31 39 0.2
Gobiidae
Gy m nogobius hept acant hus
17 14.5 25.5 83 4 1 3 1 92 0.5
Leiognat hidae
Nuchequula nuchalis
27 5.7 43.1 137 155 292 1.5
Mugilidae
Liza haem at ocheilus
28 14.4 14.4 1 1 0.0
Salangidae
Salangicht hy s ishikaw ae
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
206
Terapont idae
Terapon j arbua 34 17.7 22.7 3 3 0.0 Tet raodont idae
Takifugu niphobles
35 108.7 137.3 1 1 2 0.0
Unk now n 12 4 3 1,476 55 4 241 9 1,804 9.5
Clupeidae
Clupeidae spp. 5 4.6 12.5 5 342 347 1.8
Cy prinidae
Tribolodon spp. 9 21.6 78.6 1 4 5 0.0 Gobiidae
Gobiidae spp. 15 12.4 47.7 1 42 8 1 52 0.3
Gy m nogobius
spp.
19 27.2 27.2 1 1 0.0
Rhinogobius
spp.
23 4 14 6 1 7 0.0
Trident iger
spp.
24 5 14.4 2 1,086 39 3 1,130 5.9
Hem iram phidae
Hem iram phus
spp.
25 11.1 11.1 1 1 0.0
Mugilidae
Mugilidae spp. 29 65 36.1 5 3 156 8 172 0.9
Plat ycephidae
Plat y cephalus
spp.
30 11.5 14.7 3 3 0.0
Pleuronectidae
Kareius spp. 32 14.3 33.1 85 85 0.4 Triglidae
Chelidonicht hy s
spp.
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
207
Figur e 4. Nonm et ric m ult idim ensional scalin g for t he num er ical abundance of t he 36 fish t axa in relat ion t o t he 3 st at ions. The Bray–Curt is was t he dissim ilar it y index used t o generat e t he NMS. The bubble plot was proport ional t o t he average wat er salin it y recorded for each st at ion while t he
vect or ( arrow) was direct ed t owar ds t he st at ions wit h increasing gradient in wat er salinit y. Each num ber in t he plot corr esponds t o a code for a part icular t axon given in Table 2.
Figur e 5. Rényi’s diversit y prof ile of t he fish com m unit y in t he lower, m iddle and upper est uaries of Obit su- gawa River.
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
208
cont ent ion t hat est uaries are dom inat ed by icht hyofaunal species t hat dem ost rat e a wide t olerance lim it s t o t he fluct uat ing condit ions prev alent in t his sy st em ( Whit field 1999) .
Figur e 6. Rank abundance curve for t he proport ional abundance ( % ) of the fish t axa t hat were present in t he lower ( a) , m iddle ( b) and upper ( c) est uaries. Each num ber in t he plot corresponds
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
209
The abundance of m arine t eleost s decreased wit h increase dist ance upst ream . This group corresponded t o 76.3% ( 9,566 individuals) of t he t ot al cat ch in t he low er est uary , 15.7% ( 376 individuals) in t he m iddle est uary and 2.6% ( 104 individuals) in t he upper est uary ( Table 3) . This abundance pat t ern w as sim ilar t o t he large Sw an Est uary of t em perat e sout hw est ern Aust ralia w herein densit y of m arine fishes decreased wit h dist ance from t he m out h of t he est uary but cont rast ing t o som e degree because species richness in t he said sy st em also decreased wit h dist ance from t he riv er m out h ( Loneragan & Pot t er 1990) . I n t he current st udy how ev er, species richness of m arine fish did not changed m uch in t he low er est uary ( 7 fam ilies and 8 species) , m iddle est uary ( 7 fam ilies and 8 species) and upper est uary ( 7 fam ilies and 7 species) despit e t he m ark ed difference in w at er salinit y part icularly bet ween t he low er and upper est uaries. Ex cept for H. v alenciennei, count of m arine species in t he upper est uary w ere sparse and m ost lik ely w ere m arine st ragglers ( Elliot t et al 2007) . On t he ot her hand, a significant num ber of H. v alenciennei larv ae ( 7.5± 0.7 m m SL) w ere sam pled in t he upper est uary in July w hen t he w at er salinit y w as 0.2 psu w hile adult s ( 69.1± 16.4 m m SL) rem ained in t he low er est uary . Jut agat e et al ( 2009) described t his species as an opport unist ic m arine species t hat som et im es ent ered t he est uary for t he purpose of feeding and breeding. I t w as likely t hat t he larv al st age of t his species effect iv ely penet rat ed t he saline reaches of t he est uary for t he purpose of feeding and has a considerable t olerance t o t he oligohaline w at ers of t he upper est uary .
Alt hough act ual count of est uarine gobies did not change m uch in t he low er est uary ( 1,524 individuals) and m iddle est uary (1,362 individuals) as com pared t o t he upper est uary ( 3,486 individuals) ( Table 3) , it s proport ional cont ribut ion t o t he fish com m unit y increased considerably from 12.2% in low er est uary t o 57% in t he m iddle est uary and 85.5% in t he upper est uary . This observ at ion closely paralleled wit h t he result s in Sw an Est uary w herein t he great est densit ies of est uarine fish w ere recorded at sit es in t he m iddle and upper est uaries as com pared t o t he low er est uary ( Loneragan et al 1989) . Generally, species richness of t hese est uarine gobies did not changed across t he 3 st at ions w it h 7 species per st at ion. How ev er, t he scarcit y in t he act ual num ber of P. m asago and E. gilli in t his sit e can be at t ribut ed t o habit at preference of t hese est uarine gobies. Ok azaki et al ( 2011) collect ed a considerable num ber of P. m asago from t he t idepools on t he m udflat s in Tam a Riv er est uary of inner Tok y o Bay w it h dev elopm ent al st ages ranging from J1 t o adult suggest ing t he im port ance of t his habit at t o t he said species. The sam e can be said for E. gilli w herein a significant num ber of t he cat ch in t he low er est uary w ere predom inant ly plank t onic larvae and t here w as paucit y in t he act ual num ber of j uv enile and adult . This species w as in fact k now n t o inhabit t he est uarine t ide pools under st ones ( Masuda et al 1987) . Alt hough not show n here, sam ples collect ed from t he t idal creek and t idal pools of Obit su- gawa Riv er from July 2009 t o June 2010 at low t ide had found t hat E. gilli and P. m asago belong t o t he 5 m ost abundant species in t hese habit at s and t hat t hey form an im port ant part t he of t he fish com m unit y in t he t idal creek and soft sedim ent pools of Obit su- gaw a River Est uary .
Abundance of am phidrom ous fish w as prev alent in t he m iddle est uary ( Table 3) but species richness of t his group w as evident in t he upper est uary ( 4 species) as com pared t o t he m iddle est uary ( 2 species) and low er est uary (one species) . L. j aponicus
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
210
Table 3 Act ual count of t he 36 fish t axa in t he 3 st at ions grouped according t o lifecycle cat egory
Lifecycle Fam ily Taxon Lower Est uar y
Middle Est uar y
Upper Est uar y
Am phidr om ous 30 458 249
Clupeidae
Sicyopt er us j aponicus 1 Gobiidae
Gym nogobius ur ot aenia 2 Lat eolabr acidae
Lat eolabr ax j aponicus 452 245 Plecoglossidae
Plecoglossus alt ivelis alt ivelis 30 6 1
Est uar ine 1,524 1,362 3,486
Gobiidae
Acant hogobius flavim anus 52 284 2,166
Acant hogobius lact ipes 1 17 54
Eut aeniicht hys gilli 94 7
Favonigobius gym n auchen 144 33 58
Gym nogobius br eunigii 602 577 1,193
Gym nogobius m acr ognat hos 224 48 2
Gym nogobius uchidai 405 396 1
Pseudogobius m asago 12
Fr eshwat er 3 28 16
Cent r ar chidae
Lepom is m acr ochir us 3 28 16
Mar ine 9,566 376 104
At her inidae
Hypoat her ina valenciennei 16 78 Blenniidae
Om obr anchus elegans 1 1 Car angidae
Car nx sexfasciat us 1 Clupeidae
Konosir us punct at us 84 56
Sar dinella zunasi 9,207 91 Engr aulidae
Engr aulis j aponicus 17 8 14 Gobiidae
Gym nogobius hept acant hus 91 1 Leiognat hidae
Nuchequula nuch alis 131 158 3 Mugilidae
Liza haem at ocheilus 1 Salangidae
Salangicht hys ishikawae 19 60 3 Ter apont idae
Ter apon j ar bua 3
Tet r aodont idae
Takifugu niphobles 2
Unknow n 1,417 165 222
Clupeidae
Clupeidae spp. 346 1
Cypr inidae
Tr ibolodon spp. 1 4 Gobiidae
Gobiidae spp. 40 9 3
Gym nogobius spp. 1
Rhinogobius spp. 6 1
Tr ident iger spp. 1,023 63 44 Hem ir am phidae
Hem ir am phus spp. 1 Mugilidae
Mugilidae spp. 2 5 165
Plat ycephidae
Plat ycephalus spp. 3
Pleur onect idae
Kar eius spp. 85 Tr iglidae
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
211
Count of freshw at er species w as evident in t he m iddle est uary ( Table 3) . Generally, freshw at er fish is not capable of osm oregulat ion in high saline w at ers and are only present in est uaries w hen salinit ies decline t o v ery low lev els during t he periods of heav y freshw at er discharge ( Pot t er & Hy ndes 1999) . This w as m ost lik ely t he case for L. m acrochirus w hose abundance w as conspicuous in t he m iddle est uary in August w hen t his st at ion ex perienced low salinit y of 3 psu. This species m ost likely belong t o t he freshw at er st raggler cat egory because of it s brief occurrence at cert ain period w hen t he condit ions in t he est uary w as fav orable for t he said species ( Whit field 1999) .
The dev elopm ent al st age of gobies ranges from larv ae t o adult but t he j uv enile cont ribut ed a subst ant ial port ion ( 77.06% ) of t he sam ple. J2 corresponded t o 29.86% w hile J1 and J3 represent 26.18% and 21.06% of t he j uv eniles, respect iv ely. Specifically, t he j uv eniles of est uarine gobies w ere abundant from April t o July wit h abundance from 201 t o 1,995 individuals and w ere coherent w it h increasing w at er t em perat ure t hat w ould likely prom pt ed t he enhance product ivit y of t he est uary as w ell as grow t h of t he y oung fish ( Whit field 1999) . The presence of large num ber of j uv eniles lend support t o t he previous st udies t hat t he est uarine fish assem blage w as essent ially high in abundance part icularly for j uv enile fishes ( Whit field 1999; Ram os et al 2006) and t he est uarine t idelands of inner Tok y o Bay funct ion as an im port ant nursery habit at for som e of t he local fishes ( Kanou et al 2000, 2005b; Ok azak i et al 2011) . F. gy m nauchen, G. breunigii
and G. m acrognat hos can be furt her cat egorized as est uarine resident s because of t heir abilit y t o com plet e t heir lifecy cle wit hin t he est uary ( Whit field 1999) . On t he ot her hand, larv ae w ere not found for A. flav im anus, A. lact ipes and G. uchidai as w ell as J1 for A. lact ipes and w ere lik ely est uarine m igrant s because t he m arine larv al st age and t o a cert ain ex t ent , t he J1 st age of t hese species w ere lik ely found in t he adj acent aquat ic habit at s (Whit field 1999) .
The size of A. flav im anus increased wit h increase w at er t em perat ure ( Table 4; Figure 7) . A subst ant ial num ber of J1 w as ev ident in April w hile J2 and J3 in May . Adult on t he ot her hand w as prom inent from May t o July ( Table 5) . Size w as also found t o v ary w it h t he st at ions and w hile sm all individuals (e.g., J1) w ere prev alent in t he upper est uary , t he large individuals ( e.g., J2, J3 and adult ) w ere prom inent in t he m iddle est uary suggest ing a shift from low saline w at ers t o relat iv ely high saline w at ers w it h grow t h ( Table 6) . The size dist ribut ion of t his species can be relat ed t o t he different ial food preferences w it h grow t h ( Kanou et al 2004a) . Juv eniles wit h m ean SL of 13.5± 1.87 m m w ere k now n t o inhabit t he upper Chik ugo Est uary in Ariak e Bay , Japan charact erized by low salinit y ( 0.37- 3.1 psu) w it h st rong diet ary relat ionship on a oligohaline copepod,
Sinocalanus sinensis ( Poppe, 1889) , w hich w as an im port ant diet responsible for it upst ream dist ribut ion of t his species in spring ( I slam et al 2006) . I n t he current st udy , a subst ant ial num ber of set t ling J1 ( 14± 1.4 m m SL) w ere conspicuous in t he upper est uary in April and t hese individuals m ost lik ely subsist ed on oligohaline copepods in t his st at ion w hile t he larger J1 ( 14.5± 0.7 m m SL) m igrat ed dow nst ream w it h shift in t he diet for epiphyt ic crust aceans and poly chaet es in w hich according t o Kanou et al ( 2004a) , w ere an im port ant part of it s diet present in t he t idal m udflat s.
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
212
St at ion- w at er t em perat ure int eract ion best described t he v ariat ion in size of F. gy m nauchen ( Table 4) . While size decreased wit h increase w at er t em perat ure in t he upper est uary , t here w as an increase in size wit h increase in w at er t em perat ure in t he m iddle est uary ( Figure 7) . The upper est uary w as in fact predom inant ly inhabit ed by bot h larv ae and J1 in Sept em ber w it h t he occasional occurrence of J2 from Oct ober t o Nov em ber and J3 in Nov em ber and March. This species m ost lik ely m ov ed dow nst ream w hen t hey reached J3 st age part icularly around Decem ber and inhabit t o t he m iddle est uary w hen t hey reached t he adult st age part icularly around April and May ( Tables 5 and 6) w here t hey lik ely subsist ed on m y sids and det rit us prev alent in t he est uarine m udflat s ( Kanou et al 2004a) . Moreov er, I nui et al ( 2010) found t hat t he abundance of F. gy m nauchen in t he surf zones of nort hw est ern Ky ushu I sland, Japan responded negat iv ely t o current v elocit y and it s abundance w as show n t o decrease wit h increase dept h in t he surf zone suggest ing t he preference of t his species for shelving and calm w at er condit ions.
St at ion and w at er t em perat ure best ex plained t he v ariat ion in t he size of G. breunigii ( Table 4) . Generally , t he size decreased w it h increase in w at er t em perat ure ( Figure 7) . St ages from larv ae t o J2 w ere prev alent in May w hen t he w at er t em perat ure w as increasing w hile J3 w as present from June t o August w hen high t em perat ure w at ers w ere evident in t his sy st em . Adult on t he ot her hand w as prom inent from Sept em ber t o Oct ober w hen t he w at er t em perat ure w as decreasing ( Table 5) . Spat ial v ariat ion in size w as also evident wit h large individuals prev alent in t he m iddle and upper est uaries w hile sm all individuals in t he low er est uary suggest ing habit at shift from high saline w at ers t o low saline w at ers wit h grow t h. The larv ae and J1 w ere in fact prev alent in t he low er est uary w hile abundances of J2 t o J3 increased from t he m iddle est uary t o upper est uary. Adult s on t he ot her hand w ere prev alent in t he upper est uary ( Table 6) . The predom inance of larv ae and J1 st ages in t he low er est uary can be at t ribut ed t o t he abundance of cladocerans and plank t onic copepods in t he t idal m udflat s in w hich t his species fed ( Kanou et al 2004a) . On t he ot her hand, t he preference for t he m iddle and upper est uaries by t he lat er st ages can be at t ribut ed t o t he preference of t hese st ages t o shelving and calm w at er condit ions ( I nui et al 2010) .
Variat ion in t he size of G. m acrognat hos w as best ex plained by st at ion- w at er t em perat ure int eract ion ( Table 4) . Alt hough sm all individuals w ere prom inent in t he lower est uary and large individuals in t he m iddle est uary , t he size decreased subst ant ially wit h increase in w at er t em perat ure ( Figure 7) . J1 and J2 w ere prev alent in t he low er est uary in May w it h t he occasional presence of J3 in Decem ber. Adult w as prev alent in t he m iddle est uary from March t o May t oget her wit h J3 in April as w ell as J1 and J2 in May ( Tables 5 and 6) . Nev ert heless, t his pat t ern in size dist ribut ion indicat ed an inw ard m ov em ent wit h grow t h but at a v ery narrow habit at range from t he low er est uary t o t he m iddle est uary. According t o Kanou et al ( 2004a) , t he J1 subsist ed on calanoid and cy clopoid copepods w hile J2 t o adult s shift ed t heir diet t o errant poly chaet es, podocopid ost racods and harpact icoid copepods, w hich w ere prey it em s com m only found in t he est uarine m udflat s.
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
213
Table 4 AI C scores for different com binat ion of cov ariat es for t he st andard lengt h of select ed est uarine gobies. The cov ariat e or cov ariat e com binat ion wit h t he lowest AI C score ( * )
w as considered t he m ost appropriat e m odel t hat ex plained t he v ariat ion in size for a part icular gobiid species
Species Model Cov ariat es AI C Score Dist ribut ion fam ily Acant hogobius
flav im anus
1 Tem perat ure 3558.1 Gam m a
2 St at ion 3851.9
3 Tem perat ure
+ St at ion
3351.3*
Acant hogobius lact ipes 1 Tem perat ure 427.6 Gam m a
2 St at ion 435.3
3 Tem perat ure
+ St at ion
428.6
4 Tem perat ure: St at ion 426.1*
Fav onigobius gy m nauchen
1 Tem perat ure 657.1 Gam m a
2 St at ion 603.9
3 Tem perat ure
+ St at ion
605.4
4 Tem perat ure: St at ion 572.9*
Gy m nogobius breunigii 1 Tem perat ure 9336.7 Gaussian
2 St at ion 8413
3 Tem perat ure
+ St at ion
8384.4*
4 Tem perat ure: St at ion 8602
Gy m nogobius m acrognat hos
1 Tem perat ure 3443.6 Gam m a
2 St at ion 3011.7
3 Tem perat ure+ St at io n
2956.6
4 Tem perat ure: St at ion 2949.8*
Gy m nogobius uchidai 1 Tem perat ure 5925.3 Gam m a
2 St at ion 4437.1
3 Tem perat ure
+ St at ion
4127.8
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
214
a. Acant hogobius flav im anus b. Acant hogobius lact ipes c. Fav onigobius gy m nauchen
d. Gy m nogobius breunigii e. Gy m nogobius m acrognat hos f. Gy m nogobius uchidai
Figur e 7. St andard lengt h of est uarine gobies based on gradient s in wat er t em perat ur e in t he low er ( * ) , m iddle ( ° ) and upper (◊) estuaries. A line was drawn for t he low er ( solid line) , m iddle ( long dash) and upper ( dot t ed lin e) est uar ies t hat corresponded t o t he predict ed response of SL t o gradient s in
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
215
Table 5 Act ual count and m ean st andard lengt h ( SL) for each dev elopm ent al st age of goby in Obit su- gaw a Riv er Est uary wit hin t he 10- m ont h
sam pling period
Taxon St age Mar Apr May Jun Jul Aug Sep Oct Nov Dec
Count SL Count SL Count SL Count SL Count SL Count SL Count SL Count SL Count SL Count SL
A. flav im anus
J1 386 14.3 53 17.9 1 18.1
J2 169 26.5 7 26.8 1 31.8
J3 21 37.1 2 37
Adult 5 42.6 5 48.8 6 56.6 2 83.6 1 72.4
A. lact ipes
J2 10 16.7 3 18.9
J3 9 26.5 7 32.1 1 33 1 31.1 1 20.5 1 23.8 9 25.2
Adult 5 38.5 11 40.7 1 45.2 1 49
C. hept acant hus
Unknown 81 18.5 1 18.1
E. gilli
Larvae 90 7 1 5.5
J1 1 13.7 1 11.1
J2 2 13.5
J3 1 26.3
Adult 1 35.1 1 34.5
F. gym nauchen
Larvae 4 7.4 9 9.1
J1 14 11.3 1 12.5 3 13.3
J2 1 18.2 3 17.9 1 15 4 17.1
J3 6 27.5 1 30.8 6 28.4 6 26.2
Adult 1 43.8 4 47.6 12 51.8 1 61 1 57.4 1 50 1 45.8
Gobiidae spp.
Unknown 2 13 2 37.2
G. breunigii
Larvae 42 13.7
J1 129 17.1 5 19.3
J2 349 25.4 91 26 55 27.5 1 29.9
J3 1 36.4 75 31.1 305 34.2 142 34.2 37 36.4 97 36.7 22 37.6
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
216
G. m acrognat hos
Larvae 1 14.4 101 14.1
J1 502 16.5
J2 29 20.7
J3 5 29.4 2 29 2 27.8
Adult 13 34.4 13 32.6 11 32.5 1 32.4
G. uchidae
J1 44 14.4
J2 555 16.4 15 16.4 2 19.2 1 20
J3 41 23.8 19 24.2 66 24.1 1 20.1 1 22.9 4 23.7 21 23.4
Adult 125 27.1 45 26.7 98 26.6 1 25.9 7 25.8
P. m asago
Larvae 1 5.6
J2 1 16
J3 2 18.4
Adult 1 21.3
Rhinogobius
spp.
Larvae 6 8.4
Unknown 1 14
S. j aponicus
Unknown 1 26.6
Trident iger spp.
Larvae 2 9.1 62 8.2
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
217
Table 6 Act ual count and m ean st andard lengt h ( SL) for each dev elopm ent al st age of goby in t he
t he 3 st at ions of Obit su- gaw a Riv er Est uary wit hin t he 10- m ont h sam pling period
Taxon St age Low er est uar y Middle est uar y Upper est uar y Count SL Count SL Count SL A. flav im anus
J1 6 18.1 203 14.9 231 14.5
J2 39 27.2 92 25.8 46 27.4
J3 4 36.1 13 36.8 6 38.6
Adult 2 48.6 13 51.7 4 66.6
A. lact ipes
J2 9 17.7 4 16.2
J3 2 27.4 11 27.4 16 27.6
Adult 5 43.3 13 39.8
C. hept acant hus
Unknow n 81 18.5 1 18.1
E. gilli
Lar vae 89 7.1 2 5.6
J1 2 12.4
J2 2 13.5
J3 1 26.3
Adult 1 34.5 1 35.1
F. gym nauchen
Lar vae 3 7.3 1 7.5 9 9.1
J1 1 13 1 14.8 16 11.4
J2 4 17.1 1 18.2 4 17.2
J3 3 27.5 7 26.3 9 28.5
Adult 1 50 20 51
Gobiidae spp.
Unknow n 4 25.1
G. br eunigii
Lar vae 41 13.8 1 12.6
J1 114 16.9 9 18.6 11 19
J2 44 22.2 189 25.7 263 26.4
J3 9 34.9 200 34.1 470 34.6
Adult 1 41.9 6 45.9 170 42.7
G. m acr ognat hos
Lar vae 101 14.1 1 14.4
J1 491 16.5 10 18.5 1 15.5
J2 24 20.6 5 21.4
J3 3 29.1 6 28.9
Adult 2 30.6 36 33.3
G. uchidae
J1 44 14.4
J2 554 16.4 19 16.6
J3 14 23.6 138 24 1 22.5
Adult 7 26 269 26.8
P. m asago
Lar vae 1 5.6
J2 1 16
J3 2 18.4
Adult 1 21.3
Rhinogobius spp.
Lar vae 6 8.4
Unknow n 1 14
S. j aponicus
Unknow n 1 26.6
Tr ident iger spp.
Lar vae 62 8.2
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
218
Con clusion s. High species richness and incidence of peak in fish abundance in Obit su-gaw a Riv er Est uary w as realized in m ont hs w hen t he w at er t em perat ure w as increasing w hile m ont hs wit h v ery low t em perat ure coupled w it h low salinit y m ost lik ely lim it s fish colonizat ion in t his syst em . Species dom inance w as a coherent feat ure of t he fish com m unit y in t his est uary wit h m arine species dom inat ed t he low er est uary w hile est uarine gobies in t he m iddle and upper est uaries. The proport ional cont ribut ion of m arine t eleost s t o t he fish com m unit y decreased w it h increase dist ance upst ream w hile t hat of est uarine fishes increased wit h increase dist ance upst ream .
Marine t eleost s cont ribut ed great ly t o t he species richness and abundance of fish in t his syst em and w ere prev alent in May , July , August and Sept em ber em phasizing t he t endency of t his group t o aggregat e in t his sy st em during t he w arm m ont hs. Of part icular int erest w as t he rem ark able num ber of S. zunasi in August t hat w as in accordance wit h t he spaw ning season of t his species. I t w as likely t hat a considerable num ber of larv ae of m arine t eleost s w ere at t ract ed t o t his sy st em im m ediat ely following t he spaw ning ev ent t hat t ook place in t he adj acent euhaline w at ers and t hat t he est uary in Obit su- gaw a serv ed as a t ransient habit at for som e m arine species part icularly during t he w arm m ont hs. The reliance of m arine t eleost s in t his est uary can be cat egorized as est uarine opport unist s if t hey w ere present in subst ant ial num ber alt hough only for a brief period or m arine st ragglers because t hey w ere v ery few but nev ert heless cont ribut ed considerably t o t he species richness in t his sy st em .
The presence of am phidrom ous fish, L. j aponicus, w as coherent wit h t he spaw ning period of t his species and it s occurrence in t he est uary bet w een March and April m ost likely signaled t he com m encem ent of larv al m igrat ion t ow ards t he est uary and furt her upst ream . I n t he case of t he freshw at er fish, L. m acrochirus, it s presence in t he low er reaches of t he riv er w as at t ribut ed t o periods w hen w at er salinit y decrease t o v ery low lev el giv en t he fact t hat freshw at er fish in general is incapable of osm oregulat ion in high saline w at ers.
Est uarine fishes w ere t he second m ost abundant group t hat w as prim arily represent ed by species under fam ily Gobiidae. Gobies had t he m ost num ber of t ax a in t his sy st em m ost lik ely because of t heir abilit y t o inhabit a wide range of env ironm ent s because of t heir resilience t o fluct uat ing environm ent al gradient s in t his sy st em . Juv enile recruit m ent w as prom inent from March t o Sept em ber but m ost im port ant ly in April and May m ost likely due t o t he high product ivit y of t his sy st em during t his period and t he prev alence of w arm w at ers t hat can prom ot e t em perat ure- m ediat ed grow t h for t he y oung fish. The dev elopm ent al st ages of gobies present in t his sy st em range from larv ae t o adult but t he preponderance of j uv eniles suggest t he im port ance of t his syst em as a nursery habit at for t he est uarine gobies especially during t he j uv enile st age. F. gy m nauchen, G. breunigii and G. m acrognat hos can be furt her classified as est uarine resident s because of t heir abilit y t o com plet e t heir lifecy cle wit hin t his est uary w hile A. lact ipes w as lik ely an est uarine m igrant considering t hat t he larv al st age of t his species w as not found in t his sy st em . The dist ribut ion of dev elopm ent al st ages of est uarine gobies w ere coherent wit h t he m ont hly w at er t em perat ure and st at ion or t he int eract ion of bot h. I n general, t he size dist ribut ion of gobies across m ont hs eit her increased ( e.g.,
A. flav im anus and A. lact ipes) or decreased ( e.g., G. breunigii, G. m acrognat hos, F. gy m nauchen and G. uchidai) w it h increase in m ont hly w at er t em perat ure. Alt hough gradient in w at er salinit y increased from a freshw at er- dom inat ed- ( upper est uary ) t ow ards t he seaw at er- dom inat ed ( m iddle and low er est uaries) st at ions and t here w as a m ark v ariat ion in size of est uarine gobies across t he st at ions, it w as lik ely t hat t he size dist ribut ion of t hese est uarine gobies cannot be ex plained by w at er salinit y alone but can be at t ribut ed t o ot her relev ant fact ors such as change in prey it em s wit h grow t h as w ell as t he preference for a part icular est uarine habit at . Nev ert heless, t he dist ribut ion of t hese est uarine gobies eit her inv olv e m ov em ent from v ery low saline- ( upper est uary ) t o relat iv ely high saline ( m iddle est uary ) w at ers ( e.g., A. flav im anus and F. gy m nauchen) , m ov em ent from high saline- (low er est uary ) t o relat iv ely high saline w at ers ( e.g., G. m acrognat hos and G. uchidai) , m ov em ent fr om r elat iv ely high saline- t o v ery low saline w at ers ( e.g., A. lact ipes) and m ov em ent from high saline- t o v ery low saline w at ers ( e.g.,
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
219
est uary reflect ing t heir high t olerance t o a wide range of w at er salinit y charact erist ic in t his st at ion but nevert heless av oided t he low er est uary m ost likely due t o t he prev alence of high salinit y w at ers in t his st at ion.
Ack n ow le dge m e n t s. Grat it ude is ex pressed t o t he anony m ous referees for t heir const ruct ive com m ent s on t he m anuscript . This research w as support ed by t he Minist ry of Educat ion, Cult ure, Sport s, Science and Technology of Japan t hrough a St udent Research Scholarship grant ( Monbuk agak usho: MEXT) aw arded t o t he m ain aut hor.
Re fe r en ce s
Ak aik e H., 1981 Lik elihood of a m odel and inform at ion crit eria. Journal of Econom et rics 16: 3–14.
Ak ihisa I ., Seiichi H., 2005 Env ironm ent al assessm ent of t he Shim ant o Est uary based on biodiv ersit y of Gobioid fishes. Aquabiology 27: 39–46.
Boum an H. A., Nak ane T., Ok a K., Nak at a K., Kurit a K., Sat hy endranat h S., Plat t T., 2010 Env ironm ent al cont rols on phy t oplank t on product ion in coast al ecosy st em s: A case st udy from Tok y o Bay . Est uarine, Coast al and Shelf Science 87: 63–72.
Burnham K. P., Anderson D. R., 2004 Mult im odel inference underst anding AI C and BI C in m odel select ion. Sociological Met hods & Research 33: 261–304.
Cat t rij sse A., Mak w aia E. S., Dank w a H. R., Ham erly nck O., Hem m inga M. A., 1994 Nek t on com m unit ies of an int ert idal creek of a European est uarine brackish m arsh. Marine Ecology Progress Series 109: 195–208.
Claridge P. N., Pot t er I . C., Hardist y M. W., 1986 Seasonal changes in m ov em ent s, abundance, size com posit ion and div ersit y of t he Fish Fauna of t he Sev ern Est uary . Journal of t he Marine Biological Associat ion of t he Unit ed Kingdom 66: 229–258. Craw ley M. J., 2007 The R Book ( 1st ed) . John Wiley & Sons Lt d, The At rium , Sout hern
Gat e, Chichest er, West Sussex PO19 8SQ, England. I SBN- 13: 978- 0- 470- 51024- 7. 942 p.
Dix on P., 2003 VEGAN, a pack age of R funct ions for com m unit y ecology. Journal of Veget at ion Science 14: 927–930.
Elliot t M., Whit field A. K., Pot t er I . C., Blaber S. J. M., Cy rus D. P., Nordlie F. G., Harrison T. D., 2007 The guild approach t o cat egorizing est uarine fish assem blages: a global rev iew . Fish and Fisheries 8: 241–268.
Ellison A. M., 2004 Bay esian inference in ecology. Ecology Let t ers 7: 509–520.
Em e J., Bennet t W. A., 2009 Acut e t em perat ure quot ient responses of fishes reflect t heir divergent t herm al habit at s in t he Banda Sea, Sulaw esi, I ndonesia. Aust ralian Journal of Zoology 57: 357–362.
Fonds M., Van Buurt G., 1974 The influence of t em perat ure and salinit y on dev elopm ent and surv iv al of goby eggs ( Pisces, Gobiidae) . Aquat ic Ecology 8: 110–116.
Fuj it a S., Kinoshit a I ., Tak ahashi I ., Azum a K., 1988 Seasonal occurrence and food habit s of larv ae and j uv eniles of t w o t em perat e basses in t he Shim ant o est uary , Japan. I cht hy ological Research 35: 365–370.
Gill H., Wise B., Pot t er I ., Chaplin J., 1996 Biannual spaw ning periods and result ant divergent pat t erns of grow t h in t he est uarine goby Pseudogobius olorum: t em perat ure-induced? Marine Biology 125: 453–466.
Gill H. S., Pot t er I . C., 1993 Spat ial segregat ion am ongst goby species wit hin an Aust ralian est uary , wit h a com parison of t he diet s and salinit y t olerance of t he t w o m ost abundant species. Marine Biology 117: 515–526.
Ham ada R., Kinoshit a I ., 1988 Feeding habit of larv al and j uv enile ay u, Plecoglossus alt iv elis in t he surf zone of Tosa Bay , Japan. I cht hy ological Research 35: 382–388. Holl K. D., Crone E. E., Schult z C. B., 2003 Landscape rest orat ion: m ov ing from
generalit ies t o m et hodologies. BioScience 53: 491–502.
AACL Bi oflux , 2012, Vol um e 5, I ssue 4.
ht t p: / / w w w .bi ofl ux .com .ro/ aacl
220
I slam M., Hibino M., Tanak a M., 2006 Dist ribut ion and diet s of larv al and j uvenile fishes: I nfluence of salinit y gradient and t urbidit y m axim um in a t em perat e est uary in upper Ariak e Bay , Japan. Est uarine, Coast al and Shelf Science 68: 62–74.
I slam M. S., Tanak a M., 2005 Nut rit ional condit ion, st arv at ion st at us and growt h of early j uv enile Japanese sea bass (Lat eolabrax j aponicus) relat ed t o prey dist ribut ion and feeding in t he nursery ground. Journal of Ex perim ent al Marine Biology and Ecology 323: 172–183.
Jut agat e T., Saw usdee A., Chaidee T. T., Thongk hoa S., Chot ipunt u P., 2009 Fish in t he Pak Panang Bay and Riv er in relat ion t o t he ant i- salt dam operat ion, Part I : Assem blage pat t erns of t he m arine and brackish w at er fish. I n: 47t h Kaset sart Univ ersit y Annual Conference, Thailand, 17- 20 March 2009. pp. 120–131.
Kanou K., Kazunori A., Hit oshi I ., Ken K., Tet su K., Hiroshi K., 2002 Seasonal and spat ial changes in t he larv al and j uv enile fish fauna in surface w at ers of Tok y o Bay , cent ral Japan. Mer 40: 11–27.
Kanou K., Koik e T., Kohno H., 2000 I cht hy ofauna of t idelands in t he inner Tok y o Bay , and it s diversit y. Japanese Journal of I cht hy ology 47: 115–129.
Kanou K., Sano M., Kohno H., 2004a Food habit s of fishes on unv eget at ed t idal m udflat s in Tok y o Bay , cent ral Japan. Fisheries Science 70: 978–987.
Kanou K., Sano M., Kohno H., 2004b A net design for est im at ing t he v ert ical dist ribution of larv al and j uv enile fishes on a t idal m udflat . Fisheries Science 70: 713–715.
Kanou K., Sano M., Kohno H., 2005a Ont ogenet ic diet shift , feeding rhy t hm , and daily rat ion of j uv enile y ellow fin goby Acant hogobius flav im anus on a t idal m udflat in t he Tam a Riv er est uary, cent ral Japan. I cht hy ological Research 52: 319–324.
Kanou K., Sano M., Kohno H., 2005b Larv al and j uv enile fishes occurring wit h flood t ides on an int ert idal m udflat in t he Tam a Riv er est uary , cent ral Japan. I cht hy ological Research 52: 158–164.
Kanou K., Sano M., Kohno H., 2007 Relat ionships bet w een short - t erm v ariat ions in densit y of j uv enile y ellow fin goby Acant hogobius flav im anus and env ironm ent al v ariables on an est uarine m udflat . Fisheries Science 73: 38–45.
Kanou K., Tet su K., Koichi S., Hiroshi K., 1999 Larv ae and j uv eniles of tw o gobiids,
Chaenogobius uchidai and C. m acrognat hos, collect ed from t idelands in t he inner bay of Tok y o Bay . Mer 37: 59–68.
Kendall Jr A., Ahlst rom E. H., Moser H. G., 1984 Early life hist ory st ages of fishes and t heir charact ers. Ont ogeny and Sy st em at ics of Fishes 1: 11–22.
Kensak u A., Hiroy uki H., I zum i K., 2003 I nfluence by precipit at ion t o spat ial dispersion of ay u larv ae sw ept dow nst ream t o sea. Nippon Suisan Gak k aishi 69: 352–358.
Kindt Coe R., 2005 Tree Div ersit y Analy sis: A m anual and soft w are for com m on st at ist ical m et hods for ecological and biodiv ersit y st udies. World Agroforest ry Cent re ( I CRAF) . I SBN: 92- 9059- 179 X. 196 p.
Kneib R. T., 1997 The role of t idal m arshes in t he ecology of est uarine nekt on. Oceanography and Marine Biology an Anual Review 35: 163–220.
Kneib R. T., Wagner S. L., 1994 Nek t on use of v eget at ed m arsh habit at s at different st ages of t idal inundat ion. Marine Ecology Progress Series 106: 227–238.
Kohara M., Kohno H., 1999 Fish larv ae and j uv eniles collect ed by larv a- net in t he inner Tok y o Bay . Mer 37: 121–130.
Krück N. C., Chargulaf C. A., Saint - Paul U., Tibbet t s I . R., 2009 Early post - set t lem ent habit at and diet shift s and t he nursery funct ion of t idepools during Sillago spp. recruit m ent in Moret on Bay , Aust ralia. Marine Ecology Progress Series 384: 207–219. Laffaile P., Feunt eun E., Lefeuv re J. C., 2000 Com posit ion of fish com m unit ies in an
European m acr ot idal salt m arsh ( t he Mont Saint - Michel Bay , France) . Est uarine, Coast al and Shelf Science 51: 429–438.
Lenant on R., Pot t er I ., 1987 Cont ribut ion of est uaries t o com m ercial fisheries in t em perat e West ern Aust ralia and t he concept of est uarine dependence. Est uaries and Coast s 10: 28–35.