with Chronic Rhinosinusitis in a Chinese Population: A
Replication Study
Yuan Zhang1,2, Leandra Mfuna Endam3, Abdelali Filali-Mouhim3, Liping Zhao2, Martin Desrosiers3,4*, Demin Han1,2*, Luo Zhang1,2*
1Department of Otolaryngology Head and Neck Surgery, Beijing TongRen Hospital, Capital Medical University, Beijing, People’s Republic of China,2Key Laboratory Otolaryngology Head and Neck Surgery, Ministry of Education of China, Beijing Institute of Otolaryngology, Beijing, People’s Republic of China,3Department of Otolaryngology, Centre de Recherche du Centre Hospitalier de l’Universite´ de Montre´al (CRCHUM), Montreal, Quebec, Canada,4Department of Otolaryngology-Head and Neck Surgery, Montreal General Hospital, McGill University, Montreal, Quebec, Canada
Abstract
Background: The development of CRS is believed to be the result of combined interactions between the genetic background of the affected subject and environmental factors.
Objectives:To replicate and extend our recent findings from genetic association studies in chronic rhinosinusitis (CRS) performed in a Canadian Caucasian population in a Chinese population.
Methods:In a case-control replication study, DNA samples were obtained from CRS with (n = 306; CRSwNP) and without (n = 332; CRSsNP) nasal polyps, and controls (n = 315) in a Chinese population. A total of forty-nine single nucleotide polymorphisms (SNPs) selected from previous identified SNPs associated with CRS in Canadian population, and SNPs from the CHB HapMap dataset were individually genotyped.
Results: We identified two SNPs respectively in RYBP (rs4532099, p = 2.15E–06, OR = 2.59) and AOAH (rs4504543, p = 0.0001152, OR = 0.58) significantly associated with whole CRS cohort. Subgroup analysis for the presence of nasal polyps (CRSwNP and CRSsNP) displayed significant association in CRSwNP cohorts regarding to one SNP in RYBP (P= 3.24E–006,
OR = 2.76). Evidence of association in the CRSsNP groups in terms of 2 SNPs (AOAH_rs4504543 and RYBP_rs4532099) was detected as well. Stratifying analysis by gender demonstrated that none of the selected SNPs were associated with CRSwNP as well as CRSsNP. Meanwhile 3 SNPs (IL1A_rs17561, P = 0.005778; IL1A_rs1800587, P = 0.009561; IRAK4_rs4251513, P = 0.03837) were associated with serum total IgE level.
Conclusions: These genes are biologically plausible, with roles in regulation of transcription (RYBP) and inflammatory response (AOAH). The present data suggests the potential common genetic basis in the development of CRS in Chinese and Caucasian population.
Citation:Zhang Y, Endam LM, Filali-Mouhim A, Zhao L, Desrosiers M, et al. (2012) Polymorphisms in RYBP and AOAH Genes Are Associated with Chronic Rhinosinusitis in a Chinese Population: A Replication Study. PLoS ONE 7(6): e39247. doi:10.1371/journal.pone.0039247
Editor:Luzia Helena Carvalho, Centro de Pesquisa Rene Rachou/Fundac¸a˜o Oswaldo Cruz (Fiocruz-Minas), Brazil ReceivedFebruary 9, 2012;AcceptedMay 18, 2012;PublishedJune 19, 2012
Copyright:ß2012 Zhang et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Funding:This work was supported by grants from the National Science Fund for Distinguished Young Scholars (81025007), the National Natural Science Foundation of China (30973282 and 81100706), the Beijing Natural Science Foundation (7102030), the Special Fund of Sanitation Elite Reconstruction of Beijing (2009-2-007), and Beijing Nova Program (2010B022) to LZ and YZ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Competing Interests:The authors declare that co-author Luo Zhang is a PLoS ONE Editorial Board member. This does not alter the authors’ adherence to all the PLoS ONE policies on sharing data and materials. All other authors declare that no competing interests exist.
* E-mail: dr.luozhang@gmail.com (LZ); handm@trhos.com (DH); desrosiers_martin@hotmail.com (MD)
Introduction
Chronic rhinosinusitis (CRS) is a common inflammatory disorder of the sinus and paranasal sinus mucosa with a highly heterogeneous pathogenesis. Because of its negative impact on patients’ quality of life and the concomitant increasing social economic burden, CRS has become a global health problem [1]. The development of CRS is believed to be the result of combinations between the genetic background of the affected subject and environmental factors [1,2]. However, there is still no
clear answer as to their exact contributions to the process and the mechanisms of pathogenesis of CRS.
(Ring1A and YY1 binding protein, RYBP) [12]_ENREF_13, inflammatory response (acyloxyacyl hydroxylase, AOAH) [13] and innate immune response (IL1RL1 [5,14] and interleukin-1 receptor-associated kinase 4 (IRAK4) [6,15]), are associated with chronic rhinosinusitis (CRS) in a Canadian Caucasian population [16]. Replication of results of a genetic disease association study in independent samples has emerged as a standard for demonstrating the relevance of a candidate gene for a complex trait.
Given the evidence above, genetic backgroung plays potencial roles in the development of CRS and we hypothesized genes which were demontrated as susceptible genes for CRS in Caucasian population also exerted effects in Chinese cohort. Therefore, the aim of this study was to replicate polymorphisms in the genes performed in Canadian Caucasian population previously are associated with the Chinese population. A population-based case-control association analysis was used to assess the risk of CRS conferred by SNPs in the candidate genes in our Han Chinese cohort.
Materials and Methods
Study Subjects
306 CRS with nasal polyps (CRSwNP subjects) (180 males and 126 females) and 332 individuals affected with CRS without nasal polyps (CRSsNP) (190 males and 142 females) were prospectively recruited from the rhinology ward of Beijing Tongren Hospital between February 2008 to July 2009. A total of 315 healthy controls, of which 146 (46.3%) were female, were recruited as well. All the subjects were of Chinese Han ethnic origin and all from the north region of China. The study was approved by the Beijing Tongren Hospital Ethics Committee, and written informed consent was obtained from all participants.
Diagnosis of CRSwNP and CRSsNP was based on American Academy of Otolaryngology-Head and Neck Surgery (AAO-HNS) 2004 guidelines [17], based on assement by a single ENT doctor specialized in sinus diseases. All the CRS cases recruited in present study were unresponsive to all forms of medical therapies such as topical or intranasal corticosteroid and long term-low dose antibiotic or presented persistent signs/symptoms of CRS despite previous endoscopic sinus surgery (ESS). Patients were interviewed by trained personnel, and a standardized questionnaire was used to obtain items including demographic variables and personal and familial antecedents of allergies. Patients also underwent a standard set of laboratory tests that included measurements of total IgE to assess allergic status. Controls were recruited following two strategies: either spouses or non-blood relatives living in the same household and individuals recruited from a geographic area similar to that of CRS patients. The only attempt at matching subjects and control is their geographical location to minimize differences secondary to differences in potential environmental exposures. Nevertheless, a standardized questionnaire assessing age, sex and ethnic origin (but not smoking, history of atopy or physician diagnosed asthma) was obtained for controls. Moreover, all the controls showed negative of serum phadiatop determination.
SNP Selection
A total of forty-one single nucleotide polymorphisms (SNPs) selected from previous identified associated with CRS in Canadian population [3,5–7,9,10,16,18,19] were choose for genotying (Table 1). In addition, SNPs in IRAK4 gene from the CHB HapMap dataset were also individually genotyped. Briefly, the International Haplotype Mapping (HapMap) (www.hapmap.org) SNP databases were used to select SNPs in the IRAK4 gene region. The screened region was extended 10 kilobases upstream of the annotated transcription start site and downstream at the end
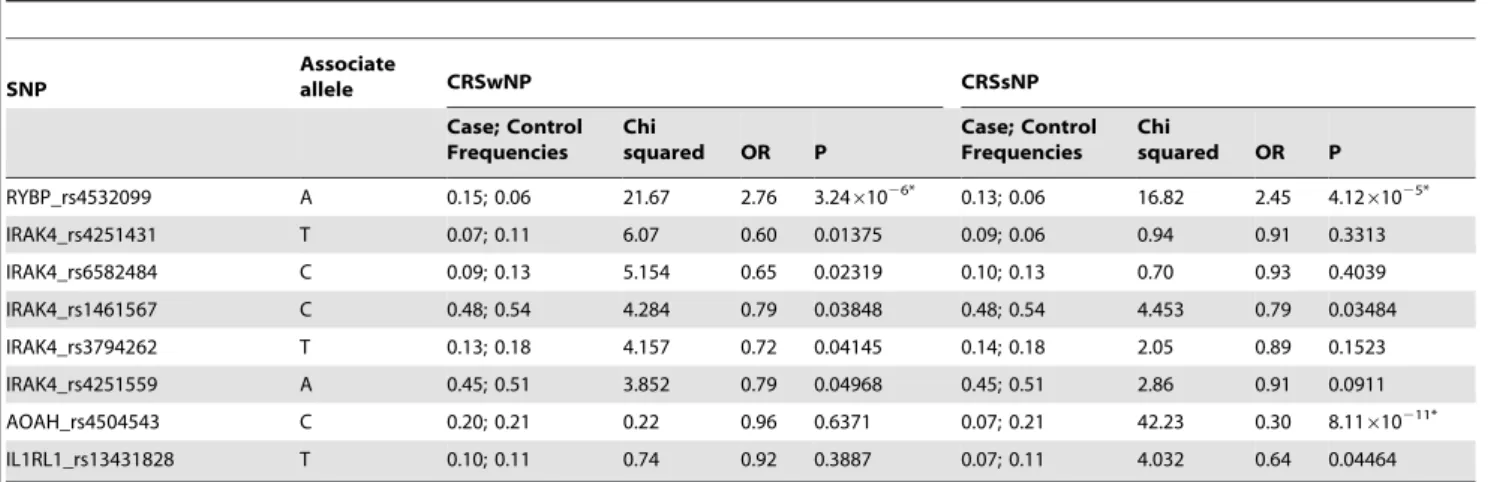
of the last IRAK4 exon. The SNPs were selected to extract the most genetic information based on CHB haplotype data using the HAPMAP database (Hapmap Data Rel 27 Phase II+III, Feb09) [20]. From this dataset, 34 SNPs in IRAK4 gene region were selected using a pairwise tagging algorithm implemented in Haploview version 4.1 program [21]. In addition, when we set Hardy-Weinberg p value cutoff, minor allele frequency and r2 thresholds at 0.01, 0.05 and 0.8, respectively, the LD pattern for IRAK4 gene in our population showed strong LD in several groups of SNPs, indicating that the SNPs in each group represent a common region (Figure 1). Consequently, we choose 10 SNPs including rs4251513, rs1461567, rs3794262, rs4251481, rs4251540, rs4251569, rs6582484, rs4251431, rs1870765 and rs12302873 to represent the entire 34 loci for eventual genotyping and the former two SNPs were composed in the selected SNPs from the previous identified associated with CRS in Canadian population. Therefore, 49 SNPs constituted the selection set to be genotyped in our patient and controls eventually.
Genotyping
DNA was collected in EDTA-treated tubes and isolated from peripheral blood leukocytes, using the DNA Isolation Kit for Mammalian Blood (Roche, Indianapolis, USA). Isolated DNA
Table 1.SNPs selected from previous identified associated with CRS in Canadian population.
Chromosome Gene SNP
1 PARS2 rs2873551
IL22RA1 rs4292900; rs4648936; rs16829225 TNFRSF1B rs235214; rs496888; rs652625; rs7550488
2 TRIP12 rs1035833
IL1RL1 rs13431828; rs10204137
IL1A rs17561; rs2856838; rs2048874; rs1800587
3 FAM79B rs13059863
RYBP rs4532099
5 TSLP rs3806932;rs2289276
6 LAMA2 rs2571584
TNFAIP3 rs3757173; rs5029938
7 LAMB1 rs4727695
AOAH rs4504543
MET rs38850
RAC1 rs836479 CACNA2D1 rs6972720
8 KIAA1456 rs11779957
MSRA rs7001821
9 MUSK rs10817091
11 PDGFD rs12574463
12 NOS1 rs1483757
NAV3 rs1726427
IRAK4 rs4251559; rs4251513; rs146567 14 SERPINA1 rs1243168; rs4900229
15 UBE3A rs1557871
20 SLC13A3 rs393990
22 CACNA1I rs3788568
from blood was stored at 4uC prior to use. To reduce genotyping cost, the majority of the selected SNPs were genotyped by the MassArray system (Sequenom) with primers and probes (Table 2) as described. One SNP (rs12302873) which was evaluated by preliminary test unsuitable to be genotyped through MassArray approach were identified by direct sequencing of PCR products of genomic DNA (Table 3). Genotyping was performed without knowledge of the case or control status. A 10% random sample was tested in duplicate by different persons, and the reproducibil-ity was 100%.
Determination of Serum total IgE and Allergen-specific IgE
Serum total and allergen-specific IgE were quantified using Phadiatop test which is based in immunoCap 100 system according to the manufactures’s directions (Pharmacia, Uppsala, Sweden). The allergen-specific IgE phadiatop covered all the common aeroallergens which included Dermatophagoides pter-onyssinus (Der p); Dermatophagoides farinae (Der f); Animal hair; Trees; Grasses; Cereals; Mugwort; Dandelion; Giant ragweed; Chenopodium album; Humulus; Locust; Blatella germanica; Pine; Plantain; Curvularia lunata; Candida albicans; Penicillium notatum; Alternaria tenuis and Aspergillus fumigatu. The CAP classification system divides results into seven categories from 0 to 6. Additional classes are scored as follows: 0.35–0.70 kU/L, class 1; 0.71–3.5 kU/L, class 2; 3.51–7.5 kU/L, class 3; 7.6–17.5 kU/ L, class 4; 17.6–50 kU/L, class 5; 50 kU/L, class 6. The units reported by CAP are in accordance with the defined WHO serum
standard IRP 75/520. For the present analyses, subjects were considered as sensitive to the allergens if the measurement of allergen-specific IgE phadiatop was equal to or above 0.35 kU/L.
Statistical Analyses
PLINK program version v1.02 was used to determine association. The association test is based on comparing allele frequencies between cases and controls using Chi-squared tests (x2). We estimated odds ratios (OR) and 95% confidence intervals
(95% CI) for the effect of polymorphisms on CRS risk. A corrected p-value of,0.05 was considered statistically significant. Bonfer-roni correction over the tested SNPs was performed for multiple adjustments. Subanalysis restricted to the presence of nasal polyps was also performed to examine whether the effect of observed associations within the population differed within the subgroups. Associations between genotype and IgE levels for all patients were assessed using an Anova test, which was performed in the R statistics software version 2.3.1. Haploview 4.1 software was used to generate the linkage disequilibrium (LD) plot.
Results
Population Characteristics
Table 4 provides a summary of the demographic characteristics of the study population. Age and gender were all well-balanced between cases and controls. The cohort of 306 CRSwNP patients had a mean age of 43 years and consisted of slightly more men (58.8%) than women (41.2%), while the 332 CRSsNP individuals Figure 1. Linkage Dysequilibrium (LD) plot for IRAK4.The LD plots were generated by Haploview 4.1. The white horizontal bar below the info track illustrates the location of SNPs on a physical scale. The shade of squares illustrates the strength of pairwise r2 values on a black and white scale where black indicates perfect LD (r2 = 1.00) and white indicates perfect equilibrium (r2 = 0). The r2 LD value is also indicated within each square. Failed SNP and SNPs not in Hardy-Weinberg equilibrium or with low minor allele frequency are not illustrated.
Table 2.Details of the primers used in the screening of SNPs by MassArray.
Gene SNP Primers (59- 39) Extension Primers (59- 39)
PARS2 rs2873551 ACGTTGGATGCAAACCACTTACAAGGTGGG CACGAGTGTCTCACCAA
ACGTTGGATGAATTACTTGCCCTGTGTGCC
IL22RA1 rs4292900 ACGTTGGATGCCTTCCGACTTGCAGAAAAC ACTTGCAGAAAACAGCAATAG
ACGTTGGATGACCACTTGGGATGAATCAGC
rs4648936 ACGTTGGATGCTGGAGTCAGCCTAAGATTG ttggaCCGGTGTGTGCAGCGCGAG ACGTTGGATGCCTTAGGAGATTGTCAAGGG
rs16829225 ACGTTGGATGGATGAAGATTCAGGCTGCTC AGGCTGCTCTCCCATCATTTTG ACGTTGGATGTCCCTTCATTCACACGAAGC
TNFRSF1B rs235214 ACGTTGGATGAAAAGCAAGGTGTTGCCAGG TTGCCAGGCCTGCTAGGCTCAAA
ACGTTGGATGCAAGGAATCAGATTCTCCCC
rs496888 ACGTTGGATGTCTCAAACCCACTGCTTGAC GCCTGGTCTTAGGACAC
ACGTTGGATGATATCCTGACCCCACAGCCT
rs652625 ACGTTGGATGTCTAGTTGTCCCCCACACAC ACACCTCAAGACCAATGGG
ACGTTGGATGATAGGGAAACTGGCAGGAGG
rs7550488 ACGTTGGATGTCCCAGCCTTTAGATTCACC CTGCTCAGCCCAACCTCC
ACGTTGGATGGGGTAGACATCTTTCTGGG
TRIP12 rs1035833 ACGTTGGATGTCGCTGTCCTGTTTTTATGC TCTTCCATTCTTACATGATCT
ACGTTGGATGGATGTGTATCTCAGATTACC
IL1RL1 rs13431828 ACGTTGGATGCGTTGTTGAGATTACTCCAG ggAGATGAGTCACTGGCATAC
ACGTTGGATGAGAGTATCACCAACTGCCTC
IL1A rs17561 ACGTTGGATGTCACATTGCTCAGGAAGCTA ATTGCTCAGGAAGCTAAAAGGTG
ACGTTGGATGATCTGCACTTGTGATCATGG
rs1800587 ACGTTGGATGTGGGAGAAAGGAAGGCATGG GGATTTTTACATATGAGCCTTCAATG ACGTTGGATGGGCCACAGGAATTATAAAAGC
FAM79B rs13059863 ACGTTGGATGTGATTGACAGGAGTCATGGG GGTGAGTGGTTAAGGATAG
ACGTTGGATGACTGGCACTATGTTAAACAC
LAMA2 rs2571584 ACGTTGGATGTAAATCTGGGCAGTTGAGGG CAGTTGAGGGATTGCTTTTAACAGAA
ACGTTGGATGTAGTATCTATATCCCCTGTC
TNFAIP3 rs3757173 ACGTTGGATGTCAGATGGAAAGAGATGGGC GGCAGTAGGAAGATTTTAAACAAA
ACGTTGGATGAGAGTCAGGCAAGCAAAAAG
rs5029938 ACGTTGGATGCTCTTGTGAAATGAGGGCAG ATGAGGGCAGTAAGTGAT
ACGTTGGATGGCCTTCACCAGCAAATCAAG
LAMB1 rs4727695 ACGTTGGATGTCCCTACTGTTCCATTTCTC CCATTTCTCTTTATTTCCATCTC
ACGTTGGATGTATTCTCACCACTGAGCCAC
MET rs38850 ACGTTGGATGGGCTACTACACTTAACCATT ACACTTAACCATTATGTAACTTC
ACGTTGGATGCCTGAGATGCAGAAGGTGTT
RAC1 rs836479 ACGTTGGATGTGGGTTTGGTTTGTTTCCCG CGCCTTCCTCCTTGTGC
ACGTTGGATGCTGCCACACCAGCAAATGTC
KIAA1456 rs11779957 ACGTTGGATGCATAATCACAACTTAAAGGC TTAAAGGCAAAAGTAGTACTC
ACGTTGGATGGGCTCTCAGCAGGAAAATAC
MSRA rs7001821 ACGTTGGATGTGAGTTGATCGATCTAGAGG TCTAGAGGTTAATGTATTATAAAGAA
ACGTTGGATGACTCACCAGCCTCCATAATC
MUSK rs10817091 ACGTTGGATGCTATTCAAAACCTATTGTC TATTTTGCATTATATACTTAATGCT
ACGTTGGATGTCTGCTAGTATTGAATCCTC
PDGFD rs12574463 ACGTTGGATGAGGAGAGTGATGCCAAACAG AGTTTACATCCAAACTATAGAGG
ACGTTGGATGATGGTGCAGTCTCATAACTC
NOS1 rs1483757 ACGTTGGATGCAACTGAGCTGATTCTCTGG ggTGGGGTTGAAATTGACTTCC
ACGTTGGATGAAAGGGACACTAGGCAAGAG
NAV3 rs1726427 ACGTTGGATGATCTATGACTTGCACAGGAG tCACAGGAGTTGTGTAGC
had a mean age of 39 years and also consisted of slightly more men (57.2%). For the 315 healthy controls mean age were 36, with 53.7% men and 46.3% women. 14.1% and 17.8% individuals were atoptic as demonstrated serum Phadiatop positive results in CRSwNP and CRSsNP cases respectively. All the subjects lived in an urbainised region the north of China and the majority of each study group belonged to Beijing and Hebei.
Association Analysis
Allele frequencies for all 49 SNPs were calculated and the significant associations between alleles and CRS phenotype were shown in Table 5. The significant associations (P,0.05) only existed among the genes coding RYBP (rs4532099), AOAH (rs4504543) and IRAK4 (rs1461567, rs4251559 and rs3794262) genes at 5 loci. Table 5 shows odds ratios for risk allele and the corresponding P values. Two SNPs respectively in RYBP (rs4532099, P = 2.15E–06, OR = 2.59) and AOAH (rs4504543, P = 0.0001152, OR = 0.58) remained significant following appli-cation of the Bonferroni correction for multiple testing for 49 simultaneous tests (P,0.001).
As for the subgroup analysis for the presence of nasal polyps (CRSwNP and CRSsNP) displayed significant association in CRSwNP cohorts regarding to one SNP in RYBP (rs4532099) and 5 SNPs IRAK4 (rs4252431, 6582484, rs1461567, rs4251559 and rs3794262) (Table 6). Among the six SNPs, only rs4532099 in RYBP (P = 3.24E–006, OR = 2.76) remained significant following
application of the Bonferroni multiple testing (P,0.001). Likewise, we detected evidence of association in the CRSsNP subgroups in terms of 4 SNPs (AOAH_rs4504543, RYBP_rs4532099, IR-AK4_rs1461567 and IL1RL1_rs13431828) as well (Table 6), while only rs4504543 in AOAH (P = 8.11E–011, OR = 0.30) and rs4532099 in RYBP (P = 4.12E–005, OR = 2.45) remained significant following application of the multiple adjustment (P,0.001).
In order to verify potential association between total serum IgE levels and the selected SNPs, a quantitative trait analysis was performed. As presented in Table 7, 3 SNPs (IL1A_rs17561, P = 0.005778; IL1A_rs1800587, P = 0.009561; IR-AK4_rs4251513, P = 0.03837) were associated with serum total IgE level.
Discussion
In this study, we replicate a number of genes in CRS in previously identified in Caucasians in a Han Chinese population. Genes associated with CRS and the Caucasian population has not yet being replicated in the Chinese population and the replication suggests a common basis.
The genes identified are of potential important biological significance. RYBP is a regulator of transcription [12]. ILIRL1 is associated with Toll-like receptor (TLR) signaling regulation [14]. SNPs in the IL1RL1 gene have previously been shown to affect
Table 2.Cont.
Gene SNP Primers (59- 39) Extension Primers (59- 39)
IRAK4 rs4251559 ACGTTGGATGGATACAGTTGGTGGTACAGG GGTACAGGCAATAAGTAAAACA
ACGTTGGATGCCTGTTGCCCCTTTCTTTAG
SERPINA1 rs1243168 ACGTTGGATGTGCCTGTGAATAATCCAACC ATAATCCAACCAAGAGCAACACAAA
ACGTTGGATGACAGATGACCTCAAACACCC
rs4900229 ACGTTGGATGAGAACTCCTCACCCAGCAGA GGGTTGTGCAGAGAGGCT
ACGTTGGATGACGAGAAGCCCTGAGAGTG
UBE3A rs1557871 ACGTTGGATGCCCACACCTGCATCAAAATC AATCTTGCAGTGCCTATTAA
ACGTTGGATGAGTCCCAAGTCTTTTCTCCC
SLC13A3 rs393990 ACGTTGGATGTAGGTGGAGCTGCTCTATTC ggaCCAGTGAAAATAATTGTCATG
ACGTTGGATGTGTCCTGCATGTGGAGTTTC
CACNA1I rs3788568 ACGTTGGATGCTTATGCCTGACATGGCACC gCACTCGGGGGAGATGGAC
ACGTTGGATGGCCTTCAGAACAAAGAGACC
doi:10.1371/journal.pone.0039247.t002
Table 3.Details of the primers used in the screening of SNPs by PCR resequencing.
Gene SNP Primers (forward) Primers (reverse)
IL1RL1 rs10204137 CCCCTCAGATCACTCACAAT AGCCAGCTAGGAGAAGTCAG
IL1A rs2856838 TGGGACTGCTATTCTTACAC CTTTCCAATTAGTTCCCTCT
rs2048874; TGTGGAGGGGCAGTCATA ACCAACACCAGCAGTATA
RYBP rs4532099 CTGTGAAGGTGGAAATACTGT GAAATGTCAAGAAGTTTACGG
AOAH rs4504543 CAACATCAGCCTACAGAA CTTTCTCCTTTCTTACCA
CACNA2D1 rs6972720 ACTACTGGTTTCCTTGCTCC CCTTCCTTCCAGTACATCTCAA
IRAK4 rs12302873 AATGTGGCATACACCTACC GTTGTGAATAGTTGGAGGC
serum level of eosinophilia and IgE in other models [5]. IRAK4 also is implicated as a signaling intermediate in the TLR signaling pathway [16,22], and SNPs in the IRAK4 gene have been documented to have a functional impact, within genotype specific effect on serum IgE level [6,23].
Importance of TLR signaling is suggested by their role in detecting and regulating responses to positive and gram-negative bacteria. Importance of IRAK-4 is suggested by the description of an enhanced susceptibility to infection with gram-positive bacteria in IRAK-4 deficient children. Previous work from the Desrosiers group has identified altered function of the TLR signaling system as key to the pathogenesis of CRS. Using complex-model analysis of pooling-based genome wide association testing on the Canadian population with CRS, they identified the polymorphisms at multiple levels of the TLR signaling cascade all confer an increased risk in CRS [2,16]. Functional support for this concept has been provided by in vitro model of epithelial cell culture documenting reduced response to TLR agonists in epithelial cells from CRS patients [24].
The identification of the AOAH gene is also of potential significant interest and suggests a novel mechanism for the development of CRS, again implicating an innate immune signaling, but in a novel fashion. AOAH is responsible for degrading lipopolysaccharide (LPS), and dysfunctional AOAH gene function leads to decreased LPS degradation with unopposed continued LPS stimulation via a TLR-4 dependant mechanism. In AOAH knockout mouse models, persistent inflammation following LPS stimulation is observed [25–27]. Supporting a role in airway disease, the AOAH gene has previously been implicated in a genome wide scan for asthma [13]. Corresponding to the above literature, here we presented that rs4504543 in the AOAH gene
played a protective role (OR = 0.58) in CRS with a strong P value (P = 0.0001152). Moreover, the AOAH_ rs4504543 loci was also revealed as a protective factor (OR = 0.30) in CRSsNP group with a stronger P value (P = 8.11–011), indicating that AOAH gene might exert a crucial protective role in the development of CRS. RYBP is a zinc finger protein with an essential role during embryonic development, which binds transcriptional factors, polycomb products, and mediators of apoptosis, suggesting roles in apparently,unrelated functions. Gene products of the RYBP gene inhibit ubiquitination and subsequent degradation of TP53, and, by interacting with MDM2, play a role in regulating transcription of TP53 target genes and promoting apoptosis. Recent findings have also suggested that RYBP may also play a role in epigenetic regulation [28], and contribute to defense against retroviruses [29]. It is thus possible that polymorphisms in the RYBP gene may be implicated in CRS by dysregulating TP53 activity in TP53 or in its target genes via alteration of RYBP gene products or by altering binding at regulatory binding sites in the RYBP gene promotor area, which contains binding sites for the following transcription factors (YY1, IRF-1, C/EBPA, GATA-1, POU2F1). This may contribute to the inflammation observed in CRS, promoting epithelial dysfunction with secondary bacterial colonization. In contrast to AOAH gene, polymorphisms in the RYBP gene was exhibited here for the first time as a significantly risk factor of CRS and either of the subgroups (CRSsNP and CRSwNP) with high OR values (ORCRS= 2.59; ORCRSsNP= 2.76; ORCRSwNP= 2.45), suggesting the variation of rs4532099 in RYBP could increase the risk of CRS development. IL1RL1 gene is involved in regulation of TLR signaling and has recently also been implicated as the receptor for IL-33. Its role in
Table 5.Single nucleotide polymorphisms associated with chronic rhinosinusitis.
SNP Associate allele Case; Control Frequencies Chi squared OR P
RYBP_rs4532099 A 0.14; 0.06 22.46 2.59 2.1561026*
AOAH_rs4504543 C 0.13; 0.21 14.87 0.58 0.0001152*
IRAK4_rs1461567 C 0.48; 0.54 5.36 0.79 0.0206
IRAK4_rs4251559 A 0.45; 0.51 5.331 0.79 0.02095
IRAK4_rs3794262 T 0.14; 0.18 4.998 0.73 0.02538
SNP: Single nucleotide polymorphisms; OR: Odd ratio; P: p-value. *: P value remains significant after Bonferroni correction. doi:10.1371/journal.pone.0039247.t005
Table 4.Demographic characteristics of the study population.
Characteristic CRSwNP (n = 306) P (vs. Controls) CRSsNP (n = 332) P (vs. Controls) Controls (n = 315)
Age Mean (Range) (years) 43616 (7–77) 0.0618 39616 (7–77) 0.0506 36615 (3–78)
Sex, No.(%) of Male 180 (58.8) 0.194 190 (57.2) 0.360 169 (53.7)
Total IgE, kU/l 120.26211.4 0.0006* 112.16277.1 0.0183* 57.46111.9
Serum phadiatop+, No.(%) 43 (14.1) 0.200#
59 (17.8) -
-Living city, No.(%)
Beijing 217 (70.9) 233 (70.2) 146 (57.0)
Hebei 25 (8.2) 29 (8.7) 19 (19.2)
Others 64 (20.9) 70 (21.1) 46 (23.8)
*: P value,0.05
#
development of CRS may be via interfering with TLR signaling, or via an alteration of IL-33 homeostasis.
Taken overall, all of the replicated genes have disparate functions, but evidence supports that dysfunctions in each these genes may conceivably contribute to development of CRS, underlining the concept that CRS represents a common morpho-logical appearance of clinical disease as an endpoint of multiple unique pathogenic mechasnisms.
Our study has obvious limitations. First and most noticeable is the small group size of the sample used. Nevertheless, given the limited number of genes genotyped in this replication study, feet corrected P value remains significant and, in the case of the RYBP
and AOAH genes, is highly significant with a high odds ratio. A second consideration is the ethnic variability in SNP frequency known for these genes. It is clear that in this study, we have replicated SNPs associated in a Caucasian population, and have not performed extensive fine mapping studies of the gene which might identify other risk SNPs. As shown in this example however, when gene coverage is adjusted to better reflect tagging SNP selection for the CHB data set, we are able to identify significant polymorphisms within the IRAK4 gene.
We replicate several genes which were proved to be associated with CRS in Caucasian population in a Chinese population, suggesting a common basis to the development of the disease in both population types. Of interest is the potential implication of inflammatory pathways, suggesting dysfunction in TLR signaling as a critical element in chronic rhinosinusitis. While these studies have significant limitations, they nevertheless offer a basis for further exploration of a role for immune signaling and response to bacteria in further studies of CRS.
Author Contributions
Conceived and designed the experiments: YZ MD L. Zhang. Performed the experiments: YZ LE AF MD L. Zhao. Analyzed the data: YZ LE AF L. Zhao MD DH L. Zhang. Contributed reagents/materials/analysis tools: YZ LE AF L. Zhao MD DH L. Zhang. Wrote the paper: YZ MD L. Zhang.
References
1. Tewfik MA, Bosse Y, Al-Shemari H, Desrosiers M (2010) Genetics of chronic rhinosinusitis: a primer. J Otolaryngol Head Neck Surg 39: 62–68. 2. Mfuna-Endam L, Zhang Y, Desrosiers MY (2011) Genetics of rhinosinusitis.
Curr Allergy Asthma Rep 11: 236–246.
3. Castano R, Bosse Y, Endam LM, Filali-Mouhim A, Desrosiers M (2010) c-MET pathway involvement in chronic rhinosinusitis: a genetic association analysis. Otolaryngol Head Neck Surg 142: 665–671 e661–662.
4. Tournas A, Mfuna L, Bosse Y, Filali-Mouhim A, Grenier JP, et al. (2010) A pooling-based genome-wide association study implicates the p73 gene in chronic rhinosinusitis. J Otolaryngol Head Neck Surg 39: 188–195.
5. Castano R, Bosse Y, Endam LM, Desrosiers M (2009) Evidence of association of interleukin-1 receptor-like 1 gene polymorphisms with chronic rhinosinusitis. Am J Rhinol Allergy 23: 377–384.
6. Tewfik MA, Bosse Y, Lemire M, Hudson TJ, Vallee-Smejda S, et al. (2009) Polymorphisms in interleukin-1 receptor-associated kinase 4 are associated with total serum IgE. Allergy 64: 746–753.
7. Mfuna Endam L, Cormier C, Bosse Y, Filali-Mouhim A, Desrosiers M (2010) Association of IL1A, IL1B, and TNF gene polymorphisms with chronic
rhinosinusitis with and without nasal polyposis: A replication study. Arch Otolaryngol Head Neck Surg 136: 187–192.
8. Nader ME, Abou-Jaoude P, Cabaluna M, Desrosiers M (2010) Using Response to a Standardized Treatment to Identify Phenotypes for Genetic Studies of Chronic Rhinosinusitis. Journal of Otolaryngology-Head & Neck Surgery 39: 69–75.
9. Kilty SJ, Bosse Y, Cormier C, Endam LM, Desrosiers MY (2010) Polymorphisms in the SERPINA1 (Alpha-1-Antitrypsin) gene are associated with severe chronic rhinosinusitis unresponsive to medical therapy. Am J Rhinol Allergy 24: e4–9.
10. Zhang Y, Endam LM, Filali-Mouhim A, Bosse Y, Castano R, et al. (2011) Polymorphisms in the nitric oxide synthase 1 gene are associated with severe chronic rhinosinusitis. Am J Rhinol Allergy 25: e49–54.
11. Lee L, Campagna DR, Pinkus JL, Mulhern H, Wyatt TA, et al. (2008) Primary ciliary dyskinesia in mice lacking the novel ciliary protein Pcdp1. Mol Cell Biol 28: 949–957.
12. Bejarano F, Gonzalez I, Vidal M, Busturia A (2005) The Drosophila RYBP gene functions as a Polycomb-dependent transcriptional repressor. Mech Dev 122: 1118–1129.
Table 6.Single nucleotide polymorphisms associated with subgroups of CRSwNP and CRSsNP.
SNP
Associate
allele CRSwNP CRSsNP
Case; Control Frequencies
Chi
squared OR P
Case; Control Frequencies
Chi
squared OR P
RYBP_rs4532099 A 0.15; 0.06 21.67 2.76 3.2461026* 0.13; 0.06 16.82 2.45 4.1261025*
IRAK4_rs4251431 T 0.07; 0.11 6.07 0.60 0.01375 0.09; 0.06 0.94 0.91 0.3313
IRAK4_rs6582484 C 0.09; 0.13 5.154 0.65 0.02319 0.10; 0.13 0.70 0.93 0.4039
IRAK4_rs1461567 C 0.48; 0.54 4.284 0.79 0.03848 0.48; 0.54 4.453 0.79 0.03484
IRAK4_rs3794262 T 0.13; 0.18 4.157 0.72 0.04145 0.14; 0.18 2.05 0.89 0.1523
IRAK4_rs4251559 A 0.45; 0.51 3.852 0.79 0.04968 0.45; 0.51 2.86 0.91 0.0911
AOAH_rs4504543 C 0.20; 0.21 0.22 0.96 0.6371 0.07; 0.21 42.23 0.30 8.11610211*
IL1RL1_rs13431828 T 0.10; 0.11 0.74 0.92 0.3887 0.07; 0.11 4.032 0.64 0.04464
SNP: Single nucleotide polymorphisms; OR: Odd ratio; P: p-value. *: P value remains significant after Bonferroni correction. doi:10.1371/journal.pone.0039247.t006
Table 7.Association between polymorphisms and IgE levels.
SNP BETA SE R2 T P
IL1A_rs17561 58.15 21 0.01112 2.769 0.005778 IL1A_rs1800587 54.14 20.84 0.009762 2.599 0.009561 IRAK4_rs4251513 31.54 15.21 0.004459 2.074 0.03837
BETA: regression coefficient; SE: standard error; R2: regression r-squared; T: Wald test (based on t-distribtion); P: p-value.
13. Barnes KC, Grant A, Gao P, Baltadjieva D, Berg T, et al. (2006) Polymorphisms in the novel gene acyloxyacyl hydroxylase (AOAH) are associated with asthma and associated phenotypes. J Allergy Clin Immunol 118: 70–77.
14. Savenije OE, Kerkhof M, Reijmerink NE, Brunekreef B, de Jongste JC, et al. (2011) Interleukin-1 receptor-like 1 polymorphisms are associated with serum IL1RL1-a, eosinophils, and asthma in childhood. J Allergy Clin Immunol 127: 750–756 e751–755.
15. Kim TW, Staschke K, Bulek K, Yao J, Peters K, et al. (2007) A critical role for IRAK4 kinase activity in Toll-like receptor-mediated innate immunity. J Exp Med 204: 1025–1036.
16. Bosse Y, Bacot F, Montpetit A, Rung J, Qu HQ, et al. (2009) Identification of susceptibility genes for complex diseases using pooling-based genome-wide association scans. Hum Genet 125: 305–318.
17. Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, et al. (2004) Rhinosinusitis: Establishing definitions for clinical research and patient care. Otolaryngol Head Neck Surg 131: S1–62.
18. Endam LM, Bosse Y, Filali-Mouhim A, Cormier C, Boisvert P, et al. (2009) Polymorphisms in the interleukin-22 receptor alpha-1 gene are associated with severe chronic rhinosinusitis. Otolaryngol Head Neck Surg 140: 741–747. 19. Cormier C, Bosse Y, Mfuna L, Hudson TJ, Desrosiers M (2009) Polymorphisms
in the tumour necrosis factor alpha-induced protein 3 (TNFAIP3) gene are associated with chronic rhinosinusitis. J Otolaryngol Head Neck Surg 38: 133– 141.
20. (2005) A haplotype map of the human genome. Nature 437: 1299–1320. 21. Barrett JC, Fry B, Maller J, Daly MJ (2005) Haploview: analysis and
visualization of LD and haplotype maps. Bioinformatics 21: 263–265.
22. Suzuki N, Saito T (2006) IRAK-4–a shared NF-kappaB activator in innate and acquired immunity. Trends Immunol 27: 566–572.
23. Zhang Y, Lin X, Desrosiers M, Zhang W, Meng N, et al. (2011) Association pattern of interleukin-1 receptor-associated kinase-4 gene polymorphisms with allergic rhinitis in a Han Chinese population. PLoS One 6: e21769. 24. C Divoy, S Rousseau, J Berube, L. Mfuna Endam, A Filali-Mouhim, et al. (2011)
Epithelial Cells From Chronic Rhinosinusitis Patients Are Hyporesponsive To Bacteria: Evidence Of Innate Immune Dysfunction In CRS. J Allergy Clin Immunol: AB2–AB3.
25. Shao B, Lu M, Katz SC, Varley AW, Hardwick J, et al. (2007) A host lipase detoxifies bacterial lipopolysaccharides in the liver and spleen. J Biol Chem 282: 13726–13735.
26. Ojogun N, Kuang TY, Shao B, Greaves DR, Munford RS, et al. (2009) Overproduction of acyloxyacyl hydrolase by macrophages and dendritic cells prevents prolonged reactions to bacterial lipopolysaccharide in vivo. J Infect Dis 200: 1685–1693.
27. Lu M, Varley AW, Ohta S, Hardwick J, Munford RS (2008) Host inactivation of bacterial lipopolysaccharide prevents prolonged tolerance following gram-negative bacterial infection. Cell Host Microbe 4: 293–302.
28. Tavares L, Dimitrova E, Oxley D, Webster J, Poot R, et al. (2012) RYBP-PRC1 complexes mediate H2A ubiquitylation at polycomb target sites independently of PRC2 and H3K27me3. Cell 148: 664–678.