Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2
Does protein binding modulate the effect of
angiotensin II receptor antagonists?
Marc P Maillard, Catherine Centeno, Åsa Frostell-Karlsson,* Hans R Brunner, Michel Burnier
Keywords: AT1-receptor antagonists, protein binding, pharmacokinetic Division of Hypertension and Vascular Medicine, Lausanne University Hospital, CH-1011 Lausanne, Switzerland
*Biacore AB,
S-754 50 Uppsala, Sweden
Correspondence to: Dr Marc P Maillard Division of Hypertension and Vascular Medicine, CHUV,
CH-1011 Lausanne, Switzerland Tel: +41 21 314 0750 Fax: +41 21 314 0761 E-mail: Marc.Maillard@ chuv.hospvd.ch
JRAAS2001;2 (suppl 1):S54-S58
Abstract
Introduction
Angiotensin II AT1-receptor antagonists are highly
bound to plasma proteins (≥99%). With some antagonists, such as DuP-532, the protein binding was such that no efficacy of the drug could be demon-strated clinically. Whether protein binding interferes with the efficacy of other antagonists is not known. We have therefore investigated in vitrohow plasma
proteins may affect the antagonistic effect of different AT1-receptor antagonists.
Methods
A radio-receptor binding assay was used to analyse the interaction between proteins and the ability of various angiotensin II (Ang II) antagonists to block
AT1-receptors. In addition, the Biacore technology, a
new technique which enables the real-time monitoring of binding events between two molecules, was used to evaluate the dissociation rate constants of five
AT1-receptor antagonists from human serum albumin.
Results
The in vitroAT1-antagonistic effects of different
Ang II receptor antagonists were differentially affected by the presence of human plasma, with rightward shifts of the IC50ranging from one to several orders of
magnitude. The importance of the shift correlates with the dissociation rate constants of these drugs from albumin. Our experiments also show that the way that AT1-receptor antagonists bind to proteins
differs from one compound to another. These results suggest that the interaction with plasma proteins appears to modulate the efficacy of some Ang II antagonists.
Conclusion
Although the high binding level of Ang II receptor antagonist to plasma proteins appears to be a feature common to this class of compounds, the kinetics and characteristics of this binding is of great importance. With some antagonists, protein binding interferes markedly with their efficacy to block AT1-receptors.
Introduction
In recent years, several specific, orally-active, non-peptide angiotensin II (Ang II) receptor antagonists have been developed and have become available clinically. These antagonists share a common mecha-nism of action, that of blockade of Ang II AT1
-recep-tors.Yet, the various antagonists differ in their
phar-macological profiles and these differences might sometimes affect their efficacy profile.1,2 One
common feature of all members of the Ang II recep-tor antagonist class is their high binding to plasma proteins. Indeed, all antagonists are generally bound to plasma proteins, mainly albumin and α1-acid
glycoprotein (AGP), by more than 99%.1 This high
affinity for proteins appears to be species-specific and may have important implications when extrap-olating animal results to humans.3 On the other
hand, the estimation of protein binding is not straightforward; thus a clear discrimination between 99 and 99.5 or 99.9% binding cannot be established precisely by conventional methods, such as ultrafil-tration or equilibrium dialysis.In addition,these mea-sures never clearly indicate the functional influence of this binding to the pharmacological activity of the drug. An extreme example of the potential interfer-ence of protein binding with the efficacy of Ang II receptor antagonist is illustrated by the develop-ment of DuP-532.When DuP-532, a potent and selec-tive AT1-receptor antagonist, was administered for
the first time to normotensive subjects in order to assess its ability to blunt the pressor responses to exogenous Ang II, no antagonism could be demon-strated.4Yet the plasma concentrations of DuP-532
measured in these volunteers were much greater than those of losartan/EXP-3174. In addition, the compound was found to be a very potent antihy-pertensive agent in animals5and
in vitro, DuP-532 and EXP-3174 (losartan's active metabolite) had similar AT1-receptor antagonistic activity.4,5
Following these initial observations, the develop-ment of DuP-532 was stopped.
The data obtained with DuP-532 suggested that the balance between the specific binding of the drug to the AT1-receptor and its association with non-specific binding sites,i.e.,plasma proteins,could play an important role in determining the pharma-cological response to the drug. In some extreme sit-uations, illustrated by DuP-532, the balance is such that the antagonist loses its capacity to bind to its specific receptor.6
The goal of the present experiments was there-fore to evaluate in vitrohow the binding to plasma proteins may affect the degree of Ang II receptor blockade induced by various AT1-receptor antago-nists in humans.
Methods
Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2
a radio-receptor assay developed recently in our laboratory.7 In this assay, the binding of
radiola-belled Ang II to a rat smooth muscle cell mem-brane preparation, expressing solely AT1
-recep-tors, is measured. To investigate the influence of proteins on the displacement of labelled Ang II by the pharmacological compounds, 6.25% human plasma was added to the binding buffer to obtain a final protein concentration of 0.4%, which approximately corresponds in vitro, to the same proportion of plasma proteins encountered in vivo in presence of ten to hundreds ng/ml of drug, which are concentrations generally achieved at peak during antihypertensive treatment. In sub-sequent studies, increasing concentrations of com-pounds known to interact strongly with protein binding, e.g. digitoxin, warfarin, diazepam, and disopyramide, were added to the binding buffer in order to characterise the nature of the binding sites to which AT1-receptor antagonists are linked.
Finally, the Biacore technology, Uppsala, Sweden (BIAtechnology) has been used to investi-gate the interaction between a set of Ang II recep-tor antagonists and immobilised human serum albumin (HSA). This technique enables the real-time monitoring of the binding events between two or more molecules without the use of labels. In addition, molecules do not need to be purified. The BIAtechnology relies on the phenomenon of surface plasmon resonance (SPR), which occurs when surface plasmon waves are excited at a metal/liquid interface. Light is directed at, and is reflected from, the side of the surface not in contact with sample, and SPR causes a reduction
in the reflected light intensity at a specific combi-nation of angle and wavelength. The binding events cause changes in the refractive index at the surface layer, which are detected as changes in the SPR signal. In our experiments, the intensity of the signal obtained from the interaction of drugs with the HSA-coated surface was correlated with the HSA-binding level. Drugs were distributed among three groups corresponding to high, medium, or low HSA-binding, based on the injection of the drug at 80 µM concentration. Binding to AGP was also investigated using the same method.Warfarin was used as a positive control. This method has been used to estimate the kinetics of the interac-tion of five Ang II receptor antagonists with HSA, and, in particular, to characterise their dissociation rate constant (KD) to HSA. Details of the exact
methodology used in these latter experiments will be published elsewhere.
Results
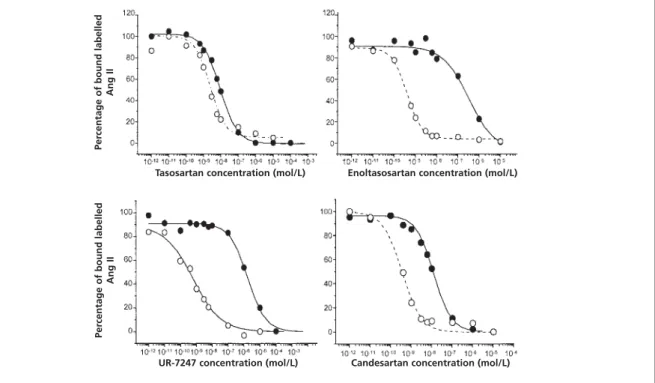
Figure 1 shows the effect of four different Ang II receptor antagonists on the binding of Ang II to AT1
-receptors, in the presence and the absence of pro-teins. The addition of human plasma induces a sig-nificant rightward shift of the displacement curve and hence an increase in the 50% inhibition constant (IC50) of some, but not all, angiotensin antagonists.
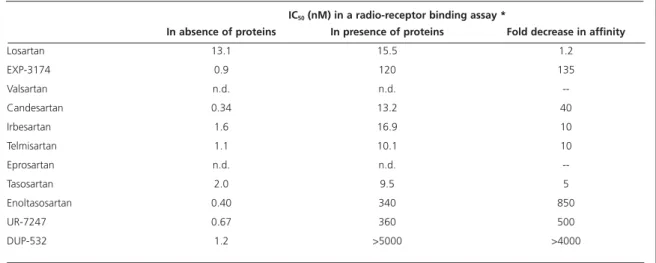
The addition of protein has no effect on the binding of Ang II itself. Table 1 summarises the protein-induced changes in IC50obtained with all
antagonists tested in our laboratory.
With antagonists like losartan, tasosartan, irbe-sartan and telmiirbe-sartan, the duration of incubation
Figure 1 Displacement of specific labelled angiotensin II binding in rat vascular smooth muscle cell preparations by different angiotensin II receptor antagonists in the absence () or in the presence (●) of 6.25% of human plasma, corresponding to 0.4% of proteins.The IC50values are summarised in Table 1. Experimental conditions: ligand: 12.5 pM 125
I-angiotensin II; binding buffer:Tris-HCl 50 mM + MgCl25 mM (pH 7.2). Each curve is the average of 2–3 experiments
performed in duplicate.
Per
centage of bound labelled
Ang II
Per
centage of bound labelled
Ang II
Tasosartan concentration (mol/L)
UR-7247 concentration (mol/L)
Enoltasosartan concentration (mol/L)
Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2 Supplement 1
does not affect the influence of proteins on the dis-placement curve (data not shown). However, with some other antagonists like candesartan, enolta-sosartan or UR-7247, the rightward shift effect of proteins is attenuated when the incubation time is increased. Thus, as shown in Figure 2 for candesar-tan, this shift is maximal after 1 hour of incubation and tends to decrease when the incubation time is prolonged. This suggests that the release of can-desartan from plasma proteins occurs slowly, gradu-ally increasing the free concentration of the drug, which in turn may compete more effectively, accord-ing to the law of mass action, with labelled Ang II at the AT1-receptor, as indicated by the progressive
decrease observed in IC50.
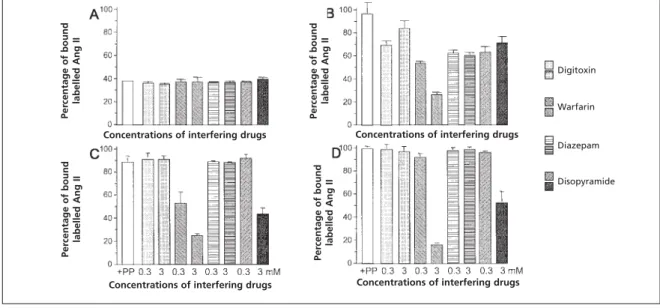
To investigate the nature of the protein binding sites, additional experiments were con-ducted in which digitoxin, warfarin, diazepam or disopyramide were added to the angiotensin binding assay. As expected, none of these com-pounds modified the curves produced by losartan, tasosartan, irbesartan or telmisartan, since the addition of protein had little, if any effect with
these agents. However, warfarin and diazepam interfered significantly and dose-dependently with the displacement of radiolabelled Ang II-induced by candesartan or UR-7247.We can thus make the hypothesis, that in these conditions, warfarin and diazepam displaced the antagonists from their non-specific protein binding sites I and II of albumin, thereby increasing their ability to block AT1-receptors (Figure 3). Using the same type of
experiments, we found that enoltasosartan binds to digitoxin, warfarin and diazepam, as well as to disopyramide-sensitive protein binding sites. Interestingly, the addition of high concentrations of either digitoxin, warfarin, diazepam or disopy-ramide does not interfere with the binding of labelled Ang II to the AT1-receptor.
The BIAtechnology allowed confirmation that all AT1-receptor antagonists are high-level HSA binders,
but showed considerable differences in AGP binding (data not shown).The albumin dissociation rate con-stants (KD) of five Ang II receptor antagonists and warfarin are presented in Table 2. Interestingly, a good relationship was found between the impor-tance of the protein-induced rightward shift of the displacement curve (characterised by the change in IC50) and the KD for albumin measured with the
BIAtechnology (Figure 4).
Figure 2 Influence of the incubation time to the kinetic of the displacement of labelled angiotensin II by candesartan. Each experiment was performed in duplicate. Same experimental conditions as in Figure 1.
Table 2 Dissociation rate constants (KD) of some
angiotensin II receptor antagonists from human serum albumin. (Warfarin has been used as positive control).
Drugs KD[µM]
Losartan 75±10
Tasosartan 45±5
Candesartan 27±2
UR-7247 20±2
Enoltasosartan 12±1
Warfarin 22
Table 1 Influence of the presence of proteins on the affinity for AT1-receptor in radio-receptor assay.
IC50(nM) in a radio-receptor binding assay *
* ligand: 5 fmol 125I-angiotensin II; binding buffer: Tris HCl 50 mM + MgCl
25 mM; +/- 6.25% human plasma; 1-hour incubation at 37oC.
In absence of proteins In presence of proteins Fold decrease in affinity
Losartan 13.1 15.5 1.2
EXP-3174 0.9 120 135
Valsartan n.d. n.d.
--Candesartan 0.34 13.2 40
Irbesartan 1.6 16.9 10
Telmisartan 1.1 10.1 10
Eprosartan n.d. n.d.
--Tasosartan 2.0 9.5 5
Enoltasosartan 0.40 340 850
UR-7247 0.67 360 500
DUP-532 1.2 >5000 >4000
Per
centage of bound labelled
Ang II
Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2
Discussion
Like many other drugs, Ang II receptor antagonists are highly protein-bound.1 Clinically, protein
binding has been claimed to be responsible for the occurrence of drug interactions between many classes of therapeutics agents, but it is rarely con-sidered a crucial pharmacological characteristic which may affect the efficacy of the drug. However, the observations made with DuP-532 have demonstrated that, in some conditions, protein binding may become an important limit-ing factor in therapeutic efficacy. The purpose of the present in vitroexperiments was therefore to further examine how plasma proteins may
modu-late the receptor blockade induced by various Ang II antagonists. Our observations show that the addition of proteins has little, if any, effect on the ability of some antagonists such as losartan, tasosartan, irbesartan or telmisartan to block AT1
-receptors, whereas proteins interfere markedly, at the receptor level, with the antagonistic activity of other compounds such as DuP-532, enoltasosar-tan, UR-7247 or even candesartan.
In recent years, we have conducted several clinical studies to assess the pharmacological properties of several orally-active Ang II antago-nists such as DuP-532,4 UR-7247,8 tasosartan,9
enoltasosartan,9 telmisartan and candesartan. In
Figure 4 Relationship between the affinity of Ang II receptor antagonists for plasma proteins, characterised by the dissociation rate constant from human serum albumin, and the changes in IC50induced by the addition of proteins to the
binding buffer when performing the displacement curves for various angiotensin II receptor antagonists.Inset:
Semi-logarithmic representation of the same data.
Figure 3 Influence of increasing concentrations of digitoxin (dotted boxes), warfarin (downward hatching boxes), diazepam (lined boxes), or disopyramide (upward hatching boxes) on blockade of the AT1-receptor by different angiotensin
II receptor antagonists in the presence of plasma proteins. (A) 10 nM tasosartan reduced bound labelled angiotensin II by 60%; digitoxin, warfarin, diazepam, or disopyramide have no influence on this displacement. (B) 10 nM enoltasosartan had little effect on the bound labelled angiotensin II. However, addition of all displacing agents enhanced the displacement of 125
I-angiotensin II in a dose-dependent manner. Displacements of bound labelled angiotensin II by 10 nM candesartan (C) or, 10 nM UR-7247 (D) were weak, but easily enhanced in the presence of warfarin or diazepam.
Per
centage of bound labelled Ang II
Concentrations of interfering drugs Concentrations of interfering drugs Concentrations of interfering drugs Concentrations of interfering drugs
Per
centage of bound labelled Ang II
Per
centage of bound labelled Ang II
Per
centage of bound labelled Ang II
Digitoxin
Warfarin
Diazepam
Journal of the Renin- Angiotensin-Aldosterone System
(Including other peptidergic systems)
March 2001 Volume 2 Supplement 1
these studies, we found that some compounds with a high in vitroaffinity for AT1-receptors, such
as DuP-532 or enoltasosartan, produced either markedly delayed or no pharmacological respons-es in humans, drespons-espite the fact that high plasma drug levels were achieved. Based on these obser-vations, we have hypothesised that these com-pounds have a high affinity for both AT1-receptors
and for non-specific protein-binding sites. Thus, with these agents there is direct competition between their ability to bind with specific and non-specific binding sites. Depending on the balance between these two possibilities the com-pounds are more or less effective in blocking AT1
-receptors.
As observed clinically, in our radio-receptor assay, the AT1antagonistic effects of Ang II receptor antagonists were differently affected by the addi-tion of human plasma. The presence of proteins shifted the concentration-response curves to the right, thus increasing the IC50.This shift was rather small for losartan, tasosartan, irbesartan and telmis-artan; a little more significant with candesartan, while the presence of plasma drastically increased the IC50of UR-7247, enoltasosartan and DUP-532.
To demonstrate that, in spite of the fact that all antagonists are highly protein-bound to a similar extent (>99%), the precise mechanisms may differ, additional experiments were performed with drugs known to interfere more or less specifically with some protein binding sites. Thus, warfarin interferes with site I, diazepam with site II and dig-itoxin with site III of albumin,10 while
disopyra-mide interferes with the AGP binding site.11 With
this approach, we have been able to show that enoltasosartan is markedly and dose-dependently displaced from proteins when warfarin, diazepam, digitoxin and disopyramide are added to the binding buffer. In contrast, only albumin sites I and II were involved in the protein binding of candesartan and UR-7247, whereas other Ang II receptor antagonists (losartan, tasosartan, irbesar-tan and telmisarirbesar-tan) were not significantly affect-ed by the presence of these compounds, even at very high pharmacological concentrations.
The BIAtechnology allowed us to confirm that all AT1-antagonists bind at a high level with albumin, but show considerable differences in AGP binding. However, the main information brought by this technique was the determination of the dissociation rate constants (KD) to HSA of five antagonists, chosen for their different behav-iour and affinity for the AT1-receptor in the pres-ence of proteins. The wide range of the KD
mea-sured in these experiments confirms that there are qualitative differences between the various antagonists in their binding to albumin. A low KD, as observed with enoltasosartan, is indicative of the presence of more stable and long-lasting AT1 -receptor antagonist/albumin complexes than it is the case for losartan. Interestingly, these experi-ments also confirmed the observation that the
AT1-receptor antagonists showing the greatest affinity for albumin are those that are bound through several binding sites, and that these agents are the most affected in vitro by the pres-ence of plasma proteins.
Taken together, these results suggest that the qualitative interaction between AT1-receptor antagonists and proteins, and not necessarily the quantitative aspect of the binding, is an important determinant which may affect the efficacy of these drugs. A detailed characterisation of the interaction of Ang II antagonists with proteins may be of interest to improve our understanding of the peculiar pharmacodynamic behaviour of some of these agents.
The interaction of proteins and AT1-receptor
antagonists only represents one parameter among others involved in the general pharmacological action of the drugs. Thus, the bioavailability and the volume of distribution,12 together with the
intrinsic functional inhibitory characteristics of the drug, i.e. dissociation constants from the AT1
-receptor, insurmountable antagonism, etc,13 are
other parameters which determine efficacy. It is therefore difficult to evaluate the relative contri-bution of protein binding to the differences between antagonists.
References
1. Burnier M, Brunner HR. Angiotensin II receptor antago-nist. Lancet2000;355:637-45.
2. Mazzolai L, Maillard M, Rossat J, Nussberger J, Brunner HR, Burnier M. Angiotensin II receptor blockade in normotensive subjects: A direct comparison of three AT1receptor
antago-nists.Hypertension1999;33:850-5.
3. Csajka C, Buclin T, Brunner HR, Biollaz J. Pharmacokinetic-pharmacodynamic profile of angiotensin II receptor antago-nists.Clin Pharmacokinet1997;32:1-29.
4. Goldberg MR, Lo MW, Christ DD et al. DuP-532, an angiotensin II receptor antagonist: first administration and com-parison with losartan.Clin Pharmacol Ther1997;61:59-69.
5. Wong PC, Hart SD, Chiu AT et al. Pharmacology of DuP-532, a selective and noncompetitive AT1receptor antagonist.
J Pharmacol Exp Ther1991;259:861-70.
6. Chiu AT, Carini DJ, Duncia JV et al. DUP-532: a second gen-eration of nonpeptide angiotensin II receptor antagonists.
Biochem Biophys Res Comm1991;177(1):209-17.
7. Maillard M, Mazzolai L, Daven V et al. Assessment of angiotensin II-receptor blockade in humans using a standard-ised angiotensin II-receptor binding assay.Am J Hypertens
1999;12:1201-8.
8. Maillard M, Rossat J, Nussberger J et al. Pharmacological profile of UR-7247, an orally active angiotensin II receptor antagonist in healthy volunteers. J Cardiovasc Pharmacol
2000;35:383-9.
9. Maillard M, Rossat J, Brunner HR, Burnier M. Tasosartan, enoltasosartan, and angiotensin II receptor blockade, the con-founding role of protein binding. J Pharmacol Exp Ther
2000;295:649-54.
10. Sjöholm I, Ekman B, Kober A, Ljungstedt-Pahlman I, Seiving B, Sjödin T. Binding of drugs to human serum albumin XI: the specificity of three binding sites as studied with albumin immo-bilized in microparticles.Mol Pharmacol1979;16:767-77. 11. Lama JJ, Haughey DB. Disopyramide binding to serum protein in man and animals.Drug Metab Disposit1981;9:582-3.
12. Israili ZH. Clinical pharmacokinetics of angiotensin II (AT1) receptor blockers in hypertension.J Hum Hypertens
2000;14:S73-S86.
13. Morsing P,Adler G, Brandt-Eliasson U et al. Mechanistic dif-ferences of various AT1-receptor blockers in isolated vessels of