Vol-7, Special Issue-Number5-July, 2016, pp965-974 http://www.bipublication.com
Research Article
Permeability of methane, carbon dioxide, oxygen and nitrogen gases using a
composite membrane of polyethersulfone/polyamide 11
Bahreini Habib, Ameri Elham* and Bahreini Ahmad
1 Department of Chemical Engineering, Faculty of Engineering,
Shahreza Branch, Islamic Azad University, P.O. Box 311-86145, Shahreza, Iran.
*Corresponding author: Email: ameri@iaush.ac.ir, Tel: +98-9132885803
ABSTRACT
With the expansion of the refining industry, processing and transportation of gas in Iran, it is of high importance to use new technologies of gas separation. Membrane methods, in particular, separation process through polymeric membranes have been growingly used in recent years. The results of multiple studies show that polyether sulfone compounds have a very good performance in manufacturing polymeric membranes in the area of gas separation. However, efforts are ongoing to improve the permeability and selectivity properties of the membranes. This study investigated separation of methane, carbon dioxide, oxygen and nitrogen gases using composite membranes including, polyethersulfone/ polyamide 11 as the polymer. The membrane was made by molding solution with a composition of 9% to 1% polyethersulfone/polyamide -11. Morphology and structure of the membrane made were examined by Fourier infrared transform (FTIR) and scanning electron microscopy (SEM). The level of permeability of the membranes was measured by a fixed volume-variable pressure. Overall results indicated that with 6 time increase of pressure, the amount of permeability of carbon dioxide reduced and then increased. Selectivity for dual gases of carbon dioxide- nitrogen increased by the increase in pressure and maximum amount of pressure is 11/187 at 8 times of increase. The selectivity of carbon dioxide- nitrogen has the greatest value among dual gases.
Key words:- membrane, composite, gas separation, polyamide-11, polyether sulfone, permeability
[I] INTRODUCTION
Many operations of chemical engineering are concerned with separation and change in concentration of solutions and mixtures. Mixture of gases is separated to extract one or more components from the mixture in pure form, and it is widely used both at small or large scale. There are four main techniques for gas separation including, absorption, adsorption, cryogenic distillation, and membranes where economy of process determines the selection of each of the methods. In membranous technique, membrane is placed against path of gaseous mixture and separation operation is done due to difference in velocity of passing various gases through the
membrane. Paradigm of gas separation dated back to one hundred years ago but gas separation techniques have been utilized and spread only 35-40 years ago. Thomas Graham measured intensity of permeability for gases. He was the first one who described solution- permeation model for polymeric membranes and his researches about porous membranes led to record Graham’s Law of Effusion [1].
it. As a result, membrane permeability was increased by the increased percent of zirconium nanoparticles [2].
Kapantaidakis et al. (1995) examined gas separation through composite alloyed membrane from two polymers of polysulfone and polyimide (PSF/PI). They obtained intensity of permeation of gases (H2, He, CO2, N2, and O2) for a series of
mixed and asymmetric membranes (PSF/PI). They concluded that gas permeability coefficient varied uniformly among permeability coefficient of the two pure polymers for gases such as (H2,
He, N2, O2) that lack mutual effect on polymer
matrix. In the case of CO2 that interacts with polyimide, permeability is slightly reduced compared to PSF and pure PI. They came to the result that alloyed membranes e.g. (PSF/PI) possessed satisfactory permeability properties that led to lower costs and create stability and resistance against chemical effects and ambient temperature. Similarly, they can provide tolerance against softening gases [3].
Pechar et al. (2002) explored membrane matrix of fluorine-content polyimide and zeolite mixture. They initially prepared membrane matrix composed of fluorine-content polyimide with 20% weight zeolite. They computed and analyzed permeability data for gases of He, CH4,
CO2, N2, and O2. The researchers concluded that
solubility coefficient has increased for all gases except N2 component that remained unchanged
because of the large-size of the gas. Permeability decreased in gases of He, CH4, and CO2 while
permeability increased in N2 and O2 gases. They
concluded that any change in rate of permeability was extremely influenced by variance of permeability coefficient [4].
Kim et al. (2007) prepared a new nanocomposite membrane based on polysulfone including Single-Walled Carbon Nanotubes and analyzed their properties. They treated carbon nanotubes by long chains of alkylamine agents and thereby improved diffusivity of nanotubes in polymer. They found that under atmospheric pressure of 4,
both permeability and diffusion increased by the increased weight fraction of nanotubes. Using SWNTs (abbreviated form of Single-Walled Nanotubes) with agents, they made octadecyl ammonium (ODA) and polysulfone as polymer matrix. The adjusted nanotubes were diffused well in polymer matrix and chloroform. Using the results of FE-SEM, which were the images of cross-section of membrane, they found that when 5% weight percent of nanotube was loaded, the adjusted nanotube were diffused well in polymer matrix while if 15% of weight was loaded in nanotube, the tubes were placed in two fields: 1. good regional diffusion; and 2. accumulated or concentrated diffusion. They found from result of DMTA analysis that there was surface area among SWNTs and polymer matrix that might be composed of polymer partial components.
However, efficiency of permeability of
membrane will more depend on the addition of nanotubes than the surface region. Moreover, rising permeability and diffusion in all tests of gas denotes the fact that the high permeability may be provided by the help of nanotubes, which result from tunnels that will be created by increase in nanotubes in polymer matrix. Kim et al. clearly found that this type of membranes might be most efficient when carbon nanotubes to be utilized with diameter smaller than 10 Angstrom in polymer matrix. They found that it required using composition of separation based on size and high permeability to overcome Rabson’s upper bound [5].
Ismail et al. (2008) examined a membrane composed of polyethersulfone/matrimid-5218 for gas separation. After making the composite membrane of flat plate from PES- PI, they explored permeability and selectivity for N2 and
O2 gases. They also analyzed the effects of
membrane. The results showed that as quantity of zeolite is added in the composite membrane, permeability increased for both gases and maximum permeability was obtained for the composed membrane with 50% weight of zeolite. Adding 30% weight of zeolite to pure membrane, selectivity of O2 increased more than N2. Ismail
et al. concluded that addition of zeolite particles to the composite membrane PES-PI would noticeably impact on the structure and properties of gas separation [6].
Ahn et al. (2008) made membrane matrix composed of polysulfone with silica nanoparticle fillers. The composite membrane matrix is employed for improving efficiency of gas separation based on which polymeric membranes are filled by mineral particles. They prepared polysulfone composite membrane matrix as a glass polymer by adding non-porous silica nanoparticles. Then, they explored the effect of the addition of fillers and large blank volume of glass polymers on the properties of gas separation. Addition of silica nanoparticles noticeably increased permeability and such a rise in the level of permeability was directly related to the quantity of silica contents; in other words, permeability was improved by increasing the silica contents. Moreover, they found that following to increase in silica nanoparticles, permeability of big gases would increase further and this results from the increase in blank volume. Rise of blank volume extremely increases effusion or diffusivity and permeability that consequently reduces selectivity. Finally, addition of silica nanoparticles will not comprehensively improve membranes. Ahn et al. found that the noticeable point was in that non-porous silica nanoparticles have caused rupture in polymeric chains and increased blank volume in glass polymers with small blank volume. Therefore, relative permeability in glass polymers with small blank volume is remarkably greater than in glass polymers with large blank volume [7].
Dorosti et al. (2011) prepared membrane matrix composed of polysulfone/polyimide that was filled by zeolite particles and explored performance of gas separation in them. They prepared membranes using soluble foundry technique and employed these membranes to determine rate of permeation intensity of CH4,
CO2, N2, and O2 in pure polymers and composite
membranes. They made different composite matrix membranes by mixing with polysulfone and polyimide filled by zeolite and analyzed the effect of loading of zeolite and ratio of two polymers on properties for permeability of membranes. The polymers were miscible and were composed of fully homogenous matrix. The researchers measured the minimum permeability among all of their tested gases and the maximum selectivity between the composed membranes with polysulfone/polyimide 50-50 ratio. The results indicated that compared to simple membranes, the composite matrix membranes could exhibit higher permeability and more acceptable selectivity. They found this point by repeated making of membranes with 20% weight of zeolite that the membranes possessed completely different properties and this event caused by the formation of uncontrollable blank pores. Dorosti et al. also found this point from the results of TGA analysis that the membranes, which were loaded by more amount of zeolite and more polyimide in their unit, possessed higher thermal resistance and this was due to the high thermal resistance in zeolite and polyimide [8].
Rafiq et al. (2011) developed and improved the
composite matric membrane using fuzzy
inversion. They loaded mineral silica
nanoparticles inside asymmetric membrane
composed of polysulfone and Polyimide
results of SEM from cross- sections suggested that accumulation and density of materials were visible in loading silica (20% weight). They
utilized acryloxypropyl trimethoxysilane
(APTMOS) as connection agent that caused adaptation among mineral and non-mineral phases. Conducting TGA test, they concluded that rise of silica contents would increase thermal stability in membranes. This finding was also confirmed by Differential Scanning Calorimtery (DSC) that indicated only one (Tg) for the
improved composite. In loadings 5.2% and 20% weight of silica, they obtained Tg = 4.2˚C and Tg
= 25˚C, respectively. They achieved the
maximum rate of permeability among
membranes at loading of silica with 20.1% weight. Therefore, they concluded that as weight percent was added in silica, permeability rate
was increased. They achieved maximum
selectivity in loading 15.2% weight for silica and at 2-10bar pressure. Rafiq et al. came to the result that the maximum selectivity was related to the membrane with 15.2% of weight from silica fillers under feeding pressure 2-10bar that again led to the formation of polymeric chains in order to be packed better and thus they might. reduce possible softening of membrane [9]. The present research is intended to separate pure gases using composite membrane of polyamide
and polyethersulfone. The unknown and
ambiguous aspects of above problem such as factor of separation of pure gases and effect of variable pressure on permeability of pure gases by the given polymeric membrane in vitro are some questions and unclear points, which are analyzed by conducting integrated tests.
[II] MATERIALS AND METHODS 2.1 Raw materials
The needed polyethersulfone (Ultrason E6020P, BASF) with molecular weight 58000g/mol was purchased from German Ludwigshafen Company and used as the main part of the membrane
Figure (1) shows the formula of general structure of the polymer.
Fig (1): Molecular structure of polyethersulfone
Polyethersulfone is a polymer with high efficiency and very similar to poly carbonate. The polar nature of polyethersulfone has provided unique and well-known properties to the polymer. One can refer to some properties such as hardness and strength, resistance against most of chemical substances, solvents, and its high temperature.
The needed polyamide-11 with molecular weight 1.05g/cm3 was prepared from German Merck Company. Figure (2) shows the formula of general structure of the polymer.
Fig (2): Molecular structure of polyamide-11
Dimethyl formamide is a solvent with chemical formula C3H7NO. Figure (3) shows molecular
Fig (3): Molecular structure of dimethyl formamide solvent.
2.2. Production method of polyethersulfone/ polyamide membrane
The given polymeric membranes are prepared by thermal fuzzy inversion technique. To make this membrane, the solution (10% weight) of a polymer is first prepared by solving 1g of each of both polymers of polyethersulfone/polyamide with ratios of 9.1 in 9g of dimethyl formaldehyde solution at temperature 50˚C under pressure 14.7psig on mixer heater for 24 hours. At the next step, the prepared solution is inserted on a clean glass plate (petri dish). The prepared membrane is dried in usual oven at 30˚C for 24h. Finally, the membranes are cleaned from solution under vacuum condition at ambient temperature for 4 hours. Thickness of the membrane was measured by a micrometer ranged from 60 to 70µm.
2.3.Devices and techniques for identifying the structure of the membrane
Fourier Infrared Spectrometry technique was employed to examine the membrane structure. Infrared optometric spectrum is widely utilized to identify organic compounds. The bipolar momentum of the molecule should be totally changed as a result of rotary motion and vibration because infrared radiation is absorbed by a molecule. If radiation frequency is the same as the natural vibratory frequency of the molecule, energy is transferred and this leads to a change in amplitude of molecular vibration. This action results in absorption of radiation. When varieties of molecules with similar nuclei such as oxygen and nitrogen vibrates and/or rotates, no
general change takes place in their bipolar momentum and therefore the compounds do not absorb infrared radiation. The built membranes are analyzed by Fourier infrared spectrometry device (JASCO FT/IR-6300 made in Japan) that is present in University of Isfahan within range of 400cm-1- 4000cm-1. SEM test was adapted to
observe morphology of cross-section in
membranes in this study. SEM device (Model: EM3200 made by KYKY Company) was utilized to conduct this test. Membrane samples were initially broken at cross-section in liquid nitrogen and then the studied surface was coated by a very thin layer of gold.
2.4. Gas permeability test with the help of membrane
In order to analyze effect of gas separation in the built membranes, permeability properties of pure gas should be determined including, oxygen, nitrogen, methane, and carbon dioxide. If the appropriate conditions are selected for execution of tests, properties of pure gases such as permeability coefficient and ideal selectivity may be assumed as criteria for efficiency of membrane in separation of mixture of gases. Delay-time method is the most common technique for testing permeation of gases in polymeric membranes [10]. In this method, gas permeation in polymer is measured and analyzed from zero-point time to the point when polymer permeation velocity approaches to a fixed number (constant). The constant pressure technique is employed to examine the amount of permeated gas through membrane and record discharge of passing gas. In constant pressure technique, ambient pressure is supposed as lower pressure and variance of volume of passing gas through the membrane is recorded.
2.5. Description of gas permeation test device
2. Regulator to adjust pressure of gas flow from cylinder includes two pressure gauges at scale (0-300bar) that indicates pressure of gas in cylinder and second pressured gauge indicates output pressure from regulator at scale (0-50bar), in which rotation of pressured regulator valve was adjusted at 2-8bar for testing.
1. Pipe and joints
2. Heater and thermostat with potential of temperature adjustment is utilized in glassy chamber in the device for regulation of temperature of input gas flow to membrane chamber, in which temperature is dropped due to reduction of pressure at output of regulator (Joule- Thomson Law).
3. Stainless steel chamber as a container for polymeric membrane and riveted value of gas flow for air release from upper part of container and receiving pure gas flow at top of membrane.
4. Graded U-shape tube is connected from one side to ambient atmosphere and from the other end to lower part of membrane container where the U-shape part is filled by water.
Fig (4): A schemata from membrane testing device 2.6. Measurement technique of gases permeation through membrane
Primarily, gas enters from upper point of membrane, and discharge rate of passed gas through membrane is measured at lower point.
Fig (5): Variance curve for quantity of gas flux from a
polymeric membrane based on delay time [10].
The time ratio of discharge of the gas passing through the membrane is obtained by connecting lower space to water column and measuring the changes in water column in the constant pressure technique. One can measure polymer diffusion and permeation coefficients for the studied gas by measurement of quantity of the gas passing through membrane in respective time. If the value of the gas passage is drawn in respective time, a similar chart will result in as Figure (5). Delay time is related to diffusion coefficient by the following formula [11]:
D l
6 2
(1)where θ denotes delay time (s), l is the membrane diameter (cm) and D expresses gas diffusion coefficient in the membrane (cm2/s). Discharge rate of passing gas will be obtained per time (Q) by measurement of gradient at static mode. Then, gas permeation coefficient in polymer is derived from the following equation:
p p
AL Q p
. .
2 1
(2)
where P: Gas permeation coefficient in polymer cm3 (STP).cm/cm2.S.cmHg
Q: Discharge of passing gas through membrane cm3 (STP)/S
L: membrane thickness (cm)
P1 and P2: Upper and lower gas pressure (cm. Hg); and A: Membrane cross-section (cm2) The permeation coefficient unit is expressed in SI- system as follows:
1barrer=10-10 cm3 (STP).cm/(cm2.S.cmHg) (3) Term STP in unit denotes volume of gas at standard temperature and pressure equivalent to 273.15 k and 760 torr.
One mole of any gas possesses 22.4 lit volume at standard temperature and pressure. Solubility coefficient of the studied gases is derived by determination of diffusion coefficient and permeation coefficient of gas in membrane using well-known solubility- diffusion formula.
D P
S (4)
S denotes coefficient of gas solubility in polymer. The ideal selectivity of membrane versus studied gases will be achieved by dividing
permeation coefficient of gases through
calculation of permeation coefficient for various gases. For example, after measurement of permeation coefficient for the two gases in membrane for both O2 and N2 gases, ideal selectivity of membrane will be calculated in relation to the two gases by following equation.
2 2
N O
P P
(5)[III] RESULTS
The properties of pure membranes and composite have been analyzed by Scanning Electron Microscopy (SEM), Fourier Infrared Spectrometry Transform, and X-Ray Diffraction (XRD) in this section. The results from measurement of permeation of pure gases CH4,
N2, O2, and CO2 in pressures of 2, 4, 6, and 8bar
and at temperature 30˚C were drawn by diagram. Similarly, the effect of pressure on permeability of carbon dioxide gas was examined in
nanocomposite membrane of
polyethersulfone/polyamide -11.
3.1. Analysis of Fourier infrared transform spectra for pure membrane and composite of polyethersulfone/polyamide 11
Figure 6 indicates Fourier in
frared transform for pure membranes and composites. The existing bonds have been explained in the structural formula of the given composite membrane and place of infrared absorption frequencies related to any bond with respect to spectrometric books and the relevant articles for analysis of the existing peaks in the given spectrum.
Fourier infrared transform spectra indicates pure membrane of polyethersulfone in which the peaks locating within the range 1105-1300 cm-1
Fig (6): FT- IR spectrum for membrane of polyethersulfone/polyamide 11
belong to S=O=S sulfone group, the peaks within the range 1245 cm-1 are related to ether group, and the peaks within the range 1480-1570cm-1 correspond to benzene aromatic ring. Likewise, the peaks within the range 3063-3300cm-1 are related to N-H and the peaks locating within the range 1100cm-1 belong to C-O in polyamide membrane. The peaks locating in the range 1450cm-1 belong to C-H.
Fig (7): Image of SEM in cross section of
polyethersulfone/polyamide-11 with thickness 65.60µ
The morphology of the composite membrane was studied by SEM test. Figures of SEM indicate images with zooms of 500 and 50’000, respectively. Figure 7 shows thickness of the built membrane (65.60µ). Moreover, a visible point in Figure 8 is that a very favorable adaptation is observed among polymer network so that no free space has been created in polymer grid and membrane surface lacks any porosity and hole.
Fig (8): Image of SEM in cross section of polyethersulfone/ polyamide with zoom of 50000
3.3. Gas permeation
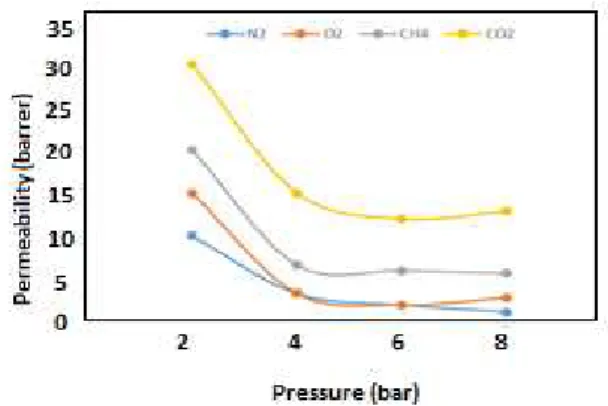
Permeability of gases (carbon dioxide, methane, nitrogen and oxygen) was measured by fixed
volume-variable pressure technique at
temperature 30˚C and under pressure 2, 4, 6, and 8bar for all of the built membranes. With respect to the existing values in the diagrams, it could be generally found that the order of permeability in gases is as follows:
2 2 4
2 CH O N
CO P P P
P (6)
Fig (9): Permeability of gases of nitrogen, oxygen, methane, and carbon dioxide in membranes of polyethersulfone/polyamide under various pressures and at temperature 30˚C
As anticipated, permeability of carbon dioxide is highly different from other studied gases. The difference results from high liquefaction potential in carbon dioxide along with the given small synthetic diameter. The carbon dioxide possesses this property that causes softening of membrane and opens polymeric chains from each other. On the other hand, glass polymers separate gases based on their molecular size. According to Figure 9, synthetic diameter in nitrogen is smaller than in methane; therefore, permeation of nitrogen should be greater than methane. Alternately, methane possesses higher liquefaction potential than nitrogen and although separation is done based on synthetic diameters in glass polymers, liquefaction potential factor in gas is also efficient. Thus, it leads to a greater permeation of methane compared to nitrogen and
oxygen in composite membrane of
polyethersulfone/polyamide-11.
The liquefaction potential rate of the gas is a determinant factor in solution-diffusion process. Therefore, high liquefaction potential in methane increases permeability of the gas compared to nitrogen.
3.4. Effect of pressure on permeability of carbon dioxide gas
gases under pressure in glass polymer. Langmuir adsorption corresponds to entrapment and solution of gas molecules in the prepared sites. Thus, less driving force (impetus) is necessary for adsorption in the areas.
Thus, it is easily done under lower pressures in
comparison to Henry adsorption. Henry
adsorption comprises of diffusion and solubility of gas molecules between polymer chains, is more difficult than adsorption in Langmuir areas and needs more impetus. According to the partial statics theory, the gas molecules adsorbed in the Langmuir areas have lower diffusion coefficient than in Henry sites. Therefore, the diffusion coefficient is lower under smaller pressures because adsorption has occurred mainly in Langmuir sites. However, diffusion coefficient increases with the increased pressure and greater adsorption in Henry sites. Since permeability coefficient is the product of solubility coefficient by diffusion coefficient and rate of reduction in solubility coefficient under increased pressure is more than the rise of diffusion, permeability coefficient is reduced as the pressure increases. Analysis of diagram indicates the effect of pressure on permeability of carbon dioxide gas in
composite membrane of
polyethersulfone/polyamide 11.
The minimal increase in permeation under pressure levels higher than 6bar stems from the increased interaction between molecules of carbon dioxide gas. In other words, interaction force is reduced between molecules of carbon dioxide gas and membrane by increasing the
pressure in composite membrane of
polyethersulfone /polyamide and softness effect takes place at higher pressure (6bar).
3.5. Analysis of results of ideal selectivity in composite membrane of polyethersulfone /polyamide-11
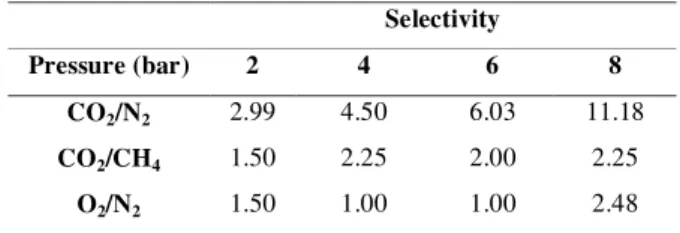
According to Table (1), selectivity of carbon dioxide gas is increased versus nitrogen by rise of pressure in composite membrane of
polyethersulfone/ polyamide and highest quantity of selectivity is 11.187 under 8bar pressure. Moreover, selectivity of carbon dioxide versus methane and oxygen gas versus nitrogen is at the highest level under 8bar pressure.
Table 1. Selectivity of various gases compared to each other under different pressures
Selectivity
8 6
4 2
Pressure (bar)
11.18 6.03
4.50 2.99
CO2/N2
2.25 2.00
2.25 1.50
CO2/CH4
2.48 1.00
1.00 1.50
O2/N2
[IV] CONCLUSION
With regard to the factors affecting rising permeability of gases in polymeric membranes, it could be generally stated that:
1. Results of Infrared Spectra (FT-IR) in
composite membrane of
polyethersulfone/polyamide indicate the
expected bonds in the built membrane.
2. Findings of SEM test denote uniform and without porosity in composite membrane of polyethersulfone/ polyamide-11.
3. Permeability of carbon dioxide is greatly different from the other studied gases. Such a difference is caused by high liquefaction potential of carbon dioxide along with its small synthetic diameter.
4. Permeability of carbon dioxide passes through a descending trend up to 6bar pressure as pressure increases and then it increases under 8bar pressure and higher due to softness effect. Selectivity of carbon dioxide gas versus nitrogen is the highest value among selectivity of other gases and it is 11.187 under 8bar pressure
REFERENCES
1. Madaeni S S (2004) Membranes and
membrane processes, University of Razi Kermanshah, Iran.
3. Kaldis S P, Kapantaidakis G C,
Sakellaropoulos G P (1995) Polymer
membrane conditioning and design for enhanced CO2/N2 separation. Coal. Sci. Technol. 24: 1927-1930.
4. Pechar T W, Tsapatsis M, Eva Marand, E,
Davis R (2002) Preparation and
characterization of a glassy fluorinated polyimide zeolite-mixed matrix membrane. Desalination. 146: 3-9.
5. Park H B, Kim J K, Nam S Y, Lee Y M (2007) Self-Assembly and Crystalline Growth
of Poly(3,4- Ethylenedioxythiophene)
Nanofilms. Adv. Mater. 19: 3501–3506. 6. Kusworo T D, Ismail F, Mustafa A (2015)
Experimental design and response surface modeling of pi/pes-zeolite 4A mixed matrix membrane for CO2 separation. J. Eng. Sci.
Technol. 10: 1116–1130.
7. Ahn J, Chung, W J, Pinnau I, Guiver M D (2008) Polysulfone/silica nanoparticle
mixed-matrix membranes for gas separation. J. Membr. Sci. 314: 123-133.
8. Sadeghi M, Vefaei Manesh A (2009)
Introduction to the membrane and membrane processes, Sepahan Publications.
9. Dorosti F, Omidkhah M R, Pedram M Z,
Moghadam F, (2011) Fabrication and
characterization of polysulfone/polyimide– zeolite mixed matrix membrane for gas separation. Chem. Eng. J. 171: 1469-1476. 10.Sikander R, Zakaria M (2012) Kinetics of
thermal degradation of polysulfone/polyimide blended polymeric membranes. Appl. Polym. 123: 3755–3763.
11.Semsarzadeh M A, Ghalei B (2012)
Characterization and gas permeability of polyurethane and polyvinyl acetate blend
membranes with polyethylene oxide–